Abstract
Background
Patients with cancer who are infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are more likely to develop severe illness and die compared with those without cancer. The impact of immune checkpoint inhibition (ICI) on the severity of COVID-19 illness is unknown. The aim of this study was to investigate whether ICI confers an additional risk for severe COVID-19 in patients with cancer.
Methods
We analyzed data from 110 patients with laboratory-confirmed SARS-CoV-2 while on treatment with ICI without chemotherapy in 19 hospitals in North America, Europe and Australia. The primary objective was to describe the clinical course and to identify factors associated with hospital and intensive care (ICU) admission and mortality.
Findings
Thirty-five (32%) patients were admitted to hospital and 18 (16%) died. All patients who died had advanced cancer, and only four were admitted to ICU. COVID-19 was the primary cause of death in 8 (7%) patients. Factors independently associated with an increased risk for hospital admission were ECOG ≥2 (OR 39.25, 95% CI 4.17 to 369.2, p=0.0013), treatment with combination ICI (OR 5.68, 95% CI 1.58 to 20.36, p=0.0273) and presence of COVID-19 symptoms (OR 5.30, 95% CI 1.57 to 17.89, p=0.0073). Seventy-six (73%) patients interrupted ICI due to SARS-CoV-2 infection, 43 (57%) of whom had resumed at data cut-off.
Interpretation
COVID-19–related mortality in the ICI-treated population does not appear to be higher than previously published mortality rates for patients with cancer. Inpatient mortality of patients with cancer treated with ICI was high in comparison with previously reported rates for hospitalized patients with cancer and was due to COVID-19 in almost half of the cases. We identified factors associated with adverse outcomes in ICI-treated patients with COVID-19.
Keywords: immunotherapy
Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).1 As of October 2020, more than 35,000,000 people have been infected with SARS-CoV-2 worldwide with more than 1,000,000 deaths.2 The clinical spectrum of COVID-19 varies enormously from asymptomatic individuals to critical illness and death.3 Risk factors for severe disease include older age, male sex and comorbidities such as cardiovascular and pulmonary diseases, diabetes and cancer.4 5 Mortality rates of COVID-19 infection in the cancer population range from 7.6% to 33%5–9 compared with 1.4%–2.3%3 10 in an unselected population.
One key question specific for the cancer population pertains to the potential impact of immune checkpoint inhibition (ICI) on the clinical course of COVID-19. Programmed cell death 1 (PD-1)–based immunotherapy releases the brakes of immune tolerance mechanisms leading to effective anti-tumor responses.11 Adaptive immune cells involved in this process, in particular CD8+ and CD4+ T cells, are also essential to control and establish immunity against viruses, and ICI may enhance immunologic control of viral infections.12 13 It is therefore theoretically possible that ICI offers protection against the development of severe COVID-19 illness. Nevertheless, these immune cells—either through direct cytotoxicity or cytokine release—can also contribute to inflammation and may aggravate the clinical course of COVID-19. An example of an immune-mediated consequence of SARS-CoV-2 is acute respiratory distress syndrome which is the leading cause of mortality in COVID-19.14 15
ICI has been associated with severe disease in some9 16 17 but not all18 studies, and the numbers of ICI-treated patients in these studies were small. Although vigilance is certainly warranted in the ICI-treated patient population, unnecessary treatment delays may compromise cancer-related outcomes and some patients may not be offered ICI therapy in areas of high COVID-19 prevalence due to concerns of infection and severe illness. With the pandemic continuing, recommendations are needed to inform ICI-related treatment decisions. In this multicentric study, we describe the clinical course, treatment and outcomes of COVID-19 infection in patients treated with ICI across different tumor types and across different geographic regions.
Methods
Study design and participants
We conducted a multicenter, retrospective, cohort study in 19 centers across 9 countries (Australia, Canada, France, Germany, Italy, Switzerland, The Netherlands, UK and USA). Between March 5 and May 15, 2020, we included 110 adult (aged ≥18 years) patients with any type of solid malignancy who had undergone treatment with ICI and had laboratory-confirmed positive SARS-CoV-2. The test could either be nucleic acid detection based (nasopharyngeal swabs) or serological. Asymptomatic patients found positive for SARS-CoV-2 were included in this study. These patients were tested according to local policies after exposure to a person with confirmed SARS-CoV-2 (transmission tracking and contact tracing). All patients must have received at least one cycle of ICI within 12 months prior to testing positive for SARS-CoV-2. Patients who received chemotherapy within 12 weeks prior to COVID-19 diagnosis were excluded as chemotherapy-induced immunosuppression may have confounded analyses. Combinations of ICI with anti-VEGF agents were allowed. Clinical and laboratory data were obtained from the medical records from each of the centers. Local Institutional Review Board approval was required for each center. All study procedures were in accordance with the precepts of Good Clinical Practice and the declaration of Helsinki.
Procedures
Clinical data were extracted from medical records and de-identified for analysis. Data were divided into the following categories: demographics and patient characteristics (age, sex, geographic region, Eastern Cooperative Oncology Group (ECOG) performance status), cancer characteristics (cancer type, American Joint Committee on Cancer stage, treatment setting), comorbidities (cardiovascular, pulmonary, renal disease or diabetes mellitus), ICI treatment (anti-PD-1/anti-PD-L1, combination anti-PD-1 plus anti-CTLA-4 or other), COVID-19 symptoms (fever, cough, dyspnea), laboratory tests (leukocytes, lymphocytes, C reactive protein (CRP) and creatinine), management (oxygen therapy, mechanical ventilation, use of vasopressors, renal replacement therapy, antivirals, antibiotics, glucocorticoids, anti-IL-6 agents and intravenous immunoglobulins) and clinical outcomes (hospital admission, intensive care unit admission and mortality).
Statistical analysis
Patient demographics and clinical characteristics are summarized by their frequency and proportion. Primary outcomes were hospital admission (coded yes/no) and overall survival (time to event). Measures for association between demographic and clinical characteristics on one hand and hospital admission on the other hand were assessed using univariate and multivariate logistic regression. ORs and associated 95% CIs are reported. Overall survival was assessed using univariate Cox proportional hazard regression; given the low number of deaths observed, multivariate Cox was not considered. HRs and associated 95% CIs are reported. For associations between demographic and clinical characteristics and intensive care unit admission, only frequencies are reported given the low absolute number of events. The selection of symptoms and laboratory findings was based on previous reports.19
Results
Patient characteristics
Between March 5 and May 15, 2020, 110 patients with cancer on ICI only who tested positive for SARS-CoV-2 were included in this study. The median follow-up since COVID-19 diagnosis was 82 days (range 1–119 days). The median age was 63 years (range 27–86) and most patients were male (65%). Seventy (64%) patients were treated in Europe (48 in Italy) and 36 (33%) in North America. Eighty-three (75%) patients had advanced cancer, including 64 (58%) with melanoma, 17 (16%) with non-small cell lung cancer (NSCLC) and 10 (9%) with renal cell carcinoma (RCC) (table 1). Most patients did not have an underlying comorbidity (55%); the most common comorbidities were cardiovascular disease (27%), diabetes mellitus (15%), pulmonary disease (12%) and/or renal disease (5%). Overall, for cancer, 90 (82%) patients were treated with anti-PD-(L)1 monotherapy (nivolumab, pembrolizumab, spartalizumab, atezolizumab or durvalumab) and 16 (15%) with combination anti-PD-(L)1 and anti-CTLA-4 (nivolumab–ipilimumab, durvalumab–tremelimumab or pembrolizumab–MK1308). Twenty-five (23%) patients had chemotherapy prior to ICI. The median time between last chemotherapy and COVID-19 diagnosis was 40 weeks (range 12–559). Out of 70 patients with response evaluation available at COVID-19 diagnosis, 33 had a partial or complete response (advanced setting) or no evidence of recurrence (adjuvant setting), 20 stable disease and 17 disease progression. Further demographic, clinical, treatment and laboratory findings are presented in table 1.
Table 1.
Demographic, disease and treatment findings at COVID-19 diagnosis
| All patients (n=110) | Admitted (n=35) | Died (n=18) | ||||
| Median age (range) years | 63 (27–86) | 65 (35–83) | 63 (42–81) | |||
| <65 | 58 | (53%) | 17 | (49%) | 9 | (50%) |
| ≥65 | 52 | (47%) | 18 | (51%) | 9 | (50%) |
| Sex | ||||||
| Female | 38 | (35%) | 11 | (32%) | 7 | (39%) |
| Male | 72 | (65%) | 24 | (68%) | 11 | (61%) |
| Region | ||||||
| Australia | 4 | (3%) | 1 | (3%) | 0 | (0%) |
| Europe | 70 | (64%) | 16 | (46%) | 5 | (27%) |
| Italy | 48 | (45%) | 6 | (17%) | 3 | (17%) |
| UK | 8 | (7%) | 5 | (14%) | 1 | (5%) |
| The Netherlands | 6 | (5%) | 2 | (6%) | 0 | (0%) |
| Germany | 4 | (3%) | 1 | (3%) | 0 | (0%) |
| France | 3 | (3%) | 2 | (6%) | 1 | (5%) |
| Switzerland | 1 | (1%) | 0 | (0%) | 0 | (0%) |
| North America | 36 | (33%) | 18 | (51%) | 13 | (73%) |
| USA | 29 | (27%) | 16 | (45%) | 11 | (62%) |
| Canada | 7 | (6%) | 2 | (6%) | 2 | (11%) |
| ICI treatment setting | ||||||
| (Neo)adjuvant | 27 | (25%) | 4 | (11%) | 0 | (0%) |
| Advanced | 83 | (75%) | 31 | (89%) | 18 | (100%) |
| Cancer type | ||||||
| Melanoma | 64 | (58%) | 17 | (49%) | 5 | (28%) |
| NSCLC | 17 | (16%) | 5 | (14%) | 4 | (22%) |
| RCC | 10 | (9%) | 4 | (11%) | 2 | (11%) |
| Other | 19 | (17%) | 9 | (26%) | 7 | (39%) |
| Coexisting disorder | ||||||
| None | 60 | (55%) | 21 | (60%) | 8 | (44%) |
| Cardiovascular | 30 | (27%) | 5 | (14%) | 2 | (11%) |
| Diabetes mellitus | 16 | (15%) | 6 | (17%) | 3 | (17%) |
| Pulmonary | 13 | (12%) | 5 | (14%) | 4 | (22%) |
| Renal | 6 | (5%) | 3 | (9%) | 3 | (17%) |
| ICI | ||||||
| Anti-PD-1/PD-L1 | 90 | (82%) | 23 | (66%) | 13 | (72%) |
| Anti-PD-1 plus anti-CTLA-4 | 16 | (15%) | 10 | (28%) | 4 | (22%) |
| Other* | 4 | (3%) | 2 | (6%) | 1 | (6%) |
| Previous chemotherapy | ||||||
| Yes | 25 | (23%) | 8 | (23%) | 6 | (33%) |
| No | 85 | (77%) | 27 | (77%) | 12 | (67%) |
| Response to ICI at COVID-19 diagnosis Adjuvant |
||||||
| NED | 4 | (4%) | 1 | (3%) | 0 | (0%) |
| Advanced | ||||||
| PR/CR | 29 | (26%) | 7 | (20%) | 3 | (17%) |
| SD | 20 | (18%) | 6 | (17%) | 4 | (22%) |
| PD | 17 | (16%) | 11 | (31%) | 6 | (33%) |
| Not reported | 40 | (36%) | 10 | (29%) | 5 | (28%) |
*Pembrolizumab–bevacizumab, pembrolizumab–anti-TIGIT, avelumab–axitinib, pembrolizumab–vopratelimab.
CR, complete response; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ICI, immune checkpoint inhibition; NED, no evidence of disease; NSCLC, non-small cell lung cancer; PD, progressive disease; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1; PR, partial response; RCC, renal cell carcinoma; SD, stable disease.
Diagnosis and management of COVID-19
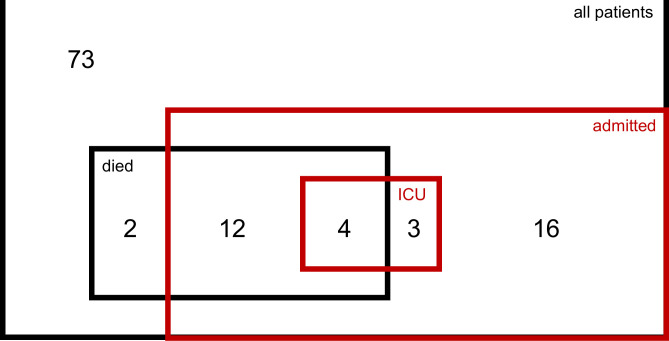
Median time between COVID-19 diagnosis and last ICI dose was 26 days (range 0–363). Sixty-seven (62%) patients were symptomatic at COVID-19 diagnosis. The most common COVID-19–related symptoms were fever (67%), cough (58%) and dyspnea (33%), and 10% of patients presented ECOG ≥2 at the time of COVID-19 diagnosis. Lymphocytopenia was the most common laboratory abnormality in 47% of the 79 patients who had laboratory tests available within 3 days of COVID-19 diagnosis, and 15 patients (14%) were on prednisone equivalent dose of ≥10 mg at diagnosis, mostly for ICI toxicity (table 2). Thirty-five (32%) patients were admitted to hospital and 7 (6%) to intensive care (figure 1). For the patients managed in hospital, 20 received oxygen therapy and 3 mechanical ventilation. Antibiotics and antiviral agents were given to 24 and 5 admitted patients, respectively. Only 10 patients were treated with glucocorticoids for COVID-19; anti-IL6 and intravenous immunoglobulin were given to 2 patients and 1 patient, respectively (table 2). Two patients required vasopressor support and one patient renal replacement therapy. Of the 110 patients, ICI was interrupted in 76 (73%) and resumed in 43 (57%).
Table 2.
Symptoms, laboratory findings and treatments for COVID-19
| All patients (n=110) | Admitted (n=35) | Died (n=18) | ||||
| Median time between last ICI and COVID-19 diagnosis, days (range) | 26 (0–363) | 36 (0–363) | 17 (0–319) | |||
| Symptomatic at COVID-19 diagnosis | ||||||
| Yes* | 67/108 | (62%) | 29/35 | (83%) | 15/18 | (82%) |
| Fever | 45/67 | (67%) | 20/29 | (69%) | 8/15 | (53%) |
| Cough | 39/67 | (58%) | 15/29 | (52%) | 8/15 | (53%) |
| Dyspnea | 22/67 | (33%) | 15/29 | (52%) | 9/15 | (60%) |
| ECOG at COVID-19 diagnosis | ||||||
| 0–1 | 99 | (90%) | 25 | (72%) | 13 | (72%) |
| 2–4 | 11 | (10%) | 10 | (28%) | 5 | (28%) |
| Laboratory tests† | ||||||
| WBC ≥10,000/mm3 | 13/79 | (16%) | 10/29 | (34%) | 3/15 | (20%) |
| Lymphocytes <1500/mm3 | 36/77 | (47%) | 19/28 | (68%) | 11/14 | (79%) |
| CRP ≥100 mg/L | 11/36 | (31%) | 11/26 | (42%) | 8/14 | (57%) |
| Creatinine ≥133 µM | 10/78 | (13%) | 6/28 | (21%) | 3/14 | (21%) |
| Prednisone ≥10 mg equivalent | 15/110 | (14%) | 10/35 | (28%) | 4/18 | (22%) |
| Admission to hospital | 35/110 | (32%) | -– | -– | 16/18 | (89%) |
| Admission to intensive care unit | 7/110 | (6%) | 7/35 | (20%) | 4/18 | (22%) |
| Oxygen therapy | 22/108 | (20%) | 20/33 | (61%) | 12/16 | (75%) |
| Mechanical ventilation | 3/108 | (3%) | 3/33 | (9%) | 2/16 | (13%) |
| Use of antibiotics | 28/108 | (26%) | 24/33 | (73%) | 13/16 | (81%) |
| Use of antivirals | 7/107 | (7%) | 5/32 | (16%) | 5/16 | (31%) |
| Use of glucocorticoids | 10/107 | (9%) | 10/32 | (31%) | 3/16 | (19%) |
| Use of anti-IL6 agents | 2/108 | (2%) | 2/33 | (6%) | 2/16 | (13%) |
| Use of intravenous immunoglobulins | 1/108 | (1%) | 1/33 | (3%) | 0/16 | (0%) |
| Use of vasopressor support | 2/108 | (2%) | 2/33 | (6%) | 2/16 | (13%) |
| Use of renal replacement therapy | 1/108 | (1%) | 1/33 | (3%) | 0/16 | (0%) |
| ICI interrupted for COVID-19‡ | ||||||
| Yes | 76/104 (73%) | 24/32 (75%) | 13/17 (77%) | |||
| No | 28/104 (27%) | 8/32 (25%) | 4/17 (23%) | |||
| ICI resumed§ | ||||||
| Yes | 43/76 (57%) | 4/24 (17%) | 2/13 (15%) | |||
| No | 33/76 (43%) | 20/24 (83%) | 11/13 (85%) | |||
*Not known for 2 patients.
†Within 3 days of COVID-19 diagnosis.
‡Information on reason for interrupting ICI was not known for 6 patients.
§Number of patients who resumed ICI after discontinuing because of COVID-19.
CRP, C reactive protein; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibition; WBC, white blood cells.
Figure 1.
Venn diagram representing all patients (110), patients admitted to hospital (35), admitted to intensive care unit (ICU) (7) and patients who died (18).
Clinical factors associated with a higher hospital admission
Factors associated with a higher risk for hospital admission for COVID-19 management included treatment with combination immunotherapy (OR 4.37, 95% CI 1.40 to 13.61, p=0.0287), ECOG ≥2 (OR 30.82, 95% CI 3.75 to 253.2, p=0.0014), treatment with corticosteroids at a prednisone equivalent dose of ≥10 mg (OR 5.04, 95% CI 1.54 to 16.48, p=0.0075), COVID-19 symptoms (OR 5.34, 95% CI 1.86 to 15.33, p=0.0018), in particular dyspnea (OR 4.74, 95% CI 1.58 to 14.21, p=0.0054), leukocyte count ≥10,000/mm3 (OR 8.70, 95% CI 2.15 to 35.29, p=0.0025) and lymphocyte count <1500/mm3 (OR 3.76, 95% CI 1.39 to 10.16, p=0.0089) (table 3). Of the 13 patients who had leukocytosis, 4 were in treatment corticosteroids (prednisone equivalent dose ≥10 mg). Factors independently associated with hospital admission were treatment with combination immunotherapy (OR 5.68, 95% CI 1.58 to 20.36, p=0.0273), ECOG ≥2 (OR 39.25, 95% CI 4.17 to 369.2, p=0.0013) and COVID-19 symptoms (OR 5.30, 95% CI 1.57 to 17.89, p=0.0073) (table 3).
Table 3.
Univariate and multivariate analysis of factors associated with hospital admission and overall survival
| Hospital admission | Overall survival | |||||
| Univariate OR (95% CI) |
P value | Multivariate OR (95% CI) |
P value | Univariate HR (95% CI) |
P value | |
| Age (years) | ||||||
| ≤65 | 1 | 0.7662 | 1 | 0.5814 | ||
| >65 | 1.13 (0.50 to 2.55) | 0.76 (0.29 to 2.01) | ||||
| Sex | ||||||
| Male | 1 | 0.5017 | 1 | 0.7924 | ||
| Female | 0.74 (0.31 to 1.78) | 1.14 (0.42 to 3.10) | ||||
| Region | ||||||
| Europe | 1 | 0.0306 | 1 | 0.0006 | ||
| Australia | 0.31 (0.13 to 0.75) | NA* | ||||
| North America | 0.35 (0.03 to 3.73) | 6.30 (2.20 to 18.00) | ||||
| Treatment setting | ||||||
| Advanced | 1 | 0.1080 | NA† | |||
| (Neo)adjuvant | 0.42 (0.14 to 1.21) | NA | ||||
| Cancer type | ||||||
| Melanoma | 1 | 0.4847 | 1 | 0.0345 | ||
| NSCLC | 1.15 (0.35 to 3.75) | 3.81 (1.02 to 14.27) | ||||
| Other | 2.21 (0.75 to 6.53) | 5.71 (1.73 to 18.83) | ||||
| RCC | 1.84 (0.46 to 7.34) | 2.65 (0.51 to 13.66) | ||||
| Coexisting disorder Cardiovascular |
||||||
| No | 1 | 0.0498 | 1 | 0.1446 | ||
| Yes | 0.34 (0.12 to 1.00) | 0.33 (0.08 to 1.46) | ||||
| Diabetes | ||||||
| No | 1 | 0.5566 | 1 | 0.6697 | ||
| Yes | 1.39 (0.46 to 4.20) | 1.31 (0.38 to 4.57) | ||||
| Pulmonary disease | ||||||
| No | 1 | 0.5482 | 1 | 0.1289 | ||
| Yes | 1.44 (0.44 to 4.79) | 2.38 (0.78 to 7.31) | ||||
| Kidney disease | ||||||
| No | 1 | 0.3183 | 1 | 0.0271 | ||
| Yes | 2.32 (0.44 to 12.15) | 4.09 (1.17 to 14.29) | ||||
| ICI | ||||||
| Anti-PD-1/anti-PD-L1 | 1 | 0.0287 | 1 | 0.0273 | 1 | 0.7585 |
| Anti-PD-1+anti-CTLA-4 | 4.37 (1.40 to 13.61) | 5.68 (1.58 to 20.36) | 1.43 (0.41 to 5.03) | |||
| Other | 2.91 (0.39 to 21.88) | 2.13 (0.17 to 26.29) | 1.77 (0.23 to 13.62) | |||
| Interval between last ICI dose and COVID-19 diagnosis | ||||||
| ≥28 days | 1 | 0.2021 | 1 | 0.6273 | ||
| <28 days | 0.59 (0.26 to 1.33) | 1.27 (0.48 to 3.34) | ||||
| Previous chemotherapy | ||||||
| No | 1 | 0.8084 | 1 | 0.4246 | ||
| Yes | 0.88 (0.33 to 2.38) | 1.53 (0.54 to 4.35) | ||||
| ECOG at COVID-19 diagnosis | ||||||
| 0–1 | 1 | 0.0014 | 1 | 0.0013 | 1 | 0.0115 |
| 2–4 | 30.82 (3.75 to 253.2) | 39.25 (4.17 to 369.2) | 3.84 (1.35 to 10.92) | |||
| Prednisone ≥10 mg/day | ||||||
| No | 1 | 0.0075 | 1 | 0.5099 | ||
| Yes | 5.04 (1.54 to 16.48) | 1.52 (0.44 to 5.30) | ||||
| Symptoms | ||||||
| No | 1 | 0.0018 | 1 | 0.0073 | 1 | 0.0526 |
| Yes | 5.34 (1.86 to 15.33) | 5.30 (1.57 to 17.89) | 4.31 (0.98 to 18.85) | |||
| Fever | ||||||
| No | 1 | 0.7839 | 1 | 0.1786 | ||
| Yes | 1.16 (0.41 to 3.25) | 0.50 (0.18 to 1.38) | ||||
| Cough | ||||||
| No | 1 | 0.3484 | 1 | 0.5760 | ||
| Yes | 0.63 (0.23 to 1.67) | 0.75 (0.27 to 2.07) | ||||
| Dyspnea | ||||||
| No | 1 | 0.0054 | 1 | 0.0182 | ||
| Yes | 4.74 (1.58 to 14.21) | 3.49 (1.24 to 9.84) | ||||
| Laboratory tests‡ WBC ≥10,000/mm3 |
||||||
| No | 1 | 0.0025 | 1 | 0.4181 | ||
| Yes | 8.70 (2.15 to 35.29) | 1.70 (0.47 to 6.12) | ||||
| Lymphocytes <1500/mm3 | ||||||
| No | 1 | 0.0089 | 1 | 0.0250 | ||
| Yes | 3.76 (1.39 to 10.16) | 4.38 (1.20 to 15.94) | ||||
| CRP ≥100 mg/L | ||||||
| No | NA§ | 1 | 0.0194 | |||
| Yes | NA | 3.70 (1.24 to 11.10) | ||||
| Creatinine ≥133 µM | ||||||
| No | 1 | 0.0879 | 1 | 0.1012 | ||
| Yes | 3.29 (0.84 to 12.88) | 2.98 (0.81 to 11.03) | ||||
| RECIST response | ||||||
| SD/PD | 1 | 0.0928 | 1 | 0.1422 | ||
| PR/CR | 0.40 (0.14 to 1.17) | 0.38 (0.10 to 1.39) | ||||
*No patients in Australia died.
†All patients who died had advanced disease.
‡Within 3 days of COVID-19 diagnosis.
§All patients with CRP ≥100 mg/L were admitted to hospital.
CR, complete response; CRP, C reactive protein; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; ECOG, Eastern Cooperative Oncology Group; ICI, immune checkpoint inhibition; NA, not available; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD, progressive disease; PD-L1, programmed cell death-ligand 1; PR, partial response; RCC, renal cell carcinoma; SD, stable disease; WBC, white blood cells.
Survival outcome
At the time of data cut-off (July 20, 2020), of the 35 patients admitted to hospital, 19 (54%) were discharged and 16 had died in hospital (46%) (figure 1). Two patients died in a nursing unit. Of all 18 (16%) patients who died, COVID-19 was the primary cause of death in 8 patients (7% total cohort), and 3 had evidence of cytokine release syndrome (table 4). Malignancy was the primary cause of death in eight patients (7% total cohort); two patients died of other causes. All 18 patients who died had advanced cancer, 15 (82%) had COVID-19–related symptoms (table 2). Only four patients were admitted to the intensive care unit; six were not admitted because of the underlying malignancy, two due to a constrained healthcare system (Italy), and no reason was specified for the remaining six patients (table 4). Factors associated with an increased risk of death were residence in North America versus Europe (HR 6.30, 95% CI 2.20 to 18.00, p=0.0006), pre-existing kidney disease (HR 4.09, 95% CI 1.17 to 14.29, p=0.0271), ECOG ≥2 (HR 3.84, 95% CI 1.35 to 10.92, p=0.0115), dyspnea (HR 3.49, 95% CI 1.24 to 9.84, p=0.0182), lymphocyte count <1500/mm3 (HR 4.38, 95% CI 1.20 to 15.94, p=0.0250) and CRP ≥100 mg/mL (HR 3.70, 95% CI 1.24 to 11.10, p=0.0194). Four (24%) out of 17 patients with lung cancer died in comparison with 5 (8%) out of 64 patients with melanoma (HR 3.81, 95% CI 1.02 to 14.27, p=0.0345).
Table 4.
Demographic and treatment findings for patients who died
| Age | Sex | Country | Cancer type | ICI type | Symptoms | Admitted to hospital | ICU | Reason not to admit to ICU | Primary cause of death | Cytokine release syndrome |
| 80 | F | USA | NSCLC | Anti-PD-1/L1 | No | Yes | Yes | – | Other | – |
| 73 | F | USA | NSCLC | Anti-PD-1/L1 | Yes | Yes | Yes | – | COVID-19 | No |
| 54 | M | USA | Melanoma | Anti-PD-1/L1 | Yes | Yes | No | Underlying malignancy | Malignancy | – |
| 51 | M | USA | RCC | Anti-PD-1/L1 | Yes | Yes | No | Not stated | Malignancy | – |
| 71 | F | USA | Urothelial | Anti-PD-1/L1 | Yes | No | No | Underlying malignancy | Malignancy | – |
| 61 | M | USA | H&NSCC | Anti-PD-1/L1 | Yes | Yes | No | Not stated | Other | – |
| 74 | M | USA | Gastric | Anti-PD-1/L1 | Yes | Yes | No | Not stated | COVID-19 | Yes |
| 81 | M | USA | Esophageal | Anti-PD-1/L1 | Yes | Yes | Yes | – | COVID-19 | No |
| 65 | M | USA | Esophageal | Anti-PD-1/L1 | Yes | Yes | No | Not stated | COVID-19 | Yes |
| 45 | F | USA | HCC | Anti-PD-1/L1 | Yes | Yes | No | Not stated | Malignancy | – |
| 49 | F | USA | Sarcoma | Anti-PD-1 plus anti-CTLA-4 | No | Yes | Yes | – | COVID-19 | Yes |
| 60 | M | Canada | NSCLC | Anti-PD-1/L1 | Yes | No | No | Not stated | Malignancy | – |
| 56 | M | Canada | RCC | Anti-PD-1 plus anti-CTLA-4 | Yes | Yes | No | Underlying malignancy | Malignancy | – |
| 65 | M | Italy | NSCLC | Anti-PD-1/L1 | Yes | Yes | No | Constrained healthcare system | COVID-19 | No |
| 73 | F | Italy | Melanoma | Anti-PD-1/L1 | Yes | Yes | No | Underlying malignancy | COVID-19 | No |
| 72 | M | Italy | Melanoma | Anti-PD-1/L1 | Yes | Yes | No | Constrained healthcare system | COVID-19 | No |
| 35 | F | UK | Melanoma | Anti-PD-1 plus anti-CTLA-4 | No | Yes | No | Underlying malignancy | Malignancy | – |
| 42 | M | France | Melanoma | Anti-PD-1 plus anti-CTLA-4 | Yes | Yes | No | Underlying malignancy | Malignancy | – |
CTLA-4, cytotoxic T-lymphocyte-associated protein 4; F, female; HCC, hepatocellular carcinoma; H&NSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibition; ICU, intensive care unit; M, male; NSCLC, non-small cell lung cancer; PD-1, programmed cell death protein 1; PD-L1, programmed cell death-ligand 1; RCC, renal cell carcinoma.
Discussion
This is the largest study of patients with confirmed COVID-19 infection while on treatment with ICI alone. We show a mortality rate due to COVID-19 (7%) higher than what is reported for an unselected COVID-19 positive population (1.4%–2.3%3 10) but on the lower side of the range documented for patients with cancer (7.6%–33%).6–9 Inpatient mortality of patients with cancer treated with ICI was high (46%) in comparison with previously reported rates for hospitalized patients with cancer (11%–28%)17 20 21 and was due to COVID-19 in almost half of the cases. These findings have implications for clinical decision-making for patients who are receiving immunotherapy.
COVID-19 has had, and continues to have, an unprecedented impact on society and healthcare services. The increased risk for severe illness and mortality in patients with cancer has raised safety concerns among patients and clinicians alike.7 8 17 The notion that a subgroup of patients with severe COVID-19 develop a cytokine storm syndrome,14 possibly reflecting a high-risk inflammatory phenotype, is of particular concern in a context of ICI.22 To better understand the impact of ICI on patients with laboratory-confirmed SARS-CoV-2, we integrated and analyzed data from 110 ICI-treated patients with different cancer types.
Almost 40% of patients had asymptomatic COVID-19 infection, which is well in the range of the reported 30%–75% of individuals with asymptomatic COVID-19 infection in the community.8 23–25 Although less permissive screening programs in some countries make it challenging to ascertain the true number of asymptomatic COVID-19 cases, patients with cancer are likely to be repeatedly tested. Our findings do not indicate a different symptomatic presentation at diagnosis of COVID-19 in ICI-treated patients. Considering the frequency at which patients on ICI visit healthcare facilities, this finding underscores the importance of transmission tracking and contact tracing in healthcare services to avoid spread of COVID-19 among vulnerable patients.
Just under a third of the patients with COVID-19 on immunotherapy were admitted to hospital in our study, most of whom were symptomatic. This is slightly lower than hospital admission rates reported for the cancer population in general.7 The presence of dyspnea was, as expected, a predictor for both hospital admission and mortality. ICU admission rate in our study was low at only 6%. While this is in line with the notion that 5% of individuals without comorbidities develop severe illness from COVID-19 infection,3 it is in stark contrast with the high mortality rates in the cancer population. In our study, 18 out of 110 patients died (16%), most not attributed to COVID-19. This fatality rate is clearly higher than the case fatality rate in the community (2.3%)3 yet within the range of what has been reported for the cancer population (7.6%–33%).5 6 None of the patients who received adjuvant ICI died. Of the 18 patients who died, 14 were not admitted to ICU. Although underlying malignancy and constrained healthcare systems were reported as factors in decision-making, further exploration of the perceptions of a cancer diagnosis will be essential in an era where increasing numbers of patients with advanced cancer achieve long-term responses to immunotherapy.
Interpreting overall mortality is inherently complicated by the divergent impact ICI may have on COVID-19–related and malignancy-related outcomes. Furthermore, several factors could have influenced mortality rates in our study. First, the effect of tumor type on COVID-19 outcomes has been recognized: higher COVID-19 mortality rates have been reported for hematological26 and thoracic malignancies.6 Our cohort included more patients with melanoma than with NSCLC and the mortality rate of patients with melanoma was lower than that of patients with NSCLC. Second, treatment with anti-PD-(L)1 plus anti-CTLA-4 was associated with a significant higher admission but not mortality rate possibly reflecting the higher adverse events rate but better oncological outcomes associated with combination immunotherapy. Third, patients who had chemotherapy in the 12 weeks prior to COVID-19 were excluded from our study. If recent chemotherapy results in worse COVID-19–related outcomes, exclusion of these patients could contribute to a difference in observed outcomes; however, recent administration of chemotherapy has not been invariably associated with worse COVID-19 outcomes.8 9 Finally, median follow-up in our study was 82 days, which is several times longer than earlier reports.6 7
Of particular relevance is the notion, consistent with other reports,9 that baseline corticosteroid use (at a prednisone equivalent dose of ≥10 mg) is associated with a higher risk for admission. The RECOVERY trial, which had not yet been published when patients in our study were treated, showed that the anti-inflammatory and immunosuppressive effects of dexamethasone were beneficial in patients requiring respiratory support.22 While the use of corticosteroids may be confounding in an ICI context (immunotherapy-related adverse events), it remains important to note that dampening immune response with corticosteroids in the early stages of COVID-19 infection can be detrimental and may also compromise cancer-related outcomes.27 In later stages, with an aberrant inflammatory response and less viral activity taking place, corticosteroids could be of benefit. This may be consistent with our finding that prednisone was associated with a higher risk for hospital admission but not with increased mortality. Lymphocytopenia, as reported by Yang et al,19 and high CRP were predictors for higher mortality but may also impact malignancy-related outcomes.28 It is, however, somewhat unexpected that a well-established risk factor such as cardiovascular disease was not associated with more severe COVID-19 illness. This is likely the result of a smaller population (ICI-only as opposed to unselected) and cardiovascular disease not being further specified. In our study, older age and male sex were also not associated with a higher mortality (4 out of 18 patients who died were younger than 50 years, 3 of whom were women).
Approximately one fourth of the patients did not interrupt ICI despite COVID-19 infection and most of these patients were asymptomatic. This subset of patients was too small to draw any conclusions regarding adverse outcomes for the patients themselves, yet it is, from a public health perspective, imperative to isolate patients who tested positive for SARS-CoV-2.
Our study has some limitations. First, there is no non-ICI control group; this would have been challenging given that treatment choices are inextricably linked to patient and disease characteristics making a retrospective comparison of treatment modalities in a matched patient population elusive. Second, patients contracted COVID-19 relatively early in the pandemic and there was no universal screening for COVID-19 among all ICI-treated patients. This may have resulted in an underestimation of the true number of COVID-19–infected patients on ICI (in particular asymptomatic patients) with possible bias toward symptomatic patients who required hospital admission. Lastly, the low ICU admission rate because of underlying malignancy (and the possible role of advance care directives) complicates case fatality rate interpretation.
In conclusion, this is, to our knowledge, the largest multicenter study to investigate the impact of COVID-19 specifically in ICI-treated patients with cancer. It is well established that patients with cancer are at a higher risk of severe COVID-19 infection in comparison with individuals without comorbidities. Based on our findings, treatment with ICI does not appear to be an additional risk factor for severe COVID-19 infection in patients with cancer. We could identify several factors predictive of a higher risk for hospital admission and/or mortality. This general observation does not exclude the possibility that individual patients at the extremes of the COVID-19 clinical spectrum experience exacerbating or mitigating effects from ICI treatment. A better understanding of high-risk inflammatory phenotypes prone to develop severe COVID-19 illness remains essential, particularly in an ICI context.
Acknowledgments
AR is supported by a Cameron fellowship at Melanoma Institute Australia. AANR acknowledges fellowship funding from a Hold’em For Life Clinician Scientist Award. AMM is supported by a Cancer Institute NSW Fellowship and Melanoma Institute Australia. GVL is supported by an NHMRC Practitioner fellowship and the University of Sydney Medical Foundation.
Footnotes
Twitter: @AljosjaRogiers, @inespiresilva, @amenzies9, @MattCarlino, @PAscierto, @OsamaRahma2, @ProfGLongMIA
AR and IPdS contributed equally.
MM and GVL contributed equally.
Correction notice: This article has been corrected since it first published. The provenance and peer review statement has been included. In addition to this, the affiliation of Paola Queirolo has been updated.
Contributors: AR, IPS, AMM, MSC and GVL were involved in study design. AR, IPS, CT, CAT, JMG, MHT, SN, LZ, MiM, PB, AE, NP, MGV, AANR, JB, SR, JM, TPM, TC, JL, AV, SDS, MOB, AMM, MSC, ME, CB, LiZ, DS, LP, PQ, CP, AH, RD, JH, CUB, CR, RJS, PAA, WHM, FSH, KS, KLR, OER, PCL, RDC, MaM and GVL were involved in data collection. AR and IPS were involved in data acquisition and management. AR, IPS, SL, MaM and GVL were involved in data analysis and interpretation. AR, IPS, SL and GVL were involved in manuscript writing. All authors reviewed and approved the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MiM: consultant/advisor for Bristol Myers Squibb, MSD, Novartis, Roche, Pierre Fabre. AANR: fellowship funding from Alamos Gold Inc. JMG: project focused consultant/advisor for Merck/Pfizer, MSD, Amgen, Novartis, Bristol Myers Squibb and Pierre Fabre; travel support from Ultrasun, L’Oreal, MSD, Bristol Myers Squibb and Pierre Fabre outside of the submitted work. TPM: fellowship funding from Alamos Gold Inc. SDS: consultant/advisor for Janssens, Novartis and Sanofi. AMM: consultant/advisor to Bristol Myers Squibb, MSD, Novartis, Roche, Pierre Fabre and QBiotics. MSC: consultant/advisor for Bristol Myers Squibb, MSD, Amgen, Novartis, Pierre Fabre, Roche, Sanofi, Merck, Ideaya, Regeneron, Nektar, Eisai; honoraria from Bristol Myers Squibb, MSD, Novartis. CB: consultant/advisor for Amgen, Bristol Myers Squibb, MSD, Novartis Pharma AG, Regeneron Pharmaceuticals Inc, Roche Pharma, Sanofi Aventis. LiZ: consultant/advisor for Bristol Myers Squibb, Novartis, Pierre Fabre, Sunpharma, Sanofi, MSD; research funding from Novartis; travel support from Bristol Myers Squibb, Pierre Fabre, Sanofi, Amgen, Novartis, Sunpharma. DS: consultant/advisor for Roche/Genentech, Novartis, Bristol Myers Squibb, MSD, Merck Serono, Amgen, Immunocore, Incyte, 4SC, Pierre Fabre, Mologen and Sanofi/Regeneron; honoraria from Roche/Genentech, Novartis, MSD, Bristol Myers Squibb, Merck Serono, Amgen, Immunocore, Incyte, 4SC, Pierre Fabre, Sysmex, Grünenthal Group, Agenus, Array BioPharma, AstraZeneca, LEO Pharma, Pfizer, Philogen, Regeneron and Mologen; travel/accommodation expenses from Roche/Genentech, Novartis, Bristol Myers Squibb, Merck Serono, Amgen and Merck; speakers bureau for Novartis, Bristol Myers Squibb, MSD, Amgen, Incyte, Pierre Fabre and Roche; research funding from Novartis and Bristol Myers Squibb; steering committee membership for Novartis, MSD and Bristol Myers Squibb. AH: consultant/advisor for Amgen, Bristol Myers Squibb, MSD/Merck, Pfizer, NeraCare, Novartis, Philogen, Pierre Fabre, Roche and Regeneron/Sanofi-Genzyme. RD: intermittent, project focused consulting and/or advisory relationships with Novartis, MSD, Bristol-Myers Squibb, Roche, Amgen, Takeda, Pierre Fabre, Sun Pharma, Sanofi, Catalym, Second Genome, Regeneron, Alligator, MaxiVAX SA and touchIME outside the submitted work. JH: consultant/advisor for AIMM, AchillesTx, Bristol Myers Squibb, BioNTech, GSK, Immunocore, Merck Serono, MSD, Neogene Tx, Novartis, Pfizer, Roche/Genentech, Sanofi, Seattle Genetics, Third Rock Ventures, Vaximm; research grants from Amgen, Bristol Myers Squibb, MSD, BioNTech, Novartis. CUB: consultant/advisor for Bristol Myers Squibb, MSD, Roche, Novartis, GSK, AZ, Pfizer, Lilly, GenMab, Pierre Fabre, Third Rock Ventures; research funding from Bristol Myers Squibb, Novartis, NanoString; stock ownership: Uniti Cars; co-founder: Immagene BV. CR: consultant/advisor for Bristol Myers Squibb, MSD, Roche, Novartis, CureVac, Sanofi, Pierre Fabre. RJS: consultant/advisor for Asana Biosciences, Astrazeneca, Bristol Myers Squibb, Eisai, Iovnace, Pfizer, Merck, Novartis, Replimune; research funding: Amgen, Merck. PAA: consultant/advisory for Bristol Myers Squibb, Roche/Genentech, MSD, Novartis, Array, Merck Serono, Pierre Fabre, Incyte, Medimmune, AstraZeneca, Syndax, Sun Pharma, Sanofi, Idera, Ultimovacs, Sandoz, Immunocore, 4SC, Alkermes, Italfarmaco, Nektar, Boehringer-Ingelheim, Eisai, Regeneron; research funding from Bristol Myers Squibb, Roche/Genentech, Array and travel support from MSD. FSH: consultancy/advisory for Bristol Myers Squibb, Merck, EMD Serono, Novartis, Surface, Compass Therapeutics, Apricity, Aduro, Sanofi, Pionyr, 7 Hills Pharma, Torque, Rheos, Kairos, Bicara, Psioxus Therapeutics, Pieris Pharmaceutical, Zumutor, Corner Therapeutics, Idera, Takeda, Genentech/Roche, Bioentre, Gossamer. KPMS: consulting/advisory for Bristol Myers Squibb, MSD, Abbvie, Pierre Fabre, Novartis; honoraria received from Novartis, Roche, MSD (all paid to institution). KLR: consultant/advisor for Teladoc. OER: consultant/advisor for Merck, Celgene, Five Prime, GSK, Bayer, Roche/Genentech, Puretech, Imvax, Sobi; research support from Merck; speaker for activities supported by educational grants from Bristol Myers Squibb and Merck; patent “Methods of using pembrolizumab and trebananib” pending. PCL: consultant/advisor for Bristol Myers Squibb, MSD, Pierre Fabre, Novartis, Amgen and Roche; travel support from Bristol Myers Squibb and MSD; research support from Bristol Myers Squibb. MaM: consultant advisor for Bristol Myers Squibb, MSD, Novartis, Roche, PierreFabre. GVL: consultant/advisor for Aduro Biotech Inc, Amgen Inc, Array Biopharma inc, Boehringer Ingelheim International GmbH, Bristol Myers Squibb, Highlight Therapeutics SL, MSD, Novartis Pharma AG, QBiotics Group Limited, Regeneron Pharmaceuticals Inc, SkylineDX BV, all declarations of interest are outside of the submitted work. AR, IPS, CT, CAT, JMG, MHT, SN, LZ, PB, AE, NP, MGV, JB, SR, TC, JL, AV, MOB, ME, LP, PQ, CP, WHM, RDC and SL have no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
All data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not required.
References
- 1.Lai C-C, Shih T-P, Ko W-C, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents 2020;55:105924. 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johns Hopkins Coronavirus Resource Center . COVID-19 Map. Available: https://coronavirus.jhu.edu/map.html [Accessed 5 Oct 2020].
- 3.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 4.Tian J, Yuan X, Xiao J, et al. Clinical characteristics and risk factors associated with COVID-19 disease severity in patients with cancer in Wuhan, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:893–903. 10.1016/S1470-2045(20)30309-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aylward B, Liang W. Report of the WHO-China joint mission on coronavirus disease 2019 (COVID-19) 2020.
- 6.Garassino MC, Whisenant JG, Huang L-C, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 2020;21:914–22. 10.1016/S1470-2045(20)30314-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907–18. 10.1016/S0140-6736(20)31187-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee LY, Cazier J-B, Angelis V, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26. 10.1016/S0140-6736(20)31173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robilotti EV, Babady NE, Mead PA, et al. Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020;26:1218–23. 10.1038/s41591-020-0979-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–86. 10.1158/2159-8290.CD-18-0367 [DOI] [PubMed] [Google Scholar]
- 12.Zheng M, Gao Y, Wang G, et al. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol 2020;17:533–5. 10.1038/s41423-020-0402-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber DL, Wherry EJ, Masopust D, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682–7. 10.1038/nature04444 [DOI] [PubMed] [Google Scholar]
- 14.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–4. 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coperchini F, Chiovato L, Croce L, et al. The cytokine storm in COVID-19: an overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev 2020;53:25–32. 10.1016/j.cytogfr.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonomi L, Ghilardi L, Arnoldi E, et al. A rapid fatal evolution of Coronavirus Disease-19 in a patient with advanced lung cancer with a long-time response to nivolumab. J Thorac Oncol 2020;15:e83–5. 10.1016/j.jtho.2020.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783. 10.1158/2159-8290.CD-20-0422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo J, Rizvi H, Egger JV, et al. Impact of PD-1 blockade on severity of COVID-19 in patients with lung cancers. Cancer Discov 2020;10:1121–8. 10.1158/2159-8290.CD-20-0596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyashita H, Mikami T, Chopra N, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 2020;31:1088–9. 10.1016/j.annonc.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta V, Goel S, Kabarriti R, et al. Case fatality rate of cancer patients with COVID-19 in a New York hospital system. Cancer Discov 2020;10:935–41. 10.1158/2159-8290.CD-20-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med 2020:NEJMoa2021436. 10.1056/NEJMoa2021436 [DOI] [Google Scholar]
- 23.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020;173:362–7. 10.7326/M20-3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louie JK, Scott HM, DuBois A, et al. Lessons from mass-testing for COVID-19 in long term care facilities for the elderly in San Francisco. Clin Infect Dis 2020. 10.1093/cid/ciaa1020. [Epub ahead of print: 20 Jul 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA 2020;323:2191–2. 10.1001/jama.2020.6887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee LYW, Cazier J-B, Starkey T, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020;21:1309–16. 10.1016/S1470-2045(20)30442-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrelli F, Signorelli D, Ghidini M, et al. Association of steroids use with survival in patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Cancers 2020;12:546. 10.3390/cancers12030546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diehl A, Yarchoan M, Hopkins A, et al. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 2017;8:114268–80. 10.18632/oncotarget.23217 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data relevant to the study are included in the article or uploaded as online supplemental information.