Abstract
Introduction
During a pandemic, there are many situations in which the first available vaccines may not have as high effectiveness as vaccines that are still under development or vaccines that are not yet ready for distribution, raising the question of whether it is better to go with what is available now or wait.
Methods
In 2020, the team developed a computational model that represents the U.S. population, COVID-19 coronavirus spread, and vaccines with different possible efficacies (to prevent infection or to reduce severe disease) and vaccination timings to estimate the clinical and economic value of vaccination.
Results
Except for a limited number of situations, mainly early on in a pandemic and for a vaccine that prevents infection, when an initial vaccine is available, waiting for a vaccine with a higher efficacy results in additional hospitalizations and costs over the course of the pandemic. For example, if a vaccine with a 50% efficacy in preventing infection becomes available when 10% of the population has already been infected, waiting until 40% of the population are infected for a vaccine with 80% efficacy in preventing infection results in 15.6 million additional cases and 1.5 million additional hospitalizations, costing $20.6 billion more in direct medical costs and $12.4 billion more in productivity losses.
Conclusions
This study shows that there are relatively few situations in which it is worth foregoing the first COVID-19 vaccine available in favor of a vaccine that becomes available later on in the pandemic even if the latter vaccine has a substantially higher efficacy.
INTRODUCTION
During a pandemic, such as the current coronavirus disease 2019 (COVID-19) coronavirus pandemic, there are many situations in which the first available or currently available vaccines1 , 2 may not have as high effectiveness as vaccines that are still under development3, 4, 5 or as those that are not yet ready for distribution, raising the question of whether it is better to go with what is available now or wait. For example, although the currently reported vaccine efficacy for the Pfizer/BioNTech and Moderna COVID-19 vaccines have been ≥90%,6 , 7 historically, vaccine effectiveness often drops when the vaccines are ultimately rolled out into the general population8 , 9 because clinical trials occur under controlled and ideal conditions and may not capture all heterogeneity within the population. Furthermore, these clinical trials were conducted during the summer/fall6 , 7 when transmission was likely lower than that in the late fall/winter,10, 11, 12 meaning that vaccine effectiveness could be lower when the infection risk is higher. It is also not clear what the vaccine efficacy may be in preventing infection and viral shedding because the reported results to date have focused on the risk of symptomatic COVID-19.6 , 7 In addition, although the recent emergence of a new variant13 , 14 may not currently affect the effectiveness of the Pfizer/BioNTech and Moderna vaccines, it does raise the possibility that a future variant may emerge that adversely affects vaccine effectiveness. This would raise the question of whether to vaccinate with currently available vaccines or wait for one with a higher effectiveness against that particular variant.
In addition, delays in the roll out of the 2-dose Pfizer/BioNTech COVID-19, Moderna COVID-19, and now the AstraZeneca/Oxford COVID-19 vaccines have led to the discussion of giving single doses to cover more people rather than giving the full-dose regimen to fewer people.15, 16, 17 A single dose could result in a far lower vaccine effectiveness (potentially ≤50%6 , 7), which does raise the question of whether it is better to split the doses now or wait until there are more doses to give everyone the full 2-dose regimen. Thus, it would be helpful to better understand the trade-off between the costs (in terms of clinical and economic outcomes) of waiting and the cost savings of a higher efficacy vaccine (whether it prevents infection or prevents worse COVID-19 outcomes). To address this question, the team developed and used a computational model representing the U.S. population and COVID-19 coronavirus spread to evaluate the impact and value of introducing different COVID-19 coronavirus vaccines at various times during the pandemic.
METHODS
Model Structure
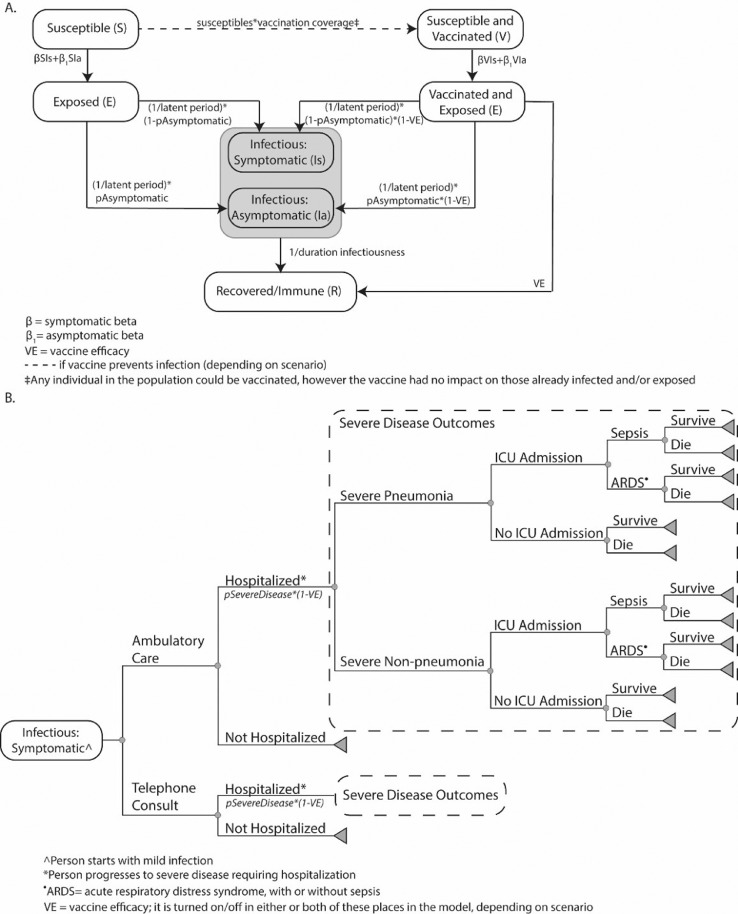
In 2020, using Microsoft Excel with the Crystal Ball add-in, the team adapted a previously described computational model representing the U.S. population (327,167,434 people), their interactions with each other, COVID-19 coronavirus spread, its potential health and economic outcomes,18 , 19 and vaccination. The model (Figure 1 ) advances in discrete, 1-day time steps for 2.5 years (Appendix, available online). On any given day, each individual is in 1 of 5 mutually exclusive COVID-19 coronavirus compartments: (1) susceptible (not infected and able to become infected), (2) exposed (infected but not able to transmit to others), (3) infectious and asymptomatic (infected but without symptoms and able to transmit to others), (4) infectious and symptomatic (infected, showing symptoms, and able to transmit to others), or (5) recovered/immune (not infected and unable to become infected). Each day, individuals randomly interact with each other, and an infectious person can potentially transmit the virus to a susceptible person. Individuals move through the compartments at various rates (Appendix, available online). The Appendix (available online) also describes the model data sources, calibration, validation, input parameters, and values.
Figure 1.
Model structure for (A) transmission and (B) clinical pathway of COVID-19 cases.
ARDS, acute respiratory distress syndrome; ICU, intensive care unit; VE, vaccine efficacy.
Each symptomatically infected person (i.e., COVID-19 case regardless of formal diagnosis) travels through a probability tree of different sequential age-specific outcomes (Appendix, available online).18 , 19 The person accrues relevant costs and health effects as they travel through the model. In addition, each vaccinated person incurs vaccination costs, which include the vaccine itself, vaccine administration, and logistics for the entire regimen (e.g., all doses). A vaccinated person experiencing minor side effects incurs the cost of over-the-counter medications; a person experiencing major side effects incurs the cost of hospitalization.
Vaccination
A total of 2 different types of vaccines are represented. One type can prevent infection and viral shedding. In this case, vaccination moves a person into the V compartment. After exposure to an infected person, these individuals have a probability of being infected that is equal to 1 minus the vaccine efficacy. The second type does not affect the risk of becoming infected but does reduce the probability of progressing to severe COVID-19 by attenuating this risk on the basis of vaccine efficacy. Once an individual is vaccinated, protection lasts throughout the simulation duration (i.e., no waning immunity). Vaccination has a probability of causing minor (e.g., fever, soreness, headache) and major (e.g., Guillain–Barre Syndrome, allergic reaction/anaphylaxis, resulting in hospitalization) side effects. Vaccination of any number of individuals can occur on any given day. Individuals who have been already infected can also be vaccinated. Because it is not yet clear how soon the protection onset will occur after vaccination, vaccination in the model is defined as the time point at which the onset of protection occurs (e.g., if the onset occurs 2 weeks after vaccination, the actual vaccination would have occurred 2 weeks before the simulated day). Because the progression of the pandemic differs depending on the virus characteristics and the implementation of different nonpharmaceutical interventions (NPIs), the vaccination day is reported as the percentage of the population that has already been infected at some point.
Nonpharmaceutical Interventions
Because NPIs (e.g., social distancing and wearing face masks) have been implemented to varying degrees through the course of the pandemic, different experimental scenarios represented what would happen if NPIs are implemented. For example, scenarios looked at what would happen if no NPIs were in place and what would happen if NPIs were implemented to approximate their use as of October 8, 202020 (Appendix, available online).
Economic Measures
The third-party payer perspective includes direct medical costs (e.g., vaccination and hospitalization), whereas the societal perspective includes direct medical costs and productivity losses owing to absenteeism. Hourly wage across all occupations21 serves as a proxy for productivity losses. Absenteeism results in productivity losses for the duration of symptoms. All COVID-19 cases accrue productivity losses, regardless of age or employment status because everyone is assumed to contribute to society.
Health effects were measured in quality-adjusted life years (QALYs). Each COVID-19 infection accrues QALY values on the basis of age-dependent healthy QALY value attenuated by infection-specific utility weights for their infection duration. Those who survive accrue the net present value of QALYs for the remainder of an individual's lifetime.22 Vaccination was considered cost effective if the incremental cost–effectiveness ratio was ≤$50,000/QALY.
Experimental Scenarios
Experiments consisted of Monte Carlo simulations of 2,000 trials, varying parameters throughout their range (Appendix Table 1, available online). The first set of scenarios consisted of vaccinating individuals to prevent infection, and the second set consisted of vaccinating to reduce severe disease. Different scenarios represent what may happen when varying vaccine efficacy (20%–80%) and varying the percentage of the population exposed before vaccination onset (5%–50%). Sensitivity analyses varied population coverage (25%–75%), vaccination cost ($45–$125), NPI use, and the probability of ambulatory care (from the distribution in Appendix Table 1, available online, to 0%). Appendix Figure 1 (available online) shows how key model outcomes change when varying all model parameter values.
RESULTS
Table 1 shows the clinical outcomes and costs from a select sample of scenarios when different COVID-19 coronavirus vaccines are introduced at different times during the pandemic. Figure 2 and Appendix Figures 2 and 3 (available online) show how 2 major outcomes (i.e., total COVID-19 coronavirus cases and costs) change when vaccines that prevent infections at different efficacies are introduced at different times during the pandemic. Figure 3 shows the similar outcomes (i.e., total hospitalizations, ventilator use, costs) for vaccines that reduce the severity of the disease. These scenarios assume that the comparison vaccine (i.e., the second available vaccine with a different efficacy from that of the first available vaccine) has a 100% probability of becoming available at the given time.
Table 1.
Clinical and Economic Outcomes From COVID-19 Coronavirus for Various Vaccination Scenarios
| Vaccination scenario | Total cases (in millions) | Symptomatic cases (in millions) | Ambulatory care visits (in millions) | Hospitalizations (in millions) | Intensive care unit admissions (in millions) | Patients requiring a ventilator (in millions) | Vaccination costs (in billions) | Direct medical costs (in billions) | Productivity losses (in billions) | QALYs (in millions) |
|---|---|---|---|---|---|---|---|---|---|---|
| No NPI use before vaccination onset | ||||||||||
| 5% have already been infected | ||||||||||
| 20% VE | 261.1 (230.8, 279.9) | 169.6 (148.9, 184.1) | 26.0 (13.6, 40.3) | 23.5 (20.6, 25.9) | 3.8 (3.3, 4.2) | 2.9 (2.4, 3.4) | 13.9 | 332.1 (289.6, 369.7) | 277.0 (120.4, 626.9) | 7,231.8 (6,633.1, 7,787.0) |
| 50% VE | 203.6 (168.0, 226.0) | 132.2 (108.0, 148.0) | 20.5 (10.5, 31.6) | 18.4 (15.0, 20.8) | 3.0 (2.4, 3.4) | 2.3 (1.8, 2.7) | 13.9 | 262.3 (214.0, 299.7) | 215.9 (92.5, 490.9) | 7,225.0 (6,652.6, 7,819.9) |
| 80% VE | 141.7 (104.2, 170.0) | 91.8 (67.6, 111.0) | 14.1 (7.1, 23.1) | 12.8 (9.3, 15.5) | 2.1 (1.5, 2.5) | 1.6 (1.1, 2.0) | 13.9 | 186.3 (138.2, 226.2) | 149.9 (64.5, 322.6) | 7,253.4 (6,696.1, 7,808.2) |
| 25% have already been infected | ||||||||||
| 20% VE | 271.6 (244.4, 289.2) | 176.3 (157.7, 190.5) | 27.1 (14.0, 42.1) | 24.4 (21.8, 26.9) | 4.0 (3.5, 4.4) | 3.1 (2.5, 3.6) | 13.9 | 344.1 (303.7, 384.1) | 288.7 (127.7, 631.8) | 7,211.9 (6,610.3, 7,750.4) |
| 50% VE | 230.5 (203.5, 250.3) | 149.9 (131.0, 164.9) | 23.1 (12.0, 36.2) | 20.8 (18.0, 23.2) | 3.4 (2.9, 3.8) | 2.6 (2.1, 3.1) | 13.9 | 295.3 (254.4, 332.5) | 246.3 (105.0, 519.8) | 7,219.1 (6,635.9, 7,764.7) |
| 80% VE | 191.2 (165.1, 212.4) | 124.7 (107.0, 139.4) | 19.3 (9.7, 29.9) | 17.2 (14.7, 19.5) | 2.8 (2.4, 3.2) | 2.2 (1.7, 2.6) | 13.9 | 246.8 (210.7, 280.8) | 205.4 (88.3, 459.6) | 7,236.9 (6,629.1, 7,778.7) |
| 50% have already been infected | ||||||||||
| 20% VE | 283.6 (257.5, 289.2) | 184.3 (166.1, 190.5) | 28.5 (14.8, 42.1) | 25.6 (22.9, 26.9) | 4.1 (3.7, 4.4) | 3.2 (2.6, 3.6) | 13.9 | 359.9 (318.7, 384.1) | 300.8 (133.8, 631.8) | 7,235.2 (6,667.9, 7,750.4) |
| 50% VE | 259.6 (236.5, 276.1) | 168.8 (152.8, 182.1) | 26.0 (13.5, 40.1) | 23.4 (21.0, 25.6) | 3.8 (3.4, 4.2) | 2.9 (2.4, 3.4) | 13.9 | 330.9 (292.7, 366.7) | 270.2 (117.8, 584.1) | 7,228.3 (6,639.2, 7,760.8) |
| 80% VE | 237.9 (217.5, 254.4) | 154.4 (140.8, 167.5) | 23.7 (12.3, 36.6) | 21.5 (19.2, 23.6) | 3.5 (3.1, 3.8) | 2.7 (2.2, 3.1) | 13.9 | 303.5 (270.3, 337.6) | 253.4 (112.5, 551.3) | 7,233.5 (6,650.7, 7,791.5) |
| NPI use, similar to current U.S. measures | ||||||||||
| 5% have already been infected | ||||||||||
| 20% VE | 220.5 (176.7, 253.6) | 142.9 (113.1, 166.2) | 21.9 (10.8, 35.3) | 19.8 (15.5, 23.2) | 3.2 (2.5, 3.8) | 2.4 (1.8, 3.1) | 13.9 | 281.2 (223.0, 334.0) | 229.6 (100.0, 500.5) | 7,240.4 (6,652.3, 7,788.3) |
| 50% VE | 165.9 (144.6, 193.5) | 107.7 (89.3, 127.1) | 16.8 (8.6, 27.2) | 15.0 (12.4, 17.7) | 2.4 (2.0, 2.9) | 1.9 (1.4, 2.4) | 13.9 | 216.6 (179.0, 258.0) | 174.1 (73.5, 382.4) | 7,263.8 (6,668.5, 7,827.6) |
| 80% VE | 112.5 (85.3, 131.5) | 72.4 (54.8, 85.7) | 11.1 (5.7, 17.9) | 10.1 (7.6, 12.0) | 1.6 (1.2, 1.9) | 1.3 (0.9, 1.6) | 13.9 | 150.4 (115.8, 178.5) | 119.2 (49.8, 256.1) | 7,262.5 (6,655.0, 7,817.4) |
| 25% have already been infected | ||||||||||
| 20% VE | 228.2 (177.4, 263.2) | 148.2 (115.3, 173.1) | 22.5 (11.7, 36.9) | 20.6 (15.5, 24.2) | 3.3 (2.5, 3.9) | 2.5 (1.9, 3.2) | 13.9 | 291.8 (227.7, 345.9) | 235.3 (103.6, 499.1) | 7,229.7 (6,667.2, 7,791.3) |
| 50% VE | 186.5 (136.0, 219.5) | 121.1 (88.4, 143.9) | 18.5 (9.1, 30.4) | 16.8 (12.3, 20.2) | 2.7 (2.0, 3.3) | 2.1 (1.5, 2.7) | 13.9 | 240.5 (179.7, 289.5) | 197.5 (84.3, 446.0) | 7,252.1 (6,648.6, 7,778.8) |
| 80% VE | 146.6 (110.8, 178.8) | 95.4 (71.7, 116.9) | 14.7 (7.2, 24.5) | 13.2 (10.0, 16.3) | 2.1 (1.6, 2.7) | 1.7 (1.2, 2.1) | 13.9 | 193.2 (146.8, 238.0) | 154.5 (65.4, 346.9) | 7,256.7 (6,649.1, 7,801.1) |
| 50% have already been infected | ||||||||||
| 20% VE | 261.1 (230.8, 279.9) | 169.6 (148.9, 184.1) | 26.0 (13.6, 40.3) | 23.5 (20.6, 25.9) | 3.8 (3.3, 4.2) | 2.9 (2.4, 3.4) | 13.9 | 332.1 (289.6, 369.7) | 277.0 (120.4, 626.9) | 7,231.8 (6,633.1, 7,787.0) |
| 50% VE | 203.6 (168.0, 226.0) | 132.2 (108.0, 148.0) | 20.5 (10.5, 31.6) | 18.4 (15.0, 20.8) | 3.0 (2.4, 3.4) | 2.3 (1.8, 2.7) | 13.9 | 262.3 (214.0, 299.7) | 215.9 (92.5, 490.9) | 7,225.0 (6,652.6, 7,819.9) |
| 80% VE | 141.7 (104.2, 170.0) | 91.8 (67.6, 111.0) | 14.1 (7.1, 23.1) | 12.8 (9.3, 15.5) | 2.1 (1.5, 2.5) | 1.6 (1.1, 2.0) | 13.9 | 186.3 (138.2, 226.2) | 149.9 (64.5, 322.6) | 7,253.4 (6,696.1, 7,808.2) |
Note: All scenarios are for a vaccine that prevents infection and assumes an $85 vaccination cost with a 50% vaccination coverage. Presented values are median (95% uncertainty interval).
NPI, nonpharmaceutical intervention; QALYs, quality-adjusted life year; VE, vaccine efficacy.
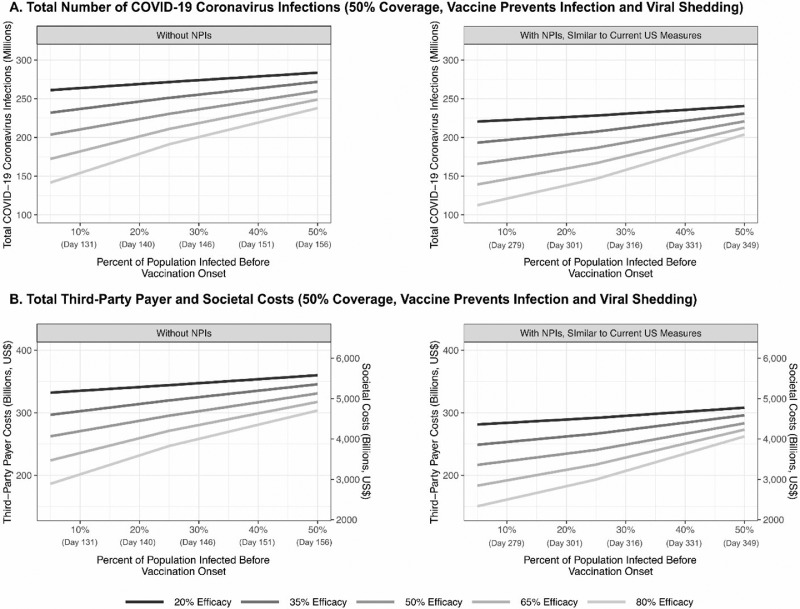
Figure 2.
Impact of vaccine efficacy and timing of vaccination onset (percentage of the population infected with COVID-19 coronavirus before vaccination onset) on (A) the total number of COVID-19 coronavirus cases and (B) total third-party payer and societal costs during the course of the pandemic for a vaccine that prevents infection (50% vaccination coverage and $85 vaccination cost) with and without NPIs.
NPI, nonpharmaceutical intervention.
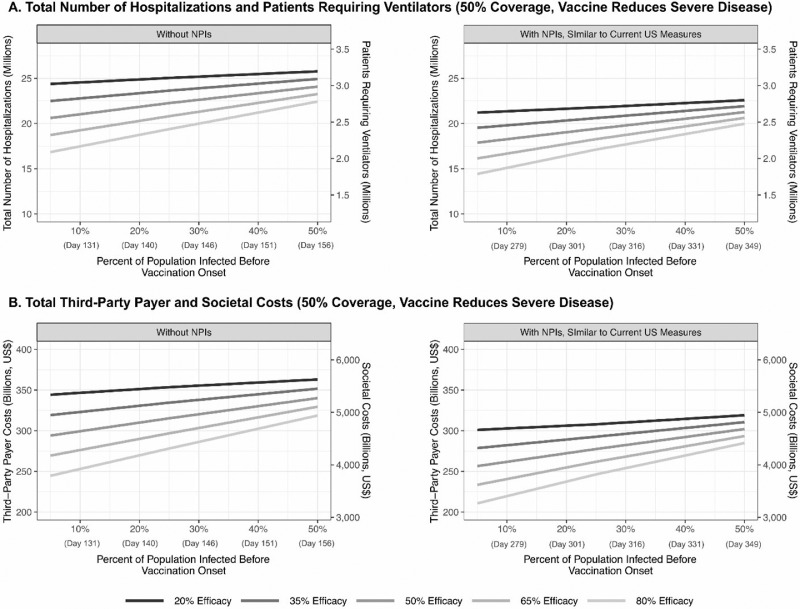
Figure 3.
Impact of vaccine efficacy and timing of vaccination onset (percentage of the population infected with COVID-19 coronavirus before vaccination onset) on (A) the total number of hospitalizations and patients requiring a ventilator and (B) total third-party payer and societal costs during the course of the pandemic for a vaccine that reduces severe disease (50% vaccination coverage and $85 vaccination cost) with and without NPIs.
NPI, nonpharmaceutical intervention.
If the First Vaccine That Prevents Infection Becomes Available Earlier in the Pandemic
Early in the pandemic (i.e., <10% of the population have been infected) and assuming that no NPIs have been implemented, if the first vaccine has an efficacy ≥50%, there are no situations when waiting for ≥1 month for a higher efficacy vaccine results in fewer cases and costs (Figure 2 and Table 1). When the efficacy of the first available vaccine is ≤35%, waiting until an additional 35% of the population becomes infected (∼30 days) for another vaccine ≥35 percentage points more effective results in fewer cases (100% probability of a second vaccine becoming available at that time).
Even with NPIs in place (Figure 2), when the first available vaccine's efficacy is >50%, there is no situation in which waiting for ≥1 month for a vaccine that is higher in efficacy results in fewer cases and costs. When the first available vaccine's efficacy is >35% to 50%, waiting until an additional 20% of the population have been infected, which translates to approximately 37 days, for a vaccine that is 30 percentage points higher in efficacy prevents cases. If the first vaccine efficacy is ≤35%, waiting until 20%–30% more people become infected (∼37–52 days) for another vaccine that is ≥15 percentage points more effective is worthwhile.
In all other cases, waiting for a higher efficacy vaccine accrues additional cases and costs. For example, if there is a vaccine with 35% efficacy when 10% of the population have been infected, waiting for a vaccine with a 50% efficacy when 35% of the population are infected can result in 4.5 million additional cases and 424,500 additional hospitalizations, costing $6.3 billion and $12.2 billion more in direct medical costs and productivity losses, respectively (with NPI use). As another example, if there is a vaccine with a 50% efficacy when 10% of the population have been infected, waiting until 40% of the population are infected for a vaccine with an 80% efficacy results in 15.6 million additional cases and 1.5 million additional hospitalizations, costing $20.6 billion and $12.4 billion more in direct medical costs and productivity losses, respectively.
Results are robust to changes in vaccination coverage and cost, the probability of seeking ambulatory care, and when the daily discount rate is applied (Appendix, available online).
If the First Vaccine That Prevents Infection Becomes Available Later in the Pandemic
Later in the pandemic (>10% of the population has been infected), even with NPIs implemented, when the first vaccine available has an efficacy ≥50%, there are no situations when waiting for ≥1 month for a higher efficacy vaccine results in fewer cases and costs. However, when the first available vaccine has an efficacy ≤35%, waiting until an additional 20% of the population becomes infected (∼30 days) for a vaccine that is ≥30 percentage points more effective is worthwhile.
Otherwise, waiting for a higher efficacy vaccine results in additional cases and costs. For example, if there is a vaccine with 35% efficacy when 20% of the population have been infected, waiting until 45% of the population are infected for a vaccine with a 50% efficacy results in 11.6 million additional cases and 1.1 million additional hospitalizations, costing $14.2 billion more in direct medical costs and $14.0 billion more in productivity losses. As another example, if there is a vaccine with a 50% efficacy available when 25% of the population have been infected, waiting until 50% of the population become infected for a vaccine with 80% efficacy results in 17.3 million more cases, 1.6 million additional hospitalizations, $21.7 billion more in direct medical costs, and $18.8 billion more in productivity losses (with NPIs) (Table 1).
If the First Vaccine That Reduces Severe Disease Becomes Available Earlier in the Pandemic
When <10% of the population has been infected and no NPIs have been in place, waiting for a higher efficacy vaccine only results in fewer hospitalizations and costs when the first available vaccine has a ≤35% efficacy. In this case, waiting until an additional 35% of the population has been infected (∼30 days) for a vaccine with a ≥30 percentage point efficacy gain results in fewer hospitalizations (Figure 3).
With NPIs, when the first available vaccine is >50% efficacious and <10% of the population has been infected, waiting until an additional 15% of the population have been infected (∼30 days) for a vaccine that is ≥15 percentage points more effective and waiting until an additional 25% of the population are infected (∼45 days) for a vaccine that is ≥30 percentage points more effective results in fewer hospitalizations (100% probability of the second vaccine becoming available at that time). When the first available vaccine has an efficacy of >35%–50%, waiting until an additional 25% of the population are infected (∼45 days) for a vaccine with ≥30 percentage point gain in efficacy is worthwhile. Similarly, when the first available vaccine has an efficacy ≤35%, waiting until an additional 20% of the population are infected (∼37 days) for another vaccine that is ≥15 percentage points more effective and until an additional 30% of the population are infected (∼52 days) for a vaccine with ≥30 percentage point gain in efficacy results in fewer hospitalizations.
Otherwise, waiting for a vaccine with a higher efficacy results in additional hospitalizations and costs (e.g., if there is a vaccine with 50% efficacy when 10% of the population have been infected, waiting until 40% become infected for a vaccine with 80% efficacy results in an additional 440,369 hospitalizations, 69,578 intensive care unit admissions, and $7.2 billion in direct medical costs).
If the First Vaccine That Reduces Severe Disease Becomes Available Later in the Pandemic
As the pandemic progresses (i.e., >10% of the population infected), there are no situations when waiting for ≥1 month for a higher efficacy vaccine results in fewer hospitalizations and costs when NPIs are implemented and the first available vaccine is >50% efficacious. However, when the first available vaccine has an efficacy <35%, waiting until an additional 20% of the population are infected (∼30 days) for another vaccine that is ≥15 percentage points higher in efficacy is worthwhile. When the first available vaccine has an efficacy between ≥35% and 50%, waiting until an additional 20% of the population are infected (∼30 days) for another vaccine that is ≥30 percentage points higher in efficacy reduces hospitalizations and costs. In all other situations, waiting for a vaccine with a higher efficacy results in worse health outcomes and higher costs (e.g., if there is a vaccine with 35% efficacy when 25% of the population have been infected, waiting until 45% of the population become infected for a vaccine with 50% efficacy results in 301,391 more hospitalizations and $3.5 billion more in direct medical costs).
DISCUSSION
This study shows that there are relatively few situations in which it is worth foregoing the first COVID-19 vaccine available in favor of a vaccine that becomes available later in the pandemic, even if the latter vaccine has a substantially higher efficacy. Thus, even if the Pfizer/BioNTech and Moderna vaccines end up having a lower than 90% effectiveness in preventing transmission (e.g., seasonal differences in infection risk and ideal conditions of trials) and another vaccine with even higher effectiveness subsequently becomes available, this study shows how vaccinating with the first available vaccine would have been justified. This is because as the pandemic progresses and there are more and more cases each day, the potential gain from a higher efficacy vaccine is offset by the rise in additional cases, hospitalizations, and associated costs. This is the case for vaccines that prevent infection and those that reduce disease severity.
As indicated earlier, delays in the Pfizer/BioNTech and Moderna vaccine roll out has prompted the United Kingdom to give single doses to cover more people rather than to give the full-dose regimen to fewer people.15, 16, 17 Results from this study do show the value of such a strategy. However, there are potential issues with just delivering 1 dose of a 2-dose vaccine (e.g., 1 dose may not have the same protection duration). Because clinical trials to date have focused on participants getting the full 2-dose course,6 , 7 it is unclear what the longer-term effectiveness may be for 1 dose. Moreover, some have worried about what partial protection may do to selection pressure and the emergence of virus variants.23
The recent emergence of the B.1.1.7 variant in which the spike protein has some amino acid changes13 , 14 but perhaps not enough to affect current COVID-19 vaccine effectiveness does raise the concern that future variants may affect vaccine effectiveness and necessitate the development and roll out of vaccines that are more specific to the future variants. Nevertheless, these results show that continuing to use vaccines with even reduced effectiveness can be preferable to halting and waiting for a vaccine with higher effectiveness against the variant.
Although there may be variations in COVID-19 coronavirus spread throughout different U.S. states and municipalities, chances are that >10% of the populations in most states will have been infected by the time vaccines are available. Thus, the general findings of administering the first vaccine available should hold both nationally and locally.
This study shows that from epidemiologic, clinical, and economic perspectives, it does not pay to wait to vaccinate. In addition, not only is vaccinating people as soon as possible ethical, but it is also utilitarian and morally necessary to protect the greatest number of people, saving lives while at the same time saving costs.
Limitations
All models, by definition, are simplifications of real life and cannot account for every possible outcome.24 Model inputs drew from various sources, and new data on COVID-19 coronavirus continue to emerge. Because the epidemic course may not be predictable, researchers explored a range of possible scenarios and parameter values. This study did not consider waning immunity (neither natural nor vaccine induced) because it is not yet well understood.25 , 26 In addition, it did not discount costs each day because this is a small fraction of overall costs; accounting for this would not impact situations in which the first vaccine available results in fewer cases and costs than a second vaccine with a higher efficacy. Because vaccines are still under development, the full profile of adverse events is not yet known. Scenarios assume the same risk profile for all vaccines, but this may vary, and a vaccine with more (or more frequent) side effects may result in higher costs, which are a small fraction of the total overall direct medical costs. These analyses did not include all costs that vaccines can avert, such as caregiver productivity losses (e.g., caring for ill family members) or declines in economic activity (e.g., job loss). To note, vaccination costs may be covered by various payers (e.g., Operation Warp Speed, third-party payers, local governments, consumers); however, this analysis did not separate out what would be covered by each payer because it is not known who will be paying these costs. This study also assumes that there are sufficient healthcare resources (e.g., intensive care unit beds and ventilators) for all patients; however, if the healthcare system is overburdened, patients with COVID-19 may not receive proper care, leading to higher mortality, whereas mortality may decrease with improvements in therapeutics and treatments.
CONCLUSIONS
This study shows that there are relatively few situations in which it is worth foregoing the first COVID-19 vaccine available in favor of a vaccine that becomes available later on in the pandemic even if the latter vaccine has a substantially higher efficacy.
ACKNOWLEDGMENTS
The authors of this manuscript are responsible for its content, including data analysis. Statements in the manuscript do not necessarily represent the official views of or imply endorsement by the NIH, the Agency for Healthcare Research and Quality (Rockville, MD), or the HHS. The funders of this study did not have any role in the study design; collection, analysis, or interpretation of data; writing the report; or the decision to submit the report for publication.
This work was supported in part by the CUNY Graduate School of Public Health & Health Policy (New York City, NY), the Agency for Healthcare Research and Quality through Grant 1R01HS028165-01, and the National Institute of General Medical Sciences (Bethestha, MD) as part of the Models of Infectious Disease Agent Study Network under Grants 1R01GM127512-01A1 and 3R01GM127512-01A1S1.
PJH and MEB codirect a center for vaccine development that develops vaccines against emerging and neglected diseases, including diseases caused by coronaviruses such as COVID-19. Baylor College of Medicine (Houston, TX) nonexclusively licensed the COVID-19 vaccine technology to Biological E, an India-based manufacturing company. These authors have no financial stakes in any COVID-19 vaccine candidates under development. No other financial disclosures were reported.
Footnotes
Supplemental materials associated with this article can be found in the online version at https://doi.org/10.1016/j.amepre.2021.01.001.
Appendix. SUPPLEMENTAL MATERIAL
REFERENCES
- 1.Hinton DM. U.S. Food and Drug Administration; Silver Spring, MD: December 23, 2020. Pfizer COVID-19 vaccine EUA letter of authorization reissued 12-23-20.https://www.fda.gov/media/144412/download [Google Scholar]
- 2.Hinton DM. U.S. Food and Drug Administration; Silver Spring, MD: December 18, 2020. Moderna COVID-19 EUA letter of authorization.https://www.fda.gov/media/144636/download [Google Scholar]
- 3.Zimmer C, Corum J, Wee SL. August 3, 2020. Coronavirus vaccine tracker. The New York Times.https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html Updated. [Google Scholar]
- 4.Heidelberg Institute for Geoinformation Technology; July 5, 2020. Mapping COVID-19 research: clinical trials map.https://covid-19.heigit.org/clinical_trials.html Updated. [Google Scholar]
- 5.COVID-19 treatment and vaccine tracker. Milken Institute, FasterCures.https://covid-19tracker.milkeninstitute.org. Updated July 31, 2020. Accessed August 3, 2020.
- 6.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. December 30, 2020 doi: 10.1056/NEJMoa2035389. In press. Online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fedson DS. Measuring protection: efficacy versus effectiveness. Dev Biol Stand. 1998;95:195–201. https://www.karger.com/Book/Home/223451 [PubMed] [Google Scholar]
- 9.Singal AG, Higgins PD, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5(1):e45. doi: 10.1038/ctg.2013.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haque SE, Rahman M. Association between temperature, humidity, and COVID-19 outbreaks in Bangladesh. Environ Sci Policy. 2020;114:253–255. doi: 10.1016/j.envsci.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meyer A, Sadler R, Faverjon C, Cameron AR, Bannister-Tyrrell M. Evidence that higher temperatures are associated with a marginally lower incidence of COVID-19 cases. Front Public Health. 2020;8:367. doi: 10.3389/fpubh.2020.00367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mallapaty S. Why COVID outbreaks look set to worsen this winter. Nature. 2020;586(7831):653. doi: 10.1038/d41586-020-02972-4. [DOI] [PubMed] [Google Scholar]
- 13.Baric RS. Emergence of a highly fit SARS-CoV-2 variant. N Engl J Med. 2020;383(27):2684–2686. doi: 10.1056/NEJMcibr2032888. [DOI] [PubMed] [Google Scholar]
- 14.Emerging SARS-CoV-2 variants. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/scientific-brief-emerging-variants.html. Updated December 29, 2020. Accessed January 5, 2021. [PubMed]
- 15.Pierson B. U.S. may cut some Moderna vaccine doses in half to speed rollout, official says. Toronto Sun. January 3, 2021 https://torontosun.com/news/world/u-s-may-cut-some-moderna-vaccine-doses-in-half-to-speed-rollout-official-says [Google Scholar]
- 16.Tufekci Z, Mina M. Can we do twice as many vaccinations as we thought? The New York Times. December 18, 2020 https://www.nytimes.com/2020/12/18/opinion/coronavirus-vaccine-doses.html [Google Scholar]
- 17.Wachter RM, Jha AK. It's time to consider delaying the second dose of coronavirus vaccine. The Washington Post. January 3, 2021 https://www.washingtonpost.com/opinions/2021/01/03/its-time-consider-delaying-second-dose-coronavirus-vaccine/ [Google Scholar]
- 18.Bartsch SM, O'Shea KJ, Ferguson MC, et al. Vaccine efficacy needed for a COVID-19 coronavirus vaccine to prevent or stop an epidemic as the sole intervention. Am J Prev Med. 2020;59(4):493–503. doi: 10.1016/j.amepre.2020.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bartsch SM, Ferguson MC, McKinnell JA, et al. The potential health care costs and resource use associated with COVID-19 in the United States. Health Aff (Millwood) 2020;39(6):927–935. doi: 10.1377/hlthaff.2020.00426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.United States COVID-19 cases and deaths by state over time. Centers for Disease Control and Prevention. https://data.cdc.gov/Case-Surveillance/United-States-COVID-19-Cases-and-Deaths-by-State-o/9mfq-cb36. Updated October 11, 2020. Accessed October 12, 2020.
- 21.U.S. Bureau of Labor Statistics; April 2, 2019. May 2018 national occupational employment and wage estimetes United Sates.https://www.bls.gov/oes/2018/may/oes_nat.htm Updated. [Google Scholar]
- 22.Human Mortality Database. University of California, Berkeley, Max Planck Institute for Demographic Research.
- 23.Kelland K. Russia vaccine roll-out plan prompts virus mutation worries. Toronto Sun. August 21, 2020 https://torontosun.com/news/world/russia-vaccine-roll-out-plan-prompts-coronavirus-mutation-worries/wcm/8f7348c6-8a37-4e01-a59b-df917a84760c/ [Google Scholar]
- 24.Lee BY. Digital decision making: computer models and antibiotic prescribing in the twenty-first century. Clin Infect Dis. 2008;46(8):1139–1141. doi: 10.1086/529441. [DOI] [PubMed] [Google Scholar]
- 25.Peeling RW, Wedderburn CJ, Garcia PJ, et al. Serology testing in the COVID-19 pandemic response. Lancet Infect Dis. 2020;20(9):e245–e249. doi: 10.1016/S1473-3099(20)30517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altmann DM, Douek DC, Boyton RJ. What policy makers need to know about COVID-19 protective immunity. Lancet. 2020;395(10236):1527–1529. doi: 10.1016/S0140-6736(20)30985-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.