Abstract
Background
Widespread lockdowns imposed during the coronavirus disease 2019 crisis may impact birth outcomes.
Objective
This study aimed to evaluate the association between the COVID-19 lockdown and the risk of adverse birth outcomes in Botswana.
Study Design
In response to the coronavirus disease 2019 crisis, Botswana enforced a lockdown that restricted movement within the country. We used data from an ongoing nationwide birth outcomes surveillance study to evaluate adverse outcomes (stillbirth, preterm birth, small-for-gestational-age fetuses, and neonatal death) and severe adverse outcomes (stillbirth, very preterm birth, very-small-for-gestational-age fetuses, and neonatal death) recorded prelockdown (January 1, 2020–April 2, 2020), during lockdown (April 3, 2020–May 7, 2020), and postlockdown (May 8, 2020–July 20, 2020). Using difference-in-differences analyses, we compared the net change in each outcome from the prelockdown to lockdown periods in 2020 relative to the same 2 periods in 2017–2019 with the net change in each outcome from the prelockdown to postlockdown periods in 2020 relative to the same 2 periods in 2017–2019.
Results
In this study, 68,448 women delivered a singleton infant in 2017–2020 between January 1 and July 20 and were included in our analysis (mean [interquartile range] age of mothers, 26 [22–32] years). Across the included calendar years and periods, the risk of any adverse outcome ranged from 27.92% to 31.70%, and the risk of any severe adverse outcome ranged from 8.40% to 11.38%. The lockdown period was associated with a 0.81 percentage point reduction (95% confidence interval, −2.95% to 1.30%) in the risk of any adverse outcome (3% relative reduction) and a 0.02 percentage point reduction (95% confidence interval, −0.79% to 0.75%) in the risk of any severe adverse outcome (0% relative reduction). The postlockdown period was associated with a 1.72 percentage point reduction (95% confidence, −3.42% to 0.02%) in the risk of any adverse outcome (5% relative reduction) and a 1.62 percentage point reduction (95% confidence interval, −2.69% to −0.55%) in the risk of any severe adverse outcome (14% relative reduction). Reductions in adverse outcomes were largest among women with human immunodeficiency virus and among women delivering at urban delivery sites, driven primarily by reductions in preterm birth and small-for-gestational-age fetuses.
Conclusion
Adverse birth outcomes decreased from the prelockdown to postlockdown periods in 2020, relative to the change during the same periods in 2017–2019. Our findings may provide insights into associations between mobility and birth outcomes in Botswana and other low- and middle-income countries.
Key words: human immunodeficiency virus, mobility, neonatal death, pregnancy outcomes, preterm birth, small-for-gestational-age fetuses, stillbirth
Introduction
Widespread lockdowns imposed during the coronavirus disease 2019 (COVID-19) crisis may have affected birth outcomes worldwide, but the magnitude and direction of these effects remain uncertain. A hospital in Ireland reported a 73% decrease in the incidence of very low birthweight infants from January 2020 to April 2020 compared with the same period in the previous 2 decades,1 a study in Denmark found a 90% decrease in the incidence of extremely preterm birth during the lockdown period from March 12 to April 14 compared with the same period during the previous 5 years,2 and a study in the Netherlands found reductions in the incidence of preterm birth across various time windows surrounding the implementation of COVID-19 mitigation measures (eg, an odds ratio of 0.77 comparing 2 months after and 2 months before March 9).3 In the United States, 1 hospital in Nashville estimated that there were 20% fewer infants in the neonatal intensive care unit in March than during that month in previous years.4 However, many hospitals around the world reported no difference in preterm births during the lockdown,4 and there is concern that lockdown restrictions could also lead to increases in more severe outcomes, such as stillbirth and neonatal death. A study using data from a London hospital found a higher incidence of stillbirth during the COVID-19 pandemic than the period immediately before the pandemic, but there was no difference in preterm birth.5 A study in 9 hospitals across Nepal found a higher incidence of stillbirth, neonatal mortality, and preterm birth during the 9.5-week lockdown than in the 12.5 weeks before the lockdown.6 Finally, a study using data from 4 hospitals in western India found a higher incidence of stillbirth during the 10 weeks following the lockdown than in the 10 weeks before the lockdown.7 The mechanisms underlying all of these reported findings are speculative and in most cases need to be considered in the context of the additional unknown effect of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection itself.8
AJOG at a Glance.
Why was this study conducted?
Widespread lockdowns imposed during the coronavirus disease 2019 (COVID-19) crisis may have affected birth outcomes worldwide.
Key findings
The postlockdown period in 2020 was associated with a 1.72 percentage point reduction (95% confidence interval [CI], −3.42% to −0.02) in the risk of any adverse outcome (stillbirth, preterm birth, small-for-gestational-age [SGA] fetuses, and neonatal death) and a 1.62 percentage point reduction (95% CI, −2.69 to −0.55) in the risk of any severe adverse outcome (stillbirth, very preterm birth, very SGA fetuses, and neonatal death). Reductions in adverse outcomes were largest among women with human immunodeficiency virus and among women delivering at urban sites, driven primarily by reductions in preterm birth and SGA fetuses.
What does this add to what is known?
Our data provide an evaluation from Sub-Saharan Africa of the impact of a COVID-19 lockdown on birth outcomes and suggest a modest reduction in preterm birth and SGA fetuses following the lockdown period.
To date, there has been no study on the impact of the COVID-19 lockdowns on adverse pregnancy outcomes in Sub-Saharan Africa, a region with one of the greatest burdens of adverse pregnancy outcomes and risk factors that are often distinct from those in high-income countries. Despite having only 3 reported SARS-CoV-2 cases at the time,9 Botswana announced a state of emergency because of COVID-19 on March 31, 2020,10 and a nationwide lockdown started at midnight on April 2, 2020.11, 12, 13 After the initial 28-day period, the lockdown was extended until May 7, 2020.14 Movement restrictions were gradually lifted between May 8, 2020, and May 22, 2020.15, 16, 17, 18, 19 Although SARS-CoV-2 swept through South Africa, infecting 364,328 people as of July 20, 2020,20 COVID-19 largely spared Botswana during the early phase of the pandemic; through July 20, 2020, there were 522 cases of SARS-CoV-2, and there was no confirmed case in pregnancy.20 This provides a unique opportunity to isolate the impact of the lockdown from any direct impact of SARS-CoV-2.
The Tsepamo study has been conducting birth outcomes surveillance at delivery hospitals throughout Botswana since August 2014 and includes data from more than 119,000 births. In this analysis, we used Tsepamo data to estimate the risk of adverse birth outcomes before (January 1, 2020–April 2, 2020), during (April 3, 2020–May 7, 2020), and after (May 8, 2020–July 20, 2020) the COVID-19 national lockdown and compared these risks with the same 3 periods in 2017–2019. We also examined whether the impact of the lockdown varied by HIV status, by urban or rural delivery hospital, and by other demographic factors.
Materials and Methods
The Tsepamo study
The Tsepamo study is a birth outcomes surveillance study in Botswana.21 Data were abstracted from the maternity obstetrical record (a record of antenatal care) at the time of delivery from all women delivering at selected hospitals throughout the country. The Tsepamo study included 8 sites (approximately 45% of all births in Botswana) from August 2014 to July 2018 and 18 sites (approximately 72% of all births nationwide) from July 2018 to July 2020. The Tsepamo study captured data on >99% of all births that occurred at the included sites because almost all women bring their antenatal medical records (“maternity card”) to delivery.21 , 22 In Botswana, approximately 95% of women deliver at a hospital.23
Eligibility criteria and exposure groups
Women who delivered a singleton baby after at least 24 weeks’ gestation in 2017–2020 between January 1 and July 20 were included in our analysis (in Botswana, pregnancies that end before 24 weeks’ gestation are considered miscarriage and admitted to the general medical wards). We defined January 1, 2020 to April 2 as the period before the lockdown (“prelockdown”), April 3, 2020 to May 7 as the period during the lockdown (“lockdown”), and May 8, 2020 to July 20 as the period following the lockdown (“postlockdown”). We compared the lockdown year, 2020, with the previous 3 years, 2017–2019.
Outcomes
Shelter-in-place adherence
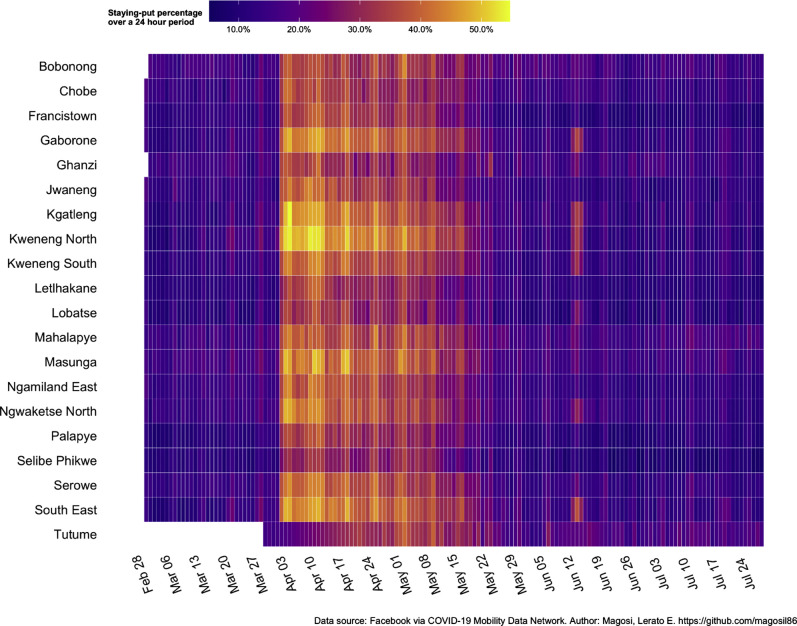
We defined the proportion of people remaining in 1 location over a 24-hour period as an indicator of shelter-in-place adherence. We calculated the average number of Facebook users with location services turned on that were present in the same 600×600-m grid location over a 24-hour period. Presence in the same location was defined as global positioning system pings in at least 3 different time blocks of the day.24, 25, 26 We created heatmaps to depict the 24-hour staying-put percentage by region of Botswana from February 28, 2020, to July 24, 2020.
Birth outcomes
The primary outcomes of interest were the combined endpoints of any adverse outcome and any severe adverse outcome. Any adverse outcome was composed of stillbirth, preterm birth, small-for-gestational-age (SGA) fetuses, or neonatal death. Any severe adverse was outcome composed of stillbirth, very preterm birth, very SGA fetuses, or neonatal death. Secondary endpoints were the individual outcomes. Stillbirth was defined as fetal death at ≥24 weeks’ gestation (summed Apgar score of 0). Preterm birth was defined as a birth at less than 37 weeks’ completed gestation and very preterm was a birth at less than 32 weeks’ completed gestation. Tertiary outcomes were birth at <34 weeks’ completed gestation27 and continuous gestational age at delivery. Gestational age was calculated at the time of delivery by the midwife using the estimated date of delivery determined during antenatal care, typically using reported last menstrual period. SGA fetuses were defined as fetuses less than the 10th percentile, and very SGA fetuses were defined as less than the 3rd percentile of birthweight by gestational age according to the International Fetal and Newborn Growth Consortium for the 21st Century norms.28 , 29 Neonatal deaths included deaths within 28 days of birth among infants who had never left the hospital.
Statistical analysis
We used a difference-in-differences analysis to assess the relationship between the lockdown and each outcome. That is we compared the change in each outcome from the prelockdown to lockdown periods in 2020 (the first difference) with the change in each outcome during the same 2 periods in 2017–2019 (the second difference). We also compared the change in each outcome from the prelockdown to postlockdown periods in 2020 with the change in each outcome during the same 2 periods in 2017–2019. We obtained 95% confidence intervals (CIs) using a linear probability model30 with robust standard errors to account for clustering within delivery sites.31 , 32 Relative risk reductions were calculated using the average baseline risk in the prelockdown period from 2017 to 2020.
We conducted separate analyses for the primary outcomes by maternal HIV status, delivery location (urban, delivery sites in Gaborone or Francistown; rural, all other delivery sites), parity (first child vs 1 or more children), and occupation (salaried vs nonsalaried). In post hoc analyses, we examined subgroups defined by multiple factors (eg, HIV status and delivery location).
In sensitivity analyses, we adjusted our estimates for individual-level demographic variables (HIV status, calendar year of delivery, age, occupation, education, parity, gravity, marital status, delivery location, smoking status, and use of alcohol), and extended the lockdown period through May 21 to include the 2-week period when restrictions were gradually lifted.
Finally, we plotted the weekly risk of the primary outcomes over a 28-week period (January 3, 2020–July 16, 2020) compared with the same period (January 2 to July 16) in 2017–2019.
Institutional approval for this study was granted by the Health Research and Development Committee in Botswana and by the institutional review board of Harvard T. H. Chan School of Public Health in Boston, Massachusetts. Maternal consent was waived because data were collected anonymously and by means of medical record abstraction.
Results
Study population
A total of 68,448 women delivered a singleton infant in 2017–2020 between January 1 and July 20 and were included in our analysis. Table 1 shows the number of births during the prelockdown, lockdown, and postlockdown periods in 2020 and during the same calendar periods in 2017–2019. Comparing 2020 with the previous year, the number of births was similar during the lockdown period (3589 vs 3432) but slightly lower during the postlockdown period (7162 vs 7413). Demographic characteristics were similar across years and across periods, except the median number of antenatal visits decreased from 10 across all periods in 2017–2019 to 9 across all periods in 2020 (Table 1). The median maternal age was 26 years, 23% were living with HIV, 38% delivered at an urban delivery site, 62% had other children, and 33% had a salaried occupation. Of the 15,767 women with HIV, the proportion who self-reported discontinuing antiretrovirals during pregnancy was less than 0.6% across all years and did not differ in 2020, including during the lockdown period (data not shown). To our knowledge, no modification to antenatal care was put in place during the lockdown period, and telemedicine was not routinely available. A food insecurity mitigation strategy was implemented in Botswana during the lockdown, with food baskets provided free of charge at locations throughout the country.
Table 1.
Characteristics of women giving birth in Botswana during the prelockdown (January 1 to April 2), lockdown (April 3 to May 7), and postlockdown (May 8 to July 20) periods in 2020 and during the same calendar periods in 2017–2019
| Characteristics | Year | Prelockdown period (Jan. 1 to April 2) | Lockdown period (April 3 to May 7) | Postlockdown period (May 8 to July 20) |
|---|---|---|---|---|
| Number of births (percentage of births during Jan. 1 to July 20 period) | 2017–2019a | 22,356 (46.5) | 8316 (17.3) | 17,396 (36.2) |
| 2017 | 6584 (46.6) | 2537 (17.9) | 5020 (35.5) | |
| 2018 | 6341 (46.5) | 2347 (17.2) | 4963 (36.4) | |
| 2019 | 9431 (46.5) | 3432 (16.9) | 7413 (36.6) | |
| 2020 | 9629 (47.3) | 3589 (17.6) | 7162 (35.1) | |
| Age | 2017–2019 | 26 (22–32) | 26 (22–32) | 26 (22–32) |
| 2020 | 26 (22–32) | 27 (22–33) | 26 (22–32) | |
| Nulliparity | 2017–2019 | 8524 (38.3) | 3215 (38.9) | 6607 (38.1) |
| 2020 | 3478 (36.3) | 1265 (35.4) | 2588 (36.2) | |
| Women living with HIV | 2017–2019 | 5164 (23.1) | 1941 (23.3) | 4032 (23.2) |
| 2020 | 2190 (22.7) | 785 (21.9) | 1655 (23.1) | |
| Delivery at urban delivery locationb | 2017–2019 | 9119 (40.8) | 3380 (40.6) | 7020 (40.4) |
| 2020 | 3146 (32.7) | 1124 (31.3) | 2284 (31.9) | |
| Salaried occupation | 2017–2019 | 7371 (33.0) | 2795 (33.6) | 5701 (32.8) |
| 2020 | 3188 (33.1) | 1197 (33.4) | 2289 (32.0) | |
| Antenatal visits | 2017–2019 | 10 (7–12) | 10 (7–12) | 10 (7–12) |
| 2020 | 9 (6–12) | 9 (6–12) | 9 (6–12) |
Data are presented as number (percentage) or median (interquartile range).
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2021.
The number of births increased in 2019 because of the expansion of the birth outcomes surveillance study in July 2018
Gaborone and Francistown.
Shelter-in-place adherence
Figure 1 shows the 24-hour staying-put percentage from February 28, 2020, to July 24, 2020, by region in Botswana. Staying-put percentage increased from 10% to 40% to 50% when the nationwide lockdown was instituted on April 3, 2020, gradually decreased following the phased relaxation of extreme social distancing measures beginning on May 8, 2020, and was consistent with prelockdown levels by June 5, 2020. Changes in staying-put percentage over time were consistent across the country.
Figure 1.
Staying-put percentage by region in Botswana, February 28, 2020 to July 24, 2020
Data are the average number of Facebook users with location services turned on that were present in the same 600×600-m grid location over a 24-hour period. Presence in the same location considered as global positioning system ping in at least 3 different time blocks of the day. Threshold: at least 300 unique users present. Baseline: average number of people staying put during the month of February 2020.
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2021.
Birth outcomes
Table 2 shows the net change in the risk of each outcome from the prelockdown to lockdown periods in 2020 relative to the same 2 periods in 2017–2019, and the net change in the risk of each outcome from the prelockdown to postlockdown periods in 2020 relative to the same 2 periods in 2017–2019. The lockdown period was associated with a 0.81 percentage point reduction (95% CI, −2.95% to 1.30%) in the risk of any adverse outcome (3% relative reduction) and a 0.02 percentage point reduction (95% CI, −0.79% to 0.75%) in the risk of any severe adverse outcome (0% relative reduction). The postlockdown period was associated with a 1.72 percentage point reduction (95% CI, −3.42% to −0.02%) in the risk of any adverse outcome (5% relative reduction) and a 1.62 percentage point reduction (95% CI, −2.69% to −0.55%) in the risk of any severe adverse outcome (14% relative reduction). The largest reduction associated with the lockdown period for an individual outcome was for preterm birth (−1.52 percentage points [95% CI, −3.14% to 0.10%] or 9% relative reduction), whereas the largest reduction associated with the postlockdown period for an individual outcome was for SGA fetuses (−1.07 percentage points [95% CI, −2.26% to 0.12%] or 7% relative reduction). There was no difference in neonatal death or stillbirth during the lockdown or postlockdown periods. Findings were similar when evaluating birth at less than 34 weeks’ completed gestation and continuous gestational age at delivery (Supplemental Table).
Table 2.
Risk difference and difference in differences (95% CI) of each adverse birth outcome during the prelockdown (January 1 to April 2), lockdown (April 3 to May 7), and postlockdown (May 8 to July 20) periods in 2020 and in the same calendar periods in 2017–2019
| Outcome | Prelockdown period(Jan. 1 to April 2); risk, n/N (%) | Lockdown period(April 3 to May 7); risk, n/N (%) | Postlockdown period(May 8 to July 20); risk, n/N (%) | Difference in differences (95% CI) |
|
|---|---|---|---|---|---|
| Lockdown vs prelockdowna | Postlockdown vs prelockdownb | ||||
| Any adverse outcome | |||||
| 2017–2019 | 6835/21,559 (31.70) | 2399/8018 (29.92) | 5040/16,827 (29.95) | ||
| 2020 | 2911/9273 (31.39) | 987/3427 (28.80) | 1925/6894 (27.92) | ||
| Difference, 2020 vs 2017–2019 | −0.31% (−1.44% to 0.82%) | −1.12% (−2.94% to 0.70%) | −2.03% (−3.29% to −0.76%) | −0.81% (−2.95% to 1.30%) | −1.72% (−3.42% to −0.02%) |
| Any severe adverse outcome | |||||
| 2017–2019 | 2451/21,540 (11.38) | 774/8015 (9.66) | 1750/16,815 (10.41) | ||
| 2020 | 1019/9271 (10.99) | 317/3427 (9.25) | 579/6890 (8.40) | ||
| Difference, 2020 vs 2017–2019 | −0.39% (−1.15% to 0.38%) | −0.41% (−1.57% to 0.76%) | −2.00% (−2.81% to −1.20%) | −0.02% (−0.79% to 0.75%) | −1.62% (−2.69% to −0.55%) |
| Stillbirth | |||||
| 2017–2019 | 530/22,354 (2.37) | 183/8316 (2.20) | 380/17,396 (2.18) | ||
| 2020 | 226/9629 (2.35) | 76/3589 (2.12) | 145/7162 (2.02) | ||
| Difference, 2020 vs 2017–2019 | −0.02% (−0.39% to 0.34%) | −0.08% (−0.65% to 0.48%) | −0.16% (−0.55% to 0.23%) | −0.06% (−0.90% to 0.78%) | −0.14% (−0.67% to 0.39%) |
| Preterm birth | |||||
| 2017–2019 | 3563/21,746 (16.38) | 1316/8075 (16.30) | 2624/16,916 (15.51) | ||
| 2020 | 1552/9332 (16.63) | 518/3448 (15.02) | 1031/6942 (14.85) | ||
| Difference, 2020 vs 2017–2019 | 0.25% (−0.66% to 1.15%) | −1.27% (−2.71% to 0.17%) | −0.66% (−1.66% to 0.34%) | –1.52% (−3.14% to 0.10%) | −0.91% (−2.57% to 0.75%) |
| Very preterm birth | |||||
| 2017–2019 | 833/21,746 (3.83) | 270/8075 (3.34) | 577/16,916 (3.41) | ||
| 2020 | 338/9332 (3.62) | 99/3448 (2.87) | 161/6942 (2.32) | ||
| Difference, 2020 vs 2017–2019 | −0.21% (−0.67% to 0.25%) | −0.47% (−1.15% to 0.21%) | −1.09% (−1.54% to −0.64%) | −0.26% (−0.80% to 0.27%) | −0.88% (−1.46% to −0.31%) |
| SGA | |||||
| 2017–2019 | 3560/21,517 (16.55) | 1173/8001 (14.66) | 2575/16,785 (15.34) | ||
| 2020 | 1464/9251 (15.83) | 493/3421 (14.41) | 932/6879 (13.55) | ||
| Difference, 2020 vs 2017–2019 | −0.72% (−1.61% to 0.17%) | −0.25% (−1.66% to 1.16%) | −1.79% (−2.77% to −0.82%) | 0.47% (−1.35% to 2.29%) | −1.07% (−2.26% to 0.12%) |
| Very SGA | |||||
| 2017–2019 | 1352/21,517 (6.28) | 415/8001 (5.19) | 940/16,785 (5.60) | ||
| 2020 | 584/9251 (6.31) | 177/3421 (5.17) | 321/6879 (4.67) | ||
| Difference, 2020 vs 2017–2019 | 0.03% (−0.56% to 0.62%) | −0.01% (−0.90% to 0.87%) | −0.93% (−1.54% to −0.33%) | −0.04% (−1.03% to 0.94%) | −0.96% (−1.87% to −0.05%) |
| Neonatal death | |||||
| 2017–2019 | 324/21,771 (1.49) | 96/8119 (1.18) | 212/16,991 (1.25) | ||
| 2020 | 104/9400 (1.11) | 32/3511 (0.91) | 76/7005 (1.08) | ||
| Difference, 2020 vs 2017–2019 | −0.38% (−0.65% to −0.12%) | −0.27% (−0.66% to 0.12%) | −0.16% (−0.46% to 0.13%) | 0.11% (−0.54% to 0.76%) | 0.22% (−0.16% to 0.60%) |
CI, confidence interval; SGA, small for gestational age.
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2021.
Calculated as the difference between the change in each outcome from the prelockdown to lockdown periods in 2020 and the change in each outcome during the same 2 calendar periods in 2017–2019
Calculated as the difference between the change in each outcome from the prelockdown to postlockdown periods in 2020 and the change in each outcome during the same 2 calendar periods in 2017–2019.
The reduction in both primary outcomes during the lockdown period was larger among women with HIV and among women with salaried employment (Table 3 ). The reduction in both primary outcomes during the postlockdown period was larger among women with HIV, women delivering at urban delivery sites, and women who already had children. The largest reductions were observed during the postlockdown period among women with HIV (−3.86 percentage points [95% CI, −6.32% to −1.39%] or 10% relative reduction for any adverse outcome and −2.26 percentage points [95% CI, −4.14% to −0.38%] or 16% relative reduction for any severe adverse outcome) and among women delivering at urban delivery sites (−3.37 percentage points [95% CI, −6.30% to −0.44%] or 10% relative reduction for any adverse outcome and −2.93 percentage points [95% CI, −5.01% to −0.85%] or 21% relative reduction for any severe adverse outcome). In a post hoc analysis, we calculated that for a woman with HIV delivering at an urban delivery site (9.3% of study population), there was a 6.31 percentage point reduction (95% CI, −14.21% to 1.59%) in the risk of having any adverse outcome (16% relative reduction) and a 2.17 percentage point reduction (95% CI, −7.88% to 3.55%) in the risk of having any severe adverse outcome (13% relative reduction) during the lockdown period. In this same subgroup, there was a 3.43 percentage point reduction (95% CI, −9.64% to 2.77%) in the risk of having any adverse outcome (9% relative reduction) and a 3.52 percentage point reduction (95% CI, −8.01% to 0.96%) in the risk of having any severe adverse outcome (22% relative reduction) during the postlockdown period.
Table 3.
Difference in differences (95% CI) of the composite adverse birth outcomes during the prelockdown (Jan. 1 to April 2), lockdown (April 3 to May 7), and postlockdown (May 8 to July 20) periods in 2020 and in the same calendar periods in 2017–2019, by key subgroups
| Outcome and subgroup | Prelockdown period risk (%) | Difference in differences (95% CI) |
|
|---|---|---|---|
| Lockdown vs prelockdowna | Postlockdown vs prelockdownb | ||
| Any adverse outcome | |||
| Overall | 31.61 | −0.81% (−2.95% to 1.30%) | −1.72% (−3.42% to−0.02%)c |
| Women with HIV | 37.69 | −3.51% (−9.40% to 2.38%) | −3.86% (−6.32% to −1.39%) |
| Women without HIV | 29.58 | 0.09% (−1.91% to 2.10%) | −0.98% (−3.12% to 1.17%) |
| Urban delivery sitesc | 33.79 | −0.60% (−4.33% to 3.13%) | −3.37% (−6.30% to −0.44%) |
| Rural delivery sitesd | 30.23 | −0.88% (−3.74% to 1.98%) | −0.83% (−2.17% to 0.52%) |
| Nulliparous women | 33.43 | −1.39% (−4.21% to 1.43%) | −0.64% (−2.98% to 1.71%) |
| Parous women | 30.39 | −0.47% (−3.14% to 2.20%) | −2.30% (−4.48% to −0.12%) |
| Women with salaried employment | 27.36 | −2.41% (−5.99% to 1.17%) | −2.45% (−5.80% to 0.90%) |
| Women without salaried employment | 33.73 | −0.03% (−2.12% to 2.06%) | −1.40% (−3.61% to 0.80%) |
| Women with HIV delivering at urban sitec | 39.67 | −6.31% (−14.21% to 1.59%) | −3.43% (−9.64% to 2.77%) |
| Any severe adverse outcome | |||
| Overall | 11.26 | −0.02% (−0.79% to 0.75%) | −1.62% (−2.69% to −0.55%) |
| Women with HIV | 13.78 | −1.05% (−3.94% to 1.85%) | −2.26% (−4.14% to −0.38%) |
| Women without HIV | 10.29 | 0.49% (−1.04% to 2.01%) | −1.33% (−2.37% to −0.28%) |
| Urban delivery sitesc | 14.08 | −0.15% (−2.79% to 2.50%) | −2.93% (−5.01% to −0.85%) |
| Rural delivery sitesd | 9.48 | 0.02% (−0.69% to 0.74%) | −0.98% (−2.28% to 0.32%) |
| Nulliparous women | 11.70 | 0.71% (−1.34% to 2.75%) | −0.96% (−3.26% to 1.35%) |
| Parous women | 10.91 | −0.33% (−1.57% to 0.91%) | −1.93% (−2.94% to −0.93%) |
| Women with salaried employment | 10.17 | −0.53% (−2.04% to 0.98%) | −1.48% (−2.73% to −0.22%) |
| Women without salaried employment | 11.81 | 0.23% (−0.87% to 1.34%) | −1.69% (−3.11% to −0.27%) |
| Women with HIV delivering at urban sitec | 16.22 | −2.17% (−7.88% to 3.55%) | −3.52% (−8.01% to 0.96%) |
CI, confidence interval.
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2021.
Calculated as the difference between the change in each outcome from the prelockdown to lockdown periods in 2020 and the change in each outcome during the same 2 calendar periods in 2017–2019
Calculated as the difference between the change in each outcome from the prelockdown to postlockdown periods in 2020 and the change in each outcome during the same 2 calendar periods in 2017–2019
Gaborone and Francistown
All other delivery sites.
Adjusting for individual-level demographic variables and extending the lockdown period by 2 weeks had no material impact on our estimates (data not shown).
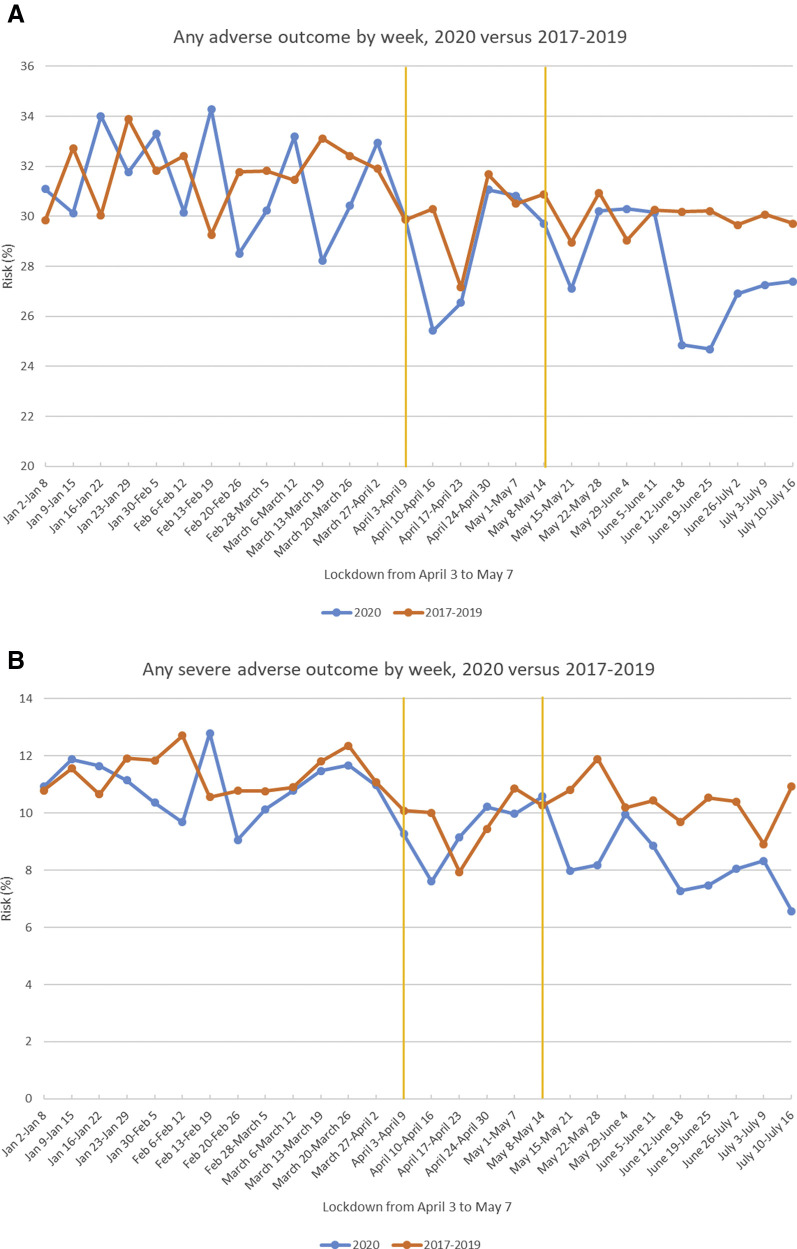
Figure 2 shows the risks of any (panel A) and any severe (panel B) adverse outcomes from January to June in 2017–2019 and in 2020. Although some seasonal or calendar time variation may have been present in all years, when comparing 2020 with 2017–2019, the weekly risks were similar during the 13 weeks before the lockdown period and the 5 weeks during the lockdown period, but lower during the 10 weeks following the lockdown period.
Figure 2.
Weekly risk of any adverse and any severe adverse outcome
Weekly risk of any adverse outcome (A) and any severe adverse outcome (B) over a 28-week period (January 3, 2020–July 16, 2020) compared with the same period (January 2 to July 16) in 2017–2019. The yellow vertical lines show the lockdown period.
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2021.
Comment
Principal findings
We utilized a large birth outcomes surveillance study in Botswana to estimate the changes in the risk of adverse birth outcomes following the COVID-19 national lockdown in 2020, compared to similar time periods in 2017-2019, to present a novel difference-in-differences analysis. During the lockdown period, the number of deliveries remained constant, there was noticeable adherence to the shelter-in-place order (40%–50%), and no meaningful difference in adverse birth outcomes was observed. However, we found modest reductions in the risk of any adverse outcome and any severe adverse outcome from the prelockdown to postlockdown periods, relative to changes during the same 2 periods in 2017–2019. These reductions were mostly driven by reductions in preterm birth, very preterm birth, SGA fetuses, and very SGA fetuses, whereas there was little evidence for a change in neonatal death or stillbirth. We found evidence for effect modification by HIV status and urban vs rural delivery site; the postlockdown period was associated with more than 3 percentage point reduction (approximately 10% relative reduction) in the risk of any adverse outcome and more than 2 percentage point reduction (16%–21% relative reduction) in the risk of any severe adverse outcome among women with HIV and among women delivering at urban sites.
Results
Our findings were consistent with some previous studies that found decreases in the risk of birth of low birthweight infants1 and preterm birth2 , 3 following COVID-19 lockdowns, although the magnitude of our findings was smaller. Our results differ from studies in London, Nepal, and India that found an increased risk of stillbirth during the lockdown period.5, 6, 7 The differences between our findings and studies conducted in Western Europe and the United States could be explained by differences in risk factors for adverse birth outcomes between high-income and low- and middle-income countries. In addition, the previous studies compared the lockdown period with the period immediately before the lockdown or to the same period in previous years, even though adverse birth outcomes vary by season and by calendar year.33, 34, 35 For example, several studies conducted in Sub-Saharan Africa have found more favorable birth outcomes in the dry season than in the rainy season,36, 37, 38, 39, 40, 41, 42 and previous work in Botswana found modest reductions in adverse birth outcomes over calendar time.43 By using a difference-in-differences approach, we were able to adjust for both seasonal and calendar variation in adverse birth outcomes. An additional strength of our study was the inclusion of more than half of all births in Botswana across multiple delivery sites, ensuring a representative sample of births in the country. Videos 1 and 2 summarize the study and key findings.
Clinical implications
Several possible explanations have been proposed for the favorable impact of COVID-19 lockdown on preterm birth and birth of low birthweight infants, including reducing inflammation, decreasing the risk of influenza and other infections, decreasing physical labor, decreasing stress, and decreasing exposure to air pollution.1 , 2 , 4 In Botswana, we found that the shelter-in-place order successfully led to more people staying put, which could have reduced physical labor, exposure to infections and air pollution, and some sources of stress. The food insecurity mitigation strategy implemented in Botswana could have increased nutritional support during the lockdown, but its impact remains unknown. It is also possible that the lockdown led to a reduction in preterm iatrogenic delivery. In addition, we did not find any evidence that the shelter-in-place order negatively affected access to medications for the 23% of women living with HIV. We saw greater reductions in adverse outcomes among women delivering at urban delivery sites, women with HIV, and women with salaried employment, suggesting that the lockdown could have affected the daily lives of these women to a larger extent. Although the greater reduction in adverse outcomes among women with HIV could be because of these women being more likely to deliver at urban deliver sites, it is also possible that sheltering in place directly affected adverse outcomes in this population, for example, through reducing inflammation. It is possible that stay-at-home orders had less of an impact on women in rural areas and women without salaried employment because these women may have continued physical labor, such as farming, during the lockdown period. It is also possible that the stay-at-home order increased stress,44 anxiety, and undernutrition (despite mitigation strategies), especially among those who were food insecure and economically disadvantaged. The reduction in adverse outcomes was greater (although modest) in the postlockdown period and negligible in the lockdown period. A plausible explanation for this finding is that the lockdown had a delayed effect on pregnancy outcomes, related to factors in the second trimester of pregnancy or early in the third trimester of pregnancy.
Research implications
Further studies are needed to identify both the mechanism and the gestational window for potential benefits related to decreasing movement during pregnancy and factors associated with pregnancy outcomes during pandemics. Although our findings may not be generalizable to other settings with different distributions of risk factors for adverse birth outcomes (such as maternal nutrition, age, and HIV prevalence45), they may also provide insight into potential interventions to reduce unknown causes of adverse outcomes.
Strengths and limitations
Difference-in-differences analyses rely on the assumption that the trend in adverse outcomes in 2017–2019 would be parallel to the trend in adverse outcomes in 2020 in the absence of the lockdown.46 Our finding that the weekly trend in adverse outcomes during the prelockdown period was similar in 2017–2019 compared with the same period in 2020 provides support that this “parallel trends” assumption may approximately hold. In addition, we found little variation in the demographic characteristics of women delivering throughout the study period. However, the parallel trends assumption would not be met if other changes occurred in Botswana at the same time as the lockdown that also affected adverse outcomes. Difference-in-differences analyses also require an assumption of “strict exogeneity” that the choice to impose a lockdown was not determined by the prelockdown risk of adverse outcomes.46 Because the lockdown was imposed exclusively to stop the spread of COVID-19, this assumption is likely to hold.
Our study has important limitations. First, our analysis only captured women delivering at a hospital included in the surveillance study. If women were more likely to deliver at home or at a local hospital not included in the surveillance study following the lockdown, our results could be biased. We found that the proportion of births during the postlockdown period in 2020 was slightly lower than the proportion of births during the same period in 2017–2019; however, it is unlikely that this approximately 1% decrease would explain our findings. Second, our analysis only captures births after at least 24 weeks’ gestation. If the risk of miscarriage changed during the lockdown period, we would not be able to capture this. Third, we were not able to assess individual-level mobility. Although staying-put percentage increased during the lockdown period, we were not able to evaluate the relationship between individual-level mobility and adverse outcomes.
Conclusions
We found a 1.72 percentage point reduction (5% relative reduction) in any adverse outcome and a 1.62 percentage point reduction (14% relative reduction) in any severe adverse outcome from the prelockdown to postlockdown periods in 2020, relative to changes during the same 2 periods in 2017–2019. We found no meaningful difference in adverse birth outcomes from the prelockdown to lockdown periods. The greatest impact was on preterm birth and SGA fetuses and among women with HIV and those delivering in urban areas. Although these reductions were modest, they may provide insights into identifying potential interventions to reduce adverse birth outcomes in Botswana and in other low- and middle-income countries throughout the world.
Acknowledgments
We would like to thank the coronavirus disease (COVID-19) mobility network and Facebook Data for Good for providing access to the aggregated anonymized mobility data and Caroline Buckee, Andrew Schroeder, Nishant Kishore, and Alex Pompe for helping us understand the data. We would also like to thank the Tsepamo study team, including our research assistants, Daphne Segobye, Tsaone Gaonakala, Cynthia Dube, Edith Moseki, Gosego Legase, Keemenao France, Mmapula Ofhentse, Naledi Kamanga, Onkabetse Mokgosi, Rosemary Moremi, Shally Morgan, Tshepang Motlotlegi, Patricia Mophutegi, Keba Rabasiako, Nametsang Thosa, Maipelo Kegakilwe, Masego Kgafela, Tshegofato Motladile, Tsholofelo Tsokunyane, Kealeboga Mmokele, Obakeng Makalane, Thuto Rabana, Seele Mafokate, Annah Bojang, Tlhabologo Baitsemi, Priscilla Mashona, and Bathoba Mabiletsa; the maternity staff and administrators at the 18 participating hospitals; the members of the Botswana Ministry of Health and Wellness, in particular, the Department of HIV/AIDS Prevention and Care and the Department of Maternal and Child Health; and Rohan Hazra and Nahida Chakhtoura of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) for their support. This study would not have been possible without the support of the leadership of the Botswana-Harvard AIDS Institute Partnership, including Ria Madison, Bernadette Kgake, Tryphinah Majutah-Lungah, and Erik van Widenfeld. We also acknowledge the funding and support from the NICHD and National Institute of Allergy and Infectious Diseases.
Footnotes
The authors report no conflict of interest.
This work was funded by the National Institutes of Health (NIH)/the Eunice Kennedy ShriverNational Institute of Child Health and Human Development (R01 HD080471, K23 HD088230-01A1, and K01 HD100222-01A1) and NIH/the National Institute of Allergy and Infectious Diseases (K24AI131924).
Cite this article as: Caniglia EC, Magosi LE, Zash R, et al. Modest reduction in adverse birth outcomes following the coronavirus disease 2019 lockdown. Am J Obstet Gynecol 2021;224:615.e1-12.
Supplementary Data
Short video for Twitter
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2020.
Long video for Facebook
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2020.
Supplementary Material
Supplemental Table.
Mean difference, risk difference, and difference in differences (95% CI) of gestational age at delivery and birth at <34 weeks’ gestation during the prelockdown (January 1 to April 2), lockdown (April 3 to May 7), and postlockdown (May 8 to July 20) periods in 2020 and in the same calendar periods in 2017–2019
| Outcome | Prelockdown period(Jan. 1 to April 2) | Lockdown period(April 3 to May 7) | Postlockdown period(May 8 to July 20) | Difference in differences (95% CI) |
|
|---|---|---|---|---|---|
| Lockdown vs prelockdowna | Postlockdown vs prelockdownb | ||||
| Gestational age at delivery | Mean (wk) | Mean (wk) | Mean (wk) | ||
| 2017–2019 | 38.33 | 38.36 | 38.38 | — | — |
| 2020 | 38.32 | 38.43 | 38.49 | — | — |
| Difference, 2020 vs 2017–2019 | −0.02 (−0.09 to 0.05) | 0.08 (−0.03 to 0.19) | 0.10 (0.03–0.18) | 0.10 (0.01–0.19) weeks | 0.12 (0.00–0.25) weeks |
| Birth at <34 wk | Risk, n/N (%) | Risk, n/N (%) | Risk, n/N (%) | ||
| 2017–2019 | 1350/21,746 (6.21) | 472/8,075 (5.85) | 941/16,916 (5.56) | — | — |
| 2020 | 553/9332 (5.93) | 175/3448 (5.08) | 312/6942 (4.49) | — | — |
| Difference, 2020 vs 2017–2019 | −0.28% (−0.86% to 0.29%) | −0.77% (−1.66% to 0.12%) | −1.07% (−1.67% to −0.47%) | −0.49% (−1.12% to 0.17%) | −0.79% (−1.67% to 0.10%) |
CI, confidence interval.
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2021.
Calculated as the difference between the change in each outcome from the pre-lockdown to lockdown periods in 2020 and the change in each outcome during the same two calendar periods in 2017–2019
Calculated as the difference between the change in each outcome from the pre-lockdown to post-lockdown periods in 2020 and the change in each outcome during the same two calendar periods in 2017–2019.
References
- 1.Philip R.K., Purtill H., Reidy E., et al. Reduction in preterm births during the COVID-19 lockdown in Ireland: a natural experiment allowing analysis of data from the prior two decades. 2020. https://www.medrxiv.org/content/10.1101/2020.06.03.20121442v1 Available at: Accessed August 1, 2020. [DOI] [PMC free article] [PubMed]
- 2.Hedermann G., Hedley P.L., Baekvad-Hansen M., et al. Changes in premature birth rates during the Danish nationwide COVID-19 lockdown: a nationwide register-based prevalence proportion study. 2020. https://www.medrxiv.org/content/10.1101/2020.05.22.20109793v1 Available at: Accessed August 1, 2020.
- 3.Been J.V., Burgos Ochoa L., Bertens L.C.M., Schoenmakers S., Steegers E.A.P., Reiss I.K.M. Impact of COVID-19 mitigation measures on the incidence of preterm birth: a national quasi-experimental study. Lancet Public Health. 2020;5:e604–e611. doi: 10.1016/S2468-2667(20)30223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The New York Times During coronavirus lockdowns, some doctors wondered: where are the preemies? 2020. https://www.healthleadersmedia.com/clinical-care/during-coronavirus-lockdowns-some-doctors-wondered-where-are-preemies Available at: Accessed August 1, 2020.
- 5.Khalil A., von Dadelszen P., Draycott T., Ugwumadu A., O’Brien P., Magee L. Change in the incidence of stillbirth and preterm delivery during the COVID-19 pandemic. JAMA. 2020;324:705–706. doi: 10.1001/jama.2020.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kc A., Gurung R., Kinney M.V., et al. Effect of the COVID-19 pandemic response on intrapartum care, stillbirth, and neonatal mortality outcomes in Nepal: a prospective observational study. Lancet Glob Health. 2020;8:e1273–e1281. doi: 10.1016/S2214-109X(20)30345-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumari V., Mehta K., Choudhary R. COVID-19 outbreak and decreased hospitalisation of pregnant women in labour. Lancet Glob Health. 2020;8:e1116–e1117. doi: 10.1016/S2214-109X(20)30319-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalil A., Kalafat E., Benlioglu C., et al. SARS-CoV-2 infection in pregnancy: a systematic review and meta-analysis of clinical features and pregnancy outcomes. EClinicalMedicine. 2020;25:100446. doi: 10.1016/j.eclinm.2020.100446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.APO Group Coronavirus—Botswana: COVID-19 cases statistics in Botswana as of March 28, 2020. 2020. https://www.africanews.com/2020/03/28/coronavirus-botswana-coronavirus-covid-19-cases-statistics-in-botswana-as-of-28-march-2020// Available at: Accessed August 1, 2020.
- 10.Republic of Botswana [NEOC Bulletin] - Issue 1. Presidential (COVID-19) Task Force Bulletin. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 11.Republic of Botswana [NEOC Bulletin] - Issue 2. Presidential (COVID-19) Task Force Bulletin. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 12.Republic of Botswana. Government Gazette Extraordinary April 2, 2020. 2020. Gaborone; 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 13.Republic of Botswana. Government Gazette Extraodinary March 31, 2020. 2020. Gaborone; 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 14.Republic of Botswana [NEOC Bulletin] - Issue 21. Presidential (COVID-19) Task Force. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 15.Republic of Botswana [NEOC Bulletin] - Issue 36. Presidential (COVID-19) Task Force. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 16.Republic of Botswana. NEOC—return to work guidelines 5 May 2020; 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 17.Republic of Botswana [NEOC Bulletin] - Issue 28. Presidential (COVID-19) Task Force. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 18.Botswana Ministry of Health . 2020. NEOC Bulletin - Issue 31. Presidential (COVID-19) Task Force. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 19.Republic of Botswana [NEOC Bulletin] - Issue 27. Presidential (COVID-19) Task Force. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 20.Republic of Botswana [NEOC Bulletin] - Issue 79. Presidential (COVID-19) Task Force. 2020. Available at: https://www.bocra.org.bw/covid19-information. Accessed August 1, 2020. [Google Scholar]
- 21.Zash R., Jacobson D.L., Diseko M., et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr. 2017;171 doi: 10.1001/jamapediatrics.2017.2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zash R., Holmes L., Diseko M., et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381:827–840. doi: 10.1056/NEJMoa1905230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization Botswana: WHO statistical profile. 2015. http://www.who.int/gho/countries/bwa.pdf?ua=1&ua=1 Available at:
- 24.COVID-19 Mobility Data Network. 2020. https://www.covid19mobility.org/ Available at:
- 25.Facebook Data for Good. 2020. https://dataforgood.fb.com/ Available at:
- 26.Buckee C.O., Balsari S., Chan J., et al. Aggregated mobility data could help fight COVID-19. Science. 2020;368:145–146. doi: 10.1126/science.abb8021. [DOI] [PubMed] [Google Scholar]
- 27.Williams J.E., Pugh Y. The late preterm: a population at risk. Crit Care Nurs Clin North Am. 2018;30:431–443. doi: 10.1016/j.cnc.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Villar J., Cheikh Ismail L., Victora C.G., et al. International standards for newborn weight, length, and head circumference by gestational age and sex: the newborn cross-sectional study of the INTERGROWTH-21st project. Lancet. 2014;384:857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 29.Villar J., Giuliani F., Fenton T.R., et al. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387:844–845. doi: 10.1016/S0140-6736(16)00384-6. [DOI] [PubMed] [Google Scholar]
- 30.Spiegelman D., Hertzmark E. Easy SAS calculations for risk or prevalence ratios and differences. Am J Epidemiol. 2005;162:199–200. doi: 10.1093/aje/kwi188. [DOI] [PubMed] [Google Scholar]
- 31.Castillo-Carniglia A., Kaufman J.S., Pizarro E., Marín J.D., Wintemute G., Cerdá M. School collective occupation movements and substance use among adolescents: a school-level panel design. Drug Alcohol Depend. 2017;176:21–27. doi: 10.1016/j.drugalcdep.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 32.Allison P.D. Second edition. SAS Institute; Cary, NC: 2012. Logistic regression using SAS: theory and application. [Google Scholar]
- 33.Bodnar L.M., Simhan H.N. The prevalence of preterm birth and season of conception. Paediatr Perinat Epidemiol. 2008;22:538–545. doi: 10.1111/j.1365-3016.2008.00971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman A., Rahman M., Pervin J., et al. Time trends and sociodemographic determinants of preterm births in pregnancy cohorts in MATLAB, Bangladesh, 1990-2014. BMJ Glob Health. 2019;4 doi: 10.1136/bmjgh-2019-001462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization Preterm birth. 2018. https://www.who.int/news-room/fact-sheets/detail/preterm-birth Available at:
- 36.Friis H., Gomo E., Nyazema N., et al. Maternal body composition, HIV infection and other predictors of gestation length and birth size in Zimbabwe. Br J Nutr. 2004;92:833–840. doi: 10.1079/bjn20041275. [DOI] [PubMed] [Google Scholar]
- 37.Chodick G., Flash S., Deoitch Y., Shalev V. Seasonality in birth weight: review of global patterns and potential causes. Hum Biol. 2009;81:463–477. doi: 10.3378/027.081.0405. [DOI] [PubMed] [Google Scholar]
- 38.Fallis G., Hilditch J. A comparison of seasonal variation in birthweights between rural Zaire and Ontario. Can J Public Health. 1989;80:205–208. [PubMed] [Google Scholar]
- 39.Ceesay S.M., Prentice A.M., Cole T.J., et al. Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. BMJ. 1997;315:786–790. doi: 10.1136/bmj.315.7111.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kinabo J. Seasonal variation of birth weight distribution in Morogoro, Tanzania. East Afr Med J. 1993;70:752–755. [PubMed] [Google Scholar]
- 41.Prentice A.M., Cole T.J., Foord F.A., Lamb W.H., Whitehead R.G. Increased birthweight after prenatal dietary supplementation of rural African women. Am J Clin Nutr. 1987;46:912–925. doi: 10.1093/ajcn/46.6.912. [DOI] [PubMed] [Google Scholar]
- 42.Rayco-Solon P., Fulford A.J., Prentice A.M. Differential effects of seasonality on preterm birth and intrauterine growth restriction in rural Africans. Am J Clin Nutr. 2005;81:134–139. doi: 10.1093/ajcn/81.1.134. [DOI] [PubMed] [Google Scholar]
- 43.Caniglia E.C., Zash R., Jacobson D.L., et al. Emulating a target trial of antiretroviral therapy regimens started before conception and risk of adverse birth outcomes. AIDS. 2018;32:113–120. doi: 10.1097/QAD.0000000000001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cousins S. COVID-19 has “devastating” effect on women and girls. Lancet. 2020;396:301–302. doi: 10.1016/S0140-6736(20)31679-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christian P., Smith E.R., Zaidi A. Addressing inequities in the global burden of maternal undernutrition: the role of targeting. BMJ Glob Health. 2020;5 doi: 10.1136/bmjgh-2019-002186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wing C., Simon K., Bello-Gomez R.A. Designing difference in difference studies: best practices for public health policy research. Annu Rev Public Health. 2018;39:453–469. doi: 10.1146/annurev-publhealth-040617-013507. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Short video for Twitter
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2020.
Long video for Facebook
Caniglia et al. Coronavirus disease 2019 lockdown and adverse birth outcomes in Botswana. Am J Obstet Gynecol 2020.