Highlights
-
•
Sensory tests revealed significant differences at salt reductions of 33% and above.
-
•
The structure of salt-reduced biscuits showed larger and more air cells.
-
•
Salt reduction has led to a decrease in water content and hardness of the biscuit.
-
•
Salt affects cereal proteins aggregation around flour particles and on bubble walls.
-
•
Salt reduction decreases the concentration of hydrophobic volatile in the headspace.
Keywords: Biscuits, Sodium reduction, Physicochemical properties, Sensory properties, Porosity, Biscuit structure
Abstract
Salt is included in many foods which consumers do not regard as salty. This “hidden-salt” may offer functional benefits but is often overlooked in sodium reduction strategies. This study investigated its role in shortbread-like sweet biscuits (1.05 g NaCl/100 g). Sensory tests revealed significant flavour and texture differences after a salt reduction of 33% (0.86 g/ 100 g). This was explained by differences in the partitioning of hydrophobic aroma compounds into the headspace and a significant impact on structure. Texture analysis and X-ray-µCT measurements revealed a reduced hardness with larger and more air cells in salt-reduced biscuits. It is suggested that salt impacts on cereal proteins by altering their aggregation around flour particles and at bubble walls and that slower water loss occurs in salted matrices during baking. Hence, this study revealed the key properties significantly affected by salt reduction and proposes an explanation which will help to develop a targeted “hidden-salt” reduction strategy.
1. Introduction
While sodium is necessary for normal body functions, it is also linked with health problems at high consumption levels. A study by Ni Mhurchu et al. showed that 77% of the total sodium intake comes from processed foods, either because they contain a high level of salt (such as ready meals, processed meats like sausages, cheese and salty snack food) or because they are consumed frequently in large amounts, such as processed cereal products, bread and biscuits (Ni Mhurchu et al., 2011). High salt intake causes an increase in blood pressure and therefore increases the risk of cardiovascular disease, stroke and coronary heart disease. The World Health Organization (WHO) recommends that adults consume less than 2 g of sodium (Na, 23 g.mol−1) daily, which corresponds to 5 g of salt (sodium chloride, NaCl, 58.5 g.mol−1) (WHO, 2020). However, average intake exceeds this level in Europe with 10.7–13.6 g/day for the highest salt consumption rates in eastern and southern European countries (Kloss, Meyer, Graeve, & Vetter, 2015). A global action program for the prevention and control of non-communicable diseases program was developed by the WHO with different voluntary global targets to reduce the rate of cardiovascular diseases, cancer and diabetes (WHO, 2014). It recommends a 30% reduction in dietary intake of sodium.
Historically salt is included in foods for 3 principal functions: preservation, sensory enhancement properties and processing (Hutton, 2002), however today it is mainly included as it is required for processing of certain products, or for its unique sensory properties. To make sure that products with low sodium content remain appealing to consumers, sodium reduction in food products should not alter the consumer experience (e.g. sensory properties). One simple strategy is the gradual reduction of the sodium content, but if this is done too quickly, the sodium reduction can result in consumers moving to other products higher in sodium or balancing the taste difference by adding back sodium chloride during preparation or consumption (Zandstra, Lion, & Newson, 2016). Sweet biscuits have been highlighted because they often contain “Hidden salt” that the consumer does not realise is present. A survey performed by Consensus Action on Salt and Health (CASH) in 2013 found that biscuits are within the top ten contributors of salt intake in the UK diet (CASH, 2013a, CASH, 2013b), and in 2015 the Pan American Health Organization (PAHO) proposed inaugural regional salt reduction targets for cookies and biscuits (Campbell, Legowski, Legetic, Nilson, & L’Abbe, 2015), therefore technical solutions for the removal of this “hidden salt” need to be developed.
Little is known about the effect of sodium reduction on sweet biscuits (shortbread-like), their structure and the aroma compounds generated/released. Some studies have investigated the impact of fat and sugar in this food matrix (Cepeda-Vázquez et al., 2018, Mamat et al., 2010), but to the best of the authors’ knowledge, the impact of sodium reduction was not explored on sweet biscuits (Israr et al., 2016, Silow et al., 2016). One study looked at the role of sodium in model cookies and showed a better browning by increasing furfural generation during caramelisation reaction (Kocadağlı & Gökmen, 2016).
During the dough preparation of sweet biscuits, high sugar and fat levels and a low water level are known to restrict gluten hydration (Gaines, 1990), which leads to limited gluten network development (protein aggregation) and to the formation of a non-elastic dough (Wade, 1988). During the baking process of the dough, a chemical reaction happens with fat, sugar, and protein. Starch granules can swell during this process but in short dough, this phenomenon is very limited. Starch particles can be partially degraded, but the high sucrose and low water levels prevent gelatinization (Chevallier, Della Valle, Colonna, Broyart, & Trystram, 2002). Gaines (1990) stated that gluten proteins remain functional during the baking process in this shortbread-like matrix (Gaines, 1990). Chevallier et al., 2000, Pareyt et al., 2009 reported that the level of extractable proteins after baking decreased significantly, suggesting the formation of an efficient gluten network in the dough during the baking process (Chevallier et al., 2000, Pareyt et al., 2009). Moreover, Chevallier et al. suggest that the structure of the matrix after baking can be attributed to the high sucrose levels (Chevallier et al., 2000). As a biscuit may be described as a complex matrix made of sugars, lipids, starch granules and protein aggregates, they propose that the structure’s cohesiveness can be mainly achieved by sugars becoming glassy during the cooling step after baking. However, it still appears that the quality of the protein aggregation and gluten network is the most important factor that affects the structural properties of the biscuits (Pareyt and Delcour, 2008, Pareyt et al., 2010). Many physico-chemical modifications occur during this baking step (Sablani, Marcotte, Baik, & Castaigne, 1998) and all these baking-induced changes are important for sensory acceptance by the consumers (Arepally, Reddy, Goswami, & Datta, 2020). Whilst lipid oxidation, caramelisation and the Maillard reaction explain many of the changes in aroma compounds, the interactions between this complex mixture of compounds are not fully known, in particular, the impact of sodium on the aggregation of protein, gluten polymerization, and the effects on the final structure and flavour after baking. The objective of this study was therefore to report on the impact of salt on the physicochemical characteristics (colour, aroma, texture) and sensory properties of sweet biscuits, and to improve our understanding of the reactions occurring in this complex matrix. This will help the biscuit producers to develop a cost-effective targeted “hidden-salt” reduction strategy maintaining good organoleptic properties and high acceptability by consumers.
2. Materials and methods
2.1. Reference dough
Reference dough (L3) was prepared following a standard recipe developed internally in our department to match commercial recipes (Ayed et al., 2018, CASH, 2013a, CASH, 2013b), from the ingredients listed below:
(1) Unsalted Butter 23.2 g/100 g of final dough (composition: fat (82.9%), polysaccharides (0.6%), fibre (0%), protein (0.6%), salt (0.11%)); (2) Caster sugar 18.6 g/100 g; (3) Semi-skimmed long life milk 11.1 g/100 g (composition: fat (1.6%) polysaccharides (4.6%), fibre (0%), protein (3.5%), salt (0.02%)); (4) Salt, 0.6 g/100 g; (5) Flour (soft wheat) containing self-raising agent 46.5 g/100 g (composition: fat (1.2%), carbohydrates (75.6%) of which starch (72.3%) and polysaccharides (1.3%), fibre (3.1%), protein (8.9%), salt (0.9%)). All the ingredients were sourced from Sainsbury’s (Supermarket company, UK) except the self-raising flour which was sourced from Morrisons (Supermarket company, UK).
2.2. Baking process
The baking process was adapted from that developed by Yang et al. (2012). The ingredients from (1) to (4) were weighed and blended manually for 1 min with a whisk. Then (5) was added and the dough was mixed with a Food processor (Multipro Home, Kenwood, UK). A homogeneous dough was formed after 1 min of continuous mixing of the ingredients. Each dough preparation was then rolled to 40 mm thickness using an industrial laminator (Fritsch, Rollfix, Germany) and shaped with a model cutter (24 mm diameter, round with smooth edge) to produce biscuits. The biscuits were placed with equal separating distances on the same tray to reduce baking variation. The tray of biscuits was placed in a Deck oven at 180 °C (Tom Chandley Compacta, UK) and baked for 12 min. Subsequently, the baking tray was removed from the oven to allow the biscuits to cool for 10 min at room temperature (20 °C). Biscuits at the edge of the tray were discarded to minimise baking variation that was observed in these positions. Biscuits on average had a height of 6 mm, diameter 32 mm and weighed 3 g. The biscuits were carefully packed and stored in sealed aluminium bags with a minimum headspace within the bag.
Four doughs, from L0 to L3, were formulated and each contained different quantities of added sodium chloride (Table 1). L3 is the reference, similar to commercial biscuits available in supermarkets with a higher quantity of salt (L3: 1.05 g of salt per 100 g of biscuits; commercial biscuits high in salt: 1.20 g ± 0.30 g of salt per 100 g). The reduction of 33% (L2), 67% (L1) and 100% (L0) of added salt was performed to cover the range of salt reduction usually considered in food industries with L0 similar to commercial biscuits low in salt (L0: 0.44 g of salt per 100 g of biscuits; commercial biscuits low in salt: 0.35 g ± 0.30 g of salt per 100 g) (CASH, 2013a, CASH, 2013b). It should be noticed that the salt contained in L0 (0.44 g of salt per 100 g of biscuits) comes naturally form the other ingredients, such as flour and milk.
Table 1.
Sodium chloride amount in sample before/after baking and after water extraction (DM = dry material); quantification performed by ICP-MS. Water content and activity of baked biscuits (measured by moisture balance and water activity meter, respectively); Rate of mass loss and temperature measured by TGA, and colour properties in CIELab colour space.
| Biscuits | L0 | L1 | L2 | L3 |
|---|---|---|---|---|
| Added NaCl in 100 g dough (g) | 0 | 0.19 | 0.37 | 0.56 |
| Reduction of Added NaCl (%) | 100 | 67 | 33 | 0 |
| Added NaCl in 100 g DM (g) | 0 | 0.21 | 0.43 | 0.64 |
| Total NaCl in 100 g of baked biscuit (g) | 0.44 | 0.66 | 0.86 | 1.05 |
| Total NaCl in 100 g DM (g) | 0.45 | 0.68 | 0.89 | 1.09 |
| Total NaCl reduced (L3 as reference) (%) | 59 | 38 | 18 | 0 |
| NaCl extracted from 100 g biscuit (g) | 0.38 | 0.59 | 0.8 | 0.99 |
| NaCl extracted from 100 g DM (g) | 0.39 | 0.62 | 0.83 | 1.03 |
| NaCl not extracted from 100 g DM (g) | 0.06 | 0.06 | 0.06 | 0.06 |
| NaCl extracted from DM (%) | 86 ± 2b | 91 ± 1b | 93 ± 1a | 94 ± 2a |
| Water content (%) | 3.1 ± 0.3c | 3.4 ± 0.3b | 3.4 ± 0.2b | 3.6 ± 0.2a |
| Water Activity at 23.5 °C | 0.25 ± 0.05 | 0.27 ± 0.04 | 0.28 ± 0.04 | 0.29 ± 0.01 |
| Temperature to reach greatest rate of loss (°C) | 104.8 ± 1.1 | 104.9 ± 0.9 | 105.2 ± 1.0 | 103.8 ± 3.2 |
| Greatest rate of mass loss measured between 30 and 180 °C (µg/s) | 4.2 ± 0.3a | 4.0 ± 0.4a | 4.1 ± 0.3a | 3.2 ± 0.3b |
| L* (lightness) | 55.4 ± 0.6 | 55.4 ± 0.6 | 55.1 ± 0.6 | 54.7 ± 0.8 |
| ΔE*cs colour difference between L3 and the other samples | 0.8 ± 0.3 | 0.8 ± 0.3 | 0.9 ± 0.2 | Ref. |
Values show average ± standard deviation. Differences between groups are shown with different letters and are significantly different according to Tukey test (p < 0.0001).
2.3. Determination of the biscuit colour
The colour of each side of each type of biscuit was determined with the Colour Quest XE (Hunter Lab, U.K.) to produce L*, a* and b* values (13 measures for each biscuits), similar to that carried out on baked goods (Chevallier et al., 2002, González-Mateo et al., 2009). The colour reading includes lightness (L*), a* which varies from green (negative) to red (positive) and b* which corresponds to a yellow-blue scale on which yellow is positive. The total colour difference (ΔE*) from a reference/control colour (L3) to a target colour (L2, L1 and L0) in the CIELAB space was performed with the Color-Difference Equation (Driver, Francis, & Clydesdale, 1976) (ISO4120:2007); (ΔE*CS = [(LC − LS)2+(aC − aS)2+(bC − bS)2]1/2, where ΔE: Euclidean distance between two colours, L: lightness of the colour, a: redness or greenness, b: yellowness or blueness, S: studied sample, C: control sample). The Colour Quest XE instrument was calibrated with a white calibration tile (serial no. CQX2614) and a light trap (serial no. CQX2614). The location in the oven and the baking time were adjusted in order to have exactly the same colour for each biscuit.
2.4. Sensory evaluation
Prior to the sensory evaluation test, ethical clearance was obtained from the School of Biosciences, University of Nottingham (SBREC160133A). A triangle test was chosen in order to determine if the reduced salt biscuit samples are perceived as sufficiently similar to the reference (ISO 4120:2004). In order to know how many panellists we needed to recruit to perform a similarity test, 3 criteria have been set up: the α risk, β risk and the ρd. The α risk (fixed to 0.2) is the probability of concluding that a perceptible difference exists when one does not. The β risk (fixed to 0.05) is the probability of concluding that no perceptible difference exists when one does. The ρd is the maximum allowable proportion of distinguishers and it was fixed to 20% according to literature recommendation for similar studies (Meilgaard, Carr, & Civille, 2006). Therefore, the minimum number of assessments required for the triangle test, was set at 96 and 108 untrained panellists (aged between 18 and 70 years old, 64 women and 44 men) were recruited to participate in this study to perform a similarity test (Meilgaard et al., 2006).
Three biscuits samples (shape: round, Φ: 32 mm, mass: 3 g per biscuit) were presented in a white disposable cup labelled with random 3-digit codes, at room temperature (19 °C). The order presentation of the samples was randomized and each panellist was asked to rinse their mouths with Evian water before and between each trial. The evaluation was conducted in sensory booths with panellists isolated from each other, conforming to ISO standards (ISO: 8589:2007).
2.5. Sodium content
A precise quantification of the total amount and the water extractable amount of sodium chloride was performed with an ICP-MS after extraction (method adapted from Khokhar et al., 2018).
2.5.1. Sample preparation for the quantification of the total amount of sodium chloride in biscuits (Total NaCl)
Dry ashing method: Prior to burning, 3 g of biscuits are ground in a mortar, transferred to a crucible bowl and placed in a muffle furnace at 550 °C for 4 h. The ashes were then digested in Digitubes (SCP SCIENCE, Canada) by adding 10 mL HNO3 and heated in heating blocks, at 95˚C for 2 h (glass watches on top of the Digitubes). Up to 50 mL of milli-Q water was added after 10 min at room temperature, and the solution was diluted 10 times with a 2% HNO3 solution.
2.5.2. Sample preparation for the quantification of the amount of sodium chloride extractable by water (NaCl extracted).
To determine the quantity of extractable sodium from the biscuits (mimicking sodium extraction by saliva), a method was developed to optimize the salt extraction process. To do so, 9 g of ground biscuits have been mixed with 6, 9 or 12 mL of ultrapure water, and then centrifuged at 5000g for 15 min at 4 °C (Thermo CR3i multifunction centrifuge, KeyWrite-DTM). The intermediary solvent layer was isolated and 3 mL of the extract was filtered (nylon syringe filter 4 mm, 0.22 µm) into glass vials. This extract was then diluted 10 times with a 2% HNO3 solution. The results showed that above 9 mL of added water it was not possible to extract more sodium from the samples. Therefore, all the extraction processes were carried out with 9 mL of water (12 samples: 4 biscuits × 3 replicates).
2.5.3. ICP-MS analysis
The sample solutions were analysed by inductively coupled plasma mass spectrometry (ICP-MS), using a Thermo-Fisher Scientific X-Series II instrument. For instrument calibration, internal standards were used as follows: Scandium (50 μg.L-1), Rhodium (10 μg.L-1) and Iridium (5 μg.L-1) in a matrix of 2% HNO3. For calibration, external standards for elements were prepared in the range 0–100 μg.L-1. Both an autosampler (Cetac ASX-520) and a concentric glass venture nebuliser (Thermo-Fisher Scientific) were used. The data processing was undertaken using Plasmalab software (version 2.5.4; Thermo-Fisher Scientific, UK).
2.6. Aroma compounds
The identification and quantification of the key volatile compounds were performed with a GC–MS using the SPME method as previously described by Liu et al. (Liu, Yang, Linforth, Fisk, & Yang, 2019) and adapted as follows:
2.6.1. GC–MS
Separation and detection of the aroma compounds of interest was achieved by injection of the sample using a 1300 series Trace GC Ultra coupled with a TSQ series mass spectrometer (Thermo Scientific, Hemel Hemptead, UK), carried out in electron ionisation mode with an ion source temperature of 200 °C, and a scanned mass range of m/z 30–300.
The volatile compounds were separated on a ZB-WAX fused silica capillary column (100% polyethylene glycol phase, 30 m × 0.25 mm × 1.0 μm; Phenomenex Inc., Macclesfield, UK) under the following conditions: injector temperature, 250 °C; flow of 5 mL/min, pressure of the carrier (helium) 18 psi. The oven temperature was held for 5 min at 30 °C, and then increased by 5 °C/min to 250 °C. Full scan mode was used to detect the volatile compounds (mass range from m/z 30 to 300). Peak identification was verified by comparing with the retention index (RI) and mass spectra of authentic spectra in a reference collection (the NIST/EPA/NIH Mass Spectral Library, version 2.0, Faircom Corporation, U.S.). The semi-quantification of volatiles collected from the headspace was calculated from GC peak areas, by comparison with the peak area of the internal standard (3-heptanone).
2.6.2. Headspace static trapping: SPME
Biscuits were ground in a mortar and weighed (3 g ± 0.001 g) in a 20 mL vial, then 6 mL of water and 20 µL of internal standard (3-heptanone, Sigma, Saint Louis, USA, at 0.01 mg/L in methanol, Laboratory reagent grade, Fisher Scientific, UK) were added. All the vials were sealed by silicon lid with PTFE septa and aluminium crimp caps.
Some fermentation issues were observed after 4 h so the sample order was randomised to avoid systematic changes during the day and each vial was analysed 1 h after preparation. 5 replicate measurements were carried out on each type of biscuit.
Before volatile extraction, the sample was pre-heated for 15 min using the GC agitator.
The extraction of volatile compounds was carried out by exposing a 1 cm length of StableFlex SPME fibre with a 50/30 μm divinylbenzene/Carboxen/polydimethylsiloxane coating (DVB/CAR/PDMS) in the headspace of the sample at 35 °C for 10 min. The fibre was then desorbed for 15 min in the injection port of the gas-chromatograph (Trace GC Ultra -Thermo Scientific) operating in split-less mode.
2.6.3. Aroma extraction and quantification
Biscuits were ground in a mortar and weighed (3 g ± 0.001 g) into 20 mL conical centrifuge tubes. 3 mL of methanol (Laboratory reagent grade, Fisher Scientific, UK) and 20 µL of internal standard (3-heptanone, Sigma, Saint Louis, USA, at 0.01 mg/L in methanol, Laboratory reagent grade, Fisher Scientific, UK) were then added. The mixture was mixed 2 min with a vortex mixer, and the conical tube centrifuged at 1000×g for 60 min at 40 °C, the latter temperature to maintain the fat phase in a liquid state. The top layer (liquid fraction) was carefully transferred into a 5 mL glass flask using a Pasteur pipette. The organic phase was diluted with a 5/95 mixture of methanol/HPLC-grade water (MeOH/Water). Prior to use, disposable cartridges with a volume of 1 mL containing 100 mg C18-bonded silica, (all cartridges were from J.T. Baker, The Netherlands) were conditioned by rinsing with 2 mL of methanol followed by 2 mL of HPLC-grade water. The aqueous solution (20 mL) containing the analytes was loaded on the Solid-Phase-Extraction (SPE) cartridge (2 mL/min). Clean-up was obtained by washing the cartridge with 1 mL of HPLC-grade water (2 mL/min). The cartridge was dried for 15 min at room temperature. Finally, the flavours trapped on the column, were desorbed with 2 mL of methanol (0.5 mL/min) and the extract dried by adding 1 g of anhydrous sodium sulfate. A 1 µL volume was injected with an autosampler in the injection port of the gas-chromatograph (Trace GC Ultra – Thermo Scientific) operating in split mode (methods described in paragraph 2.6.1). Randomisation of the samples has been made in order to avoid systematic change during the day. Each extraction was analysed for 3 replicates.
2.7. Water loss, moisture content and water activity
2.7.1. Thermogravimetry (TGA)
The weight loss of doughs during baking was measured with a Mettler-Toledo TGA/SDTA 851e thermal gravimetric analyser under a nitrogen atmosphere (3 replicates). A total of 10.0 ± 0.2 mg of sample was placed in an aluminium pan and sealed with a pierceable lid. Immediately before measurement the lid was pierced and the sample heated from 30 to 200 °C at a heating rate of 10 °C/min. The initial, final and maximal loss-rate temperatures for the moisture loss step, together with the overall percentage weight loss were calculated using STARe Thermal Analysis Software.
2.7.2. Moisture content
Moisture content was analysed as previously described by Fisk et al. by drying the biscuit using an OHAUS MB25 moisture balance (Fisk, Linforth, Taylor, & Gray, 2011). 2 g of sample were ground in a mortar and then put on the moisture balance pan and the balance was programmed to run at 120 °C for 12 min. 12 replicates of each type of biscuit were tested.
2.7.3. Water activity
An Aqualab water activity meter (series 3TE) was used to measure the Water Activity (Aw) of the biscuits as previously described by Laguna, Vallons, Jurgens, and Sanz (2013). Calibration was performed with 2 references: ultrapure water and saturated magnesium chloride (MgCl2) solution which correspond to a water activity of 1.000 and 0.328 respectively. To perform an accurate measurement a piece of biscuit was crushed with a mortar and 1 g was put into a plastic pan. The pan was then put in the Aqualab to measure the water activity. 16 replicates were run.
2.8. Instrumental texture evaluation
2.8.1. Microscopy
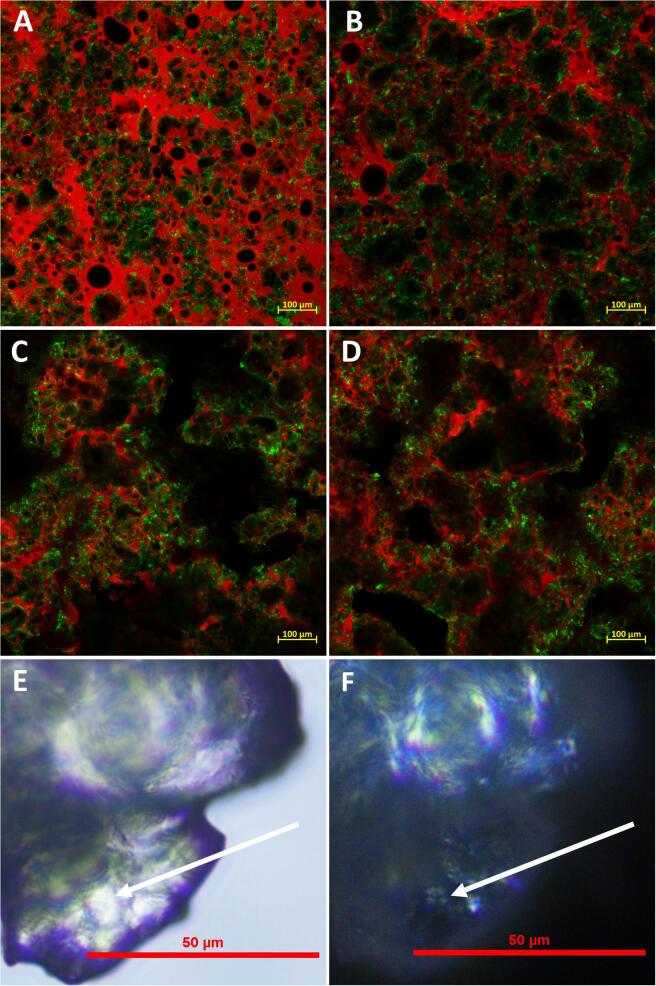
Confocal scanning laser microscopy was used as previously described to visualise the proteins and the fat phase contained in the structure of L0 and L3 biscuit before and after baking (Ayed et al., 2020). Stains were simultaneously premixed in the doughs to visualise the distribution of the protein and fat components. Nile Red, 0.01 mg/kg of final dough mass, was used to label lipids, and Fast Green FCF, 0.01 mg/kg of final dough mass, was used to bind to proteins.
Samples were cut in the form of a cuboid (approximately 10 × 10 × 6 mm), mounted on glass bottom microwell dishes (MatTek P35G-1.5-20-C), and examined using a Zeiss LSM880 Laser Scanning Microscope. A band pass filter between 550 and 620 nm was selected for the detection of Nile red; when excited at 488 nm, and 640–710 nm for the detection of fast green FCF; when excited at 633 nm. An overlay of the two channels, of the same area, gave the final image of the microstructure seen in the biscuits; showing in red the fat, in green the protein and in black the starch component. Starch granules are seen embedded in a continuous fat matrix. Images of representative areas of each sample were taken using a ×10 and ×20 magnification objective with an argon, and Helium-Neon laser respectively.
A light microscope, Nikon Eclipse Ci (Shinagawa, Tokyo, Japan), was used to estimate the size and confirm non-gelatinized starch granules in the samples after baking. The micrographs were acquired using transmitted and polarized light at ×20 magnification.
2.8.2. Three-point bend
A Texture Analyser (TAXT Texture Analyser, Stable Micro Systems) was used to measure the force in Newtons (N) required to fracture biscuits in the compression mode. A three-point bend test was carried out using a three-point bending rig (HDP/3PB), a heavy-duty platform (HDP/90) and a load cell of 5 kg.
The force required to break a sample in two was measured by loading a biscuit, placed on 2 parallel bars 18 mm apart, by a third bar, or upper blade linked to the probe, equidistant between the 2 support bars. The upper blade moved vertically downwards with a pre-test speed of 3 mm s−1, a test speed of 3 mm s−1, and a post-test speed of 10 mm s−1. The initial distance between the upper blade and the biscuit was 5 mm; the trigger force was 0.49 N; contact force 1 g, and the return distance and speed 10 mm and 10 mm/s respectively.
The force required to break the biscuit was calculated as the absolute peak force from the fracture curve (three-point break force; 3PBF). 15 replicates of each type of biscuits were performed.
2.8.3. X-ray µCT analysis
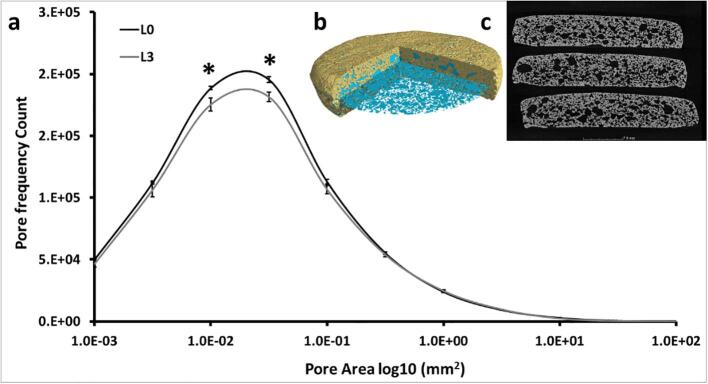
The microstructure of two biscuits (L0 and L3) was analysed by X-ray computed microtomography (X-ray μCT) using a Phoenix v|tome|x m 240 kV X-ray μCT system (GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany) at The Hounsfield Facility, University of Nottingham. Triplicate biscuits were fixed together in order to be scanned as one stack (Fig. 4c). The scan consisted of 2160 projection images collected over a 360° rotation using an electron acceleration energy of 80 kV, a current of 250 µA and a scan resolution of 27 µm. Four images were averaged per angular projection acquired with a detector timing of 200 ms and a sensitivity of 4 for each image resulting in a total scan time of 27 min. Projection images were reconstructed into 3D volumes using Datos|x REC software (GE Sensing & Inspection Technologies GmbH, Wunstorf, Germany) based on a filtered back-projection algorithm, and then rendered and exported as 16 bit grayscale .tiff images (XY axis) using VG StudioMAX v2.2 software (Volume Graphics GmbH, Germany). The microstructure of the biscuits was quantified using a modified procedure of Mathers et al. (2018). Briefly, an image mask of the total biscuit volume was prepared using Avizo Fire software (FEI Company). The original and mask XY slice images of the sample was used to subtract the pores from the biscuit and quantify total porosity and pore size distribution using the ‘Analyse Particles’ tool in FIJI software (Schindelin et al., 2012).
Fig. 4.
(a) Pore size distribution showing a significant difference (*: p < 0.01) between 1.0E-02 and 5.0E-02 log10 mm2. (b) A 3D rendered model of the biscuit after reconstruction showing the biscuit structure (porous cavity highlighted in blue). (c) A sample X-ray μCT ZX image showing the arrangement of three biscuits that were scanned together. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
2.9. Statistical analysis
The data obtained from colour, moisture content, water activity, texture analyser and aroma release experiments were statically evaluated using the software Microsoft® Excel 15/XLSTAT©-Pro (2018, France). Data were subjected to univariate analysis of variance (ANOVA). The significance level was set at p < 0.05. Significant differences among means of treatments were evaluated by the post-hoc multiple comparisons Fisher test.
For the sensory study, the statistical analyses were done with the Clopper-Pearson test and the Thurstonian model (d-prime values) in order to compare the three sets of samples. d-prime is the sensory distance between two samples, where one unit represents a standard deviation. If the proportion of correct answers increases, the distance between the two products increases and consequently the d-prime value increases (d-prime > 1: significantly different).
3. Results and discussion
There are several key quality attributes of sweet biscuits that control consumer liking. These are visual appearance, flavour perception, and texture. Therefore, after baking, sample biscuits with 33%, 67% and 100% levels of salt reduction were evaluated for their colour, sensory perception, flavour and physical properties.
3.1. Colour of the biscuits
In this study, there was no significant difference in colour between the biscuits with varying salt levels (p > 0.05); similar lightness (L = 55.2 ± 0.7) and ΔE < 1 (Table 1 and Table S1) means that the human eye cannot perceive the difference of colour (Bodart, de Peñaranda, Deneyer, & Flamant, 2008). This was surprising as sodium chloride has previously been shown to significantly impact the colour of dry snack foods (Driver et al., 1976, Moreau et al., 2009, Yang et al., 2012). It is believed that the lack of effect of sodium chloride on the colour is due to the relatively low sodium chloride concentration and high sucrose concentrations in this class of biscuits. Sugars are the main controlling factor for moisture availability in the biscuits and therefore salt reduction did not affect significantly the colour generating reactions under these baking conditions.
3.2. Sensory perception
All the biscuits produced with salt reduction (L2, L1, L0; 33%, 67%, 100% reduction respectively) were perceived as different from control (L3) by untrained panellists. The effective difference between each sample was expressed as d-prime (a sensory distance between two samples). L0, L1 and L2 (100%, 67% and 33% salt reduction, respectively) were perceived significantly different (p < 0.05, d-prime: 2.4, 1.5 and 1.0, respectively) from L3 (control with highest salt content). This result was confirmed with the panellist’s answers on the questionnaire, as 65 out of 108 found the difference between L2 and L3 hard to determine (see Fig. S1 in supplemental data). Therefore, a sodium chloride reduction of 33% is already significantly perceived and panellists discriminate it in terms of flavour (sweetness) and texture (brittleness), but not saltiness. In order to explain the sensory results, the second part of the study focused on the identification of the physicochemical properties affected by the salt reduction.
3.3. Salt impact on volatile compounds
Volatile compounds were detected in the headspace above all biscuits, of which 15 compounds were selected as key odour active compounds commonly identified in baked confectionery products (Garvey et al., 2020, Giarnetti et al., 2015, Matsakidou et al., 2010, Pasqualone et al., 2014, Pozo-Bayón et al., 2007), found to be stable with good reproducibility and were therefore used for interpretation. The compounds are shown in Table 2 and represent a number of classes of volatile aroma compounds: 5 aldehydes, 5 ketones, 3 furans and 2 pyrazines were identified.
Table 2.
List of the aroma compounds identified by SPME-GC–MS.
| N° | Aroma | Functional group | log P* | Odour description | Retention time (min) | Retention Index (RI) | RI NIST** |
|---|---|---|---|---|---|---|---|
| 1 | 2,3-pentanedione | Ketone | −0.85 | Buttery | 12.4 | 1078 | 1058 ± 9 |
| 2 | 2-furanmethanol | Furan | 0.45 | Bready/caramel | 30.9 | 1683 | 1660 ± 9 |
| 3 | Methylpyrazine | Pyrazine | 0.49 | Nutty | 19.6 | 1286 | 1266 ± 10 |
| 4 | 2-pentanone | Ketone | 0.75 | Fruity | 9.1 | 989 | 981 ± 8 |
| 5 | Furfural | Furan | 0.83 | Bready | 25.8 | 1493 | 1461 ± 11 |
| 6 | Ethylpyrazine | Pyrazine | 0.98 | Nutty | 21.8 | 1356 | 1337 ± 12 |
| 7 | 2-methylbutanal | Aldehyde | 1.23 | Bready | 6.6 | 923 | 914 ± 8 |
| 8 | 3-methylbutanal | Aldehyde | 1.23 | Fruity | 6.8 | 928 | 918 ± 8 |
| 9 | Benzaldehyde | Aldehyde | 1.71 | Fruity/nutty | 27.7 | 1560 | 1520 ± 14 |
| 10 | 2-heptanone | Ketone | 1.73 | Buttery | 16.7 | 1198 | 1182 ± 8 |
| 11 | Hexanal | Aldehyde | 1.80 | Fruity | 13.1 | 1096 | 1083 ± 8 |
| 12 | 2-nonanone | Ketone | 2.17 | Fruity | 23.4 | 1407 | 1390 ± 7 |
| 13 | Nonanal | Aldehyde | 3.27 | Fruity | 23.5 | 1413 | 1391 ± 8 |
| 14 | 2-undecanone | Ketone | 3.69 | Fruity | 29.3 | 1619 | 1598 ± 6 |
| 15 | 2-pentylfuran | Furan | 3.87 | Fruity | 18.4 | 1248 | 1231 ± 9 |
*Estimated by EPI suite software; **from NIST/EPA/NIH Mass Spectral Library.
3.3.1. Total volatile aroma compounds analysed by solid-phase extraction (SPE)
The total aroma of the biscuits was extracted and analysed by SPE-GCMS. The total aroma present in the biscuits did not vary with different levels of salt. This suggests that there is no impact of the reduced salt content on aroma formation, which supports the colour results as biscuit colour is a good proxy for heat exposure and subsequent flavour generation (González-Mateo et al., 2009).
3.3.2. Availability of volatile aroma compounds in the headspace above the biscuits by gas phase SPME-GC–MS
Aroma compounds were grouped into two major classes, hydrophobic (predominantly water insoluble) and hydrophilic (predominantly water-soluble). There was no impact of salt reduction on the total headspace availability of hydrophilic aroma compounds (logP < 1.7), however there was a significant impact on the hydrophobic compound group (logP > 1.7, p < 0.005) shown in Fig. 1. The headspace intensity of three hydrophobic compounds was significantly reduced in the salt reduced biscuits: hexanal (p < 0.001), nonanal (p < 0.05) and 2-pentylfuran (p < 0.05). A fourth compound (2-heptanone) was slightly impacted but not significantly (p = 0.113). As discussed in an earlier section, there was no difference in the concentration of these hydrophobic compounds in the biscuits, but their headspace intensity varied suggesting that the sodium chloride directly affects the partitioning of these hydrophobic aroma compounds into the headspace.
Fig. 1.
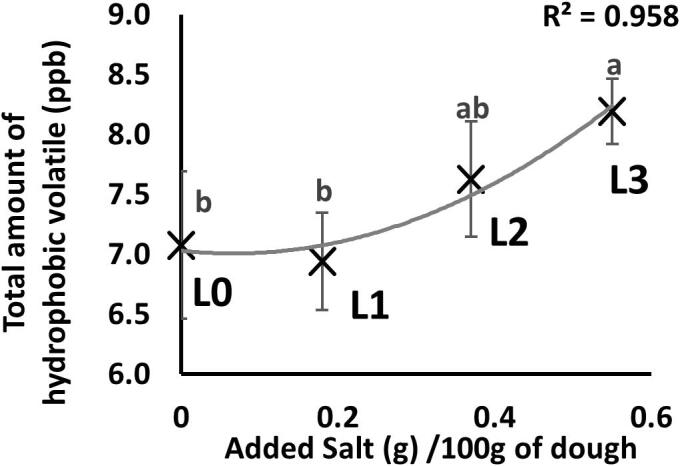
Total headspace intensity of hydrophobic volatile aroma compounds in samples with varying levels of salt addition. Statistical parameters were calculated by ANOVA (p < 0.005) and letters denote Fisher test mean comparison classification, error bars represent the standard deviation.
The differential partitioning of aroma compounds could be through a salting out process. Previously Diler et al. has shown that sodium chloride could directly impact aroma release as sodium chloride interacts with free water and reduces its availability to act as a solvent (Diler, Le-Bail, & Chevallier, 2016). This loss of free water will directly impact aroma compounds, as many aroma compounds are present at concentrations close to their limit of solubility. There therefore could be a consequential increase in the gas–liquid partition coefficient (Flores, Gianelli, Perezjuan, & Toldra, 2007). The fact that the hydrophilic aroma compounds were not affected apparently contradicts this, however the explanation for this could lie in the high amount of sucrose present. The hydrophilic aroma compounds may be dissolved in a high solute, water-sugar matrix, the water activity of which is not impacted by the addition or removal of small amounts of salt (de Roos, 2003); the significant impact of salt on the hydrophobic compounds may therefore be due to a change in the physical association of the fat in the biscuit rather than through a salting out phenomenon.
3.4. Sodium quantification (ICP-MS)
The total amount of sodium chloride contained in the biscuits after baking was quantified by ICP-MS (Table 1). The biscuits with no added salt (L0) contained 0.44 g of sodium chloride in 100 g of biscuits that presumably is the sodium present naturally in the flour and milk. The full sodium biscuits (L3) contained 1.05 g NaCl/100 g of biscuits corresponding to an extra 0.61 g NaCl/100 g in accordance with the recipe (0.64 g/100 g added NaCl in 100 g of DM, Table 1). As expected, the reduced salt biscuits had a lower level of total salt with L2 (Total NaCl reduction 18%, added NaCl reduction 33%), L1 (Total NaCl reduction 38%, added NaCl reduction 67%), and L0 (Total NaCl reduction 59%, added NaCl reduction 100%) which is within the range of industrial formulations.
The water extraction of sodium chloride showed that in L0 around 86% of the salt was extracted. As L0 does not contain any added salt, the extraction efficiency of the non-added salt is about 86% (0.38 g/100 g of biscuits). By comparing the amount of total salt in the DM with the amount of salt extracted from the DM, it appears that 14% (0.06 g) of the non-added NaCl cannot be extracted which applies to all biscuits (Table 1). This is explained by an irreversible binding of the sodium to the un-dissolvable phase of the biscuits. Mosca et al. identified that matrices containing protein and salt have a fraction of bound sodium ions probably related to the presence of negatively charged groups in proteins (Mosca, Andriot, Guichard, & Salles, 2015).
This also confirms that adding salt to biscuit dough does not affect the amount of unextractable salt. Therefore, the modifications of the perception and physicochemical properties observed in this study are only due to the amount of free salt in the biscuits.
3.5. Water loss, moisture content and water activity
3.5.1. Moisture content
During the baking process most of the water present in the dough was evaporated, resulting in a dry and porous product. (Chevallier et al., 2000, Walker et al., 2012). There was a significant impact of salt concentration on the moisture content of the biscuits (ANOVA and Fisher pairwise comparison test) with L0 and L3 having moisture contents of 3.1% and 3.6% respectively (Table 1). There was no difference observed between L1 and L2 but the global trend shows a higher moisture content in the samples containing salt. This reduction in moisture content in the lower salt biscuits may imply that the water is less retained in the low salt biscuits and therefore more easily lost during baking.
3.5.2. Water activity
There is no significant difference (p = 0.540) in water activity between any of the samples (AwL3: 0.286; AWL0: 0.255). This was surprising as addition of sodium chloride has been previously shown to impact water activity of starch rich products such as breads (0.974 for bread with 1.2% of sodium chloride and 0.989 for bread without sodium chloride) (McCann & Day, 2013). It is hypothesised that the high levels of sugars are the main regulator for water activity and the that low levels of salt do not significantly impact water activity, although it may play a role during the baking process when there is more moisture present, which could explain the difference in water content shown previously.
3.5.3. Thermogravimetry (TGA)
When analysed by thermogravimetric analysis (Table 1, Fig. 2A) rapid weight reduction occurred as the temperature exceeded 90 °C. Comparison between the biscuits with no added salt (L0) and the control samples (L3: with 0.56 g of added salt in 100 g of wet dough) showed the maximum rate of weight loss was least for the control sample. No significant differences were observed between L0, L1 and L2 (similar shape and intensity, Fig. 2B), but the rate of loss TGA curve for L3 revealed a broadened and skewed curve where the peak occurred at ~104 °C. At the end of the TGA run when the (10 mg) biscuit dough samples had reached 180 °C the total weight losses measured for the samples were not discernibly different (L0:83.2, L1:83.7, L2:83.3, L3:83.7%). But Table 1 shows that when the ~3.5 g doughs had been baked at 180 °C for 12 min in an industrial oven, the moisture contents of the final baked biscuits were significantly greater for the highest salt samples (L3). The differences between the rates and amounts of water lost on heating for samples with differing salt levels may be explained by: 1. the molecular interactions of the matrix materials with the salt (see Chen, MacNaughtan, Jones, Yang, and Foster (2020) for a more detailed consideration of the degree of water association with the matrix) and 2. variation in the physical characteristics of the samples created by the change in salt level (Bottom, 2008). As water is lost from the system, the concentration of the salt in the remaining water will increase. This will increase the boiling point for the remaining fraction of solution and so reduce the rate of water loss. However, this is a relatively small effect and will only be seen at the highest salt levels. The salt is also likely to impact on the behaviour of the proteins and therefore affect the interactions between water and both the protein and starch networks. This was recently confirmed by Avramenko et al. as they showed that increasing the amount of added NaCl in wheat flour doughs led to a slight decrease in the water loss during the baking process (Avramenko, Hopkins, Hucl, Scanlon, & Nickerson, 2020).
Fig. 2.
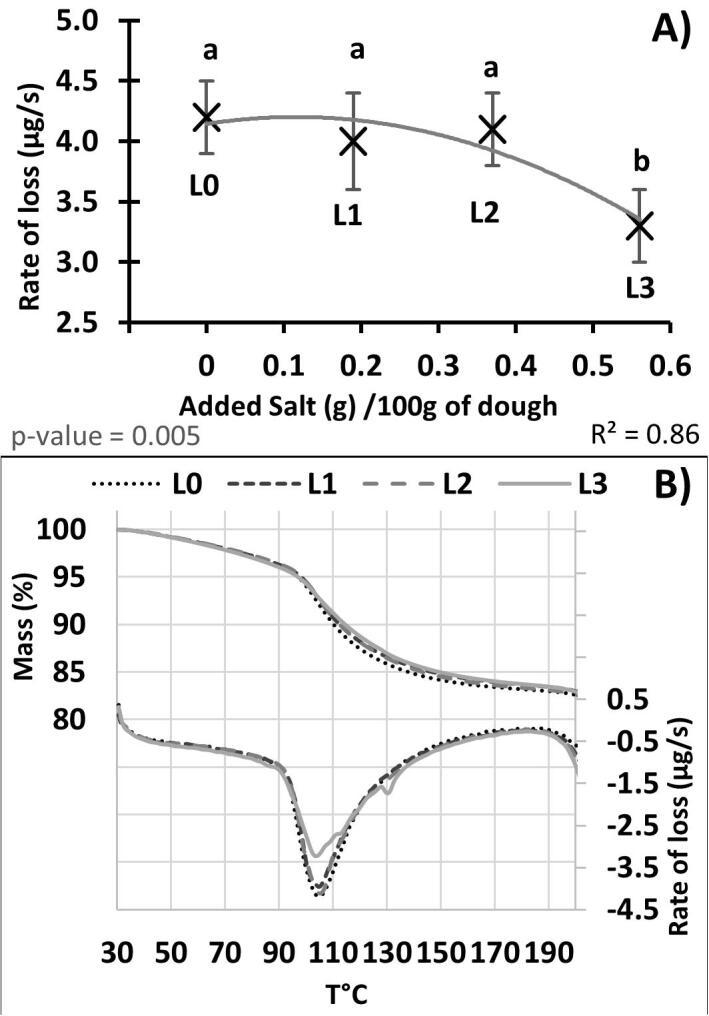
A) Greater rate of mass change (µg/s) measured between 30 °C and 180 °C by TGA during a model baking process at a rate of 10°Cmin-1 as a function of added salt in the dough. Absolute values were used for the Y axis and error bars are standard deviations. B) The upper lines are mass evolution measured by TGA in short dough biscuit with different salt levels (L3 the reference dough, L2 33% salt reduction, L1 67% salt reduction and L0 dough without added salt) upon increasing the temperatures. The lower lines are rates of mass change (µg/s) upon increasing the temperatures.
For water vapour to be lost it must diffuse through the system. In the small dough samples, it would be expected that this would be occur in a predictable steady fashion, but the change in shape of the mass rate curves (Fig. 2B lower curves) suggests that in the high salt samples mass loss is more difficult. When the water content is low, apparently random small events of vapour release appear to occur. If the water is trapped within the matrix, and the matrix suddenly breaks releasing the water vapour peaks with sharp leading edges might be expected to appear. During the baking of the ~3.5 g biscuits the pathway through which the water must diffuse is greater. In addition to the factors impacting on the vaporisation of the water, i.e. the molecular interactions and stability of the matrix, the presence of the air pockets is likely to be relevant. The different bubble structure created in the high salt samples may well be related to the higher moisture content during the baking period as indicated by the final higher moisture content.
3.6. Salt impact on texture
3.6.1. Microscopy
Microscopy images of stained biscuit doughs and the baked biscuits are shown in Fig. 3. These images indicate a very heterogeneous system with the red stained fat phase looking to be the more continuous phase with small aggregates of protein (stained green) surrounding the dark inclusions. These dark inclusions could represent the starch, sugar or voids within the matrix. The dark regions appear to be smaller within the dough containing the higher salt levels (L3) compared to the no added salt sample (L0). The protein distribution also appears to be different in these samples as it appears to be more associated with the edges of the voids in the no salt samples compared to the reference dough (L3). The low moisture content in these doughs (≈17%), a high fat level (23.4%) and sugar level (18.6%) and minimal mixing would restrict protein hydration leading to a non-continuous protein network (Manley, Pareyt, & Delcour, 2011). This is considered optimal in biscuits as full gluten formation would result in a very hard inedible product (Chevallier et al., 2002, Zheng et al., 2020). Exclusion of the water from the fat phase does seem to have concentrated the proteins at the edges of the more hydrophilic carbohydrate surfaces. This appears to be greater for the lower salt containing samples and could reflect the relevant solubilities of the prolamins in the available hydration media.
Fig. 3.
Confocal scanning laser micrographs before baking of A) L3 the reference dough; B) L0 dough without added salt; and after baking C) L3 the reference biscuit and D) L0 biscuit without added salt. Fat is stained red and proteins are stained green; starch granules are observed as black ovoid surrounded by green layer (diameter < 100 nm). The micrograph of a baked biscuit E) L3 is obtained with an optical microscope under normal light and F) L3 under cross-polarized light confirming that with cross-polarized light a “Maltese cross” typical of non-gelatinised starch was observed in the biscuits after baking. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The micrographs 3c and 3d show the stained microstructures of the biscuits after baking. The areas of black have increased and could reflect the void spaces in the matrix as well as the expansion of areas of carbohydrate. The proteins still appear to be aggregating and are associated with the voids (black areas). During the baking process, the proteins contained in flour (glutenin and gliadin) react with water and can lead to the formation of a partially cross-linked gluten network (Doescher et al., 1987, Pareyt and Delcour, 2008, Pareyt et al., 2010). However, in short biscuits full gluten formation is undesirable and the images indicate that the visualised protein remains as aggregates.
The dominant material (by weight) in the matrix would be the starch granules. The low levels of water in the dough and loss of water as the samples bake may render the plasticiser levels in the mixture to be insufficient to cause loss of order in the semi crystalline starch. Partial degradation of starch particles has previously been reported by Chevallier et al. but high levels of sucrose and low water prevent the typical gelatinization behaviour of the starch (Chevallier et al., 2002). Lineback and Wongsrikasem (1980) have shown that starch isolated from comparable systems (cookies) was 4% gelatinised, due to the low amount of free water (Lineback & Wongsrikasem, 1980). This was indicated by the presence of intact starch granules in the microscopy images of the baked products in Fig. 3 (E-F).
The biscuit matrix appears to be fat continuous with large areas that represent the carbohydrate and air cells. The protein is scattered across the matrix in discrete clumps of approximately 1 µm in diameter. In the no added salt sample, the protein seems to be more associated with the edges of the black filler phases. Due to the elevated temperatures, immediately after baking the baked matrix could be amorphous with the fat and sugar structures being flexible. It is expected that the sugars would be well distributed within the matrix and these, on removal from the oven, would be above their glass transition temperature (Ruiz-Cabrera & Schmidt, 2015). During cooling, the soft solid matrix would be expected to change; the fats may recrystallise and sugar components become glassy as they go below the original glass transition temperature and form the final biscuit structure (Kalichevsky et al., 1992, Laguna et al., 2013). The solid heterogeneous matrix obtained appears to contain both lipids and amorphous glassy carbohydrates with embedded starch granules (both complete and partially gelatinized) and protein aggregates associated with the more hydrophilic surfaces (Chevallier et al., 2000, Chevallier et al., 2002). Variation within the structures would give rise to differences in air inclusions, rates of water loss on heating and the final texture of the biscuit.
3.6.2. Evaluation of biscuit hardness by a three-point bend test
The general hardness of the biscuit was measured by the three-point bending test. To perform this test a three-point bend rig was utilized and the force required to fracture the samples evaluated. The force required for fracture decreased significantly (p < 0.05) at lower salt levels in the biscuit, indicating that the higher salt samples were stronger (L3: 13.0 ± 1.0 Newton) and more resistant to fracture and that the low salt samples (L0: 11.7 ± 0.9 Newton) were more brittle with less force being required to break the biscuits. The increased strength found in the biscuits containing higher levels of salt could be explained by more uniform water release and hence smaller and more uniform bubble structures within the biscuits.
Lynch et al. has previously shown that sodium chloride increases the strength of the gluten network in bread doughs (protein networks with a larger diameter) (Lynch, Dal Bello, Sheehan, Cashman, & Arendt, 2009). It is believed that salt masks the proteins surface charges and therefore reduces electro-repulsion between the proteins, thereby increasing the chance of protein–protein interactions (McCann & Day, 2013). In these low moisture systems, the variation in salt levels may induce proteins to move to the hydrophilic interfaces and help provide surface activity for bubble formation and growth.
3.6.3. X-ray µCT analysis
It has previously been shown that X-ray CT is a powerful technique to study the microstructure of aerated baked products e.g. porosity and pore size (Sylvie Chevallier et al., 2014, Yang et al., 2012). The effects of sugar and fat levels on biscuit porosity have previously been studied by X-ray CT (Bram Pareyt et al., 2009) and showed that increasing sugar levels resulted in linear increases in porosity, mean cell size, and mean cell wall thickness. We further developed this technique to observe the impact of added sodium on biscuit internal pore structure, to obtain reproducible figures for internal porosity of the entire biscuit. This method was able to identify clearly internal pores, interconnected channels and the variation in pore structure across the biscuit X-Y-Z plane. This showed that there was a significant effect of salt reduction on the pore structure of the biscuits. With the L0 having a greater porosity compared to L3 (p < 0.01, 47.7% and 44.7%, respectively). This porosity is due to a significant difference in the pore size distribution (Fig. 4) with the number of intermediary sized pores (0.01 to 0.03 mm2) being higher in L0.
The precise role of the salt in causing the overall differences observed is difficult to say. It would appear that not only does the salt have a role on the water handling capacity of the system it does impact on the protein behaviour in terms of location and aggregation of the main wheat flour proteins. Proteins in these limiting water environments may well play important roles in acting at the gas wall boundaries during gas production from the baking powders and water vapour liberation. Added salt may well allow a better distribution of the proteins throughout the network, achieving a more homogenous network and allowing fewer air cells and more homogenous bubble growth. By decreasing the amount of NaCl in the dough there was a decrease in resistance to extension which led to bigger bubbles. This was confirmed by Avramenko et al. (2020) on model doughs. More and larger bubbles, as occur in the no added salt sample (L0), would encourage water loss at a faster rate as mass transfer in the gaseous phases would be rapid and lead to a thinner cell wall. As observed by Pareyt et al. (2009) the cell wall thickness is correlated with the break strength of biscuits and therefore, the consequences of salt acting on the water content are both molecular and microstructural. In summary, lower levels of added salt results in a more friable product that has bigger gas cells in the matrix which develop during the baking process.
4. Conclusions
This study reports and explains for the first time, the impact of “hidden salt” reduction on sweet biscuits in terms of their sensory perception, physicochemical characteristics such as colour, aroma, and structure. Salt addition had a significant impact on the water content of the final biscuit, the microstructure of the biscuit in terms of positioning of the proteins in the doughs and air inclusions in the baked biscuits. The biscuits without added salt were easier to break, as measured by a texture analyser structure, and a sensory panel. Instrumental measures of the colour of the biscuits were not affected by the salt levels, nor were the total volatiles measured. The sensory panel could taste the difference in the samples, although they did not suggest that the tastant differences were due to salt perception. The structuring of the cooked salt-reduced biscuits showed larger and more air cells. This could have given rise to the changes in the texture and may have influenced the partitioning of the volatiles and differences in the water contents. However, delayed release of water could have also caused changes to microstructure with lower vapour pressures being exerted in the presence of the salts. What makes this biscuit system different from typical systems in salt reduction studies are the high levels of fat and sugar present. The lipids seem to form the continuous matrix phase. There are indications that the starch may be only marginally impacted by the cooking regime and the protein clusters appear similar in both the raw dough and cooked biscuits. Some interaction between the salt and the other ingredients would seem to occur as full extraction of the sodium seems difficult. In the limiting aqueous phase present in the biscuit matrix, it is suggested that cereal proteins tend to aggregate around flour particles and on bubble walls. This tendency appears greater for the low salt systems. Surface active agents would allow for enhanced bubble nucleation and growth, causing smaller bubbles with thinner walls and therefore would give rise to a less fracturable biscuit (L3). In the presence of increased salt, more of the gluten proteins would enter the matrix rather than sitting at the interface and therefore reinforce the main matrix of the biscuit. This work also shed light on the amount of bound sodium in the baked matrix, which is potentially essential for the structure cohesiveness but not necessarily perceived during the chewing process.
Numerous countries have not undertaken a robust strategy of salt intake reduction and this work can contribute to the development of an efficient salt reduction strategy in sweet baked goods with potentially a high positive impact on younger populations.
This study showed that salt plays a significant role in sweet biscuit structure formation (elasticity and porosity) and flavour availability (hydrophobic volatiles are retained with added salt). Therefore, a future global cost-effective salt reduction strategy would have a better chance of success by preserving these properties and maintaining good acceptability by consumers.
CRediT authorship contribution statement
Charfedinne Ayed: Conceptualization, Methodology, Formal analysis, Methodology, Investigation, Validation, Writing - original draft, Resources, Funding acquisition, Project administration, Supervision. Mui Lim: Formal analysis, Methodology, Validation, Writing - review & editing. Khatija Nawaz: Formal analysis, Methodology, Investigation, Validation, Writing - review & editing. William Macnaughtan: Formal analysis, Methodology, Investigation, Validation, Writing - review & editing. Craig J. Sturrock: Formal analysis, Methodology, Investigation, Validation, Writing - review & editing. Sandra E. Hill: Investigation, Validation, Writing - review & editing. Robert Linforth: Investigation, Validation, Writing - review & editing. Ian D. Fisk: Writing - review & editing, Resources, Funding acquisition, Project administration, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This work was supported by the Biotechnology and Biological Sciences Research Council (grant number BB/N021126/1) and the CASCADE-Funding scheme (FP7-PEOPLE-2012-COFUND-600181, University of Nottingham). The work was carried out in the Flavour Research Group, Division of Food, Nutrition and Dietetics in the School of Biosciences, University of Nottingham. The Hounsfield Facility received funding from European Research Council (FUTUREROOTS. Grant Agreement ID: 294729), the Biotechnology and Biological Sciences Research Council of the United Kingdom and The Wolfson Foundation. The authors would like to acknowledge Dr Rebecca Ford, Dr Qian Yang and Elizabeth Starr from the University of Nottingham for their help and support with the sensory study, as well as Saul Vazquez Reina and Scott Young from the Environmental Science department, University of Nottingham, for their help and support with technical analysis with the ICP-MS. We also would like to thank Delphine Ecouellan, Robin Leaper for their help in carrying out the experiments during their Masters and undergraduate projects and Val Street for her help and support with data acquisition with the TGA.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2021.100115.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Arepally D., Reddy R.S., Goswami T.K., Datta A.K. Biscuit baking: A review. Lwt-Food Science and Technology. 2020;131 doi: 10.1016/j.lwt.2020.109726. [DOI] [Google Scholar]
- Avramenko N.A., Hopkins E.J., Hucl P., Scanlon M.G., Nickerson M.T. Role of NaCl level on the handling and water mobility in dough prepared from four wheat cultivars. Journal of Texture Studies. 2020;51(5):766–778. doi: 10.1111/jtxs.12525. [DOI] [PubMed] [Google Scholar]
- Ayed C., Bramante F., Nwaiwu O., MacNaughtan W., Bakalis S., Foster T. Water penetration into mixed and un-mixed carbohydrate powders. Carbohydrate Polymer Technologies and Applications. 2020;1:100007. doi: 10.1016/j.carpta.2020.100007. [DOI] [Google Scholar]
- Ayed, C., Lim, M., Macnaughtan, W., Linforth, R., & Fisk, I. D. (2018). Understanding the impact of sodium on the structural properties of sweet biscuits. In B. Siegmund & E. Leitner (Eds.), Flavour science: Proceedings of the XV Weurman Flavour Research Symposium (pp. 73-76): Graz University of Technology, Institut für Informationssysteme und Computer Medien. https://doi.org/10.3217/978-3-85125-593-5-14.
- Bottom, R. (2008). Thermogravimetric analysis. In P. Gabbott (Ed.), Principles and applications of thermal analysis (pp. 87-118): Blackwell Publishing Ltd.
- Campbell N., Legowski B., Legetic B., Nilson E., L’Abbe M. Inaugural Maximum Values for Sodium in Processed Food Products in the Americas. Journal of Clinical Hypertension. 2015;17(8):611–613. doi: 10.1111/jch.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASH, (2013). Biscuit Survey 2013. Retrieved from: http://www.actiononsalt.org.uk/news/surveys/2013/biscuits/, Accessed 15th September 2020.
- CASH. (2013). New research reveals salt hidden in biscuits. Retrieved from: http://www.actiononsalt.org.uk/news/surveys/2013/Biscuits/100901.html Accessed 5th June 2020.
- Cepeda-Vázquez M., Rega B., Descharles N., Camel V. How ingredients influence furan and aroma generation in sponge cake. Food Chemistry. 2018;245:1025–1033. doi: 10.1016/j.foodchem.2017.11.069. [DOI] [PubMed] [Google Scholar]
- Chen Y., MacNaughtan W., Jones P., Yang Q., Foster T. The state of water and fat during the maturation of Cheddar cheese. Food Chemistry. 2020;303:125390. doi: 10.1016/j.foodchem.2019.125390. [DOI] [PubMed] [Google Scholar]
- Chevallier S., Colonna P., Buleon A., Della Valle G. Physicochemical behaviors of sugars, lipids, and gluten in short dough and biscuit. Journal of Agricultural and Food Chemistry. 2000;48(4):1322–1326. doi: 10.1021/jf990435+. [DOI] [PubMed] [Google Scholar]
- Chevallier S., Della Valle G., Colonna P., Broyart B., Trystram G. Structural and chemical modifications of short dough during baking. Journal of Cereal Science. 2002;35(1):1–10. doi: 10.1006/jcrs.2001.0388. [DOI] [Google Scholar]
- Chevallier S., Réguerre A.-L., Le Bail A., Della Valle G. Determining the cellular structure of two cereal food foams by X-ray micro-tomography. Food Biophysics. 2014;9(3):219–228. doi: 10.1007/s11483-014-9336-5. [DOI] [Google Scholar]
- de Roos K.B. Effect of texture and microstructure on flavour retention and release. International Dairy Journal. 2003;13(8):593–605. doi: 10.1016/S0958-6946(03)00108-0. [DOI] [Google Scholar]
- Diler G., Le-Bail A., Chevallier S. Salt reduction in sheeted dough: a successful technological approach. Food Research International. 2016;88:10–15. doi: 10.1016/j.foodres.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Doescher, L. C., Hoseney, R. C., & Milliken, G. A. (1987). A mechanism for cookie dough setting. Cereal Chemistry, 64(3), 158-163. <Go to ISI>://WOS:A1987H530800006.
- Driver M.G., Francis F.J., Clydesdale F.M. Colorimetry of dry breakfast-type cereals. Journal of Food Science. 1976;41(6):1353–1356. doi: 10.1111/j.1365-2621.1976.tb01169.x. [DOI] [Google Scholar]
- Fisk I.D., Linforth R.S.T., Taylor A.J., Gray D.A. Aroma encapsulation and aroma delivery by oil body suspensions derived from sunflower seeds (Helianthus annus) European Food Research and Technology. 2011;232(5):905–910. doi: 10.1007/s00217-011-1459-z. [DOI] [Google Scholar]
- Flores M., Gianelli M., Perezjuan M., Toldra F. Headspace concentration of selected dry-cured aroma compounds in model systems as affected by curing agents. Food Chemistry. 2007;102(2):488–493. doi: 10.1016/j.foodchem.2006.04.011. [DOI] [Google Scholar]
- Gaines C.S. Influence of chemical and physical modification of soft wheat protein on sugar-snap cookie dough consistency, cookie size, and hardness. Cereal Chemistry. 1990;67(1):73–77. <Go to ISI>://WOS:A1990CN06900015. [Google Scholar]
- Garvey E.C., O’Sullivan M.G., Kerry J.P., Kilcawley K.N. Optimisation of HS-SPME parameters for the analysis of volatile compounds in baked confectionery products. Food Analytical Methods. 2020;13(6):1314–1327. doi: 10.1007/s12161-020-01740-4. [DOI] [Google Scholar]
- Giarnetti M., Paradiso V.M., Caponio F., Summo C., Pasqualone A. Fat replacement in shortbread cookies using an emulsion filled gel based on inulin and extra virgin olive oil. LWT - Food Science and Technology. 2015;63(1):339–345. doi: 10.1016/j.lwt.2015.03.063. [DOI] [Google Scholar]
- González-Mateo S., González-SanJosé M.L., Muñiz P. Presence of Maillard products in Spanish muffins and evaluation of colour and antioxidant potential. Food and Chemical Toxicology. 2009;47(11):2798–2805. doi: 10.1016/j.fct.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Hutton T. Sodium: Technological functions of salt in the manufacturing of food and drink products. British food journal (Croydon, England) 2002;104(2):126–152. doi: 10.1108/00070700210423635. [DOI] [Google Scholar]
- Israr T., Rakha A., Sohail M., Rashid S., Shehzad A. Salt reduction in baked products: Strategies and constraints. Trends in Food Science & Technology. 2016;51:98–105. doi: 10.1016/j.tifs.2016.03.002. [DOI] [Google Scholar]
- Kalichevsky M.T., Jaroszkiewicz E.M., Blanshard J.M.V. Glass transition of gluten. 1: Gluten and gluten—sugar mixtures. International Journal of Biological Macromolecules. 1992;14(5):257–266. doi: 10.1016/S0141-8130(05)80038-8. [DOI] [PubMed] [Google Scholar]
- Khokhar J.S., Sareen S., Tyagi B.S., Singh G., Wilson L., King I.P., Broadley M.R. Variation in grain Zn concentration, and the grain ionome, in field-grown Indian wheat. Plos One. 2018;13(1) doi: 10.1371/journal.pone.0192026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss L., Meyer J.D., Graeve L., Vetter W. Sodium intake and its reduction by food reformulation in the European Union—a review. NFS Journal. 2015;1:9–19. doi: 10.1016/j.nfs.2015.03.001. [DOI] [Google Scholar]
- Kocadağlı T., Gökmen V. Effects of sodium chloride, potassium chloride, and calcium chloride on the formation of α-dicarbonyl compounds and furfurals and the development of browning in cookies during baking. Journal of Agricultural and Food Chemistry. 2016;64(41):7838–7848. doi: 10.1021/acs.jafc.6b03870.s001. [DOI] [PubMed] [Google Scholar]
- Laguna L., Vallons K.J.R., Jurgens A., Sanz T. Understanding the effect of sugar and sugar replacement in short dough biscuits. Food and Bioprocess Technology. 2013;6(11):3143–3154. doi: 10.1007/s11947-012-0968-5. [DOI] [Google Scholar]
- Lineback D.R., Wongsrikasem E. Gelatinization of starch in baked products. Journal of Food Science. 1980;45(1):71–74. doi: 10.1111/j.1365-2621.1980.tb03873.x. [DOI] [Google Scholar]
- Liu C., Yang Q., Linforth R., Fisk I.D., Yang N.i. Modifying Robusta coffee aroma by green bean chemical pre-treatment. Food Chemistry. 2019;272:251–257. doi: 10.1016/j.foodchem.2018.07.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch E.J., Dal Bello F., Sheehan E.M., Cashman K.D., Arendt E.K. Fundamental studies on the reduction of salt on dough and bread characteristics. Food Research International. 2009;42(7):885–891. doi: 10.1016/j.foodres.2009.03.014. [DOI] [Google Scholar]
- Mamat H., Abu Hardan M.O., Hill S.E. Physicochemical properties of commercial semi-sweet biscuit. Food Chemistry. 2010;121(4):1029–1038. doi: 10.1016/j.foodchem.2010.01.043. [DOI] [Google Scholar]
- Manley, D., Pareyt, B., & Delcour, J. A. (2011). Short dough biscuits. In D. Manley (Ed.), Manley's technology of biscuits, crackers and cookies, 4th Edition (pp. 331-346).
- Mathers A.W., Hepworth C., Baillie A.L., Sloan J., Jones H., Lundgren M., Sturrock C.J. Investigating the microstructure of plant leaves in 3D with lab-based X-ray computed tomography. Plant Methods. 2018;14(1) doi: 10.1186/s13007-018-0367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsakidou A., Blekas G., Paraskevopoulou A. Aroma and physical characteristics of cakes prepared by replacing margarine with extra virgin olive oil. LWT - Food Science and Technology. 2010;43(6):949–957. doi: 10.1016/j.lwt.2010.02.002. [DOI] [Google Scholar]
- McCann T.H., Day L. Effect of sodium chloride on gluten network formation, dough microstructure and rheology in relation to breadmaking. Journal of Cereal Science. 2013;57(3):444–452. doi: 10.1016/j.jcs.2013.01.011. [DOI] [Google Scholar]
- Meilgaard M.C., Carr B.T., Civille G.V. CRC Press; 2006. Sensory evaluation techniques. [Google Scholar]
- Moreau L., Lagrange J., Bindzus W., Hill S. Influence of sodium chloride on colour, residual volatiles and acrylamide formation in model systems and breakfast cereals. International Journal of Food Science and Technology. 2009;44(12):2407–2416. doi: 10.1111/j.1365-2621.2009.01922.x. [DOI] [Google Scholar]
- Mosca A.C., Andriot I., Guichard E., Salles C. Binding of Na+ ions to proteins: Effect on taste perception. Food Hydrocolloids. 2015;51:33–40. doi: 10.1016/j.foodhyd.2015.05.003. [DOI] [Google Scholar]
- Ni Mhurchu C., Capelin C., Dunford E.K., Webster J.L., Neal B.C., Jebb S.A. Sodium content of processed foods in the United Kingdom: Analysis of 44,000 foods purchased by 21,000 households. American Journal of Clinical Nutrition. 2011;93(3):594–600. doi: 10.3945/ajcn.110.004481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareyt B., Brijs K., Delcour J.A. Sugar-snap cookie dough setting: The impact of sucrose on gluten functionality. Journal of Agricultural and Food Chemistry. 2009;57(17):7814–7818. doi: 10.1021/jf9010774. [DOI] [PubMed] [Google Scholar]
- Pareyt B., Bruneel C., Brijs K., Goesaert H., Delcour J.A. Flour sodium dodecyl sulfate (SDS)-extractable protein level as a cookie flour quality indicator. Journal of Agricultural and Food Chemistry. 2010;58(1):353–360. doi: 10.1021/jf902879c. [DOI] [PubMed] [Google Scholar]
- Pareyt B., Delcour J.A. The role of wheat flour constituents, sugar, and fat in low moisture cereal based products: A review on sugar-snap cookies. Critical Reviews in Food Science and Nutrition. 2008;48(9):824–839. doi: 10.1080/10408390701719223. [DOI] [PubMed] [Google Scholar]
- Pareyt B., Talhaoui F., Kerckhofs G., Brijs K., Goesaert H., Wevers M., Delcour J.A. The role of sugar and fat in sugar-snap cookies: Structural and textural properties. Journal of Food Engineering. 2009;90(3):400–408. doi: 10.1016/j.jfoodeng.2008.07.010. [DOI] [Google Scholar]
- Pasqualone A., Bianco A.M., Paradiso V.M., Summo C., Gambacorta G., Caponio F. Physico-chemical, sensory and volatile profiles of biscuits enriched with grape marc extract. Food Research International. 2014;65:385–393. doi: 10.1016/j.foodres.2014.07.014. [DOI] [Google Scholar]
- Pozo-Bayón M.A., Ruíz-Rodríguez A., Pernin K., Cayot N. Influence of eggs on the aroma composition of a sponge cake and on the aroma release in model studies on flavored sponge cakes. Journal of Agricultural and Food Chemistry. 2007;55(4):1418–1426. doi: 10.1021/jf062203y. [DOI] [PubMed] [Google Scholar]
- Ruiz-Cabrera M.A., Schmidt S.J. Determination of glass transition temperatures during cooling and heating of low-moisture amorphous sugar mixtures. Journal of Food Engineering. 2015;146:36–43. doi: 10.1016/j.jfoodeng.2014.08.023. [DOI] [Google Scholar]
- Sablani S.S., Marcotte M., Baik O.D., Castaigne F. Modeling of simultaneous heat and water transport in the baking process. LWT - Food Science and Technology. 1998;31(3):201–209. doi: 10.1006/fstl.1997.0360. [DOI] [Google Scholar]
- Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T., Cardona A. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silow C., Axel C., Zannini E., Arendt E.K. Current status of salt reduction in bread and bakery products – A review. Journal of Cereal Science. 2016;72:135–145. doi: 10.1016/j.jcs.2016.10.010. [DOI] [Google Scholar]
- Wade, P. (1988). Biscuits, cookies and crackers. Vol. 1. London: Elsevier Applied Science.
- Walker S., Seetharaman K., Goldstein A. Characterizing physicochemical changes of cookies baked in a commercial oven. Food Research International. 2012;48(1):249–256. doi: 10.1016/j.foodres.2012.04.003. [DOI] [Google Scholar]
- WHO . WHO Library; Geneva, Switzerland: 2014. Global action plan for the prevention and control on NCDs. [Google Scholar]
- WHO. (2020). Recommendations for salt reduction. Retrieved from: https://www.who.int/news-room/fact-sheets/detail/salt-reduction, Accessed 15th September 2020.
- Yang N., Fisk I.D., Linforth R., Brown K., Walsh S., Mooney S., Hort J. Impact of flavour solvent on biscuit micro-structure as measured by X-ray micro-Computed Tomography and the distribution of vanillin and HMF (HPLC) European Food Research and Technology. 2012;235(6):1083–1091. doi: 10.1007/s00217-012-1837-1. [DOI] [Google Scholar]
- Zandstra E.H., Lion R., Newson R.S. Salt reduction: Moving from consumer awareness to action. Food Quality and Preference. 2016;48:376–381. doi: 10.1016/j.foodqual.2015.03.005. [DOI] [Google Scholar]
- Zheng B., Zhao H., Zhou Q., Cai J., Wang X., Cao W., Jiang D. Relationships of protein composition, gluten structure, and dough rheological properties with short biscuits quality of soft wheat varieties. Agronomy Journal. 2020;112(3):1921–1930. doi: 10.1002/agj2.20127. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.