Abstract
It can be a diagnostic challenge to identify patients with coronavirus disease 2019 in whom antibiotics can be safely withheld. This study evaluated the effectiveness of a guideline implemented at Sheffield Teaching Hospitals NHS Foundation Trust that recommends withholding antibiotics in patients with low serum procalcitonin (PCT), defined as ≤0.25 ng/mL. Results showed reduced antibiotic consumption in patients with PCT ≤0.25 ng/mL with no increase in mortality, alongside a reduction in subsequent carbapenem prescriptions during admission. The results support the effectiveness of this guideline, and further research is recommended to identify the optimal cut-off value for PCT in this setting.
Keywords: Antimicrobial stewardship, COVID-19, Procalcitonin, SARS-CoV-2, Bacterial co-infection, Superadded infection
Introduction
In patients with coronavirus disease 2019 (COVID-19), the presentation of fever, tachypnoea and hypoxia, together with lung infiltrates on chest imaging and a frequent rise in biomarkers such as C-reactive protein [1], presents a challenge to rational use of antimicrobials as it is difficult to exclude bacterial co-infection with confidence. Rates of true bacterial co-infection are estimated to be 7–14% [2,3]. Despite this, early in the pandemic, 80% of patients with COVID-19 received antibiotic treatment [4]. Strategies for accurate identification of patients with COVID-19 who do not have bacterial co-infection are needed to reduce antimicrobial prescription and promote antimicrobial stewardship [5]. National Institute for Health and Care Excellence guidance on pneumonia in the context of COVID-19 has recommended further research into the use of procalcitonin (PCT) for this purpose.
This study aimed to evaluate whether the inclusion of measurement of PCT in a hospital guideline for antibiotic prescription in COVID-19 contributed to: (1) antibiotic usage and (2) outcomes in confirmed cases of COVID-19 at a large NHS foundation trust hospital in the UK.
Methods
Study design, study site and population
This retrospective observational study was undertaken at two sites of Sheffield Teaching Hospitals NHS Foundation Trust (STHNFT).
Eligible patients were aged ≥18 years, diagnosed with COVID-19 between 5th March and 15th April 2020 with a positive severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) reverse transcriptase polymerase chain reaction (RT-PCR) result on nose and/or throat swabs and/or deep respiratory samples [6], and had a PCT assay undertaken within 48 h of collection of the first positive SARS-CoV-2 sample. Patients with both community and nosocomial acquisition of COVID-19 were included. STHNFT guidelines recommend that antibiotics can be withheld in patients with COVID-19 with PCT ≤0.25 ng/mL unless felt necessary by a senior clinician, as concomitant bacterial infection is considered unlikely below this level [7].
Patients diagnosed before 5th March 2020 were excluded as COVID-19 was managed as a high-consequence infectious disease at this point, and patients were admitted regardless of the severity of symptoms. The enrolment end date of 15th April 2020 was before the introduction of mandatory SARS-CoV-2 screening of all patients admitted to hospital.
This study was granted approval by STHNFT Clinical Effectiveness Unit (Ref: 9863).
Data collection and outcomes
Demographic and clinical characteristics of patients were drawn from existing laboratory, pharmacy and clinical databases, and from examination of physical and electronic patient notes. Data were entered into an electronic case report form (Access 2010, Microsoft Corp., Redmond, WA, USA).
The primary outcome was antibiotic consumption in World Health Organization defined daily doses (DDDs) per day alive over 28 days following diagnosis of COVID-19, defaulting to that for intravenous drug where DDD differs by route of administration. Days of treatment were also calculated, representing the number of days in the 28-day period for which any antibiotics were prescribed.
Data on antibiotic-associated adverse events were collected, including hospital-acquired pneumonia/ventilator-associated pneumonia (see online Supplementary material for definition), Clostridioides difficile infection, meticillin-resistant Staphylococcus aureus acquisition, and isolation of an extended-spectrum beta-lactamase or AmpC beta-lactamase-producing organism from a clinical sample.
Statistical analysis
All values from patients meeting the eligibility criteria were summarized using the most appropriate form, either frequency and percentage or median and interquartile range. Differences between demographics were analysed using a suitable significance test, depending on whether or not parametric assumptions were met, as detailed in each table. To investigate the relationship between PCT positivity and total DDD and between antibiotic receipt at 48 h post-diagnosis and meropenem prescription, linear and logistic regression models were explored, adjusting for demographic confounders (age, sex, ethnicity and comorbidities.) All statistical analyses were performed using Stata Version 16.1 (StataCorp. College Station, TX, USA).
Results
Study population
In total, 629 patients received inpatient care at STHNFT during the study period. Of these, 368 patients met the eligibility criteria and were included in the analysis. Excluded patients either did not have PCT measured (N=146, 23%) or had PCT measured outside the 48-h window for inclusion (N=115, 18%). Of the 368 patients included, 60% were male with a median age of 75 years. Of these, 218 (59%) patients had PCT ≤0.25 ng/mL (negative) and 150 (41%) had PCT >0.25 ng/mL (positive).
Patient demographics and comorbidities stratified by PCT are seen in Table I . There was no significant difference in demographics between the two groups in terms of age, sex, body mass index or ethnicity. Comorbidities between the two groups were similarly distributed with the exception of malignancy, which was more common in the negative PCT group. There were no pregnant women in the cohort.
Table I.
Baseline demographics of patients stratified by procalcitonin level
| Procalcitonin level | ≤0.25 ng/mL | >0.25 ng/mL | Total | P-value | |
|---|---|---|---|---|---|
| Total N (%) | 218 (59) | 150 (41) | 368 (100) | ||
| Age at admission (years), median (IQR) | 75 (61–84) | 74 (60–82) | 75 (60–83) | 0.417b | |
| Age group (years) | 18–39 | 13 (6) | 9 (6) | 22 | 0.849a |
| 40–49 | 13 (6) | 6 (4) | 19 | ||
| 50–59 | 26 (12) | 22 (15) | 48 | ||
| 60–69 | 32 (15) | 27 (18) | 59 | ||
| 70–79 | 51 (23) | 33 (22) | 84 | ||
| ≥80 | 83 (38) | 53 (35) | 136 | ||
| Sex | Male | 123 (56) | 98 (65) | 221 | 0.086c |
| Female | 95 (44) | 52 (35) | 147 | ||
| BMI (kg/m2) (N=330) | <20 | 16 (8) | 9 (7) | 25 | 0.885c |
| 20–25 | 51 (26) | 40 (30) | 91 | ||
| 25–30 | 66 (34) | 44 (33) | 110 | ||
| ≥30 | 62 (32) | 42 (31) | 104 | ||
| Ethnicity | White | 172 (79) | 112 (75) | 284 | 0.428a |
| Black | 13 (6) | 13 (9) | 26 | ||
| Asian | 11 (5) | 5 (3) | 16 | ||
| Mixed | 1 (0) | 1 (1) | 2 | ||
| Other | 3 (1) | 3 (2) | 6 | ||
| Not stated | 11 (5) | 14 (9) | 25 | ||
| Missing | 7 (3) | 2 (1) | 9 | ||
| Any comorbidity | No | 38 (17) | 31 (21) | 69 | 0.435c |
| Yes | 180 (83) | 119 (79) | 299 | ||
| Hypertension | No | 140 (64) | 96 (64) | 236 | 0.965c |
| Yes | 78 (36) | 54 (36) | 132 | ||
| Diabetes (type 1 or 2) | No | 154 (71) | 110 (73) | 264 | 0.573c |
| Yes | 64 (29) | 40 (27) | 104 | ||
| Cardiovascular disease | No | 134 (61) | 101 (67) | 235 | 0.250c |
| Yes | 84 (39) | 49 (33) | 133 | ||
| Asthma | No | 195 (89) | 134 (89) | 329 | 0.972c |
| Yes | 23 (11) | 16 (11) | 39 | ||
| Malignancy | No | 183 (84) | 140 (93) | 323 | 0.007c |
| Yes | 35 (16) | 10 (7) | 45 | ||
| Immunosuppressed | No | 199 (91) | 136 (91) | 335 | 0.839c |
| Yes | 19 (9) | 14 (9) | 33 | ||
| Chronic lung disease | No | 177 (81) | 122 (81) | 299 | 0.973c |
| Yes | 41 (19) | 28 (19) | 69 | ||
| Chronic renal impairment | No | 192 (88) | 125 (83) | 317 | 0.196c |
| Yes | 26 (12) | 25 (17) | 51 | ||
| Pregnancy | No | 218 (100) | 150 (100) | 368 | N/A |
| Yes | 0 (0) | 0 (0) | 0 | ||
IQR, interquartile range; BMI, body mass index; N/A, not applicable.
Fisher's Exact test.
Mann–Whitney U-test.
Chi-squared test.
Compliance with guideline
Seventy-three (33%) patients in the negative PCT group were on antibiotics 48 h following diagnosis of COVID-19 compared with 126 (84%) patients in the positive PCT group (P<0.001), suggesting good compliance with the guideline.
Antibiotic usage
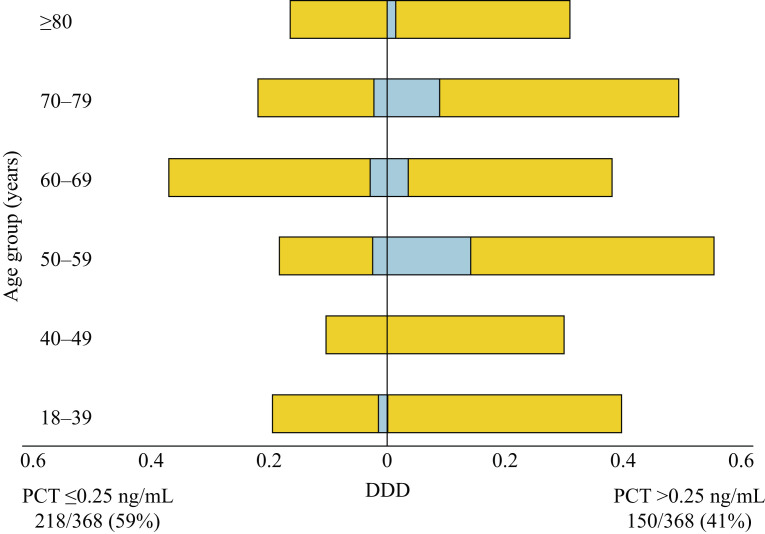
Data on total DDD of antibiotics received in the 28-day follow-up period and DDD per alive day are presented in Figure 1 and Table S1 (see online supplementary material). Patients in the negative PCT group received significantly fewer DDDs of antibiotics (both total and per alive day) compared with patients in the positive PCT group (median DDD 3.0 vs 6.8; P<0.001). A log-linear model was computed to explore the relationship with PCT positivity after adjustment for demographic confounders (comorbidities, age, sex, ethnicity) to ensure that regression assumptions were met. A significant relationship between PCT and total DDDs remained after accounting for these confounders; on average, a patient with PCT >0.25 ng/mL had almost three-fold more DDDs of antibiotics compared with patients with PCT ≤0.25 ng/mL [coefficient 2.72, 95% confidence interval (CI) 2.03–3.62; P<0.001] (Table S2, see online supplementary material).
Figure 1.
Antibiotic consumption as demonstrated by average defined daily dose (DDD, yellow bars) and average meropenem DDD (blue bars) between positive (>0.25 ng/mL) and negative (≤0.25 ng/mL) procalcitonin (PCT) groups, stratified by age.
28-day outcomes
Over the 28-day follow-up period, 116 (32%) patients died, 229 (62%) were discharged and 23 (6%) remained in hospital. The median length of stay was 8.35 days. Forty-seven (13%) patients were admitted to the intensive care unit (ICU), and of these, 32 (68%) were intubated and ventilated. PCT, age and 28-day mortality distributions of the patients are shown in Figure S1 (see online supplementary material). Sixty-two (28%) patients in the negative PCT group died compared with 54 (36%) patients in the positive PCT group (P=0.021), and 19 (9%) patients in the negative PCT group were admitted to the ICU compared with 28 (19%) patients in the positive PCT group (P=0.007).
Meropenem was the only carbapenem used in the study population. With specific reference to meropenem consumption, positive PCT was associated with a three-fold increase in the odds of receiving any meropenem during the course of hospital admission (odds ratio 3.16, 95% CI 1.50–6.65; P=0.002) after adjustment for demographic confounders (Figure 1 and Table S3, see online supplementary material).
There was no significant difference in rates of infective complications between positive and negative PCT groups (Table S2, see online supplementary material).
Discussion
This observational study reveals the contribution of a local guideline advising against the use of antibiotics for confirmed cases of COVID-19 with PCT ≤0.25 ng/mL, leading to reduced antibiotic consumption compared with national statistics [4] with no negative impact on 28-day outcome.
Clinicians were encouraged to request PCT measurement for any patient requiring admission to hospital with COVID-19. The guideline was discussed with relevant admitting specialities, particularly the accident and emergency and acute medicine departments. The inclusion of PCT in an electronic ‘COVID order set’ also promoted its measurement.
The 28-day mortality figures in this study (28% PCT ≤0.25 ng/mL, 36% PCT >0.25 ng/mL) are similar to data published by the International Severe Acute Respiratory and Emerging Infection Consortium, the largest COVID-19 patient registry in the UK, suggesting that implementation of the guideline did not cause harm [4].
The adopted PCT threshold of 0.25 ng/mL was intentionally conservative, and it may be possible to safely adopt a higher threshold. Further research to evaluate the optimal cut-off value for PCT at which antibiotics can be withheld safely is recommended.
Although the guideline was well received and implemented, a proportion of patients with negative PCT still received antibiotics. Local investigations of the rationale for antibiotic prescription in these patients need to be undertaken.
The higher mortality seen in patients with PCT >0.25 ng/mL supports the findings of other authors demonstrating an association between higher PCT values and severe disease or death [8,9]. It is likely that higher PCT in these patients reflects bacterial superinfection and consequent impairment in outcome in many cases. It is also possible that PCT may be raised in severe COVID-19 independent of bacterial infection, which would open the possibility for further improvement in antimicrobial stewardship through use of a higher PCT threshold or other parameters.
Reducing the unnecessary use of antibiotics through this guideline is a key component to mitigating the risk of antimicrobial resistance. The risk of severe COVID-19 increases with age, and the elderly are also at greater risk of the adverse consequences of excessive antibiotic use [10].
This study found a three-fold increase in the odds of carbapenem prescription in the positive PCT group. This is important in the context of the increasing global incidence of carbapenemase-producing Enterobacteriales. This study shows the impact of early rationalized antimicrobial therapy on later prescription of broad-spectrum agents.
The limitations of this study include the fact that it was from a single centre and was retrospective in design. While the study showed an association between PCT and antimicrobial consumption, it was not possible to determine whether it was the PCT result alone that informed clinical practice with relation to antimicrobial administration. Both the clinical picture and other infection markers may have affected the decision. A more comprehensive, prospective study would enable such questions to be resolved. Such a study could also evaluate the use of PCT with varying cut-off values as a diagnostic marker to improve antimicrobial stewardship in COVID-19.
In conclusion, this study found that a PCT-based guideline can be a useful tool for rationalizing the use of antibiotics in patients with COVID-19.
Acknowledgements
The authors wish to thank Dr Eirini Koutoumanou, University College London for discussion around the transformation analysis.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.01.006.
Conflict of interest statement
None declared.
Funding sources
None.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Henry B.M., De Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58 doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 2.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence . NICE; London: 2020. COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital. NICE Guideline 173. [PubMed] [Google Scholar]
- 4.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seaton R.A., Gibbons C.L., Cooper L., Malcolm W., McKinney R., Dundas S. Survey of antibiotic and antifungal prescribing in patients with suspected and confirmed COVID-19 in Scottish hospitals. J Infect. 2020;81:952–960. doi: 10.1016/j.jinf.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colton H., Ankcorn M., Yavuz M., Tovey L., Cope A., Raza M. Improved sensitivity using a dual target, E and RdRp assay for the diagnosis of SARS-CoV-2 infection: experience at a large NHS foundation trust in the UK. J Infect. 2021;82:159–198. doi: 10.1016/j.jinf.2020.05.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schuetz P., Batschwaroff M., Dusemund F., Albrich W., Bürgi U., Maurer M. Effectiveness of a procalcitonin algorithm to guide antibiotic therapy in respiratory tract infections outside of study conditions: a post-study survey. Eur J Clin Microbiol Infect Dis. 2010;29:269–277. doi: 10.1007/s10096-009-0851-0. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Z., Peng F., Xu B., Zhao J., Liu H., Peng J. Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81:e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beckett C.L., Harbarth S., Huttner B. Special considerations of antibiotic prescription in the geriatric population. Clin Microbiol Infect. 2015;21:3–9. doi: 10.1016/j.cmi.2014.08.018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.