Highlights
-
•
Legionella pneumophila can cause severe atypical community acquired pneumonia.
-
•
L. pneumophila is associated with exposure to industrial water systems, like air conditioning.
-
•
L. longbeachae causes same illness but is uncommon in the United States.
-
•
L. longbeachae is associated with exposure to compose or potting soil.
-
•
L longbeachae is difficult to diagnose given rarity, lack of rapid test, and need for specialized culture.
Keywords: Legionella longbeachae, Legionellosis, Legionnaire’s disease, Pontiac fever, Potting soil, Gardening
Abstract
Legionella longbeachae pneumonia is much less common than Legionella pneumophila pneumonia in most of the world and may evade timely diagnosis in settings that rely primarily on urine antigen testing, which detects Legionella pnuemophila serogroup 1 only. It is, however, widely recognized in Australia and New Zealand, where it is endemic and associated with exposure to compost and potting soils, rather than contaminated water systems as seen with L. pneumophila. L. longbeachae can cause a similar spectrum and severity of illness as L. pneumophila. Here we present a case of a 47-year-old man with L. longbeacheae necrotizing pneumonia following exposure to possibly contaminated soil from a wastewater treatment facility. Initial presentation included cough, chest pain, and dyspnea, and progressed to hypoxic respiratory failure, tension pneumothorax, and cardiac arrest. L. pneumophila urine antigen was negative, but bronchioalveolar lavage samples grew L. longbeachae on buffered charcoal yeast extract agar. A review of cases reported in the literature in non-endemic regions over a 20-year period identified 38 cases in Europe, 33 in Asia, and 8 in North America. Average age was 65, 65 % were male, and 35 % had potentially relevant environmental exposures. L. longbeachae should be considered in cases of severe community acquired pneumonia, particularly following a consistent environmental exposure or if initial testing for other pathogens is unrevealing. A thorough exposure history including questions about contact with potting soil or compost, and utilization of specialized agar for culture can both be key in identifying this pathogen.
Introduction
Legionella is a genus of facultative intracellular Gram-negative bacteria first recognized as a public health concern in 1977, following identification of Legionella pneumophila, the causative pathogen of the 1976 pneumonia outbreak in attendees of the American Legion annual meeting in Philadelphia, PA. Legionella pneumophila is widely known in the US as an atypical cause of community acquired pneumonia, often severe, affecting patients of increased age, and those with underlying comorbidities, tobacco use, and immunosuppression [1]. Legionella longbeachae is a far less common species in the US despite its discovery in Long Beach, California in 1980, estimated to account for 5 % of legionellosis [2]. However, L. longbeachae is endemic in Australia and New Zealand, equal at least in incidence to L. pneumophila cases there. Infection with L. longbeachae is associated with exposure to compost and potting soil, dissimilar to that of L. pneumophila and its association with man-made water systems [3,4]. Here we describe a case of a severe necrotizing pneumonia complicated by hypoxic respiratory failure, tension pneumothorax, and cardiac arrest, in a 47-year-old previously healthy man who was exposed to large amounts of dirt from a wastewater treatment facility. We also review the literature to aggregate data on cases reported in non-endemic countries in the last 20 years.
Case report
A 47-year-old man with hypertension and migraine headaches presented to an outside hospital with one week of worsening cough and small volume hemoptysis, chest congestion, dyspnea, and pleuritic non-radiating anterior chest pain. He reported dark urine and occasional hematuria without back pain, dysuria, urinary urgency or frequency, and denied fevers and chills. He was born in West Africa and immigrated to the US more than 10 years ago, denied recent travel and sick contacts, and had no history of tobacco, alcohol, or illicit substance use.
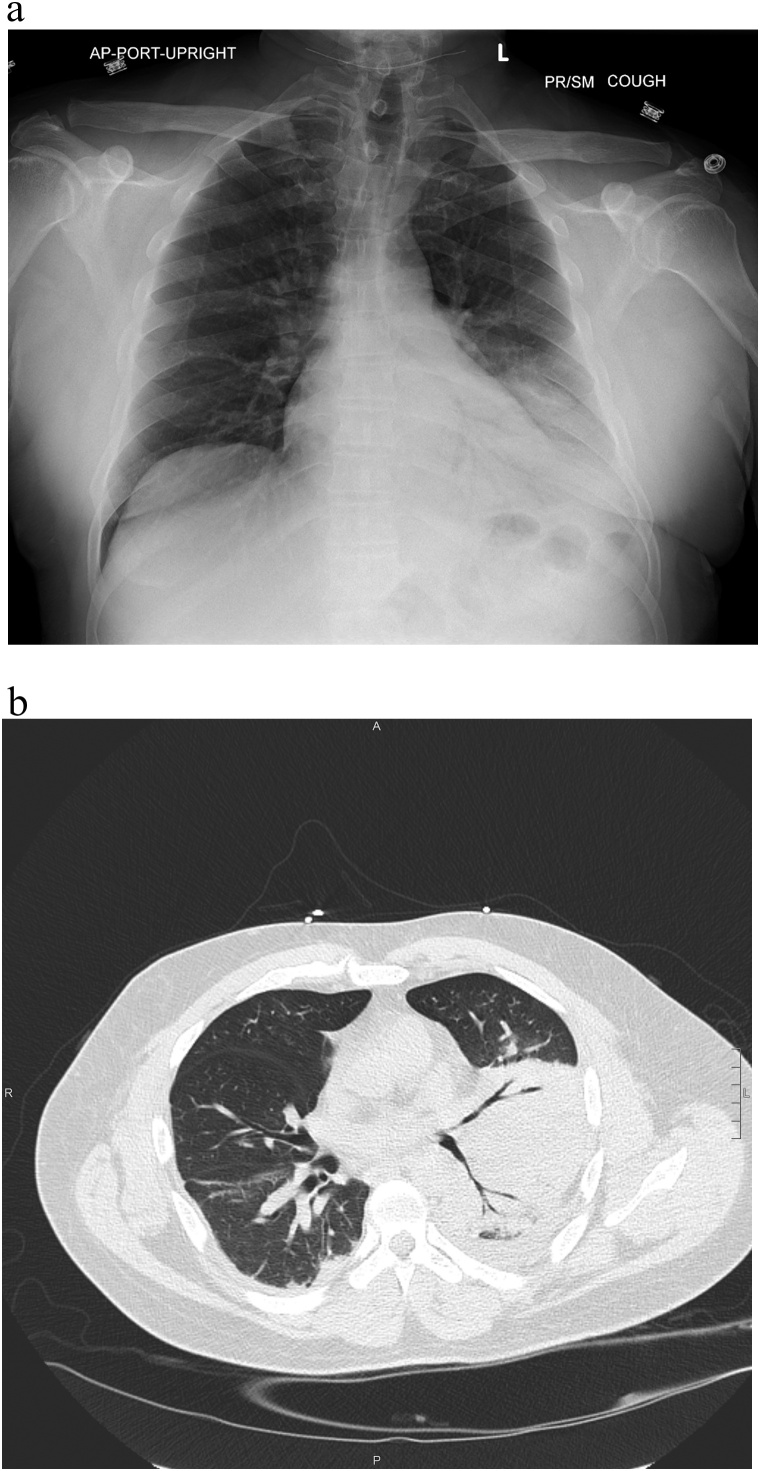
Initial vital signs included blood pressure of 92/57 mmHg, heart rate of 88 beats per minute, temperature of 37.1 °Centigrade, respiratory rate of 16 breaths per minute, and oxygen saturation of 95 % while breathing ambient air. On exam, he was not in distress and cardiopulmonary examination revealed normal heart rate, regular rhythm, and no murmurs, with normal respiratory effort and breath sounds. He was noted to have intermittent non-productive cough during examination, and had icteric sclera. Electrocardiogram showed no evidence of myocardial ischemia. White blood cell count was 10,100/μL with 92.9 % neutrophils, and the remainder of the complete blood count was unremarkable. Abnormalities on metabolic panel included sodium of 133 mEq/L, glucose of 299 mg/dL, albumin of 2.8 g/dL, total bilirubin of 7.5 mg/dL, alkaline phosphatase of 279 U/L, aspartate aminotransferase of 75 U/L, and alanine aminotransferase of 114 U/L. Renal indices were within normal limits. NT-proBNP was 225 pg/mL and D-dimer was 3,848 ng/mL. A left pleural effusion and left basilar consolidation versus atelectasis were visualized on chest x-ray, and subsequent computed tomography (CT) identified left lower lobe collapse or dense consolidation without evidence of an obstructive bronchial lesion; segmental pulmonary embolus was excluded (Fig. 1). CT of the abdomen and pelvis was unremarkable. The patient was discharged from the emergency department with a 5-day course of azithromycin and a 7-day course of cefuroxime for community acquired pneumonia (CAP).
Fig. 1.
a Chest x-ray on day of initial presentation: left basilar consolidaton with left pleural effusion. b. Chest computed tomography on day 4 after initial presentation: left lower lobe collapse or dense consolidation.
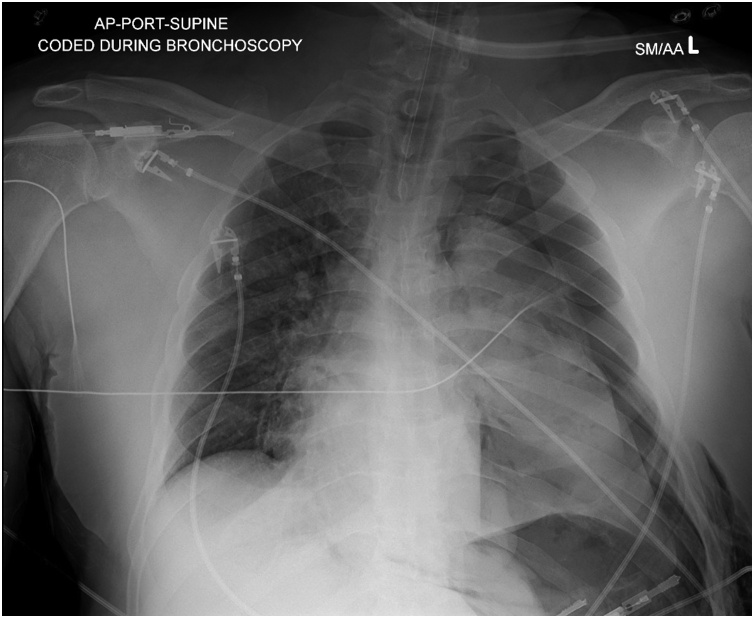
He returned two days later with progressive dyspnea, cough, and jaundice, with new lower extremity swelling. He remained afebrile and normotensive, however he was tachypneic with respiratory rate of 26 and oxygen saturation of 93 %. He had developed leukocytosis to 13.7 × 103/mm3. Right upper quadrant ultrasound did not reveal an etiology for his previously noted abnormal liver function tests. He was admitted to the hospital. Despite treatment with ceftriaxone and doxycycline he had a worsening leukocytosis (peak 16.5 × 103/mm3) and on hospital day 5 he was transferred to the intensive care unit (ICU) for hypoxemic respiratory failure requiring ventilator support. During bronchoscopy, the patient became bradycardic and had a pulseless electrical activity arrest. Return of spontaneous circulation was achieved after one minute of cardiopulmonary resuscitation. The patient underwent needle decompression followed by chest tube insertion for a left sided tension pneumothorax seen on chest x-ray (Fig. 2). Antimicrobial coverage was broadened to piperacillin-tazobactam and doxycycline on hospital day 6. He continued to have intermittent low-grade fevers, but on hospital day 9, the patient developed higher fever to 39.1 °Centrigrade and antimicrobial coverage was further broadened to linezolid and meropenem for possible hospital acquired pneumonia. He was then transferred to our tertiary care facility ICU, where bronchoscopy was performed and antimicrobial coverage was narrowed to ampicillin-sulbactam for treatment of presumed aspiration pneumonia with lung abscess. He became afebrile the following day.
Fig. 2.
Chest x-ray following cardiac arrest and cardiopulmonary resuscitation: left sided tension pneumothorax.
Initial evaluation for infectious pathogens was unremarkable. Blood cultures were sterile. Bronchoalveolar lavage (BAL) Gram stain revealed many white blood cells, few gram-positive cocci, and rare gram-negative rods, considered to be normal upper respiratory flora. Both fungal culture and stains for acid-fast bacilli from BAL were negative, as was a respiratory viral PCR panel (adenovirus, prevalent coronaviruses including SARS CoV-2, human metapneumovirus, influenza A and B, parainfluenza viruses 1–4, rhinovirus, enterovirus, and respiratory syncytial virus). BAL PCR for Chlamydia pneumoniae, Mycoplasma pneumoniae, and Bordetella pertussis were all negative. Aspergillus galactomannan, 1,3 βD glucan, Pneumocystis jirovici antigen, MRSA PCR from nares, HIV antigen/antibody, and urinary tests for antigens to Histoplasma capsulatum, Streptococcus pneumonia, and Legionella pneumophila were also negative. Mycobacterium tuberculosis interferonγrelease assay was indeterminate due to failure of the mitogen to elicit a reaction. Tests for acute hepatitis A and B, and chronic hepatitis B and C were also unrevealing.
The patient improved and was extubated on hospital day 14 and transitioned to high flow nasal cannula and then nasal cannula. His chest tube was removed with chest x-ray confirmation of resolved pneumothorax. He was transferred to the general medicine floor on hospital day 18. That day, the BAL ‘Legionella culture’ (buffered charcoal yeast extract agar plate; BCYE) was reported to be growing a Gram negative rod. Given presumed Legionnaires’ disease, he was started on a 7-day course of oral levofloxacin. Matrix assisted laser desorption/ionization – time of flight (MALDI-TOF) speciated the bacteria as Legionella longbeachae; the state microbiology lab confirmed a non-pneumophila Legionella species.
Exposure history had revealed that the patient worked for a water treatment company that excavated, pressure treated, and reinstalled underground water supply pipelines. This was initially thought to be a fitting explanation for exposure to L. longbeachae. When presented with this theory, the patient clarified that his role did not include being underground or working with the pipes directly, as he mostly worked from an office or supervising from a truck on job sites. The patient did, however, recall that he utilized dirt from near the work site as fertilizer for his lawn. Beginning 40 days prior to initial presentation, the patient and his son collected dirt, which included filtered human waste and wastewater from the filtration process. While holding bags for his son to shovel the dirt into, the patient noted he inhaled a significant amount of the dry, dusty dirt. He even noted that he found dirt in his nostrils later that day. After returning home, he aerated the lawn and used the collected dirt to fertilize the property. This project was completed 35 days prior to initial presentation. The son did not and has not had any symptoms.
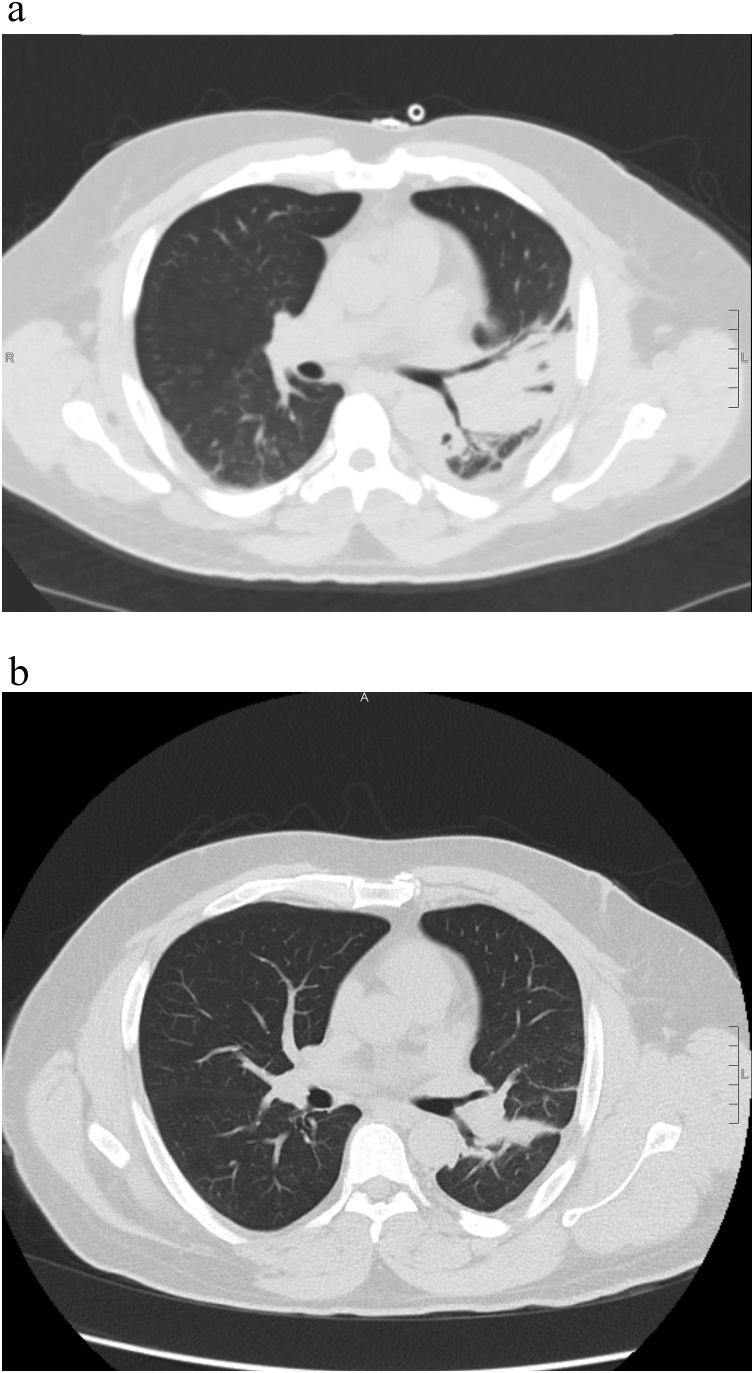
The patient continued to improve after starting levofloxacin. He remained slightly hyponatremic to 135 mEq/L with an improvement in AST and ALT to 122 U/L and 261 U/L, respectively. On day 20, a repeat CT chest showed decreased density of the left lower lobe predominant consolidation with slightly increased aeration but residual areas of necrosis (Fig. 3a). Although stable at rest, the patient exhibited oxygen desaturation with ambulation. On hospital day 21 he was discharged with supplemental oxygen via nasal cannula and the remainder of his course of levofloxacin. He followed up two months after hospitalization; he no longer required supplemental oxygen, had resolution of all symptoms except for minor pleuritic sternal chest pain, and had returned to his baseline level of functioning. Chest CT demonstrated near complete resolution of the dense necrotizing left lower lobe consolidation with possible residual organizing pneumonia or scarring, and bronchiectasis (Fig. 3b).
Fig. 3.
a Chest computed tomography hospital day 20: dense consolidation involving the entire left lower lobe and posterior left upper lobe/lingula with areas of lucency suggesting necrotizing pneumonia. Chest tube present. b. Chest computed tomography 2-months following discharge: significant interval improvement in previously noted consolidation. Residual linear opacities with internal mild bronchiectasis likely representing scarring or mild organizing pneumonia.
Review of reported cases
A review of the medical literature was performed using PubMed (US National Library of Medicine, Bethesda, Maryland) and the search term “Legionella longbeachae”, with publication date between January 1, 2000 and December 31, 2019. All publications were cross-referenced to eliminate redundant cases. References cited in the identified publications were also screened. Case series and case reports were evaluated for the following variables: patient age and sex, clinical outcome, and documented exposure history. Any publications with at least English-language abstract available were included.
One hundred forty two articles were reviewed. Forty-two articles including at least one case of L. longbeachae infection were identified, encompassing 293 cases over a 20-year period. The majority were reported from New Zealand (177) and Australia (37), where it is known to be endemic, and are not included here. Other cases were reported in Europe (Scotland 25 [[5], [6], [7], [8]]; Netherlands 6 [[9], [10], [11]]; France 2 [12,13]; Finland 1 [14]; Germany 1 [15]; Spain 1 [16]; Switzerland 1 [17]; United Kingdom 1 [18]), Asia (Thailand 20 [19]; Taiwan 6 [20]; Japan 6 [[21], [22], [23], [24], [25], [26]]; Israel 1 [27]), and North America (United States 5 [[28], [29], [30]]; Canada 3 [31,32]).
Of cases where individual or aggregate demographic data was provided, average age was 65 and 34 of 52 patients (65 %) were male. Infections other than pneumonia included infective endocarditis [12], osteomyelitis [29], and cutaneous [14,18]. Exposures mentioned included gardening or contact with potting soil or compost in 23 [[6], [7], [8],11,12,17,20,24,30,32], work at a metal recycling plant in 2 [31], touching a leaking potted plant in 1 [18], being cut by a broken flower pot in 1 [14], and sweeping tombs in 1 [20]. Several were noted to be significantly immunocompromised, with systemic lupus erythematosus [13,16,29], polymyalgia rheumatic [17], asplenia [15,27,28], chronic lymphocytic leukemia [18], and acute myelogenous leukemia [28]. Fourteen cases resulted in death [5,8,9,11,16,17,20,23,[28], [29], [30]] though data on survival was not reported in many of the cases.
Discussion
There are 58 species and 3 subspecies within the Legionella genus, 30 of which cause human infection. All replicate intracellularly within protozoa that exist in natural and artificial aquatic environments. Human infection with L. pneumophila occurs only if Legionella-harboring protozoa within water droplets are aerosolized and inhaled, which can occur via air conditioning units in hotels and hospitals. The pneumonic form of legionellosis that follows is referred to as Legionnaires’ disease, and results in mild to severe pneumonia after an incubation period of 2–14 days. While presentation can be similar to pneumococcal pneumonia, certain features can be more suggestive of Legionnaires’ disease: gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal pain), neurological symptoms (headache, seizure, focal neurological findings, obtundation), and laboratory abnormalities (low sodium and phosphorus, elevated creatine kinase and inflammatory markers, myoglobinurea, microscopic hematuria, and relative lymphopenia) [1], many of which were present in our patient. There are also several unique features of the case reported here. First, this patient had minimal chronic medical problems and was a lifelong non-smoker. He did however appear to inhale a significant amount of dry dusty soil, presumably contaminated with L. longbeachae, supporting inoculum load as a contributing factor to his illness [33]. Second, the severity of pulmonary findings and clinical course were notable. Pleural effusion has been reported in 15–50 % of cases, and nodular opacities progressing to cavitation in 10 % [1]. However, we found no report in our review of the literature of pneumothorax associated with this infection.
Legionellosis is treated with macrolides, doxycycline, or fluoroquinolones [3]. There are no clinical studies demonstrating anti-microbial resistance and in-vitro studies show equal susceptibility to macrolides and fluoroquinolones [34]. Our patient received two days of azithromycin as an outpatient and 8 days of doxycycline during his hospital course, of which 3 days were during intubation. Azithromycin has poor bioavailability (37 %), and doxycycline can have poor gastrointestinal absorption due to interactions with various other agents administered enterally. This, combined with the severity of disease, could have led to inadequate clearance of the infection initially.
The clinical manifestations and treatment of legionellosis from L. longbeachae do not differ from that of L. pneumophila, though the environmental incidence, reservoir, and diagnosis do. L. longbeachae is estimated to account for only 5 % of legionellosis in the US [2], but is much more common in Australia and New Zealand, equal or greater in incidence to L. pneumophila cases there [3]. In the last 20 years, cases have been reported in Europe, Asia, and North America also [[5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. While Legionella pneumophila is a transmitted via water sources, L. longbeachae has been found in soil samples, particularly gardening soil, compost, and potting soils; warning labels are included on commercial potting soil in some endemic regions. Studies in Australia and New Zealand have identified eating or drinking after gardening without hand hygiene, hanging flower pots that drip water, and facial contact with compost and potting soils as risk factors, in addition to classically known legionellosis risk factors of chronic pulmonary disease, heavy smoking history, older age, and immunocompromised state [[35], [36], [37]].
Legionellosis in general is thought to be under-diagnosed due to inadequate recognition and diagnostic limitations; it is estimated that less that 5 % of cases of are reported to the US CDC [2]. It would stand to reason that a much less prevalent species, such a L. longbeachae would be even more under-recognized in non-endemic regions. Legionella spp. are fastidious, requiring specialized media such as buffered charcoal yeast extract agar (BCYE), which includes iron and l-cysteine, both required for growth. Successful diagnosis through culture requires an adequate lower respiratory tract sample (though about half of patients do not have a productive cough), 3–7 days to grow, and usually a specific request by a clinician. Due to lack of purulence in samples from some patients, expectorated samples may also be rejected as inadequate. Sensitivity ranges from <10 % to 80 % [38]. The combined American Thoracic Society and Infectious Disease Society of America recommend obtaining the urinary antigen test in cases of CAP that occur in association with travel or a Legionella outbreak, and obtaining both the urinary antigen test and specialized culture of lower respiratory tract specimen in cases of severe CAP [39]. If adequate respiratory samples cannot be obtained, or if the urinary antigen test is relied upon to exclude legionellosis, any Legionella spp. that is not L. pneumophila serotype 1 might be falsely excluded. While the urinary antigen test produces rapid results and detects the majority of cases of L. pneumophila serotype 1, there is concern that its broad availability has also resulted in a bias towards this species being the only clinically relevant cause of legionellosis. PCR assays for multiple serotypes of Legionella have high sensitivity and specificity, and rapid turnaround times. They have been show to increase diagnoses by four-fold, but are not yet commercially available [38].
Conclusion
Legionella spp. can cause severe lower respiratory illness leading to fatal outcomes. L. pneumophila and non-pneumophila serotypes have different environmental risk factors, which are sometimes identified only retrospectively through scrupulous history taking. High clinical suspicion is needed to identify legionellosis, particularly that caused by non-pneumophila species such as L. longbeachae.
CRediT authorship contribution statement
Harrison Bell: Conceptualization, Writing - original draft. Sai Chintalapati: Writing - original draft. Preet Patel: Writing - original draft. Ameer Halim: Writing - original draft. Andrew Kithas: Writing - review & editing. Sarah A. Schmalzle: Conceptualization, Writing - original draft, Data curation, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgement
None.
References
- 1.Cunha B.A., Burillo A., Bouza E. Legionnaires’ disease. Lancet. 2016;387(10016):376–385. doi: 10.1016/S0140-6736(15)60078-2. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . 2020. Legionella (Legionnaire’s disease and pontiac fever)https://www.cdc.gov/legionella/clinicians/diagnostic-testing.html (Accessed 10/20/20) [Google Scholar]
- 3.Whiley H., Bentham R. Legionella longbeachae and legionellosis. Emerg Infect Dis. 2011;17(4):579–583. doi: 10.3201/eid1704.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie S.L., Beattie T.K. Compost and Legionella longbeachae: an emerging infection? Perspect Public Health. 2015;135(6):309–315. doi: 10.1177/1757913915611162. [DOI] [PubMed] [Google Scholar]
- 5.Cameron R.L., Pollock K.G.J., Lindsay D.S.J. Comparison of Legionella longbeachae and Legionella pneumophila cases in Scotland; implications for diagnosis, treatment and public health response. J Med Microbiol. 2016;65(2):142–146. doi: 10.1099/jmm.0.000215. [DOI] [PubMed] [Google Scholar]
- 6.Potts A., Donaghy M., Marley M. Cluster of legionnaires disease cases caused by Legionella longbeachae serogroup 1, Scotland, August to September 2013. Euro Surveill. 2013;18(50):20656. doi: 10.2807/1560-7917.es2013.18.50.20656. [DOI] [PubMed] [Google Scholar]
- 7.Lindsay D.S.J., Brown A.W., Brown D.J., Pravinkumar S.J., Anderson E., Edwards G.F.S. Legionella longbeachae serogroup 1 infections linked to potting compost. J Med Microbiol. 2012;61(Pt 2):218–222. doi: 10.1099/jmm.0.035857-0. [DOI] [PubMed] [Google Scholar]
- 8.Pravinkumar S.J., Edwards G., Lindsay D. A cluster of legionnaires’ disease caused by Legionella longbeachae linked to potting compost in Scotland, 2008–2009. Euro Surveill. 2010;15(8):19496. doi: 10.2807/ese.15.08.19496-en. [DOI] [PubMed] [Google Scholar]
- 9.De Bruin L., Timmerman C.P., Huisman P.M. Legionella longbeachae; don’t miss it! Neth J Med. 2018;76(6):294–297. [PubMed] [Google Scholar]
- 10.Diederen B.M.W., van Zwet A.A., van der Zee A., Peeters M.F. Community-acquired pneumonia caused by Legionella longbeachae in an immunocompetent patient. Eur J Clin Microbiol. 2005;24(8):545–548. doi: 10.1007/s10096-005-1368-9. [DOI] [PubMed] [Google Scholar]
- 11.den Boer J.W., Yzerman E.P., Jansen R. Legionnaires’ disease and gardening. Clin Microbiol Infect. 2007;13(1):88–91. doi: 10.1111/j.1469-0691.2006.01562.x. [DOI] [PubMed] [Google Scholar]
- 12.Leggieri N., Gouriet F., Thuny F., Habib G., Raoult D., Casalta J.P. Legionella longbeachae and endocarditis. Emerg Infect Dis. 2012;18(1):95–97. doi: 10.3201/eid1801.110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin M., Keller N., Martin A. A non-fatal pneumonia due to Legionella longbeachae in a patient with systemic lupus erythematosus. Lupus. 2016;25(13):1503–1504. doi: 10.1177/0961203316643599. [DOI] [PubMed] [Google Scholar]
- 14.Mentula S., Pentikäinen J., Perola O., Ruotsalainen E. Legionella longbeachae infection in a persistent hand-wound after a gardening accident. JMM Case Rep. 2014;1(4) doi: 10.1099/jmmcr.0.004374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kümpers P., Tiede A., Kirschner P. Legionnaires’ disease in immunocompromised patients: a case report of Legionella longbeachae pneumonia and review of the literature. J Med Microbiol. 2008;57(Pt 3):384–387. doi: 10.1099/jmm.0.47556-0. [DOI] [PubMed] [Google Scholar]
- 16.García C., Ugalde E., Campo Ab, Miñambres E., Kovács N. Fatal case of community-acquired pneumonia caused by Legionella longbeachae in a patient with systemic lupus erythematosus. Eur J Clin Microbiol. 2004;23(2):116–118. doi: 10.1007/s10096-003-1071-7. [DOI] [PubMed] [Google Scholar]
- 17.Reynaldos Canton Migotto T., Györik Lora S., Gaia V., Pagnamenta A. ARDS with septic shock due to Legionella longbeachae pneumonia in a patient with polymyalgia rheumatica. Heart Lung Vessel. 2014;6(2):114–118. [PMC free article] [PubMed] [Google Scholar]
- 18.Grimstead D., Tucker D., Harris K., Turner D. Cutaneous Legionella longbeachae infection in immunosuppressed woman, United Kingdom. Emerg Infect Dis. 2015;21(8):1426–1428. doi: 10.3201/eid2108.140828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Phares C.R., Wangroongsarb P., Chantra S. Epidemiology of severe pneumonia caused by Legionella longbeachae, Mycoplasma pneumoniae, and Chlamydia pneumoniae: 1-year, population-based surveillance for severe pneumonia in Thailand. Clin Infect Dis. 2007;45(12):e147–55. doi: 10.1086/523003. [DOI] [PubMed] [Google Scholar]
- 20.Wei S.H., Tseng L.R., Tan J.K. Legionnaires’ disease caused by Legionella longbeachae in Taiwan, 2006-2010. Int J Infect Dis. 2014;19:95–97. doi: 10.1016/j.ijid.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita K., Hijikuro K., Arita S., Kaneko Y., Isozaki M. A case of severe Legionella longbeachaepneumonia and usefulness of LAMP assay. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi. 2017;27(2):57–63. [PubMed] [Google Scholar]
- 22.Maniwa K., Taguchi Y., Ito Y., Mishima M., Yoshida S. Retrospective study of 30 cases of Legionella pneumonia in the Kansai region. J Infect Chemother. 2006;12(5):272–276. doi: 10.1007/s10156-006-0463-x. [DOI] [PubMed] [Google Scholar]
- 23.Ito A., Ishida T., Washio Y., Yamazaki A., Tachibana H. Legionella pneumonia due to non-Legionella pneumophila serogroup 1: usefulness of the six-point scoring system. BMC Pulm Med. 2017;17(1):211. doi: 10.1186/s12890-017-0559-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kubota M., Tomii K., Tachikawa R. Legionella longbeachae pneumonia infection from home garden soil. Nihon Kokyuki Gakkai Zasshi. 2007;45(September (9)):698–703. [PubMed] [Google Scholar]
- 25.Yamamoto K., Noda Y., Gonda H., Oishi T., Tanikawa Y., Yabuuchi E. A survival case of severe Legionella longbeachae pneumonia. Kansenshogaku Zasshi. 2001;75(3):213–218. doi: 10.11150/kansenshogakuzasshi1970.75.213. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki K., Tachibana A., Hatakeyama S., Yamaguchi K., Tateda K. Clinical characteristics in 8 sporadic cases of community-acquired legionella pneumonia. Nihon Kokyuki Gakkai Zasshi. 2002;40(April (4)):282–286. [PubMed] [Google Scholar]
- 27.Gorelik O., Lazarovich Z., Boldur I. Legionella in two splenectomized patients. Coincidence or causal relationship? Infection. 2004;32(3):179–181. doi: 10.1007/s15010-004-3005-4. [DOI] [PubMed] [Google Scholar]
- 28.Han X.Y., Ihegword A., Evans S.E. Microbiological and clinical studies of legionellosis in 33 patients with cancer. J Clin Microbiol. 2015;53(7):2180–2187. doi: 10.1128/JCM.00380-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McClelland M.R., Vaszar L.T., Kagawa F.T. Pneumonia and osteomyelitis due to Legionella longbeachae in a woman with systemic lupus erythematosus. Clin Infect Dis. 2004;38(May (10)):e102–6. doi: 10.1086/386322. [DOI] [PubMed] [Google Scholar]
- 30.Duchin J.S., Koehler J., Kobayashi J.M. Legionnaires’ disease associated with potting soil—California, Oregon, and Washington, May-June 2000. JAMA- J Am Med Assoc. 2000;284(12):1510. doi: 10.1001/jama.284.12.1510-JWR0927-3-1. [DOI] [PubMed] [Google Scholar]
- 31.Picard-Masson M., Lajoie É, Lord J. Two related occupational cases of Legionella longbeachae infection, Quebec, Canada. Emerg Infect Dis. 2016;22(7):1289–1291. doi: 10.3201/eid2207.160184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wright A.J., Humar A., Gourishankar S., Bernard K., Kumar D. Severe legionnaire’s disease caused by Legionella longbeachae in a long-term renal transplant patient: the importance of safe living strategies after transplantation. Transpl Infect Dis. 2012;14(4):E30–3. doi: 10.1111/j.1399-3062.2012.00755.x. [DOI] [PubMed] [Google Scholar]
- 33.Benin A.L., Benson R.F., Arnold K.E. An outbreak of travel-associated legionnaires disease and pontiac fever: the need for enhanced surveillance of travel-associated legionellosis in the United States. J Infect Dis. 2002;185(2):237–243. doi: 10.1086/338060. [DOI] [PubMed] [Google Scholar]
- 34.Miyashita N., Kobayashi I., Higa F. In vitro activity of various antibiotics against clinical strains of Legionella species isolated in Japan. J Infect Chemother. 2018;24(5):325–329. doi: 10.1016/j.jiac.2018.01.018. [DOI] [PubMed] [Google Scholar]
- 35.O’Connor B., Carman J., Eckert K. Does using potting mix make you sick? Results from a Legionella longbeachae case-control study in South Australia. Epidemiol Infect. 2006;135(1):34–39. doi: 10.1017/S095026880600656X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kenagy E., Priest P.C., Cameron C.M. Risk factors for Legionella longbeachae legionnaires’ disease, New Zealand. Emerg Infect Dis. 2017;23(7):1148–1154. doi: 10.3201/eid2307.161429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruehlemann S.A., Crawford G.R. Panic in the potting shed. The association between Legionella longbeachae serogroup 1 and potting soils in Australia. Med J Australia. 1996;164(1):36–38. doi: 10.5694/j.1326-5377.1996.tb94110.x. PMID: 8559094. [DOI] [PubMed] [Google Scholar]
- 38.Murdoch D.R. Diagnosis of legionella infection. Clin Infect Dis. 2003;36(1):64–69. doi: 10.1086/345529. [DOI] [PubMed] [Google Scholar]
- 39.Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019;200:e45–67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]