Abstract
Cerebral small vessel disease (SVD) is a common global brain disease that causes cognitive impairment, ischemic or hemorrhagic stroke, problems with mobility, and neuropsychiatric symptoms. The brain damage, seen as focal white and deep grey matter lesions on brain magnetic resonance imaging (MRI) or computed tomography (CT), typically accumulates “covertly” and may reach an advanced state before being detected incidentally on brain scanning or causing symptoms. Patients have typically presented to different clinical services or been recruited into research focused on one clinical manifestation, perhaps explaining a lack of awareness, until recently, of the full range and complexity of SVD.
In this review, we discuss the varied clinical presentations, established and emerging risk factors, relationship to SVD features on MRI or CT, and the current state of knowledge on the effectiveness of a wide range of pharmacological and lifestyle interventions. The core message is that effective assessment and clinical management of patients with SVD, as well as future advances in diagnosis, care, and treatment, will require a more “joined-up”’ approach. This approach should integrate clinical expertise in stroke neurology, cognitive, and physical dysfunctions. It requires more clinical trials in order to improve pharmacological interventions, lifestyle and dietary modifications. A deeper understanding of the pathophysiology of SVD is required to steer the identification of novel interventions. An essential prerequisite to accelerating clinical trials is to improve the consistency, and standardization of clinical, cognitive and neuroimaging endpoints.
Keywords: Dementia, Magnetic resonance imaging, Mild cognitive impairment, Risk factors, Small vessel disease, Stroke, Symptoms, Treatment
Introduction
Cerebral small vessel disease (SVD) is a global brain disease affecting multiple clinical domains by disrupting normal function of the perforating cerebral arterioles, capillaries, venules, and brain parenchyma, manifesting on magnetic resonance imaging (MRI) as white matter hyperintensities (WMH), small subcortical infarcts, microinfarcts, lacunes, enlarged perivascular spaces (PVS), microbleeds, superficial siderosis, intracerebral hemorrhage (ICH), and atrophy.[1,2] The core clinical manifestations include lacunar ischemic stroke, intracerebral hemorrhage and cognitive decline, including vascular cognitive impairment and amplification of pathological and cognitive Alzheimer's disease manifestations.[3–5] There is increasing recognition that its multidomain involvement extends beyond stroke and dementia [Figure 1] to include gait and balance dysfunction, behavioral and neuropsychiatric symptoms, and subtle, non-focal neurological features [Figure 2],[6–8] resulting in presentations to diverse general and specialist services [Table 1].
Figure 1.
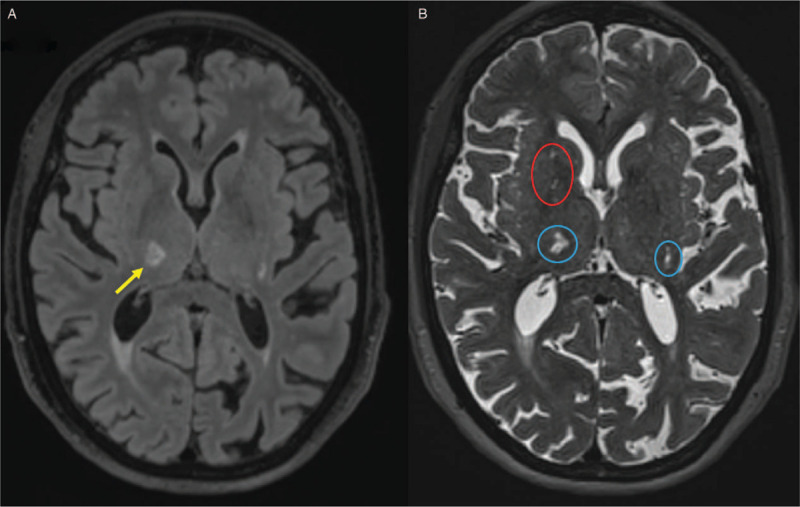
Case vignette. A 75-year-old female presents to the acute medical assessment unit with recurrent falls. She has a past medical history of hypertension, hypercholesterolaemia and recent lacunar stroke 3 months ago. She is an ex-smoker of 40 pack-years. On examination her blood pressure is 169/85 mmHg, without postural hypotension, and pulse is regular. She is objectively apathetic and mildly bradyphrenic. She has a slow, shuffling gait, with preserved arm swing, and is unsteady on initiation and turning. She has mild dysarthria and a left pronator drift. She scores 22/30 on MoCA, with deficits in executive function, attention and abstraction. Her daughter reports progressive cognitive and functional decline over the past 2 years and subtle behavioural changes developing over the past month: she has become apathetic, finds it increasingly difficult to manage her finances, and is easily fatigued performing minor household tasks. Full blood count, urea and electrolytes, and C-reactive protein are normal, serum cholesterol is 5 mmol/L, HbA1c is 40 mmol/mol and electrocardiogram shows sinus rhythm, normal conduction intervals, and mild left ventricular hypertrophy. Brain MRI shows a subacute right thalamic small subcortical infarct, periventricular and deep white matter hyperintensities, enlarged perivascular spaces, and a chronic lacune: (A) Subacute small subcortical infarct in the right thalamus (yellow arrow, on FLAIR) relating to clinical lacunar stroke three months ago and white matter hyperintensities (hyperintense on FLAIR); (B) Lacunes and enlarged perivascular spaces (blue and red circles respectively, hyperintense on T2-weighted imaging. MoCA: Montreal cognitive assessment scale; MRI: Magnetic resonance imaging; FLAIR: Fluid-attenuated inversion recovery.
Figure 2.
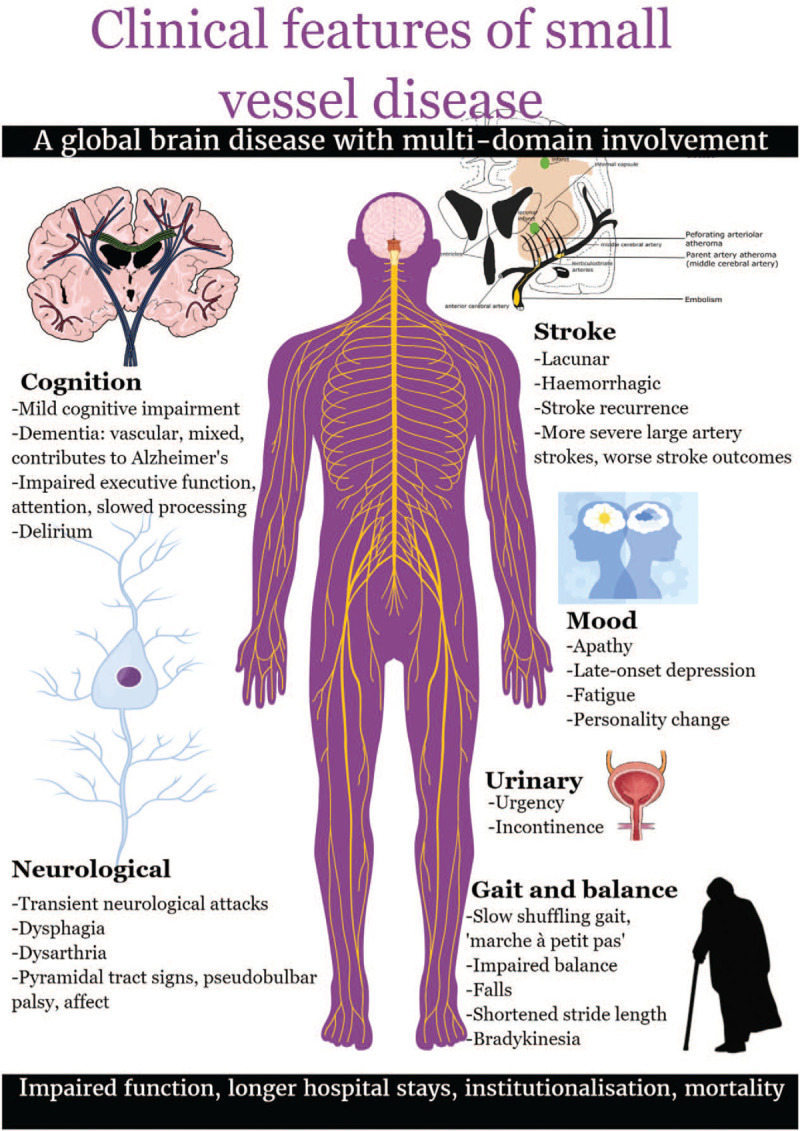
Clinical features of small vessel disease.
Table 1.
Diverse presentations: clinical small vessel disease encounters with general and specialist services.
| Clinical service | Presenting symptoms |
| General practitioner | All below presentations + informant reports of altered behavior, deteriorating cognition and function |
| Physiotherapist | Deteriorating mobility, falls |
| Speech and language therapist | Dysphagia, dysarthria |
| Occupational therapist | Functional decline requiring social support |
| Geriatric medicine service∗ | Inpatient admissions including unexplained falls, gait deterioration, delirium +/– obvious precipitant, stroke, functional and cognitive declineFalls clinicsParkinson's disease clinicsDay hospital and rehabilitationDementia clinicsResidential and nursing home assessments |
| Stroke service∗ | Stroke, transient ischaemic attacks, transient neurological attacks |
| Neurology service∗ | Stroke, transient neurological attacks, cognitive assessment clinics, referrals for declining mobility, Parkinson's disease, pseudobulbar palsy |
| Psychiatry service∗ | Dementia clinics: cognitive impairment diagnosis, management of behavioral and psychological symptoms of dementiaMood and personality changesSevere or persistent unexplained delirium |
| Urology/gynecology | Urinary symptoms |
| Accident and Emergency∗ | Falls, delirium, acute neurological symptoms including stroke |
| Acute medical assessment unit and General internal medicine∗ | Acute neurological symptoms including stroke, falls, delirium |
| Orthopedic serviceOsteoporosis service | Falls resulting in fractures |
Services with readily available access to neuroimaging investigations.
The onset of sporadic SVD typically occurs during mid to late life and although the disease, its associated risk factors, and clinical features such as gait dysfunction and cognitive decline are more prevalent with advancing age, these are not just inevitable consequences of ageing. SVD often arises on a background of other complex comorbidities, and untangling SVD symptoms from those attributable to other conditions requires careful clinical judgment including neuroimaging review. Adopting a more integrated, holistic approach to identifying early and intermediate clinical brain damage markers is essential to permit prognostication, supportive management strategies, identification of patients for emerging treatment trials, and future refinement of targeted prevention and management strategies.
Here we present an evidence-based overview of the literature on clinical aspects of SVD, discussed in the context of our clinical and research experience of caring for these patients.
Methods for Searching, Identifying, Selecting, and synthesizing Data
We searched Ovid MEDLINE using the terms “Cerebral Small Vessel Diseases/” or “White matter hyperintens∗” and “Clinical” from inception to April 3, 2020. We separately searched “Lacunar state” or “Binswanger”. On risk factors for SVD and its progression, we searched Ovid MEDLINE using the terms “Cerebral small vessel disease” OR “White matter hyperintens∗” AND “vascular risk factor” OR “risk factor” AND “disease progress∗” OR “outcome” up to June 5th 2020. On therapeutic approaches to SVD, we searched Ovid MEDLINE using the terms “Cerebral small vessel disease” OR “White matter hyperintense∗” OR “lacunar” OR “vascular cognitive impairment” up to 12th May 2020. We supplemented the electronic search with the authors’ personal files and searched reference lists of identified papers. We screened 2169 papers for clinical diagnosis, 1094 for risk factors and progression, and 7695 for interventions in SVD, including the most relevant papers reporting SVD associations.
Defining the Natural History of Clinical Cerebral Small Vessel Disease
The earliest clinicopathological reports by Binswanger[9] in 1894, based on eight post-mortem cases, described “encephalitis subcorticalis chronica progressiva”, characterized pathologically by pronounced white matter atrophy and cortical thinning and clinically by a progressive, fluctuating course, arising predominantly in males in their 50s, characterized by chronic cognitive and emotional symptoms, and occasionally punctuated by acute hemiplegic episodes.
In 1901, Marie[10] described ‘l’état lacunaire’ or “the lacunar state”, involving one or more lacunes on neuropathology, characterized by progressive neurological decline, episodes of mild hemiparesis, and later, dysarthria, marche à petit pas (gait with little steps), imbalance, incontinence, pseudobulbar signs, and dementia.
Much remains unknown about its precise natural clinical history: the disease is elusive in its early stages unless the patient has overt symptoms that are easily recognized from the current neurological lexicon for stroke or dementia [Figure 3]. Proposed pathophysiological mechanisms underlying SVD are outside the scope of this review but are described in detail elsewhere.[2,11,12] We describe acute and chronic clinical and neuroimaging manifestations at various SVD stages.
Figure 3.
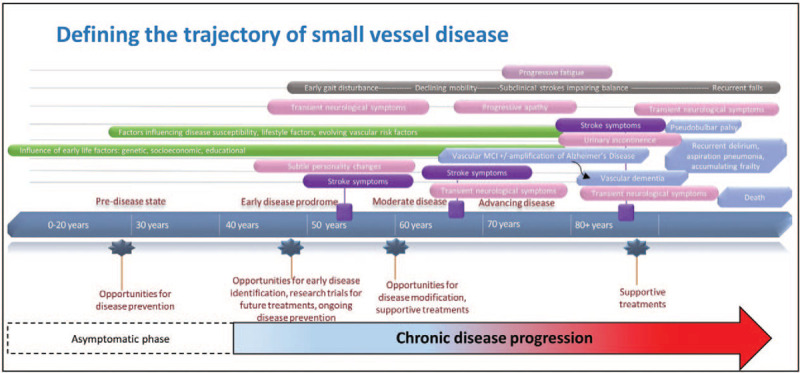
Defining the trajectory of small vessel disease. MCI: Mild cognitive impairment.
Modes of presentation
“Silent” small vessel disease
“Silent” or “covert” SVD refers to disease incidentally detected on neuroimaging without the patient apparently having overt symptoms. While some lesions are truly clinically silent, for instance if small or located in less eloquent regions,[13] careful questioning about historical stroke or transient ischemic attack (TIA) symptoms is recommended, as a positive history may render such individuals eligible for secondary stroke prevention.[14] Furthermore, a comprehensive history and examination, including collateral history from an informant, may yield more subtle, associated features such as apathy, abrupt or insidious cognitive decline, fatigue or gait disturbances that do not necessarily meet diagnostic criteria for stroke or dementia but have been linked temporally with acute lesions on Diffusion-Weighted Imaging (DWI) MRI (n = 6/649 community sample, n = 10/30 vascular dementia population).[7,15] How patients report, and clinicians interpret, these symptoms is poorly understood and inter-individual factors influencing accurate reporting are complex. For instance, a “threshold effect” of sufficient SVD burden might accumulate before triggering symptoms[16] and this might vary between individuals and at different ages [Figure 4]. Similarly, physical reserve is likely to play a role: the fitter an individual, the more compensatory mechanisms can be employed despite accumulating deficits. Whether initially silent infarcts due to SVD are clinically “unmasked” later by increasing SVD burden and/or increasing physical frailty, revealing delayed typical or atypical symptoms, is a target for future research.
Figure 4.
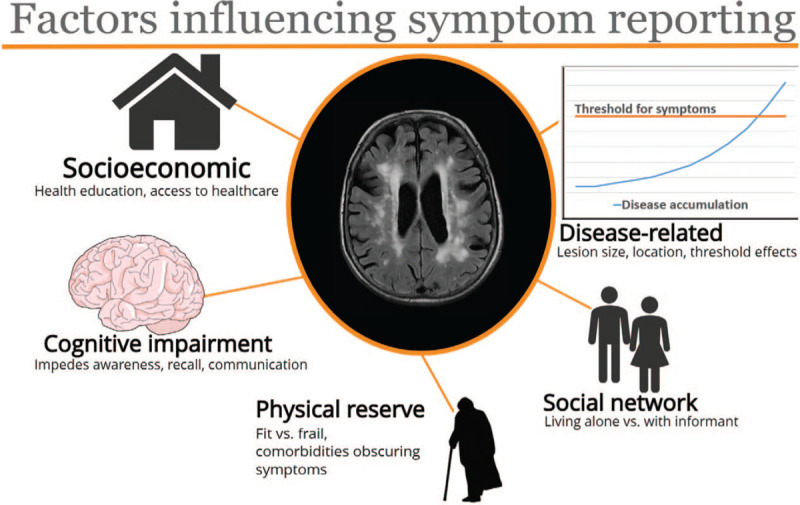
Factors influencing reporting of symptoms related to disease accumulation.
Subtle neurological symptoms
To uncover whether “non-stroke” symptoms may be associated with acute infarcts on brain imaging, some studies have focused on transient neurological attacks (TNAs). Almost one-quarter of TNA patients (n = 13/56) have corresponding DWI hyperintense lesions.[8] Moreover, both TNAs and Transient Focal Neurological Episodes, a subset of TNAs typified by spreading, recurrent, stereotyped episodes and associated with cerebral amyloid angiopathy (CAA),[17] herald a higher risk of future ischemic and hemorrhagic stroke, while TNAs also associate with chronic SVD features and dementia.[4,18,19] Other neurological symptoms associated with SVD include dysphagia,[20] dysarthria,[21] pyramidal tract signs, and pseudobulbar palsy.[22]
Neuropsychiatric symptoms
Neuropsychiatric symptoms are common post-stroke and in individuals with vascular dementia, but whether there is a shared neuroanatomical substrate remain unclear and longitudinal studies are sparse. More severe WMH are associated with apathy, fatigue, and delirium but not subjective memory complaints or anxiety (submitted). There is inadequate evidence to determine whether other symptoms including delusions or emotional lability are associated with SVD due to insufficient data and mixed approaches to symptom assessments.
Future research should target whether emotional liability, delusions, and other neuropsychiatric symptoms relate to disease severity including progression.
Whether depression contributes to, or results from, SVD is unclear. Further pathological, clinical, and imaging relationships need investigation, focusing on interactions with shared vascular risk factors, medications, treatment resistance, neurotransmitter alterations, and associations with cognitive impairment.[23]
Lacunar stroke presentations
Lacunar stroke clinical syndrome (LACS) is a key SVD manifestation.[3] While specific syndromes including pure motor/hemisensory stroke and ataxic hemiparesis are more strongly associated with acute small subcortical infarcts,[24] LACS classification is imprecise[24,25] and one-third of minor strokes are not accompanied by a corresponding acute infarct radiologically, even on the most sensitive diffusion MRI (n = 264).[26] Non-lacunar pathology, for example, cortical infarcts, may manifest as LACS and conversely, small subcortical infarcts may present with other non-LACS syndromes[25,27] in around 15% to 20% (n = 137), or develop silently.[13] While some LACS may masquerade as cortical stroke syndromes when the responsible brain lesion is close to the cortex,[27] or in specific locations such as the thalamus. Other cases where LACS and partial anterior circulation stroke (PACS) are confused may simply reflect disappearance of, or failure to recognize, cortical symptoms, mistaking dysarthria for dysphasia, or overlooking visual field defects.[25] Furthermore, other comorbidities may alter or obscure stroke presentations [Figure 4], for example, a patient with arthritis and peripheral neuropathy may not notice an ataxic hemiparesis.
Associated short-term with infarct growth (n = 61)[28] and poor functional outcomes (n = 4011)[29] in stroke, SVD effects outlast the acute phase, contributing increased risk long-term of recurrent ischaemic stroke, disability, dementia, and death (n = 71,298).[30]
Mobility and movement
Gait and balance dysfunction, shortened stride length (n = 431),[6] unexplained dizziness (n = 122),[31] falls (n = 187),[32] and features of vascular parkinsonism such as bradykinesia, rigidity, and gait disturbances (n = 503 community-dwelling)[33] are all associated with SVD.
Urinary symptoms
Eight studies, mostly in older community dwelling-subjects, detected urinary symptom associations with WMH (total n = 1944),[34–41] while two did not (n = 648).[42,43] These findings need to be reproduced in large prospective blinded studies, adjusting for mobility, frailty and co-morbidities.
Vascular cognitive impairment: clinical features
Vascular cognitive impairment (VCI) is a broad term, encompassing mild cognitive impairment and dementia. We focus on the clinically sensitive DSM-V diagnostic criteria,[44] which require evidence of cognitive decline from a previous performance level in one or more domains including: (a) concern about decline from a patient, knowledgeable informant or clinician, and (b) objective impairment or decline on testing. To establish a vascular etiology, either a temporal association with stroke/s or prominent decline in complex attention/processing speed and frontal-executive functions is required, although it is increasingly apparent that SVD is not confined to specific domains,[45] in contrast to previous thinking that focused on domain-specific impairments. Further discrimination between mild cognitive impairment and dementia is based on whether cognition is sufficiently impaired to result in loss of functional independence.[44] This may be described by either patient or informant, e.g. non-specific reports of “not managing at home” or deficits in instrumental activities of daily living, e.g. inability to independently manage one's finances. Clinicians frequently rely on the informant account, which is invaluable, as many individuals with cognitive impairment lack insight or minimise their symptoms.
Distinguishing the subcortical subtype of vascular cognitive impairment
The small vessel contribution to dementia exceeds that of large vessel disease, with incident lacunes thought to herald the highest dementia risk at least in community-dwelling subjects.[46] Cognitive features include slow thought processing, poor memory retrieval, and executive dysfunction.[47] The subcortical vascular cognitive impairment (VCI) subtype is supported by symptoms such as impaired problem-solving, personality changes including apathy, mood disorders, pseudobulbar palsy, dysarthria, subtle sensory and motor deficits, urinary symptoms, and gait deterioration including postural instability.[47,48] Although these clinical symptoms are frequently cited as subcortical VCI features, many of these correlations are based on older, small, clinicopathological and CT-based studies. There is a scarcity of MRI studies confirming these associations in VCI populations, with recent studies’ main clinical focus on cognitive tests and vascular risks. Abrupt cognitive impairment due to single strategic small subcortical infarcts has been described rarely,[47] is understudied, and requires further characterization.
The neurological examination provides clues to subtyping VCI: subtle abnormalities including dysarthria, dysphagia, and parkinsonian, rather than hemiplegic gait, are all more prevalent in subcortical vascular dementia (n = 706).[22] Subcortical may also be differentiated from cortical VCI and Alzheimer's disease by the absence of aphasia, apraxia, agnosia, amnesia, and hemianopia[48] although cortical and subcortical lesions, with or without Alzheimer's disease, frequently coexist so the specificity of these symptoms will be limited. Supportive findings on neuroimaging raise diagnostic certainty from possible to probable when there is no clear temporal relationship to stroke events,[44] although the extent of radiological SVD considered sufficient to contribute to a VCI diagnosis is debated.[49] Neuroimaging is particularly important for distinguishing SVD-related VCI, where stepwise cognitive decline is often absent, instead characterized by insidious, fluctuating cognitive decline, punctuated by neurological deficits [Figure 3].[48]
Function
SVD substantially limits independence, contributing to functional impairment,[29] stroke recurrence, dementia, and mortality after stroke,[30] as well as functional decline and mortality in non-disabled adults.[50] SVD is associated with longer hospital lengths of stay in cognitively impaired,[51] and earlier institutionalization in stroke patients.[52]
Recommended approaches to patients presenting with the SVD syndrome
Many clinical features described in this review are non-specific when considered in isolation. However, clinical presentations are frequently multifactorial, particularly in older people in whom SVD is highly prevalent [Table 1]. When faced with these features in combination, supported by previous neuroimaging, and especially in individuals with a history of lacunar stroke or cognitive impairment, one should consider SVD presence and/or progression as a contributor.
Risk Factors for SVD and its Progression
Risk factors for progression in SVD include “traditional vascular risk factors” such as age and hypertension, and MRI biomarkers, which not only represent the cornerstone for SVD diagnosis but also identify risk of progression, provide a feasible strategy for monitoring patients, and a therapeutic target.
Vascular risk factors
Several vascular risk factors are associated with SVD, but the two major ones are advancing age and hypertension.[50,53] Specifically, In community-based samples, WMH prevalence was low before 55 years of age but increased sharply with age thereafter, from 11% to 21% in the subjects 64 years of age on average to 94% in individuals 82 years of age on average.[14] Cerebral microbleeds (CMB), CAA, PVS and lacunes also increase with age.[17,50,54–56]
The most important modifiable vascular risk factor for SVD is arterial hypertension (defined as blood pressure greater than 140/90 mmHg).[57] Ambulatory blood pressure (BP) provides more accurate data on BP status than office-based BP measurements and may help BP control in patients with extensive SVD.[58] In addition, abnormal circadian BP variations during sleep, specifically non-dipping (<10% fall in nocturnal BP) and reverse-dipping patterns (rise in nocturnal BP) are associated with WMH.[59] Hypertension is also associated with CMBs in adults with and without established cerebrovascular disease.[60] SVD lesions can occur in individuals without hypertension,[61] plus recent data from large consortia genetic analyses indicate that some patients with more severe SVD may be particularly sensitive to any BP elevation (in press). Since it is currently difficult to identify individuals whose small vessels may be particularly sensitive to even minor BP elevations, it remains uncertain how intensively blood pressure should be lowered.[50]
Diabetes mellitus types 1 (relative ratio [RR] 7.2, 95% confidential interval [CI] 3.2–16.1) and 2 (RR 2.8, 95% CI 2.3–3.5) are associated with lacunar infarction[62] and other biomarkers of SVD on MRI, including atrophy[63] and CMBs.[60] Because the duration of diabetes is important in determining ischemic stroke risk, early onset of type 1 diabetes confers a cumulatively higher lacunar stroke risk in such patients. Furthermore, fasting glucose level (odds ratio [OR] 1.27, 95% CI 1.10–1.46) and high insulin resistance scores (OR 1.33, 95% CI 1.05–1.68) are also associated with increased incident lacunes.[54] People with type 2 diabetes have a 1.5 times increased risk of dementia, and high HbA1c, concentration and glucose variability are negatively associated with cognitive function.[63] Interestingly, type 2 diabetes is associated with a greater increase in depressive symptoms, which SVD may contribute to.[23,64]
Additionally, metabolic syndrome is associated with silent brain infarction and incident lacunes.[53,54] The potential impact of dyslipidemia remains uncertain. In the atherosclerosis risk in communities (ARIC) study, high triglycerides increased the risk of incident lacunes (OR 1.24, 95% CI 1.04–1.47), while elevated high-density lipoproteins (HDL) reduced the risk (OR 0.77, 95% CI 0.59–0.99).[65] Moreover, the use of lipid-lowering medications was associated with fewer incident lacunes (OR 0.15, 95% CI 0.04–0.61) in an observational study,[55] but higher total (OR 1.67, 95% CI 1.20–2.31) and lobar (OR 1.52, 95% CI 1.02–2.27) CMB presence in a separate community-based study.[66] In contrast, lower HDL may predict WMH volume increase in people aged between 73 and 76 years[67] so the relationship between HDL and SVD needs further research.
Lifestyle risk factors
Regular exercise, healthy diet (Mediterranean diet, folic acid and vitamin B12),[68] and avoiding adverse lifestyle factors such as smoking, excess alcohol or high dietary sodium, are all associated with having fewer SVD features in observational studies.[69–71] Alcohol intake is associated with worse WMH in patients with minor stroke.[72] High dietary sodium (>5 g/d) increases stroke risk (crucially lacunar stroke) and worsens WMH and total SVD burden.[68,69] Disappointingly, a subsequent systematic review of lifestyle interventions including exercise did not slow cognitive decline.[73]
Sleep dysfunction is an important and so far largely overlooked risk factor for adverse brain health. Whether unusual sleep patterns increase the risk of SVD lesions is unclear although disordered night-time sleep is associated with brain atrophy and increased daytime sleep is associated with increased PVS on MRI.[74] Abnormal sleep, such as obstructive sleep apnea, may be associated with more WMH and silent lacunar infarction,[75] although inability to correct for co-associated factors like smoking and hypertension may have overestimated the association.
Environmental, lifetime, and cultural risk factors
Genetic, environmental/lifestyle and cultural risk factors are likely related to SVD burden and to its associated outcomes such as cognitive impairment.[54] Data are currently unclear on male-female differences, and apparent differences may reflect age or recruitment bias, rather than a true difference in SVD burden, However, some hospital-based studies suggest that males have a higher burden of both sporadic[70] and monogenic SVDs,[71] but further research is needed to differentiate any true male-female difference in incidence or severity and the reasons behind any difference observed.
Regarding ethnic or geographical differences, it is difficult to disentangle effects of socioeconomic, dietary and medical histories, and use of different protocols, from true ethnic or geographical differences in the prevalence of SVD.[76]
Brain and cognitive reserves in later life are influenced by lifetime experiences, including those early in life.[77] Early life exposures could explain some of the variation between SVD and cognitive function2 and include childhood cognitive ability, with lower cognitive ability in childhood being associated with increased total WMH scores (r = –0.07, 95% CI, –0.12 to –0.02, I2 = 0%) in later life. Similarly, adverse childhood socioeconomic status (SES) increases the risk of worse deep (r = –0.181) and periventricular (r = –0.146) WMH, and lower educational attainment is associated with more WMH in later life (OR 1.24; 95% CI, 1.05–1.47). The trends were similar for other SVD markers although sample sizes were not large enough to determine if similar associations are present for other SVD markers.[77] Consistent with this, in patients presenting with minor stroke, premorbid intelligence quotient (IQ) and educational attainment predict post-stroke cognitive impairment more than stroke severity or vascular risk factors.[72]
Use of brain imaging appearances to predict risk of SVD progression
The lesions seen on MRI adopted as biomarkers of SVD include recent small subcortical (or lacunar) infarct (RSSI), WMH, lacune, CMB, visible PVS, and cerebral atrophy.[78] All of these lesions have been associated with dysfunction of the cerebral small vessels when measured in patients using MRI, including blood-brain barrier leakage, impaired cerebral vasoreactivity and increased vascular pulsatility, reflecting impaired endothelial function and related effects on the glia and neurons.[2] These lesions are individually and collectively associated with increased risk of stroke, cognitive decline and dementia, and poor functional outcomes after stroke, and are highly heritable.[29,30,50,79]
The single strongest risk factor for SVD lesion progression identified so far is having a severe SVD lesion burden at presentation.[2] Potential advances in neuroimaging of SVD based on MRI, e.g. diffusion tensor imaging (DTI) metrics such as fractional anisotropy (FA) and mean diffusivity (MD), show promise in research for detecting early white matter damage and may in future become widely used clinical applications.[80]
Although SVD lesions were previously considered to be “focal” and “permanent”, it is now clear that they represent more dynamic global disease. Thus, WMH progression is worse in those with increased baseline WMH volume,[81,82] and worsening WMH burden associates with brain atrophy including cortical thinning.[83] Since WMH may have some clinically meaningful reversible components,[81,82] the concept that prevention of worsening WMH-related brain damage may translate into long-term benefits for brain health is important.
Since the common SVD lesions are mostly visible on routine clinical brain MRI and computed tomography (CT) scanning (excluding CMB and PVS), greater use could be made of their potential for predicting prognosis. Several MRI scoring systems can be easily applied by clinicians to characterize SVD severity, many of which can predict clinical outcomes. The Fazekas scale is commonly used to evaluate WMH on MRI and can be used on CT.[78] Similarly, while less sensitive than MRI-based scores, equivalent CT-based scores for total SVD and “brain frailty”[29] predict poor functional outcome and cognitive impairment after stroke.[29,30] A simple and pragmatic score that may provide a more complete estimate of the full impact of SVD on the brain is the total SVD score (counting the presence of WMH, lacunes, CMB, and PVS on MRI as an ordinal score of 0 to 4), which could have potential for patient risk stratification.[70] Despite the increasing availability of MRI and limitations of CT, CT continues to be the most widely used neuroimaging tool in patients with neurological or neuropsychiatric symptoms, and can provide valuable information for SVD assessment.[29]
Therapeutic Approaches
Given the chronic nature and insidious progression of SVD, potential treatments will likely be required over the longer term as is done for the secondary prevention of vascular diseases. Due to the worldwide prevalence of SVD and association with increasing age, potential therapeutic agents will need to be affordable, easy to administer, safe, simple and have limited drug-drug interactions.[84,85] Currently, there is considerable variability in selection and definitions of end-points for SVD trials including of imaging endpoints and clinically relevant magnitudes of change, cognitive and functional outcomes, recurrent stroke, bleeding, and death. Hence, we report several outcomes depending on available data.
Lifestyle interventions
Lifestyle and behavioral interventions may have potential benefit in patients with SVD and are currently under investigation [Table 2]. Two trials have assessed aerobic exercise and found no difference in WMH volume[86,87] but did demonstrate improved cognitive scores at 6 months in those randomized to aerobic exercise as compared with those receiving usual care.[88] In a subgroup of a small trial (n = 54), resistance training was associated with reduced WMH volume at 12 months as compared with twice-weekly balance and tone exercises.[89] Several ongoing trials intend to build upon this data.
Table 2.
Therapeutic approaches for SVD.
| Intervention | Study type | Population | Notes |
| Lifestyle interventions | |||
| Aerobic exercise | RCTNCT01027858[86,88]AIBL[87] | 70 patients with WMH and cognitive impairment98 patients with subjective memory problems or mild cognitive impairment and at least 1 vascular risk factor | No difference in WMH volume in substudy (n = 30), but improved cognitive scores at 6 monthsNo difference in WMH volume at 2 years |
| Resistance training | RCTNCT00426881[89] | 54 patients with WMH but no cognitive impairment | Reduced WMH volume at 12 months |
| Smoking | Observational studyMild Stroke Study 2 (MSS-2)[70] Paris-Munich CADASIL[71]Lothian Birth Cohort[90] | 264 patients with stroke290 patients with CADASIL504 community-dwelling older patients | Smoking increases:- SVD burden on imaging- risk of stroke and dementia- rate of cortical thinning |
| Dietary sodium | Observational studyMSS-2[69] | 264 patients with stroke | High dietary sodium increased risk of: lacunar > cortical stroke, WMH & total SVD burden on imaging. |
| Traditional stroke prevention | |||
| Antiplatelet | Meta-analysis[93]RCTSPS3 [94]RCT subgroupRESTART[97] | 42,234 patients with lacunar stroke3020 patients with lacunar stroke254 patients with spontaneous ICH taking antithrombotic therapy | Single antiplatelet therapy reduced recurrent stroke across 17 trials.Chronic aspirin + clopidogrel vs. aspirin stopped early due to excess bleeding and death in dual antiplatelet groupSingle antiplatelet therapy vs. avoiding antiplatelet therapy did not increase hazard of recurrent ICH in the presence of CMB |
| BP lowering | Meta-analysis[58]RCTSPS3[98,99]RCT subgroupSPRINT-MIND[100]RCT substudyPRESERVE[101,102] | 1369 stroke patients3020 patients with lacunar stroke454 hypertensive patients with WMH111 hypertensive patients with lacunar stroke and established SVD | Less WMH progression with intensive BP reduction.No difference in recurrent stroke or long-term cognition with intensive BP loweringLess progression of WMH but no difference in brain volume over 4 years with intensive vs. standard BP treatmentNo difference in white matter damage on DTI with intensive vs. standard BP lowering. CBF did not fall with BP reduction in a further subgroup (n = 62) |
| Lipid lowering | RCTHeart Protection Study[103]ROCAS[104]PROSPER[105]RCT SubstudyVITATOPS[106] | 20,536 patients with vascular risk factors208 patients with TIA5804 patients with vascular risk factors81 patients with stroke and pre-stroke statin | Simvastatin did not influence cognitive outcomesSimvastatin did not influence WMH progressionPravastatin did not influence cognitive function (n = 5804) or WMH progression (n = 535)Less WMH progression with pre-stroke statin |
| Pharmacological agents under investigation | |||
| Cilostazol | Meta-analysis[107]RCTLACI-1[108]LACI-2[109] (ongoing) | 10,449 patients with ischaemic stroke57 patients with lacunar strokePatients with lacunar stroke (target 400) | Cilostazol reduced recurrent stroke (ischaemic and haemorrhagic) particularly in trials with larger populations of lacunar stroke patients.Cilostazol was associated with less WMH progressionEffect of cilostazol on recurrent stroke, death and dependency, cognition and imaging SVD markers |
| NO donors | RCTENOS[110,111]LACI-1[108]LACI-2[109] (ongoing) | 4011 patients with stroke57 patients with lacunar strokePatients with lacunar stroke (target 400) | GTN given under 6 hours of onset (n = 273) was associated with improved clinical outcomes at 90 daysPilot trial of ISMN on cerebrovascular reactivityEffect of ISMN on recurrent stroke, death and dependency, cognition and imaging SVD markers |
| Vitamins | RCT SubstudyVITATOPS[112] | 359 patients with stroke | Vitamin B supplements for 2 years in patients with severe WMH had slower WMH progression |
| Allopurinol | RCTXILO-FIST (NCT02122718) | Patients with ischaemic stroke (target 464) | Effect of Allopurinol on recurrent stroke and WMH progression |
| RIC | |||
| RIC | RCTWang et al[113] | 30 patients with SVD | RIC twice daily for 1 year reduced WMH and improved visuospatial and executive function |
AIBL: Australian Imaging Biomarkers and Lifestyle study; BP: blood pressure; CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CBF: cerebral blood flow; CMB: cerebral microbleeds; DTI: diffuse tensor imaging; ENOS: Efficacy of Nitric Oxide in Stroke Trial; GTN: glyceryl trinitrate; ICH: intracerebral haemorrhage; ISMN: isosorbide mononitrate; LACI-1/2: LACunar Intervention Trial -1 or 2; MSS-2: Mild Stroke Study-2; NO: Nitric oxide; PRESERVE is the name of a trial, it is not an acronym; PROSPER: Prospective Study of Pravastatin in the Elderly at Risk; RCT: randomised controlled trial; RESTART: Restart or Stop Antithrombotics Randomised Trial; RIC: Remote ischaemic conditioning; ROCAS: Regression of Cerebral Artery Stenosis study; SPRINT-MIND: Systolic blood PRessure INtervention Trial-MIND; SPS3: Secondary Prevention of Small Subcortical Stroke Trial; SVD: small vessel disease; VITATOPS: VITAmins TO Prevent Stroke; WMH: white matter hyperintensities; XILO-FIST: Xanthine oxidase Inhibition for the Improvement of Long-term Outcomes Following Ischaemic Stroke and Transient ischaemic attack.
Smoking is strongly associated with an increased burden of SVD and cortical loss in observational studies,[70,71,90] and therefore, smoking cessation should be strongly encouraged.
High dietary sodium was associated with increased stroke, particularly lacunar events, WMH and SVD burden in patients with stroke[69] and with risk of stroke in population studies.[91] Trials assessing the effect of dietary sodium in SVD are lacking, as they are for other vascular disease, but reduction in dietary salt is good general health advice.
Encouragingly, exercise and a healthy Mediterranean diet with folic acid and vitamin B12, combined with guideline based vascular risk reduction (ie, multidomain intervention), slowed cognitive decline in older people at risk of dementia compared with vascular risk factor reduction alone.[92]
Traditional stroke prevention treatments
Antiplatelet medication
Single antiplatelet therapy reduced recurrent stroke as compared with no antiplatelet agent in a meta-analysis of 17 trials totaling 42,234 patients with previous lacunar ischemic stroke.[93] The secondary prevention of small subcortical stroke (SPS3) trial randomized 3020 patients with a symptomatic lacunar stroke to chronic aspirin and clopidogrel versus aspirin alone and was stopped early due to excess bleeding and death in the dual antiplatelet group.[94] In observational studies, antiplatelet therapy has been associated with prevalent CMBs (OR 1.21; 95% CI 1.07–1.36)[95] while anticoagulants have been associated with prevalent and incident CMBs (OR 1.72, 95% CI 1.22–2.44; I2 = 19%).[96] Given the shared pathophysiology between CMB and ICH, the use of antiplatelet and anticoagulant therapy in the presence of CMB remains under study. A subgroup analysis from the randomized, controlled RESTART trial reported that individuals with a history of ICH taking antiplatelets in the presence of CMB did not experience increased hazard (hazard ratio [HR] 0.30, 95% CI 0.08–1.13 vs. 0.7, 95% CI 0.13–4.61).[97] Further randomized trials are needed to establish which treatments are beneficial or harmful to CMBs and ICH, both in stroke and non-stroke populations.
BP lowering
Intensive lowering of BP (<120 mmHg) in a subgroup (n = 454) of the large Systolic blood PRessure INtervention Trial (SPRINT) with WMH was associated with reduce WMH progression and decreased risk of mild cognitive impairment (HR 0.81; 95% CI 0.69–0.95) but no difference in brain volume neither risk of dementia over a 4 year period compared with standard BP management.[100] Similarly, a meta-analysis of trials including 1,369 patients with prior stroke found less WMH progression (standardized mean difference –0.19; 95% CI –0.32 to –0.06; I2 = 20%) with intensive BP lowering as compared with usual care.[58] The SPS3 trial also assessed intensive BP reduction but, in patients with prior lacunar ischemic stroke specifically, found reduced hemorrhagic stroke, however no difference in stroke recurrence[98] or long-term cognition[99] with intensive compared with standard BP lowering. In the PRESERVE trial, 111 hypertensive patients with lacunar ischemic stroke and established SVD were randomized to intensive BP lowering (<125 mmHg) vs. standard care and demonstrated no difference in white matter damage on diffusion tensor imaging,[101] while in a further subgroup cerebral blood flow was not compromised by intensive BP lowering.[102]
Lipid lowering
Unfortunately, there are no trial data pertaining to statins exclusively in lacunar stroke. The SPARCL trial revealed that atorvastatin reduced stroke recurrence in separate subgroups of patients with large artery atherosclerotic stroke and those with lacunar ischemic stroke.[114]
The effect of statins on other outcomes specific to SVD have had mixed results to date. Simvastatin did not influence cognitive outcome in the Heart Protection Study (n = 20,536),[103] nor WMH progression in the ROCAS study,[115] whilst pravastatin did not impact cognitive function (n = 5804) or WMH progression (n = 535) in the PROSPER study.[105] In contrast, patients with stroke and severe WMH had less progression of WMH if they were on a statin pre-stroke in the VITATOPS study.[106]
Pharmacological agents under investigation
Cilostazol
Cilostazol, a phosphodiesterase 3’ inhibitor, is commonly used for stroke prevention in the Asia-Pacific region. As well as its weak antiplatelet effects, cilostazol may be beneficial in preventing SVD accumulation through endothelial stabilization,[116] myelin repair,[117] neuroprotective and anti-inflammatory mechanisms.[118] A meta-analysis including 10,449 patients with prior ischemic stroke, predominantly from the South Asian-Pacific region, found that cilostazol reduced recurrent ischemic stroke (OR 0.68, 95% CI 0.57 to 0.81), intracerebral hemorrhage (OR 0.43, 95% CI 0.29 to 0.64), and death (OR 0.64, 95% CI 0.49 to 0.83) as compared with either placebo, aspirin or clopidogrel.[107] When given longer term (>6 months), cilostazol reduced recurrent ischemic stroke to a greater degree than when given short-term without increasing bleeding, and particularly in trials with larger populations of lacunar stroke patients.[107]
Cilostazol's effects on cognition, death and dependency, and imaging are unclear. In 130 participants with acute lacunar stroke, the ECLIPSE trial found no difference in WMH volume change at 90 days between those randomized to cilostazol vs. placebo, but did demonstrate that cilostazol reduced cerebral arterial pulsatility measured using transcranial Doppler.[119] The small LACI-1 trial (n = 57) found that cilostazol was well tolerated over a 11 week period in patients with lacunar stroke and was associated with less progression of WMH as compared with patients randomised to no cilostazol.[108] The ongoing LACI-2 trial seeks to assess the effect of cilostazol on recurrent stroke, cognition, imaging markers of SVD and death and dependency in 400 participants with prior lacunar stroke.[109]
Nitric oxide donors
Nitric oxide (NO) and its donors, for example, organic nitrates (eg, glyceryl trinitrate [GTN] and isosorbide mononitrate [ISMN]), has multiple effects that might be beneficial in patients with SVD.[84] Transdermal GTN given within 6 h of stroke onset improved functional outcome and cognition at 90 days in a subgroup of a large randomized trial[111]; GTN administered between 6 and 48 hours did not improve outcome.[110] However, when administered within 4 h of stroke onset in the pre-hospital arena in a subsequent trial, GTN had a neutral effect on clinical outcomes.[120] Despite, ISMN being commonly used in the management of ischemic heart disease, data regarding its use in SVD and stroke are scanty. The previously mentioned LACI-1 trial randomized patients to ISMN, in addition to Cilostazol, in a factorial design. ISMN was well-tolerated and safe, but did not influence clinical or radiological outcomes in this small trial.[108] The ongoing LACI-2 trial is also assessing ISMN and its effects on safety and efficacy in clinical and radiological outcomes.[109]
Vitamins
The vitamins of interest in SVD include vitamins B6, B12 and folate. Low levels of B12 have been associated with more severe WMH.[121] A substudy from the VITATOPS trial suggested that patients with severe WMH who received B vitamins for 2 years had slower WMH progression.[112]
Xanthine oxidase inhibitors
Allopurinol, a xanthine oxidase inhibitor, has multiple effects that may be beneficial in SVD.[84] A trial of 80 patients with ischemic stroke (1/2 lacunar etiology) demonstrated reduced BP, augmentation index and carotid intima-media thickness progression following one year of receiving allopurinol.[122] Larger trials assessing allopurinol, including Xilo-FIST (ClinicalTrials.gov: NCT02122718), are ongoing.
Remote ischemic conditioning
Remote ischemic conditioning (RIC)—transient ischemia induced to a limb using a BP cuff—has been shown to be neuroprotective in pre-clinical models.[123] In a small study of 30 patients with SVD, RIC delivered twice daily for 1 year improved visuospatial and executive function and reduced WMH compared with sham.[113] The effects of RIC in lacunar stroke are unclear; the planned RECAST-3 [ISRCTN63231313] and Remote Ischemic Conditioning in Patients With Acute Stroke [RESIST, NCT03481777] trials will shed more light on this area.
Discussion and Conclusions
We recommend a holistic, multidisciplinary assessment of individual needs in patients with suspected SVD. This includes rigorous management of modifiable risk factors including smoking cessation, dietary improvements, and appropriate evidence-based medications while balancing risks of side effects. In advancing disease, onwards referral to relevant services should be considered to maximize independence including cognitive clinics, physiotherapists, occupational therapists, and social care. We suggest highlighting awareness of practical issues including driving, accessible home environments, appointing power of attorney, and advance care planning. We support close liaison with patients, family members and general practitioners to monitor for clinical deterioration. We note wide variability in choice and definitions of end-points used in trials in SVD that would benefit from some standardization. Finally, we advocate for more clinical trials to identify effective lifestyle and pharmaceutical interventions.
Future targets for clinical practice and research
We need better recognition of symptoms that best predict disease progression in longitudinal clinical-imaging-pathological studies across healthy, cognitively impaired, and stroke populations, establishing the natural history of SVD. Serial imaging studies assessing neuropsychiatric symptoms are especially lacking. Further work on interactions between SVD, depression, and their confounders will help to clarify the vascular depression hypothesis. Urinary symptom relationships with SVD require appropriate adjustment for confounders. Research should give greater prominence to informants, paralleling clinical practice.
We need to determine whether widely-accepted clinical features of subcortical VCI described in early pathological and CT studies still hold true on longitudinal MRI studies in VCI populations. How lesion volume, location, background SVD burden and rate of lesion change interact with symptoms, cognition, function, and physical and cognitive reserves needs to be determined. The natural history of VCI including subcortical subtypes needs to be better defined, for example, prevalence of stepwise vs. progressive cognitive decline.
Further work is needed to understand the pathophysiology of SVD, using advanced preclinical, neuroimaging, and pathological research methods. We need more trials of medications and simple lifestyle modifications, or combinations thereof. Further, detailed, observational research on modifiable and non-modifiable factors is required, integrating these into clinical trial design, determining whether using different treatment strategies for individuals with non-modifiable risk factors produces any additional benefit.
Integrating approaches to research and clinical care of patients with SVD
We should empower patients and informants to self-monitor symptoms, signs, vascular risk factors, and cognitive test performance, e.g. using mobile phone applications, virtual clinics, and evolving smart technology that recognizes alterations in gait or speech patterns. We should use healthcare encounters to opportunistically seek features of SVD progression, for example, screening during vascular risk factor reviews. We should devise electronic record-based alerts based on notification of relevant healthcare referrals [Table 1], combined with existing imaging data. We should devise composite prediction scores of SVD progression for use as screening tools in everyday clinical settings, incorporating available symptom, risk factor, cognitive, demographic, and imaging reports, similar to those used for estimating cardiovascular or fracture risks.
Efforts to refine an SVD phenotype including, but extending beyond, stroke and cognitive impairment, are necessary. Apart from initial identification, we need to recognize those at the highest risk of SVD progression, tracking which clinical and imaging features herald progression. This will allow us to research targeted interventions earlier in the SVD course, preventing progression before its most disabling manifestations develop.
Acknowledgements
The authors acknowledge academic research funding sources as listed below. We are grateful to Ms Nicole Porter for administrative assistance in organizing the manuscript for submission.
Funding
This work is supported by the UK Dementia Research Institute (JMW, CA) which receives its funding from DRI Ltd, funded by the UK MRC, Alzheimer's Society and Alzheimer's Research UK; the Fondation Leducq Network for the Study of Perivascular Spaces in Small Vessel Disease (JMW; 16 CVD 05); The European Union Horizon 2020, SVDs@Target (JMW, FD, PHC-03-15, project No 666881); The Row Fogo Charitable Trust Centre for Research into Aging and the Brain (JMW); The British Heart Foundation (LACI-2 and Centre for Research Excellence; CS/15/5/31475, RE/18/5/34216); The Chief Scientist Office of Scotland (CZB/4/281, ETM/326, and Clinical Academic Fellowship UC; CAF/18/08); Chest Heart Stroke Scotland (Res14/A157); NHS Research Scotland (FND); Stroke Association (Garfield Weston Foundation Senior Clinical Lectureship FND, TSALECT 2015/04; ‘Small Vessel Disease-Spotlight on Symptoms, FD, JMW, UC, SVD-SOS; SAPG 19\100068; R4VaD, JMW, FD, PMB, 16 VAD 07; Princess Margaret Research Development Fellowship, UC, 2018; and Stroke Association Professor of Stroke Medicine PMB); PMB is a NIHR Senior Investigator.
Conflicts of interest
Conflicts of interest: The authors declare academic grants for research as listed above; JMW chairs the ESOC 2021 Planning Group, and participates in two ESO Guidelines; CA, JPA and UC have no conflicts to disclose. PMB has received honoraria as Chief Investigator or Steering Committee Chair of trials (DiaMedica, Phagenesis) and attending Advisory Boards (Moleac, Nestle, Sanofi).
Footnotes
How to cite this article: Clancy U, Appleton JP, Arteaga C, Doubal FN, Bath PM, Wardlaw JM. Clinical management of cerebral small vessel disease: a call for a holistic approach. Chin Med J 2021;134:127–142. doi: 10.1097/CM9.0000000000001177
References
- 1.Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol 2010; 9:689–701. doi: 10.1016/S1474-4422(10)70104-6. [DOI] [PubMed] [Google Scholar]
- 2.Wardlaw JM, Smith C, Dichgans M. Small vessel disease: mechanisms and clinical implications. Lancet Neurol 2019; 18:684–696. doi: 10.1016/S1474-4422(19)30079-1. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Dong YH, Lyu PY, Chen WH, Li R. Hypertension-induced cerebral small vessel disease leading to cognitive impairment. Chin Med J 2018; 131:615–619. doi: 10.4103/0366-6999.226069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos D, Wolters FJ, Darweesh SKL, Vernooij MW, de Wolf F, Ikram MA, et al. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimers Dement 2018; 14:1482–1492. doi: 10.1016/j.jalz.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. Vascular dysfunction-The disregarded partner of Alzheimer's disease. Alzheimers Dement 2019; 15:158–167. doi: 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Laat KF, van Norden AG, Gons RA, van Oudheusden LJ, van Uden IW, Bloem BR, et al. Gait in elderly with cerebral small vessel disease. Stroke 2010; 41:1652–1658. doi: 10.1161/STROKEAHA.110.583229. [DOI] [PubMed] [Google Scholar]
- 7.Saini M, Ikram K, Hilal S, Qiu A, Venketasubramanian N, Chen C. Silent stroke: not listened to rather than silent. Stroke 2012; 43:3102–3104. doi: 10.1161/strokeaha.112.666461. [DOI] [PubMed] [Google Scholar]
- 8.van Rooij FG, Vermeer SE, Goraj BM, Koudstaal PJ, Richard E, de Leeuw FE, et al. Diffusion-weighted imaging in transient neurological attacks. Ann Neurol 2015; 78:1005–1010. doi: 10.1002/ana.24539. [DOI] [PubMed] [Google Scholar]
- 9.Binswanger O. Die Abgrenzung der allgemeinen progresiven Paralyse. Berl Klin Wochenschr 1894; 31:1103–1105. 1137-1139, 1180-1186. [Google Scholar]
- 10.Marie P. Des foyers lacunaires de désintégration et de différents autres états cavitaires du cerveau. Paris, FR: Félix Alcan; 1901. [Google Scholar]
- 11.Regenhardt RW, Das AS, Lo EH, Caplan LR. Advances in Understanding the Pathophysiology of Lacunar Stroke: A Review. JAMA Neurol 2018; 75:1273–1281. doi: 10.1001/jamaneurol.2018.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown R, Benveniste H, Black SE, Charpak S, Dichgans M, Joutel A, et al. Understanding the role of the perivascular space in cerebral small vessel disease. Cardiovasc Res 2018; 114:1462–1473. doi: 10.1093/cvr/cvy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valdes Hernandez MC, Maconick LC, Munoz Maniega S, Wang X, Wiseman S, Armitage PA, et al. A comparison of location of acute symptomatic versus 'silent’ small vessel lesions. Int J Stroke 2015; 10:1044–1050. doi: 10.1111/ijs.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, et al. Prevention of stroke in patients with silent cerebrovascular disease: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017; 48:e44–e71. doi: 10.1161/STR.0000000000000116. [DOI] [PubMed] [Google Scholar]
- 15.Choi SH, Na DL, Chung CS, Lee KH, Na DG, Adair JC. Diffusion-weighted MRI in vascular dementia. Neurology 2000; 54:83.doi: 10.1212/WNL.54.1.83. [DOI] [PubMed] [Google Scholar]
- 16.Boone KB, Miller BL, Lesser IM, Mehringer CM, Hill-Gutierrez E, Goldberg MA, et al. Neuropsychological correlates of white-matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol 1992; 49:549–554. doi: 10.1001/archneur.1992.00530290141024. [DOI] [PubMed] [Google Scholar]
- 17.Banerjee G, Carare R, Cordonnier C, Greenberg SM, Schneider JA, Smith EE, et al. The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry 2017; 88:982–994. doi: 10.1136/jnnp-2016-314697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oudeman EA, Greving JP, Van den Berg-Vos RM, Biessels GJ, Bron EE, van Oostenbrugge R, et al. Nonfocal transient neurological attacks are associated with cerebral small vessel disease. Stroke 2019; 50:3540–3544. doi: 10.1161/strokeaha.119.025328. [DOI] [PubMed] [Google Scholar]
- 19.Bos MJ, van Rijn MJ, Witteman JC, Hofman A, Koudstaal PJ, Breteler MM. Incidence and prognosis of transient neurological attacks. JAMA 2007; 298:2877–2885. doi: 10.1001/jama.298.24.2877. [DOI] [PubMed] [Google Scholar]
- 20.Fandler S, Gattringer T, Eppinger S, Doppelhofer K, Pinter D, Niederkorn K, et al. Frequency and predictors of dysphagia in patients with recent small subcortical infarcts. Stroke 2017; 48:213–215. doi: 10.1161/STROKEAHA.116.015625. [DOI] [PubMed] [Google Scholar]
- 21.Urban PP, Wicht S, Vukurevic G, Fitzek C, Fitzek S, Stoeter P, et al. Dysarthria in acute ischemic stroke: lesion topography, clinicoradiologic correlation, and etiology. Neurology 2001; 56:1021–1027. doi: 10.1212/wnl.56.8.1021. [DOI] [PubMed] [Google Scholar]
- 22.Staekenborg SS, van der Flier WM, van Straaten EC, Lane R, Barkhof F, Scheltens P. Neurological signs in relation to type of cerebrovascular disease in vascular dementia. Stroke 2008; 39:317–322. doi: 10.1161/STROKEAHA.107.493353. [DOI] [PubMed] [Google Scholar]
- 23.Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, et al. Vascular depression consensus report - a critical update. BMC Med 2016; 14:161.doi: 10.1186/s12916-016-0720-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gan R, Sacco RL, Kargman DE, Roberts JK, Boden-Albala B, Gu Q. Testing the validity of the lacunar hypothesis: the northern Manhattan stroke study experience. Neurology 1997; 48:1204–1211. doi: 10.1212/WNL.48.5.1204. [DOI] [PubMed] [Google Scholar]
- 25.Mead GE, Lewis S, Wardlaw JM, Dennis MS, Warlow CP. Should computed tomography appearance of lacunar stroke influence patient management? J Neurol Neurosurg Psychiatry 1999; 67:682–684. doi: 10.1136/jnnp.67.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Makin SDJ, Doubal FN, Dennis MS, Wardlaw JM. Clinically confirmed stroke with negative diffusion-weighted imaging magnetic resonance imaging: longitudinal study of clinical outcomes, stroke recurrence, and systematic review. Stroke 2015; 46:3142–3148. doi: 10.1161/STROKEAHA.115.010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Potter G, Doubal F, Jackson C, Sudlow C, Dennis M, Wardlaw J. Associations of clinical stroke misclassification (’clinical-imaging dissociation’) in acute ischemic stroke. Cerebrovasc Dis 2010; 29:395–402. doi: 10.1159/000286342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ay H, Arsava EM, Rosand J, Furie KL, Singhal AB, Schaefer PW, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke 2008; 39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 29.Appleton JP, Woodhouse LJ, Adami A, Becker JL, Berge E, Cala LA, et al. Imaging markers of small vessel disease and brain frailty, and outcomes in acute stroke. Neurology 2020; 94:e439–e452. doi: 10.1212/WNL.0000000000008881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgakis MK, Duering M, Wardlaw JM, Dichgans M. WMH and long-term outcomes in ischemic stroke: a systematic review and meta-analysis. Neurology 2019; 92:e1298–e1308. doi: 10.1212/WNL.0000000000007142. [DOI] [PubMed] [Google Scholar]
- 31.Ahmad H, Cerchiai N, Mancuso M, Casani AP, Bronstein AM. Are white matter abnormalities associated with “unexplained dizziness”? J Neurol Sci 2015; 358:428–431. doi: 10.1016/j.jns.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Callisaya ML, Beare R, Phan T, Blizzard L, Thrift AG, Chen J, et al. Progression of white matter hyperintensities of presumed vascular origin increases the risk of falls in older people. J Gerontol A Biol Sci Med Sci 2015; 70:360–366. doi: 10.1093/gerona/glu148. [DOI] [PubMed] [Google Scholar]
- 33.van der Holst HM, van Uden IW, Tuladhar AM, de Laat KF, van Norden AG, Norris DG, et al. Cerebral small vessel disease and incident parkinsonism: the RUN DMC study. Neurology 2015; 85:1569–1577. doi: 10.1212/WNL.0000000000002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poggesi A, Pracucci G, Chabriat H, Erkinjuntti T, Fazekas F, Verdelho A, et al. Urinary complaints in nondisabled elderly people with age-related white matter changes: the Leukoaraiosis And DISability (LADIS) Study. J Am Geriatr Soc 2008; 56:1638–1643. doi: 10.1111/j.1532-5415.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- 35.Sakakibara R, Hattori T, Uchiyama T, Yamanishi T. Urinary function in elderly people with and without leukoaraiosis: relation to cognitive and gait function. J Neurol Neurosurg Psychiatry 1999; 67:658–660. doi: 10.1136/jnnp.67.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuchel GA, Moscufo N, Guttmann CR, Zeevi N, Wakefield D, Schmidt J, et al. Localization of brain white matter hyperintensities and urinary incontinence in community-dwelling older adults. J Gerontol A Biol Sci Med Sci 2009; 64:902–909. doi: 10.1093/gerona/glp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Itoh Y, Yamada S, Konoeda F, Koizumi K, Nagata H, Oya M, et al. Burden of overactive bladder symptom on quality of life in stroke patients. Neurourol Urodyn 2013; 32:428–434. doi: 10.1002/nau.22336. [DOI] [PubMed] [Google Scholar]
- 38.Clarkson BD, Griffiths D, Resnick NM. Do brain structural abnormalities differentiate separate forms of urgency urinary incontinence? Neurourol Urodyn 2018; 37:2597–2605. doi: 10.1002/nau.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogama N, Yoshida M, Nakai T, Niida S, Toba K, Sakurai T. Frontal white matter hyperintensity predicts lower urinary tract dysfunction in older adults with amnestic mild cognitive impairment and Alzheimer's disease. Geriatr Gerontol Int 2016; 16:167–174. doi: 10.1111/ggi.12447. [DOI] [PubMed] [Google Scholar]
- 40.Tadic SD, Griffiths D, Murrin A, Schaefer W, Aizenstein HJ, Resnick NM. Brain activity during bladder filling is related to white matter structural changes in older women with urinary incontinence. NeuroImage 2010; 51:1294–1302. doi: 10.1016/j.neuroimage.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wakefield DB, Moscufo N, Guttmann CR, Kuchel GA, Kaplan RF, Pearlson G, et al. White matter hyperintensities predict functional decline in voiding, mobility, and cognition in older adults. J Am Geriatr Soc 2010; 58:275–281. doi: 10.1111/j.1532-5415.2009.02699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wehrberger C, Jungwirth S, Fischer P, Tragl KH, Krampla W, Marlies W, et al. The relationship between cerebral white matter hyperintensities and lower urinary tract function in a population based, geriatric cohort. Neurourol Urodyn 2014; 33:431–436. doi: 10.1002/nau.22419. [DOI] [PubMed] [Google Scholar]
- 43.Yee CH, Leung C, Wong YY, Lee S, Li J, Kwan P, et al. Lower urinary tract symptoms in subjects with subclinical cerebral white matter lesions. J Aging Res 2018; 2018:1582092.doi: 10.1155/2018/1582092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.American Psychiatric Association. Diagnostic and Statistical manual of mental Disorders. Washington, DC: American Psychiatric Publishing; 2013. [Google Scholar]
- 45.Hamilton O K L, Backhouse E V, Janssen E, Jochems A C C, Maher C, Stevenson A J, et al. Cognitive impairments in sporadic cerebral small vessel disease (SVD): a systematic review and meta-analysis of cohorts with stroke, dementia and non-clinical presentations of SVD. medRxiv 2002; 2010. 20020628.20202020. doi: 10.1101/2020.02.10.20020628. [Google Scholar]
- 46.Sigurdsson S, Aspelund T, Kjartansson O, Gudmundsson EF, Jonsdottir MK, Eiriksdottir G, et al. Incidence of brain infarcts, cognitive change, and risk of dementia in the general population: The AGES-Reykjavik Study (Age Gene/Environment Susceptibility-Reykjavik Study). Stroke 2017; 48:2353–2360. doi: 10.1161/strokeaha.117.017357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC. Subcortical ischaemic vascular dementia. Lancet Neurol 2002; 1:426–436. doi: 10.1016/S1474-4422(02)00190-4. [DOI] [PubMed] [Google Scholar]
- 48.Cummings JL. Vascular subcortical dementias: clinical aspects. Dementia 1994; 5:177–180. doi: 10.1159/000106718. [DOI] [PubMed] [Google Scholar]
- 49.Sachdev P, Kalaria R, O’Brien J, Skoog I, Alladi S, Black SE, et al. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014; 28:206–218. doi: 10.1097/WAD.0000000000000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Debette S, Schilling S, Duperron MG, Larsson SC, Markus HS. Clinical significance of magnetic resonance imaging markers of vascular brain injury: a systematic review and meta-analysis. JAMA Neurol 2019; 76:81–94. doi: 10.1001/jamaneurol.2018.3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang KJ, Lee S, Lee Y, Lee KS, Back JH, Jung YK, et al. Severity of white matter hyperintensities and length of hospital stay in patients with cognitive impairment: a CREDOS (Clinical Research Center for Dementia of South Korea) study. J Alzheimers Dis 2015; 46:719–726. doi: 10.3233/jad-142823. [DOI] [PubMed] [Google Scholar]
- 52.Sibolt G, Curtze S, Melkas S, Pohjasvaara T, Kaste M, Karhunen PJ, et al. Severe cerebral white matter lesions in ischemic stroke patients are associated with less time spent at home and early institutionalization. Int J Stroke 2015; 10:1192–1196. doi: 10.1111/ijs.12578. [DOI] [PubMed] [Google Scholar]
- 53.Fanning JP, Wong AA, Fraser JF. The epidemiology of silent brain infarction: a systematic review of population-based cohorts. BMC Med 2014; 12:119.doi: 10.1186/s12916-014-0119-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ling Y, Chabriat H. Incident cerebral lacunes: a review. J Cereb Blood Flow Metab 2020; 40:909–921. doi: 10.1177/0271678X20908361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gyanwali B, Shaik MA, Tan BY, Venketasubramanian N, Chen C, Hilal S. Risk factors for and clinical relevance of incident and progression of cerebral small vessel disease markers in an Asian memory clinic population. J Alzheimers Dis 2019; 67:1209–1219. doi: 10.3233/JAD-180911. [DOI] [PubMed] [Google Scholar]
- 56.Biffi A, Greenberg SM. Cerebral amyloid angiopathy: a systematic review. J Clin Neurol 2011; 7:1–9. doi: 10.3988/jcn.2011.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hilal S, Mok V, Youn YC, Wong A, Ikram MK, Chen CL. Prevalence, risk factors and consequences of cerebral small vessel diseases: data from three Asian countries. J Neurol Neurosurg Psychiatry 2017; 88:669–674. doi: 10.1136/jnnp-2016-315324. [DOI] [PubMed] [Google Scholar]
- 58.van Middelaar T, Argillander TE, Floris HBM, Deinum J, Richard E, Klijn CJM. Effect of antihypertensive medication on cerebral small vessel disease: a systematic review and meta-analysis. Stroke 2018; 49:1531–1533. doi: 10.1161/strokeaha.118.021160. [DOI] [PubMed] [Google Scholar]
- 59.Chokesuwattanaskul A, Cheungpasitporn W, Thongprayoon C, Vallabhajosyula S, Bathini T, Mao MA, et al. Impact of circadian blood pressure pattern on silent cerebral small vessel disease: a systematic review and meta-analysis. J Am Heart Assoc 2020; 9:e016299.doi: 10.1161/JAHA.119.016299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007; 130:1988–2003. doi: 10.1093/brain/awl387. [DOI] [PubMed] [Google Scholar]
- 61.Vinters HV, Zarow C, Borys E, Whitman JD, Tung S, Ellis WG, et al. Review: Vascular dementia: clinicopathologic and genetic considerations. Neuropathol Appl Neurobiol 2018; 44:247–266. doi: 10.1111/nan.12472. [DOI] [PubMed] [Google Scholar]
- 62.Janghorbani M, Hu FB, Willett WC, Li TY, Manson JE, Logroscino G, et al. Prospective study of type 1 and type 2 diabetes and risk of stroke subtypes: the Nurses’ Health Study. Diabetes Care 2007; 30:1730–1735. doi: 10.2337/dc06-2363. [DOI] [PubMed] [Google Scholar]
- 63.Geijselaers SL, Sep SJ, Stehouwer CD, Biessels GJ. Glucose regulation, cognition, and brain MRI in type 2 diabetes: a systematic review. Lancet Diabetes Endocrinol 2015; 3:75–89. doi: 10.1016/S2213-8587(14)70148-2. [DOI] [PubMed] [Google Scholar]
- 64.Rensma SP, van Sloten TT, Ding J, Sigurdsson S, Stehouwer CD, Gudnason V, et al. Type 2 diabetes, change in depressive symptoms over time, and cerebral small vessel disease: longitudinal data of the AGES-Reykjavik Study. Diabetes Care 2020; 43:1781–1787. doi: 10.2337/dc19-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dearborn JL, Schneider AL, Sharrett AR, Mosley TH, Bezerra DC, Knopman DS, et al. Obesity, insulin resistance, and incident small vessel disease on magnetic resonance imaging: atherosclerosis risk in communities study. Stroke 2015; 46:3131–3136. doi: 10.1161/STROKEAHA.115.010060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke 2014; 45:1492–1494. doi: 10.1161/STROKEAHA.114.004130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dickie DA, Ritchie SJ, Cox SR, Sakka E, Royle NA, Aribisala BS, et al. Vascular risk factors and progression of white matter hyperintensities in the Lothian Birth Cohort. Neurobiol Aging 2016; 42:116–123. doi: 10.1016/j.neurobiolaging.2016.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hankey GJ. The role of nutrition in the risk and burden of stroke: an update of the evidence. Stroke 2017; 48:3168–3174. doi: 10.1161/strokeaha.117.016993. [DOI] [PubMed] [Google Scholar]
- 69.Heye AK, Thrippleton MJ, Chappell FM, Valdes Hernandez MC, Armitage PA, Makin SD, et al. Blood pressure and sodium: association with MRI markers in cerebral small vessel disease. J Cereb Blood Flow Metab 2016; 36:264–274. doi: 10.1038/jcbfm.2015.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Staals J, Makin SDJ, Doubal F, Dennis M, Wardlaw JM. Stroke subtype, vascular risk factors and total MRI brain small vessel disease burden. Neurology 2014; 83:1228–1234. doi: 10.1212/WNL.0000000000000837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol 2009; 8:643–653. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- 72.Makin SD, Doubal FN, Shuler K, Chappell FM, Staals J, Dennis MS, et al. The impact of early-life intelligence quotient on post stroke cognitive impairment. Eur Stroke J 2018; 3:145–156. doi: 10.1177/2396987317750517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kivipelto M, Mangialasche F, Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat Rev Neurol 2018; 14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 74.Aribisala BS, Riha RL, Valdes Hernandez M, Munoz Maniega S, Cox S, Radakovic R, et al. Sleep and brain morphological changes in the eighth decade of life. Sleep Med 2020; 65:152–158. doi: 10.1016/j.sleep.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 75.Huang Y, Yang C, Yuan R, Liu M, Hao Z. Association of obstructive sleep apnea and cerebral small vessel disease: a systematic review and meta-analysis. Sleep 2020; 43:1–10. doi: 10.1093/sleep/zsz264. [DOI] [PubMed] [Google Scholar]
- 76.Chauhan G, Adams HHH, Satizabal CL, Bis JC, Teumer A, Sargurupremraj M, et al. Genetic and lifestyle risk factors for MRI-defined brain infarcts in a population-based setting. Neurology 2019; 92:e486–e503. doi: 10.1212/WNL.0000000000006851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Backhouse EV, McHutchison CA, Cvoro V, Shenkin SD, Wardlaw JM. Cognitive ability, education and socioeconomic status in childhood and risk of post-stroke depression in later life: a systematic review and meta-analysis. PLoS One 2018; 13:e0200525.doi: 10.1371/journal.pone.0200525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duperron MG, Tzourio C, Sargurupremraj M, Mazoyer B, Soumare A, Schilling S, et al. Burden of dilated perivascular spaces, an emerging marker of cerebral small vessel disease, is highly heritable. Stroke 2018; 49:282–287. doi: 10.1161/STROKEAHA.117.019309. [DOI] [PubMed] [Google Scholar]
- 80.Beaudet G, Tsuchida A, Petit L, Tzourio C, Caspers S, Schreiber J, et al. Age-related changes of peak width skeletonized mean diffusivity (PSMD) across the adult lifespan: a multi-cohort study. Front Psychiatry 2020; 11:342.doi: 10.3389/fpsyt.2020.00342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wardlaw JM, Chappell FM, Valdes Hernandez MDC, Makin SDJ, Staals J, Shuler K, et al. White matter hyperintensity reduction and outcomes after minor stroke. Neurology 2017; 89:1003–1010. doi: 10.1212/WNL.0000000000004328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.van Leijsen EMC, van Uden IWM, Ghafoorian M, Bergkamp MI, Lohner V, Kooijmans ECM, et al. Nonlinear temporal dynamics of cerebral small vessel disease: the RUN DMC study. Neurology 2017; 89:1569–1577. doi: 10.1212/WNL.0000000000004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.De Guio F, Duering M, Fazekas F, De Leeuw FE, Greenberg SM, Pantoni L, et al. Brain atrophy in cerebral small vessel diseases: extent, consequences, technical limitations and perspectives: The HARNESS initiative. J Cereb Blood Flow Metab 2020; 40:231–245. doi: 10.1177/0271678X19888967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bath PM, Wardlaw JM. Pharmacological treatment and prevention of cerebral small vessel disease: a review of potential interventions. Int J Stroke 2015; 10:469–478. doi: 10.1111/ijs.12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Smith EE, Markus HS. New treatment approaches to modify the course of cerebral small vessel diseases. Stroke 2020; 51:38–46. doi: 10.1161/STROKEAHA.119.024150. [DOI] [PubMed] [Google Scholar]
- 86.Dao E, Barha CK, Best JR, Hsiung GY, Tam R, Liu-Ambrose T. The effect of aerobic exercise on white matter hyperintensity progression may vary by sex. Can J Aging 2019; 38:236–244. doi: 10.1017/s0714980818000582. [DOI] [PubMed] [Google Scholar]
- 87.Venkatraman VK, Sanderson A, Cox KL, Ellis KA, Steward C, Phal PM, et al. Effect of a 24-month physical activity program on brain changes in older adults at risk of Alzheimer's disease: the AIBL active trial. Neurobiol Aging 2020; 89:132–141. doi: 10.1016/j.neurobiolaging.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 88.Liu-Ambrose T, Best JR, Davis JC, Eng JJ, Lee PE, Jacova C, et al. Aerobic exercise and vascular cognitive impairment: A randomized controlled trial. Neurology 2016; 87:2082–2090. doi: 10.1212/WNL.0000000000003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bolandzadeh N, Tam R, Handy TC, Nagamatsu LS, Hsu CL, Davis JC, et al. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J Am Geriatr Soc 2015; 63:2052–2060. doi: 10.1111/jgs.13644. [DOI] [PubMed] [Google Scholar]
- 90.Karama S, Ducharme S, Corley J, Chouinard-Decorte F, Starr J, Wardlaw JM, et al. Cigarette smoking and thinning of the brain's cortex. Mol Psychiatry 2015; 20:778–785. doi: 10.1038/mp.2014.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gardener H, Rundek T, Wright CB, Elkind MS, Sacco RL. Dietary sodium and risk of stroke in the Northern Manhattan Study. Stroke 2012; 43:1200–1205. doi: 10.1161/STROKEAHA.111.641043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 93.Kwok CS, Shoamanesh A, Copley HC, Myint PK, Loke YK, Benavente OR. Efficacy of antiplatelet therapy in secondary prevention following lacunar stroke: pooled analysis of randomized trials. Stroke 2015; 46:1014–1023. doi: 10.1161/STROKEAHA.114.008422. [DOI] [PubMed] [Google Scholar]
- 94.Investigators TS, Benavente OR, Hart RG. al. e. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med 2012; 367:817–825. doi: 10.1056/NEJMoa1204133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiu J, Ye H, Wang J, Yan J, Wang J, Wang Y. Antiplatelet therapy, cerebral microbleeds, and intracerebral hemorrhage: a meta-analysis. Stroke 2018; 49:1751–1754. doi: 10.1161/STROKEAHA.118.021789. [DOI] [PubMed] [Google Scholar]
- 96.Cheng Y, Wang Y, Song Q, Qiu K, Liu M. Use of anticoagulant therapy and cerebral microbleeds: a systematic review and meta-analysis. J Neurol 2019; 15:1–14. doi: 10.1007/s00415-019-09572-x. [DOI] [PubMed] [Google Scholar]
- 97.Al-Shahi Salman R, Minks DP, Mitra D, Rodrigues MA, Bhatnagar P, du Plessis JC, et al. Effects of antiplatelet therapy on stroke risk by brain imaging features of intracerebral haemorrhage and cerebral small vessel diseases: subgroup analyses of the RESTART randomised, open-label trial. Lancet Neurol 2019; 18:643–652. doi: 10.1016/S1474-4422(19)30184-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, et al. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet 2013; 382:507–515. doi: 10.1016/S0140-6736(13)60852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pearce LA, McClure LA, Anderson DC, Jacova C, Sharma M, Hart RG, et al. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol 2014; 13:1177–1185. doi: 10.1016/S1474-4422(14)70224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.SPRINT MIND Investigators for the SPRINT Research Group, Nasrallah IM, Pajewski NM, Auchus AP, Chelune G, Cheung AK, et al. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019; 322:524–534. doi: 10.1001/jama.2019.10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Croall ID, Lohner V, Moynihan B, Khan U, Hassan A, O’Brien JT, et al. Using DTI to assess white matter microstructure in cerebral small vessel disease (SVD) in multicentre studies. Clin Sci (Lond) 2017; 131:1361–1373. doi: 10.1042/CS20170146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Croall ID, Tozer DJ, Moynihan B, Khan U, O’brien JT, Morris RG, et al. Effect of standard vs intensive blood pressure control on cerebral blood flow in small vessel disease: The PRESERVE randomized clinical trial. JAMA Neurol 2018; 75:720–727. doi: 10.1001/jamaneurol.2017.5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Collins R, Armitage J, Parish S, Sleight P, Peto R. Effects of cholesterol-lowering with simvastatin on stroke and other major vascular events in 20536 people with cerebrovascular disease or other high-risk conditions. Lancet 2004; 363:757–767. doi: 10.1016/s0140-6736(04)15690-0. [DOI] [PubMed] [Google Scholar]
- 104.Mok VC, Lam WW, Fan YH, Wong A, Ng PW, Tsoi TH, et al. Effects of statins on the progression of cerebral white matter lesion: Post hoc analysis of the ROCAS (Regression of Cerebral Artery Stenosis) study. J Neurol 2009; 256:750–757. doi: 10.1007/s00415-009-5008-7. [DOI] [PubMed] [Google Scholar]
- 105.ten Dam VH, van den Heuvel DM, van Buchem MA, Westendorp RG, Bollen EL, Ford I, et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology 2005; 64:1807–1809. doi: 10.1212/01.WNL.0000161844.00797.73. [DOI] [PubMed] [Google Scholar]
- 106.Xiong Y, Wong A, Cavalieri M, Schmidt R, Chu WW, Liu X, et al. Prestroke statins, progression of white matter hyperintensities, and cognitive decline in stroke patients with confluent white matter hyperintensities. Neurotherapeutics 2014; 11:606–611. doi: 10.1007/s13311-014-0270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.McHutchison C, Blair GW, Appleton JP, Chappell FM, Doubal F, Bath PM, et al. Cilostazol for secondary prevention of stroke and cognitive decline: Systematic review and meta-analysis. Stroke 2020; 51:2374–2385. doi: 10.1161/STROKEAHA.120.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blair GW, Appleton JP, Flaherty K, Doubal F, Sprigg N, Dooley R, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: The LACunar Intervention-1 (LACI-1) trial, a randomised clinical trial. EClinicalMedicine 2019; 11:34–43. doi: 10.1016/j.eclinm.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wardlaw JM, Bath PMW, Doubal F, Heye A, Sprigg N, Woodhouse LJ, et al. Protocol: The Lacunar Intervention Trial 2 (LACI-2). A trial of two repurposed licenced drugs to prevent progression of cerebral small vessel disease. Eur Stroke J 2020; doi: 10.1177/2396987320920110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.The ENOS Trial Investigators. Efficacy of nitric oxide, with or without continuing antihypertensive treatment, for management of high blood pressure in acute stroke (ENOS): a partial-factorial randomised controlled trial. Lancet 2015; 385:617–628. doi: 10.1016/S0140-6736(14)61121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Woodhouse L, Scutt P, Krishnan K, Berge E, Gommans J, Ntaios G, et al. Effect of hyperacute administration (within 6 hours) of transdermal glyceryl trinitrate, a nitric oxide donor, on outcome after stroke: subgroup analysis of the Efficacy of Nitric Oxide in Stroke (ENOS) Trial. Stroke 2015; 46:3194–3201. doi: 10.1161/STROKEAHA.115.009647. [DOI] [PubMed] [Google Scholar]
- 112.Cavalieri M, Schmidt R, Chen C, Mok V, de Freitas GR, Song S, et al. B vitamins and magnetic resonance imaging-detected ischemic brain lesions in patients with recent transient ischemic attack or stroke: the VITAmins TO Prevent Stroke (VITATOPS) MRI-substudy. Stroke 2012; 43:3266–3270. doi: 10.1161/STROKEAHA.112.665703. [DOI] [PubMed] [Google Scholar]
- 113.Wang Y, Meng R, Song H, Liu G, Hua Y, Cui D, et al. Remote ischemic conditioning may improve outcomes of patients with cerebral small-vessel disease. Stroke 2017; 48:3064–3072. doi: 10.1161/STROKEAHA.117.017691. [DOI] [PubMed] [Google Scholar]
- 114.Amarenco P, Goldstein LB, Messig M, O’Neill BJ, Callahan A, III, Sillesen H, et al. Relative and cumulative effects of lipid and blood pressure control in the Stroke Prevention by Aggressive Reduction in Cholesterol Levels trial. Stroke 2009; 40:2486–2492. doi: 10.1161/STROKEAHA.108.546135. [DOI] [PubMed] [Google Scholar]
- 115.Mok VC, Lam WW, Chen XY, Wong A, Ng PW, Tsoi TH, et al. Statins for asymptomatic middle cerebral artery stenosis: the regression of cerebral artery stenosis study. Cerebrovasc Dis 2009; 28:18–25. doi: 10.1159/000215939. [DOI] [PubMed] [Google Scholar]
- 116.Rajani RM, Quick S, Ruigrok SR, Graham D, Harris SE, Verhaaren BFJ, et al. Reversal of endothelial dysfunction reduces white matter vulnerability in cerebral small vessel disease in rats. Sci Transl Med 2018; 10:eaam9507.doi: 10.1126/scitranslmed.aam9507. [DOI] [PubMed] [Google Scholar]
- 117.Miyamoto N, Pham LD, Hayakawa K, Matsuzaki T, Seo JH, Magnain C, et al. Age-related decline in oligodendrogenesis retards white matter repair in mice. Stroke 2013; 44:2573–2578. doi: 10.1161/STROKEAHA.113.001530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hasel P, Dando O, Jiwaji Z, Baxter P, Todd AC, Heron S, et al. Neurons and neuronal activity control gene expression in astrocytes to regulate their development and metabolism. Nat Commun 2017; 8:15132.doi: 10.1038/ncomms15132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Han SW, Song TJ, Bushnell CD, Lee SS, Kim SH, Lee JH, et al. Cilostazol decreases cerebral arterial pulsatility in patients with mild white matter hyperintensities: subgroup analysis from the Effect of Cilostazol in Acute Lacunar Infarction Based on Pulsatility Index of Transcranial Doppler (ECLIPse) study. Cerebrovasc Dis 2014; 38:197–203. doi: 10.1159/000365840. [DOI] [PubMed] [Google Scholar]
- 120.Bath PM, Scutt P, Anderson CS, Ankolekar S, Appleton JP, Berge E, et al. Prehospital transdermal glyceryl trinitrate in patients with ultra-acute presumed stroke (RIGHT-2): an ambulance-based, randomised, sham-controlled, blinded, phase 3 trial. Lancet 2019; 393:1009–1020. doi: 10.1016/S0140-6736(19)30194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.de Lau LM, Smith AD, Refsum H, Johnston C, Breteler MM. Plasma vitamin B12 status and cerebral white-matter lesions. J Neurol Neurosurg Psychiatry 2009; 80:149–157. doi: 10.1136/jnnp.2008.149286. [DOI] [PubMed] [Google Scholar]
- 122.Higgins P, Walters MR, Murray HM, McArthur K, McConnachie A, Lees KR, et al. Allopurinol reduces brachial and central blood pressure, and carotid intima-media thickness progression after ischaemic stroke and transient ischaemic attack: a randomised controlled trial. Heart 2014; 100:1085–1092. doi: 10.1136/heartjnl-2014-305683. [DOI] [PubMed] [Google Scholar]
- 123.Chen G, Thakkar M, Robinson C, Doré S. Limb remote ischemic conditioning: mechanisms, anesthetics, and the potential for expanding therapeutic options. Front Neurol 2018; 9:40.doi: 10.3389/fneur.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]


