Dear editor,
Immunomodulatory and immunosuppressant disease-modifying drugs (DMD) may increase the infectious risk in people with multiple sclerosis (MS) (Moiola et al., 2020). A growing bulk of evidence, however, does not suggest an increased severity of COVID-19 disease in DMD-treated patients (Zabalza et al., 2020). Along with the forthcoming anti-COVID-19 vaccination program, questions are increasing concerning the possibility of mounting an immune response to SARS-CoV-2 in DMD-exposed people.
In a recent paper, Zabalza et al. observed a decreased serological response to SARS-CoV-2 in MS patients treated with anti-CD20 therapies or alemtuzumab (Zabalza et al., 2020). In that cohort, however, no data were available regarding antibody response to COVID-19 after cladribine treatment.
Cladribine is a highly effective oral DMD exerting its action through a sustained but transient lymphocyte depletion, with negligible effects on innate immunity (Dersch et al., 2020). Few papers reported favorable COVID-19 outcomes in MS patients treated with cladribine (Dersch et al., 2020; Jack et al., 2020; Celius, 2020; Preziosa et al., 2020; De Angelis et al., 2020).
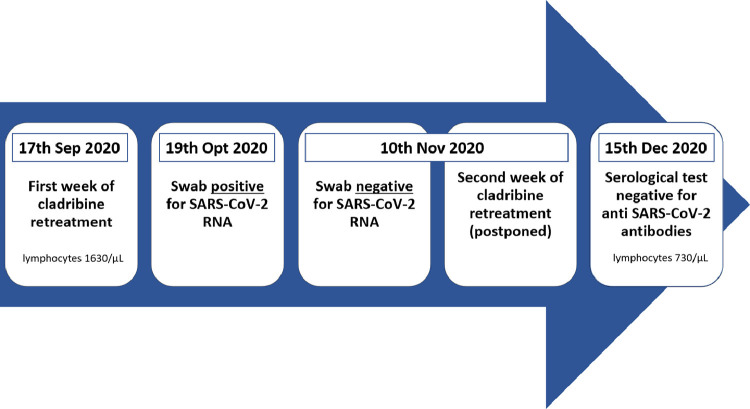
Here, we report the case of COVID-19 occurring in a 32-years-old female MS patient. Diagnosed with MS in 2012 and formerly treated with fingolimod, she was switched to cladribine treatment in 2019 following disease activity. She received two cladribine five-day courses in September and October 2019. Following cladribine, the patient experienced no further disease activity. When the patient started the planned cladribine retreatment on the 17th of September 2020 her lymphocyte count was 1630/µL. The second course of cladribine treatment, planned for the 17th of October, was postponed because of a close contact with a person tested positive for SARS-CoV-2. The patient tested positive with a nasopharyngeal swab for SARS-CoV-2 on the 19th of October. Two days later, she experienced fever (<38 °C), cough, ageusia, anosmia, nasal congestion, and diarrhea. These symptoms fully recovered after a few days, without requiring hospitalization. The second course of cladribine was then administered on the 10th of November, after the patient tested negative for SARS-CoV-2 RNA on a nasopharyngeal swab. On the 15th of December, the patient tested negative for IgM and IgG anti- SARS-CoV-2 antibodies measured with quantitative chemiluminescence immunoassay (Technogenetics, Italy. Sensitivity of 100% in samples collected at least 21 days after infection, according to manufacturer datasheet. This sensitivity value is in line with what observed for other quantitative chemiluminescence commercial kits (La Marca et al., 2020)). Blood test performed the same day showed a lymphocyte count of 730/µL (Fig. 1 ).
Fig. 1.
Timeline of the events described in the present report.
The reported patient had a self-limiting COVID-19 disease with a favorable outcome. This is in line with previous observations by other authors (Dersch et al., 2020; Jack et al., 2020; Celius, 2020; Preziosa et al., 2020; De Angelis et al., 2020). COVID-19 has been reported in 52 patients treated with cladribine; only in 23 of them, SARS-CoV-2 infection was confirmed by a laboratory test (i.e., nasopharyngeal swab and/or serological test) (Dersch et al., 2020; Jack et al., 2020; Celius, 2020; Preziosa et al., 2020; De Angelis et al., 2020). In the remaining 39 patients, COVID-19 was suspected according to clinical symptoms and laboratory tests were not performed or tested negative (Jack et al., 2020; Preziosa et al., 2020). In all reports, cladribine-treated patients had favorable outcomes, even in the presence of severe lymphopenia (Dersch et al., 2020; De Angelis et al., 2020), with no deaths nor need for mechanical ventilation. Serological data were available only for eight patients; and only one of them did not develop antibody response following infection (Preziosa et al., 2020). In that report, however, a nasal swab was not performed (Preziosa et al., 2020).
Considering the available literature, COVID-19 disease developed between two treatment weeks of the same year only in five patients (Dersch et al., 2020; Jack et al., 2020; Celius, 2020; Preziosa et al., 2020). As in our report, the second week of treatment was postponed until the recovery from COVID-19. In all cases, no severe COVID-19 disease was observed. In this subgroup, serological data were available only for two patients (Celius, 2020; Preziosa et al., 2020). While Celius reported a normal antibody response (Celius, 2020), another paper described a patient who tested negative for SARS-CoV-2 antibodies three months after the suspected infection (Preziosa et al., 2020). Also in our case, we found no antibody response two months after the infection. Condsidering that COVID-19 occurred during the expected nadir of B-cell count in cladribine-treated patients (Comi et al., 2019), this may explain the lack of anti-SARS-CoV-2 antibody response. However, the rapid decay of antibodies observed in patients with mild COVID-19 symptoms in the first 90 days after infection may have contributed to our findings (Ibarrondo et al., 2020).
Our report confirms the safety of cladribine during the COVID-19 pandemic, even when infection occurs in the weeks following treatment. The finding of no antibody response, however, might hint at a reduced immunization in recently treated patients. Should this be the case, cladribine treatment might have an impact on vaccination efficacy. The nature of our observation, however, limits its generalizability and we only considered antibody response.
Data regarding the efficacy of vaccinations in patients treated with cladribine are still lacking (Riva et al., 2020). Studies are needed to test the response to vaccines at different timepoints after cladribine administration. These studies should take into account also cell-mediated immune response that is not routinely tested and may be preserved in these patients (Riva et al., 2020). Current guidelines do not provide indications regarding the timing of inactivated vaccine administration in DMD-treated patients (Riva et al., 2020). Considering lymphocyte-depleting DMD, however, a blunted antibody response to vaccination has been observed in the first six months after alemtuzumab treatment (McCarthy et al., 2013). Against this background, it is possible to speculate that waiting until the recovery of lymphocyte count after cladribine treatment may lead to an optimized response to vaccines against SARS-CoV-2.
The patient provided her consent for this report.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
S Gelibter and M Orrico have no conflicts of interest to report.
L Moiola has received speaker's honoraria from the following companies: Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and TEVA.
M Filippi is Editor-in-Chief of the Journal of Neurology; received compensation for consulting services and/or speaking activities from Bayer, Biogen Idec, Merck-Serono, Novartis, Roche, Sanofi Genzyme, Takeda, and Teva Pharmaceutical Industries; and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA)
References
- Moiola L., Barcella V., Benatti S., Capobianco M., Capra R., Cinque P., et al. The risk of infection in patients with multiple sclerosis treated with disease-modifying therapies: a Delphi consensus statement. Mult. Scler. 2020;(September) doi: 10.1177/1352458520952311. 17135245852095231. [DOI] [PubMed] [Google Scholar]
- Zabalza A., Cárdenas-Robledo S., Tagliani P., Arrambide G., Otero-Romero S., Carbonell-Mirabent P., et al. COVID-19 in MS patients: susceptibility, severity risk factors and serological response. Eur. J. Neurol. 2020;(December) doi: 10.1111/ene.14690. 19;ene.14690. [DOI] [PubMed] [Google Scholar]
- Dersch R., Wehrum T., Fähndrich S., Engelhardt M., Rauer S., Berger B. COVID-19 pneumonia in a multiple sclerosis patient with severe lymphopenia due to recent cladribine treatment. Mult. Scler. 2020;26(10 September):1264–1266. doi: 10.1177/1352458520943783. [DOI] [PubMed] [Google Scholar]
- Jack D., Nolting A., Galazka A. Favorable outcomes after COVID-19 infection in multiple sclerosis patients treated with cladribine tablets. Mult. Scler. Relat. Disord. 2020;46(November) doi: 10.1016/j.msard.2020.102469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celius E.G. Normal antibody response after COVID-19 during treatment with cladribine. Mult. Scler. Relat. Disord. 2020;46(November) doi: 10.1016/j.msard.2020.102476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preziosa P., Rocca M.A., Nozzolillo A., Moiola L., Filippi M. COVID-19 in cladribine-treated relapsing-remitting multiple sclerosis patients: a monocentric experience. J. Neurol. [Internet] 2020;(November) doi: 10.1007/s00415-020-10309-4. 20 [cited 2020 Dec 28]; Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M., Petracca M., Lanzillo R., Brescia Morra V., Moccia M. Mild or no COVID-19 symptoms in cladribine-treated multiple sclerosis: two cases and implications for clinical practice. Mult. Scler. Relat. Disord. 2020;45(October) doi: 10.1016/j.msard.2020.102452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Marca A., Capuzzo M., Paglia T., Roli L., Trenti T., Nelson S.M. Testing for SARS-CoV-2 (COVID-19): a systematic review and clinical guide to molecular and serological in-vitro diagnostic assays. Reprod. Biomed. Online. 2020;41(3 September):483–499. doi: 10.1016/j.rbmo.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comi G., Cook S., Giovannoni G., Rieckmann P., Sørensen P.S., Vermersch P., et al. Effect of cladribine tablets on lymphocyte reduction and repopulation dynamics in patients with relapsing multiple sclerosis. Mult. Scler. Relat. Disord. 2019;29(April):168–174. doi: 10.1016/j.msard.2019.01.038. [DOI] [PubMed] [Google Scholar]
- Ibarrondo F.J., Fulcher J.A., Goodman-Meza D., Elliott J., Hofmann C., Hausner M.A., et al. Rapid decay of Anti–SARS-CoV-2 antibodies in persons with mild Covid-19. N. Engl. J. Med. 2020;383(11 September):1085–1087. doi: 10.1056/NEJMc2025179. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva A., Barcella V., Benatti S.V., Capobianco M., Capra R., Cinque P., et al. Vaccinations in patients with multiple sclerosis: a Delphi consensus statement. Mult Scler. 2020;(September) doi: 10.1177/1352458520952310. 17. [DOI] [PubMed] [Google Scholar]
- McCarthy C.L., Tuohy O., Compston D.A.S., Kumararatne D.S., Coles A.J., Jones J.L. Immune competence after alemtuzumab treatment of multiple sclerosis. Neurology. 2013;81(10 September):872–876. doi: 10.1212/WNL.0b013e3182a35215. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]