Abstract
We investigated the gut microbiota in patients with non-alcoholic fatty liver disease (NAFLD) and its correlation with fibrosis and steatosis stratified by body mass index, as reflected in the controlled attenuation parameter and transient elastography values. A cross-sectional study was performed on 37 patients with NAFLD at Cipto Mangunkusumo National General Hospital from December 2018 to March 2019. The gut microbiota was investigated in fecal samples with 16S RNA sequencing using the MiSeq next-generation sequencing platform (Illumina). NAFLD was more common in patients with metabolic syndrome. Firmicutes, Bacteroidetes, and Proteobacteria were the predominant phyla. Bacteroides was more dominant than Prevotella, contrary to the results of previous studies on healthy populations in Indonesia. Microbiota dysbiosis was observed in most samples. The gastrointestinal microbiota diversity was significantly decreased in patients with NAFLD, high triglyceride levels, and central obesity. The Firmicutes/Bacteroidetes ratio correlated with steatosis and obesity, whereas some of the other species in lower taxonomy levels were mostly associated with steatosis and obesity without fibrosis. Proteobacteria was the only phylum strongly correlated with fibrosis in patients with an average body mass index. The gut microbiota diversity was decreased in patients with NAFLD, high triglyceride levels, and central obesity, and certain gut microbes were correlated with fibrosis and steatosis.
Keywords: NAFLD, ratio of Firmicutes/Bacteroidetes, dysbiosis of gut microbiota
INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is a fatty liver condition that can lead to hepatocellular carcinoma (HCC) and has globally become a major health problem with a prevalence of 25.24% (95% CI: 22.10–28.65) [1]. However, Hasan and Machmud [2] reported a NAFLD prevalence of 30.6% in 965 subjects from West Java, Indonesia. Diet, insulin resistance, and metabolic syndrome have key roles in NAFLD pathogenesis, resulting in a complex multifactorial disease [3, 4]. Gut microbiota were also found to differ in the NAFLD population, yet the mechanism for this is unclear. Changes in gut microbiota increase fatty acid absorption and the number of inflammasomes and pro-inflammatory cytokines in the blood [5, 6].
There are only four major phyla, Firmicutes, Bacteroidetes, Actinobacteria, and Proteobacteria, in the human intestinal microbiome, 90% of which is dominated by gram-positive Firmicutes and gram-negative Bacteroidetes [7]. Some studies have used the ratio of the two dominant phyla (Firmicutes and Bacteroidetes) as a marker for microbial dysbiosis [8,9,10]. Changes in this ratio have also been found in several metabolic disorders [9].
A study conducted by Zhu et al. [11] reported that the gut microbiota composition in patients with non-alcoholic steatohepatitis (NASH) and obesity showed a certain pattern related to disease progression when compared with that of normal control subjects. They observed an increased abundance of alcohol-producing bacteria in the microbiomes of patients with NASH, which suggested a relationship between the gut microbiota composition and the severity of NAFLD. Lelouvier et al. [12] found specific differences in the proportions of several bacterial taxa in both blood and feces that correlated with the presence of liver fibrosis, thus defining a specific signature of the liver disease, but this study did not show any correlation with liver steatosis.
The microbiota in patients with NAFLD experiences changes in composition associated with dysbiosis, loss of gut barrier integrity, and an increase in pro-inflammatory immune responses, which are responsible for the pathogenesis and progression of NAFLD [6]. Some studies on the microbiota profile of patients with NAFLD and healthy controls identified microbiota at different taxonomy levels. However, past studies have been inconsistent in determining the association between the microbiota profile and NAFLD, although it is thought that there is a relationship between the microbiome composition and host health [13, 14]. The inconsistent results are probably due to variable gut microbiota compositions across geographically distinct populations.
The relationship between the Firmicutes/Bacteroidetes ratio (as a dysbiosis marker) and the severity of NAFLD, especially in Indonesia, is still unknown. The microbiota composition can be a roadblock in NAFLD management, especially in interventions at the gut microbiota level. The goal of this study was to investigate the configuration of the gut microbiota in patients with NAFLD and its correlation with fibrosis and steatosis condition, as reflected in the controlled attenuation parameter (CAP) and transient elastography (TE) values. TE (FibroScan®, Echosens) measures the velocity of sound waves passing through the liver and then converts that measurement into a liver stiffness measurement; the entire process is often referred to as liver ultrasonographic (USG) elastography. The CAP specifically targets liver steatosis using a process based on TE. It measures the degree of ultrasound attenuation by hepatic fat at the central frequency of the FibroScan® M probe while simultaneously measuring liver stiffness .
MATERIALS AND METHODS
We conducted a cross-sectional study of NAFLD patients from Cipto Mangunkusumo Hospital between December 2018 until March 2019. NAFLD was diagnosed by ultrasonography . Patients who presented with excess fat in the liver and absence of excessive alcohol consumption were recruited into this study. Patients with NAFLD who were between 18 and 60 years old were targeted as the eligible subjects for this study. We excluded patients who were pregnant or lactating, had other chronic liver diseases (hepatitis B, hepatitis C, autoimmune hepatitis, or history of alcohol consumption [>40 g/day]), had a history of intestinal resection surgery, had chronic intestinal inflammation (inflammatory bowel disease [IBD]), had hepatocellular carcinoma (HCC), had a history of use of antibiotics or probiotics in the past month, or had been on a special diet in the past month.
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by an Institutional Review Board (Medical Faculty Universitas Indonesia Ethical Committee; no. 1336/UN2.F1/ETIK/2018). We obtained written informed consent from the study subjects and conducted measurements of the levels of fibrosis and steatosis, medical history examinations, food recall interviews, and fecal examinations. All data were kept confidential.
The correlations between the the ratio of the mean relative abundance (%) of Firmicutes to that of Bacteroidetes (Firmicutes/Bacteroidetes ratio and other gut microbiota profiles with the levels of fibrosis (kPa) and steatosis (dB/m) were analyzed in this study. The minimal sample size was calculated by correlation formula with correlation coefficient (r=0.5). A total sample size of 32 was required for testing in this study. During the study period, there were 60 patients in our database who were reported to have NAFLD, and we systematically selected a sample of these patients to meet the minimal sample size. Ultimately, 37 subjects were enrolled in this study. The patients included in the study were similar in terms of body mass index (BMI) to the eligible patients who were not enrolled in the study. Further, we stratified the subjects by BMI, as a potential confounder, into three categories, with five subjects classified as normal (BMI 18.5–23 kg/m2), 12 subjects classified as pre-obese (BMI 23–24.9 kg/m2), and 25 subjects classified as obese (BMI ≥ 25 kg/m2).
CAP and TE examinations were performed for all patients using a FibroScan® (Echosens). An ultrasonographic probe was used to measure the level of fibrosis (in kPa) and steatosis (in dB/m), with the values for TE and CAP ranging from 1.5 to 75 kPa and from 100 to 400 dB/m, respectively. Higher results for TE and CAP indicate higher levels of fibrosis and steatosis. Valid results for measurements of TE and CAP were obtained with a success rate of more than 60%, and the interquartile range did not exceed 30% of the median. Physical and other medical history information for less than one month was examined to collect data with respect to BMI, AST, ALT, lipid profile, HbA1c, fasting blood glucose, 2-hr postprandial blood glucose, assessment of alcohol use, and comorbidities. Patients were defined as having significant fibrosis if they had a fibrosis level of more than 7 kPa. They were defined as having significant steatosis if they had a steatosis level of more than 280 dB/m. Central obesity was defined as an increase in waist circumference ≥80 cm in women or ≥90 cm in men according to the WHO criteria for the Asian population. Increased triglyceride and low-density lipoprotein (LDL) levels were defined as elevated serum levels of more than 150 mg/dL and 100 mg/dL, respectively. Decreased high-density lipoprotein (HDL) was defined as a serum level reduced to lower than 40 mg/dL. Diabetes mellitus was defined as fasting blood sugar ≥126 mg/dL or 2-hr postprandial blood sugar ≥200 mg/dL. Dysbiosis was defined based on an increase in the Firmicutes/Bacteroidetes ratio (cut off ≥1.09, based on the 25th percentile of the data) and a decrease in bacterial diversity beyond normal values (cut off ≤4.41, based on the 50th percentile of the data).
Food recall interviews were conducted using a food frequency questionnaire. The aim of recall of the consumption frequency of selected food items in less than one month was to estimate the intake of nutrients and to assure that patients did not have any special eating patterns with respect to diet (described in detail in the supplementary data). At the time of the interviews, the patients provided stool samples, which were collected into sterile tubes. The stool samples were initially stored at 2–8°C and then stored at −80°C within 4 hr after sample collection. Gut microbiota profiles are described in terms of mean relative abundance values (%) ranging from 0 to 100%.
DNA extraction and sequencing
Bacterial genomes were extracted at the laboratory of the Child Health Department, Cipto Mangunkusumo Hospital, and sequenced by Biosains Medika (BioSM) Indonesia using a stool DNA kit, Herculase II Fusion DNA Polymerase, and a Nextera XT Index Kit V2 with the 16S Metagenomic Sequencing Library Preparation Rev. B Protocol (Part No. 15044223). The MiSeq NGS platform (Illumina) was used for 16S rRNA sequencing with four steps: sample preparation, library construction, sequencing, and raw data collection. The detail process was used according to the manufacturer’s instructions.
Statistical analysis
The data were analyzed using IBM SPSS Statistics version 23. Numerical data normality was examined using the Shapiro-Wilk test. Data were not normally distributed if the p value <0.05. Patient characteristics were analyzed according to BMI by using the Kruskal-Wallis test as a non-parametric test, ANOVA as a parametric test, and the χ2 test for categorical data. The Shannon-Weaver (H) Index, Simpson’s (D) Diversity Index, or OTU referred to as alpha diversity, was used to analyze microbiota diversity according to the levels of fibrosis, steatosis, BMI, central obesity, triglyceride, HDL, LDL, and diabetes mellitus. Comparisons between microbiota diversity and other parameters were analyzed by Mann-Whitney test. The correlation between the Firmicutes/Bacteroidetes ratio and CAP and TE values was analyzed by Spearman’s correlation test. Correlation was determined based on the correlation coefficient (r value) and considered significant if the p value <0.05.
RESULTS
Patient characteristics
We divided the subjects into three groups based on BMI: normal (5 subjects), pre-obese (7 subjects), and obese (25 subjects). The characteristics of each group are presented in Table 1. Women dominated the groups, as 23 of the subjects were female and only 14 were male, and the average age was 50 ± 7.93 years old. The average waist circumference was 96.65 cm ± 10.02, and 24 subjects experienced dyslipidemia; 30 subjects had type 2 diabetes mellitus. However, based on the median HbA1c value of 6.6 (4.8–14), it was clear that blood glucose was under control, as also shown by the moderate values for fasting blood glucose and 2-hr postprandial blood glucose, which were 108 and 149 mg/dL, respectively. The lipid profile, including the triglyceride, LDL, and HDL levels, also showed moderate values, while the results of a liver function test (aspartate aminotransferase, alanine aminotransferase, and serum albumin) also appeared normal. In all three groups, most patients had central obesity (normal, 4/5; pre-obese, 5/7; and obese, 25/25). Dyslipidemia was dominant in the pre-obese and obese groups (5/7 and 18/25, respectively), while 4/5 subjects in the normal BMI group did not have dyslipidemia. Diabetes mellitus was dominant in all groups, even in the normal BMI group.
Table 1. Demographic data and clinical characteristics of study subjects based on body mass index.
| Variables | Total (n=37) | Normal (n=5) | Pre-obese (n=7) | Obese (n=25) | p value |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 50 ± 7.93 | 48 ± 9.76 | 50 ± 3.74 | 50 ± 8.63 | 0.907α |
| Gender male, n (n/N) | 14 (14/37) | 2 (2/5) | 4 (4/7) | 8 (8/25) | 0.477γ |
| Waist circumference, mean ± SD | 96.65 ± 10.02 | 90.80 ± 12.51 | 88.85 ± 4.60 | 100 ± 9.08 | 0.009 α* |
| Central obesity, n (n/N) | 34 (34/37) | 4 (4/5) | 5 (5/7) | 25 (25/25) | 0.029 γ* |
| HbA1c, median (range) | 6.6 (4.8–14) | 5.4 (5.0–7.2) | 7.8 (5.8–9.8) | 6.6 (4.8–14) | 0.061β |
| Fasting blood glucose, median (range) | 108 (51–291) | 99 (59–141) | 118 (81–222) | 108 (51–291) | 0.748 β |
| 2 hr postprandial blood glucose, median (range) | 149 (76–473) | 110 (81–357) | 155 (93–199) | 149 (76–473) | 0.632 β |
| Type 2 DM, n (n/N) | 30 (30/37) | 5 (5/5) | 5 (5/7) | 20 (20/25) | 0.447 γ |
| Dyslipidemia, n (n/N) | 24 (24/37) | 1 (1/5) | 5 (5/7) | 18 (18/25) | 0.078 γ |
| Triglyceride, median (range) | 124 (73–282) | 105 (86–191) | 126 (73–282) | 125 (75–259) | 0.556 β |
| HDL (mmol/L), median (range) | 45 (29–111) | 49 (40–58) | 51 (35–59) | 43 (29–111) | 0.309 β |
| LDL (mmol/L), median (range) | 126 (60–213) | 93 (90–175) | 126 (60–164) | 131 (68–213) | 0.840 β |
| AST (U/L), median (range) | 20 (12–78) | 24 (18–36) | 19 (12–33) | 20 (12–78) | 0.551 β |
| ALT (U/L), median (range) | 19 (10–61) | 17 (14–28) | 28 (11–57) | 19 (10–61) | 0.686 β |
| Albumin (mg/dL), median (range) | 4.5 (2.7–5.3) | 4.3 (4.2–4.5) | 4.6 (2.7–4.9) | 4.4 (4–5.3) | 0.485 β |
| Fibrosis, median (range) | 5.6 (3.1–18.2) | 5.1 (3.3–6.7) | 4.3 (3.6–8.0) | 6.0 (3.1–18.2) | 0.600 β |
| Steatosis, mean ± SD | 273 ± 64 | 212 ± 18 | 277 ± 59 | 285 ± 66 | 0.069 α |
| Firmicutes/Bacteroidetes ratio ≥1.09 , n (n/N) | 26 (26/37) | 4 (4/5) | 4 (4/7) | 18 (18/25) | 0.657 γ |
| Microbiota diversity ≤4.41, n (n/N) | 25 (25/37) | 3 (3/5) | 3 (3/7) | 19 (19/25) | 0.235 γ |
| Firmicutes/Bacteroidetes ratio ≥1.09 and microbiota diversity ≤4.41, n (n/N) | 18 (18/37) | 2 (2/5) | 2 (2/7) | 14 (14/25) | 0.403 γ |
SD: standard deviation; BMI: body mass index; Type 2 DM: type 2 diabetes mellitus; HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; AST: aspartate aminotransferase; ALT: alanine aminotransferase. *Statistically significant. αANOVA test. βKruskal-Wallis test. γχ2 test.
Intestinal microbiota
Characteristics of intestinal microbiota in terms of mean relative abundance of bacteria based on taxonomy
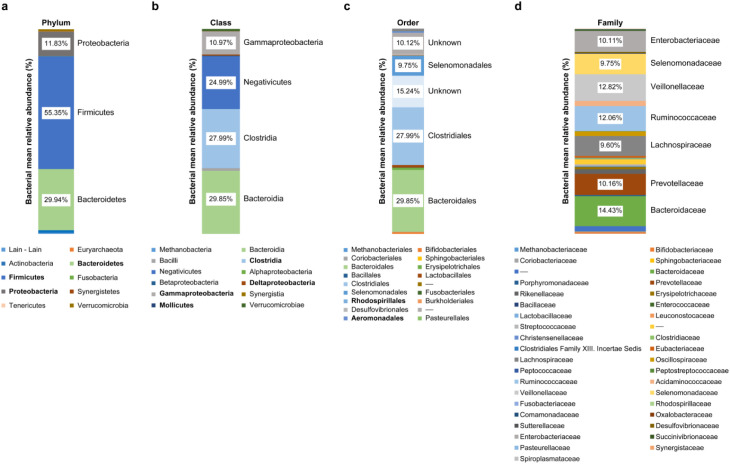
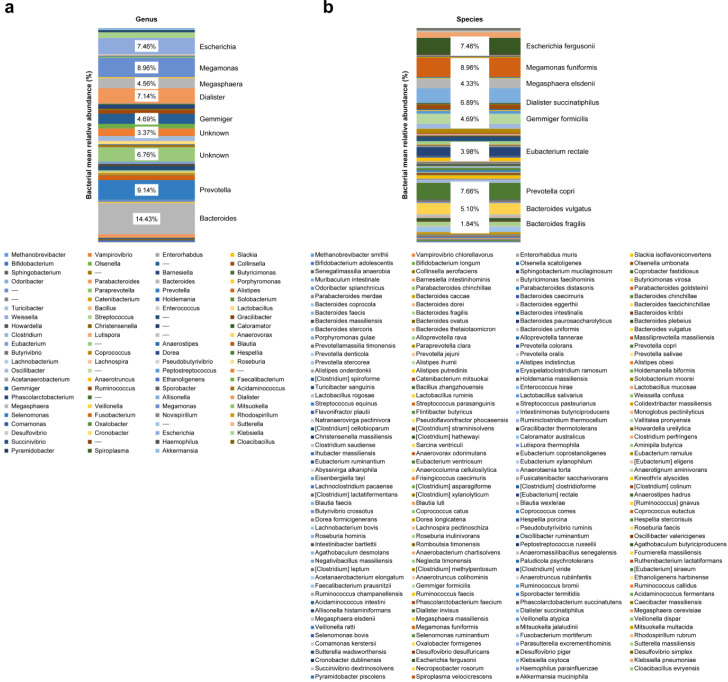
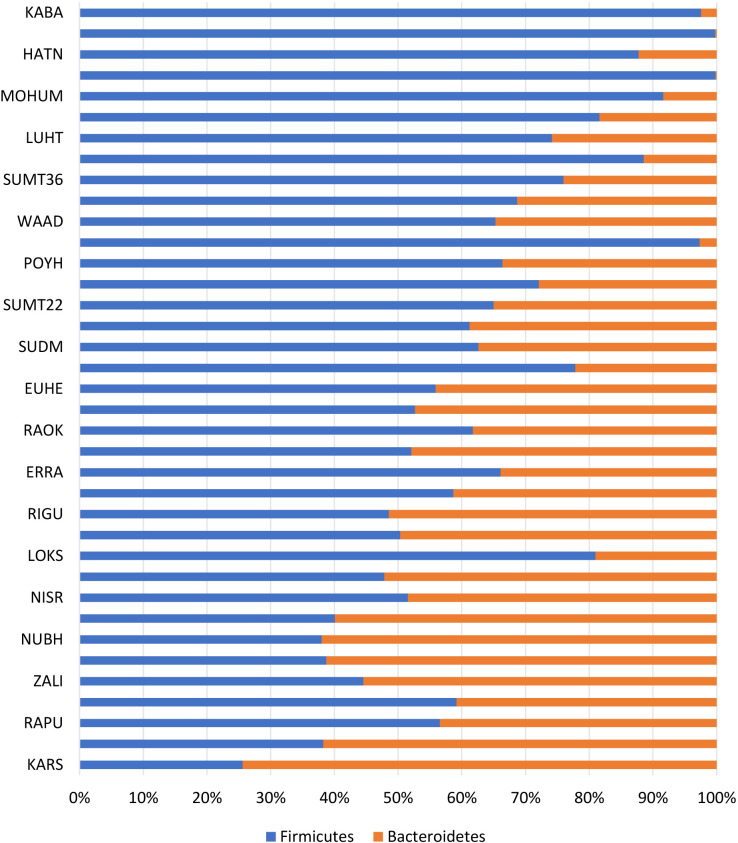
Intestinal microbiota were dominated by three major phyla: Firmicutes, 55.35%; Bacteroidetes, 29.94%; and Proteobacteria, 11.83% (Figs. 1 and 2). Figures 1 and 2 show the characteristics of gut microbiota in terms of mean relative abundance of bacteria. Firmicutes was predominant in the majority of the subjects (20 of 37 subjects), whereas Bacteroidetes was predominant in the other 17 subjects (Fig. 3). The proportions of bacteria in phyla according to mean relative abundances of bacteria detected in the NAFLD patients by BMI are shown in Table 2.
Fig. 1.
Characteristics of gut microbiota in terms of mean relative abundance bacteria.
a: Phylum; b: Class; c: Order; d: Family.
Fig. 2.
Characteristics of gut microbiota in terms of mean relative abundance of bacteria.
a: Genus; b: Species.
Fig. 3.
Characteristics of the Firmicutes/Bacteroidetes ratio in NAFLD patients.
Table 2. The proportions of bacteria in phyla according to mean relative abundances of bacteria detected in the NAFLD patients by BMI.
| Variables | Total (n=37) | Normal (n=5) | Pre-obese (n=7) | Obese (n=25) |
|---|---|---|---|---|
| Firmicutes | 55.35 ± 18.28 | 56.91 ± 28.77 | 52.08 ± 12.45 | 55.96 ± 17.91 |
| Bacteroidetes | 29.94 ± 17.09 | 23.16 ± 20.06 | 31.78 ± 12.58 | 30.78 ± 17.89 |
| Firmicutes Bacteroidetes Ratio | 36.05 ± 135.59 | 16.14 ± 20.44 | 2.05 ± 1.24 | 49.54 ± 164.00 |
| Proteobacteria | 11.83 ± 12.79 | 17.49 ± 16.87 | 8.72 ± 7.86 | 11.57 ± 13.15 |
| Actinobacteria | 1.07 ± 1.58 | 0.84 ± 1.05 | 1.15 ± 1.85 | 1.09 ± 1.63 |
| Euryarchaeota | 0.02 ± 0.05 | 0.060 ± 0.13 | 0.002 ± 0.01 | 0.011 ± 0.03 |
| Fusobacteria | 0.11 ± 0.42 | 0.03 ± 0.05 | 0.02 ± 0.03 | 0.15 ± 0.50 |
| Synergistetes | 0.11 ± 0.62 | 0.77 ± 1.69 | 0 ± 0 | 0.01 ± 0.04 |
| Tenericutes | 0.08 ± 0.33 | 0.01 ± 0.01 | 0.30 ± 0.75 | 0.04 ± 0.09 |
| Verrucomicrobia | 0.96 ± 3.74 | 0.001 ± 0.001 | 4.28 ± 8.12 | 0.22 ± 0.75 |
| Other | 0.51 ± 1.16 | 0.73 ± 0.95 | 1.66 ± 2.27 | 0.15 ± 0.20 |
NAFLD: non-alcoholic fatty liver disease; BMI: body mass index.
Microbiota diversity index
Table 3 shows the results of comparisons between alpha microbiota diversity indexes and other parameters, including fibrosis, steatosis, BMI, central obesity, triglyceride, HDL, LDL, and diabetes mellitus, by Mann-Whitney test. The table also shows the quantity of bacteria for each taxonomy level relative to its community. Diversity was determined by richness or amount (how numerous a component was found to be in a population) and evenness or distribution (comparison of the amount of a component with the number of other components in a population). The Shannon-Weaver (H) Index or Simpson’s (D) Diversity Index was used for alpha diversity. Both indicate a measure of the richness and evenness of microbiota, but the Shannon-Weaver index focuses on richness, while Simpson’s Diversity index focuses on evenness. Both used phylotype data or operational taxonomic units (OTUs), which are clusters of similar DNA sequences to be later identified with a database. The alpha diversity was significantly different in the patients with central obesity . All three diversity tests (OTU, Shannon-Weaver index, and Simpson’s Diversity index) were significantly different between the patients with and without increased triglyceride levels (p=0.010 vs. 0.005 vs. 0.003).
Table 3. Characteristics of NAFLD patients according to microbiota diversity index (OTU, Shannon-Weaver Index, and Simpson’s Diversity Index).
| OTU | p value | Shannon-Weaver Index | P value | Simpson Diversity Index | p value | |
|---|---|---|---|---|---|---|
| Fibrosis | ||||||
| Non Significant | 155 (90–331) | 0.141 | 3.94 (1.63–5.43) | 0.245 | 0.88 (0.38–0.95) | 0.473 |
| Significant | 190 (101–296) | 4.39 (2.93–5.59) | 0.90 (0.76–0.96) | |||
| Steatosis | ||||||
| Non Significant | 155 (93–331) | 0.808 | 3.94 (1.63–5.33) | 0.447 | 0.86 (0.38–0.95) | 0.213 |
| Significant | 177 (90–269) | 4.22 (2.58–5.59) | 0.90 (0.75–0.96) | |||
| BMI | ||||||
| Non Obese | 177 (99–331) | 0.527 | 4.45 (1.63–5.43) | 0.183 | 0.91 (0.38–0.95) | 0.080 |
| Obese | 176 (90–296) | 3.92 (2.31–5.59) | 0.87 (0.60–0.96) | |||
| Central obesity | ||||||
| No | 192 (155–214) | 0.404 | 4.54 (4.06–4.98) | 0.221 | 0.91 (0.88–0.94) | 0.266 |
| Yes | 176 (90–331) | 4.11 (1.63–6.59) | 0.88 (0.38–0.96) | |||
| Triglyceride | ||||||
| Normal | 183 (101–331) | 0.010* | 4.33 (3.06–5.59) | 0.005* | 0.91 (0.76–0.96) | 0.003* |
| Increase | 118 (90–201) | 3.68 (1.63–4.41) | 0.83 (0.38–0.90) | |||
| HDL | ||||||
| Normal | 188 (97–296) | 0.158 | 4.38 (1.63–5.59) | 0.064 | 0.91 (0.38–0.96) | 0.114 |
| Decrease | 151 (90–331) | 3.84 (2.31–5.33) | 0.87 (0.60–0.94) | |||
| LDL | ||||||
| Normal | 169 (106–331) | 0.929 | 4.15 (3.06–5.33) | 0.546 | 0.90 (0.80–0.94) | 0.339 |
| Increase | 180 (90–296) | 4.14 (1.63–5.59) | 0.88 (0.38–0.96) | |||
| Diabetes mellitus | ||||||
| No | 181 (112–220) | 0.684 | 4.06 (3.76–4.96) | 0.816 | 0.88 (0.84–0.95) | 0.485 |
| Yes | 172 (90–331) | 4.18 (1.63–5.59) | 0.88 (0.38–0.96) | |||
Data are presented as medians (minimum-maximum). NAFLD: non-alcoholic fatty liver disease; OTU: operational taxonomic unit; BMI: body mass index; HLD: high-density lipoprotein; LDL: low-density lipoprotein. *Statistically significant by Mann-Whitney test.
Correlation of intestinal microbiota with fibrosis and steatosis in NAFLD patients based on BMI
Table 4 shows the Spearman correlation between the Firmicutes/Bacteroidetes ratio and each microbiota based on BMI. When divided into groups based on BMI, medium positive correlation was found between the Firmicutes/Bacteroidetes ratio and steatosis (r=0.435; p=0.030) in the obesity group only. At the phylum level, medium positive correlation (r=0.528; p=0.007) was also found between Firmicutes and steatosis. The only phylum strongly correlated with fibrosis in the normal BMI group was Proteobacteria (r=0.921; p=0.026). In the pre-obese BMI group, steatosis showed very strong positive correlation with Lachnospiraceae (r=0.883; p=0.008) and Intestinimonas butyriciproducens (r=0.847; p=0.016).
Table 4. Correlation of intestinal microbiota with fibrosis and steatosis according to BMI of patients with NAFLD.
| Microbiota | Normal (n=5) | Pre-obese (n=7) | Obese (n=25) | |||||||||
| Fibrosis | Steatosis | Fibrosis | Steatosis | Fibrosis | Steatosis | |||||||
| r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | |
| Firmicutes | −0.667 | 0.219 | 0.700 | 0.188 | −0.357 | 0.432 | −0.072 | 0.878 | 0.235 | 0.257 | 0.528 | 0.007* |
| Bacteroidetes | 0.410 | 0.493 | 0.000 | 1.000 | −0.071 | 0.879 | 0.234 | 0.613 | −0.106 | 0.613 | −0.228 | 0.272 |
| Proteobacteria | 0.921 | 0.026* | −0.872 | 0.054 | 0.222 | 0.632 | 0.505 | 0.248 | −0.104 | 0.621 | −0.308 | 0.134 |
| Actinobacteria | −0.103 | 0.870 | −0.100 | 0.873 | 0.000 | 1.000 | −0.505 | 0.248 | 0.360 | 0.077 | 0.275 | 0.184 |
| Firmicutes/Bacteroidetes Ratio | −0.667 | 0.219 | 0.400 | 0.505 | −0.179 | 0.702 | −0.523 | 0.229 | 0.250 | 0.228 | 0.435 | 0.030* |
| Firmicutes/Negativicutes/Selemonadales | ||||||||||||
| Selemonadaceae/Megamonas | 0.205 | 0.741 | −0.100 | 0.873 | 0.559 | 0.192 | −0.200 | 0.667 | 0.091 | 0.664 | −0.469 | 0.018* |
| Acidaminococcaceae/Phascolarcto bacterium | 0.553 | 0.334 | −0.975 | 0.005* | 0.144 | 0.758 | 0.645 | 0.117 | 0.149 | 0.477 | 0.368 | 0.070 |
| Veillonellaceae/Dialister/D. succinatiphilus | 0.205 | 0.741 | −0.100 | 0.873 | −0.107 | 0.819 | 0.036 | 0.939 | 0.228 | 0.273 | 0.424 | 0.035* |
| Veillonellaceae/Megashphaera/M. elsdenii | 0.553 | 0.334 | −0.975 | 0.005* | 0.144 | 0.758 | 0.645 | 0.117 | 0.136 | 0.515 | 0.204 | 0.328 |
| Selenomonadaceae/Megamonas/M. funiformis | −0.975 | 0.005* | 0.800 | 0.104 | −0.288 | 0.531 | 0.355 | 0.435 | −0.190 | 0.364 | 0.241 | 0.246 |
| Firmicutes/-/Erysipelotrichales | −0.229 | 0.710 | −0.447 | 0.450 | 0.306 | 0.504 | −0.109 | 0.816 | 0.335 | 0.101 | −0.238 | 0.251 |
| Erysipelotrichaceae/Holdemania/H. massiliensis | ||||||||||||
| Firmicutes/Bacilli/Lactobacillales | −0.053 | 0.922 | 0.359 | 0.553 | −0.094 | 0.842 | 0.312 | 0.496 | −0.047 | 0.822 | −0.416 | 0.038* |
| Lactobacillaceae/Lactobacillus | ||||||||||||
| Firmicutes/Clostridia/Clostridiales | ||||||||||||
| Lachnospiraceae | 0.359 | 0.553 | −0.600 | 0.285 | −0.071 | 0.879 | 0.883 | 0.008* | −0.079 | 0.707 | −0.454 | 0.022* |
| Peptostreptococcaceae | −0.821 | 0.089 | 0.300 | 0.624 | −0.464 | 0.294 | 0.414 | 0.355 | −0.114 | 0.588 | 0.417 | 0.038* |
| Clostridiaceae/Clostridium | −0.821 | 0.089 | 0.900 | 0.037 | 0.216 | 0.641 | −0.309 | 0.500 | 0.122 | 0.562 | 0.134 | 0.523 |
| Lachnospiraceae/Roseburia | 0.359 | 0.553 | −0.300 | 0.624 | −0.054 | 0.908 | −0.400 | 0.374 | 0.215 | 0.301 | 0.418 | 0.037* |
| Ruminococcaceae/Gemmiger | 0.554 | 0.334 | −0.975 | 0.005 | 0.144 | 0.758 | 0.645 | 0.117 | 0.149 | 0.477 | 0.368 | 0.070 |
| Microbiota | Normal (n=5) | Pre-obese (n=7) | Obese (n=25) | |||||||||
| Fibrosis | Steatosis | Fibrosis | Steatosis | Fibrosis | Steatosis | |||||||
| r | p value | r | p value | r | p value | r | p value | r | p value | r | p value | |
| -/Flintibacter/F. butyricus | 0.287 | 0.640 | −0.112 | 0.858 | −0.127 | 0.786 | 0.679 | 0.094 | 0.264 | 0.202 | 0.562 | 0.003* |
| -/Intestinimonas/I. butyriciproducens | 0.526 | 0.362 | −0.359 | 0.553 | 0.393 | 0.383 | 0.847 | 0.016* | −0.478 | 0.016* | −0.246 | 0.230 |
| Lachnospiraceae/Clostridium/C. clostridioforme | −0.574 | 0.312 | 0.894 | 0.041* | −0.074 | 0.875 | 0.112 | 0.811 | −0.205 | 0.326 | 0.209 | 0.316 |
| Lachnospiraceae/Eubacterium/E. rectale | −0.308 | 0.614 | −0.500 | 0.391 | −0.667 | 0.102 | −0.509 | 0.243 | 0.359 | 0.078 | 0.440 | 0.208* |
| Ruminococcaceae/Ruminococcus/R. champanellensis | 0.821 | 0.089 | −0.300 | 0.624 | 0.090 | 0.848 | 0.000 | 1.000 | −0.188 | 0.368 | −0.426 | 0.034* |
| Actinobacteria/_/Coriobacteriales | −0.821 | 0.089 | 0.900 | 0.037* | 0.039 | 0.933 | −0.765 | 0.045 | 0.007 | 0.974 | −0.077 | 0.713 |
| Coriobacteriaceae | ||||||||||||
| Coriobacteriaceae/Collinsella | −0.821 | 0.089 | 0.900 | 0.037* | 0.039 | 0.933 | −0.765 | 0.045 | 0.006 | 0.976 | −0.033 | 0.875 |
| Coriobacteriaceae/Collinsella/C. aerofaciens | −0.821 | 0.089 | 0.900 | 0.037* | 0.039 | 0.933 | −0.765 | 0.045 | 0.006 | 0.976 | −0.033 | 0.875 |
| Proteobacteria/Betaproteobacteria/ | 0.803 | 0.102 | −0.112 | 0.858 | −0.234 | 0.613 | 0.418 | 0.350 | 0.567 | 0.003* | −0.015 | 0.943 |
| Burkholderiales | ||||||||||||
| Sutterellaceae/Sutterella | ||||||||||||
| Proteobacteria/Gammaproteobacteria/- | ||||||||||||
| Enterobacteriaceae/Escherichia | 0.667 | 0.219 | −1.000 | 0.001* | 0.429 | 0.337 | 0.360 | 0.427 | −0.129 | 0.539 | −0.297 | 0.149 |
| Enterobacteriaceae/Escherichia/E. fergusonii | 0.667 | 0.219 | −1.000 | 0.001* | 0.429 | 0.337 | 0.360 | 0.427 | −0.129 | 0.539 | −0.297 | 0.149 |
*Data are presented as correlation coefficients (with an r value of 0.4–0.59 indicating medium correlation, 0.6–0.79 indicating strong correlation, and 0.8–1 indicating very strong correlation) and considered significant (p<0.05). Spearman’s correlation test (r) was used to evaluate the correlation of the Firmicutes/Bacteroidetes ratio with CAP and TE values.
DISCUSSION
Study subject characteristics
There were more women than men in this study, similar to a population-based study in Thailand by Summart et al. [15]. Metabolic syndrome was dominant in the study subjects, which aligned with many other studies stating an apparent relationship among NAFLD, obesity, diabetes mellitus, and metabolic syndrome. In this study, 25 out of 37 subjects were obese, and 30 of the 37 subjects had type 2 diabetes mellitus. Such characteristics were similar to those of other previous studies, which stated that there was a higher prevalence of NAFLD in adults with obesity (65.7%) and type 2 diabetes mellitus (74%) [16, 17]. Individuals with NAFLD have a five times higher risk of developing diabetes [18, 19]. The association between NAFLD and type 2 diabetes mellitus can be explained by insulin resistance, dyslipidemia, and the accumulation of liver triglycerides in NAFLD and β-cell defect in type 2 diabetes mellitus [20].
Although there have been many previous studies on the microbiota, their results have been inconsistent. A study by Sohail et al. [21] reported an increase in Firmicutes in obese patients with NAFLD compared with patients without obesity and NAFLD. In another study, Jiang et al. [22] found no significant microbiota differences between NAFLD and normal control groups. Our study found that on average, Firmicutes was more abundant than Bacteroidetes, while at the genus level, Bacteroides (14.43%) was more abundant than Prevotella (9.14%), confirming that Bacteroides dominated other genera from the Firmicutes phylum.
Rahayu et al. [23] studied the microbiota profiles of young Indonesian adults and reported that in order of abundance, the most abundant bacteria were Clostridium, Prevotella, Atopobium, Bifidobacterium, and Bacteroides. The results were quite similar to other local studies showing that Prevotella was dominant but were different from the results of our study. This may be due to the different study populations, as the subjects in this study lived in Jakarta and represented an urban population with high protein and animal fat in their diet [24].
We attempted to identify the prevalence of dysbiosis by looking at the diversity of microbiota and/or increase in the ratio of Firmicutes/Bacteroidetes. Using the Firmicutes/Bacteroidetes ratio as the sole criterion for dysbiosis, 26 out of 37 subjects had dysbiosis. With decreased microbiota diversity as the sole criterion, 25 subjects had dysbiosis. By combining the two criteria, 18 subjects were determined to have dysbiosis.
Microbiota diversity index in NAFLD
The microbiota diversity in our study was assessed using the alpha diversity index through OTUs, the Shannon-Weaver index, or the Simpson’s Diversity index. The results showed significant differences in microbial diversity between the patients with and without central obesity and between the patients high and normal triglyceride levels . The patients with central obesity and those with high triglyceride levels showed a reduction in diversity compared with the other subject. This is similar to the results of studies by Turnbaugh et al. [25] and Le Chatelier et al. [26], who showed total reductions in bacterial diversity in obesity. This is also in concordance with the theory commonly used in many studies that the dysbiosis condition is a marker related to diseases.
Correlation of Firmicutes/Bacteroidetes with fibrosis and steatosis based on BMI
We analyzed the correlation of the Firmicutes/Bacteroidetes ratio and microbiota in each taxonomy level with fibrosis and steatosis based on BMI. Analysis of the NAFLD patients in each BMI group showed that the Firmicutes/Bacteroidetes ratio was only positively correlated with steatosis in the obese group. There was no significant correlation with fibrosis or steatosis in groups other than the obese group. We also found that Firmicutes showed strong positive correlation with steatosis in the obese group. This is similar to many previous studies that highlighted the role of Firmicutes in obesity [8, 21].
Further analysis in lower taxonomy levels for each microbiota revealed that only the bacteria from the phylum Proteobacteria were correlated with fibrosis in the obese and normal BMI groups. This is similar to the results of a study by Loomba et al. [27]. The higher the degree of fibrosis, the higher the abundances of Proteobacteria and Bacteroidetes. Based on the correlation analyses in this study, we can see that Proteobacteria has a role in the process of liver fibrosis, although the exact mechanism is still unknown.
Most of the microbiota examined in this study showed a positive correlation with steatosis, especially in obese patients. Some of them were from the orders Clostridiales and Selemonodales in the Firmicutes phylum. On the other hand, those correlated with steatosis in the normal BMI group were from the Actinobacteria phylum, and the mechanisms underlying steatosis caused by these microbiota were via several pathways, especially those related to fat metabolism [25]. Lactobacillus was very consistent in protecting against steatosis, while the Enterobacteriaceae family in our study showed a very strong positive correlation with steatosis in the normal BMI group. These findings differed from those of previous studies [11]. However, a study by Rahayu et al. [23] in a healthy Indonesian population showed that the Enterobacteriaceae family, especially Escherichia coli, is part of the normal flora that increases in old age.
At present, there are very few studies that can show a direct cause and effect relationship between microbiota and NAFLD pathogenesis. However, some interventional studies in animals showed the important role of intestinal microbiota, especially in triggering a metabolic response. The intestinal microbiota from obese subjects can induce liver steatosis through modulation of fat metabolism. This is probably why most intestinal microbiota in our study were correlated with steatosis but not with fibrosis. The process of steatosis becoming fibrosis requires more complex pathways and involves more factors besides intestinal microbiota [28].
We acknowledge that the limitations of this study include its small sample size, because of which we could not demonstrate that small variations in the bacterial counts were statistically significant. However, this single-center cross-sectional study was unable to view in detail the change in microbiota in relation to disease progression. We did not include normal healthy controls because it was difficult to find a population in the urban setting that was absolutely healthy, free of metabolic disorders, and not affected by an extreme diet.
In conclusion, we assumed that the bigger the difference between the subgroups studied, the stronger the potential effect of the bacteria on the phenotype. This is the first study in Indonesia to thoroughly profile the microbiota in patients with NAFLD using next-generation sequencing and try to determine the correlation of each microbiota with fibrosis and steatosis. There was a strong positive correlation between the Firmicutes/Bacteroidetes ratio and steatosis in the obese group. Some microbiota showed positive and negative correlations with fibrosis and steatosis. We suggest that future studies examine microbiota profiles in the general Indonesian population, the microbiota population in patients with NAFLD based on groups with other metabolic syndrome comorbidities, and the relationships between microbiota metabolism products and NAFLD.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available on request to the corresponding author. The data are not publicly available because they contain information that could compromise research participants’ privacy/consent.
AUTHOR CONTRIBUTIONS
Chyntia Olivia Maurine Jasirwan proposed and conducted the study, collected and analyzed the data, and provided the idea for the first draft of the manuscript. Akhmadu Muradi, Irsan Hasan, Marcellus Simadibrata, and Ikhwan Rinaldi performed the research and supervised the study. All authors contributed to the design of the study, interpretation of the results, and writing of the final manuscript. Chyntia Olivia Maurine Jasirwan is the guarantor of this study.
FUNDING
This study received one-third of its funding from a university (Medical Faculty Universitas Indonesia) and the rest of its funding from private funding.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgments
We would like to thank Ms. Anugrah Dwi Handayu for helping with stool collection and PCR analyses and Ms. Gita Aprilicia for helping with the datasets and analyses.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. 2016. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64: 73–84. [DOI] [PubMed] [Google Scholar]
- 2.Hasan I, Machmud R. 2002. Prevalence and risk factors for nonalcoholic fatty liver in Indonesia. J Gastroenterol Hepatol 17: A30. [Google Scholar]
- 3.Shulman GI. 2000. Cellular mechanisms of insulin resistance. J Clin Invest 106: 171–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarantino G, Finelli C. 2013. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol 19: 3375–3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. 2010. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des 16: 1941–1951. [DOI] [PubMed] [Google Scholar]
- 6.Kirpich IA, Marsano LS, McClain CJ. 2015. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem 48: 923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mokhtari Z, Gibson DL, Hekmatdoost A. 2017. Nonalcoholic fatty liver disease, the gut microbiome, and diet. Adv Nutr 8: 240–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. 2006. Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 9.Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, Corthier G, Furet JP. 2009. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol 9: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, Zadeh M, Gong M, Qi Y, Zubcevic J, Sahay B, Pepine CJ, Raizada MK, Mohamadzadeh M. 2015. Gut dysbiosis is linked to hypertension. Hypertension 65: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, Gill SR. 2013. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 57: 601–609. [DOI] [PubMed] [Google Scholar]
- 12.Lelouvier B, Servant F, Païssé S, Brunet AC, Benyahya S, Serino M, Valle C, Ortiz MR, Puig J, Courtney M, Federici M, Fernández-Real JM, Burcelin R, Amar J. 2016. Changes in blood microbiota profiles associated with liver fibrosis in obese patients: a pilot analysis. Hepatology 64: 2015–2027. [DOI] [PubMed] [Google Scholar]
- 13.Lau E, Carvalho D, Freitas P. 2015. Gut microbiota: association with NAFLD and metabolic disturbances. BioMed Res Int 2015: 979515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, McGilvray ID, Allard JP. 2013. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 58: 120–127. [DOI] [PubMed] [Google Scholar]
- 15.Summart U, Thinkhamrop B, Chamadol N, Khuntikeo N, Songthamwat M, Kim CS. 2017. Gender differences in the prevalence of nonalcoholic fatty liver disease in the Northeast of Thailand: a population-based cross-sectional study. F1000 Res 6: 1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. 2011. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology 140: 124–131. [DOI] [PubMed] [Google Scholar]
- 17.Paquissi FC. 2016. Immune imbalances in non-alcoholic fatty liver disease: from general biomarkers and neutrophils to interleukin-17 axis activation and new therapeutic targets. Front Immunol 7: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jäger S, Jacobs S, Kröger J, Stefan N, Fritsche A, Weikert C, Boeing H, Schulze MB. 2015. Association between the fatty liver index and risk of type 2 diabetes in the EPIC-Potsdam Study. PLoS One 10: e0124749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazlehurst JM, Woods C, Marjot T, Cobbold JF, Tomlinson JW. 2016. Non-alcoholic fatty liver disease and diabetes. Metabolism 65: 1096–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forlani G, Giorda C, Manti R, Mazzella N, De Cosmo S, Rossi MC, Nicolucci A, Di Bartolo P, Ceriello A, Guida P, AMD-Annals Study Group2016. The burden of NAFLD and its characteristics in a nationwide population with type 2 diabetes. J Diabetes Res 2016: 2931985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sohail MU, Althani A, Anwar H, Rizzi R, Marei HE. 2017. Role of the gastrointestinal tract microbiome in the pathophysiology of diabetes mellitus. J Diabetes Res 2017: 9631435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. 2015. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 5: 8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahayu ES, Utami T, Mariyatun M, Hasan PN, Kamil RZ, Setyawan RH, Pamungkaningtyas FH, Harahap IA, Wiryohanjoyo DV, Pramesi PC, Cahyanto MN, Sujaya IN, Juffrie M. 2019. Gut microbiota profile in healthy Indonesians. World J Gastroenterol 25: 1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daya M, Pujianto DA, Witjaksono F, Priliani L, Susanto J, Lukito W, Malik SG. 2019. Obesity risk and preference for high dietary fat intake are determined by FTO rs9939609 gene polymorphism in selected Indonesian adults. Asia Pac J Clin Nutr 28: 183–191. [DOI] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI. 2008. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3: 213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Chatelier E, Nielsen T, Qin J, Prifti E, Hildebrand F, Falony G, Almeida M, Arumugam M, Batto JM, Kennedy S, Leonard P, Li J, Burgdorf K, Grarup N, Jørgensen T, Brandslund I, Nielsen HB, Juncker AS, Bertalan M, Levenez F, Pons N, Rasmussen S, Sunagawa S, Tap J, Tims S, Zoetendal EG, Brunak S, Clément K, Doré J, Kleerebezem M, Kristiansen K, Renault P, Sicheritz-Ponten T, de Vos WM, Zucker JD, Raes J, Hansen T, Bork P, Wang J, Ehrlich SD, Pedersen O, MetaHIT consortium2013. Richness of human gut microbiome correlates with metabolic markers. Nature 500: 541–546. [DOI] [PubMed] [Google Scholar]
- 27.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB, Sirlin CB, Schnabl B, Brinkac L, Schork N, Chen CH, Brenner DA, Biggs W, Yooseph S, Venter JC, Nelson KE. 2017. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 25: 1054–1062.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wieland A, Frank DN, Harnke B, Bambha K. 2015. Systematic review: microbial dysbiosis and nonalcoholic fatty liver disease. Aliment Pharmacol Ther 42: 1051–1063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request to the corresponding author. The data are not publicly available because they contain information that could compromise research participants’ privacy/consent.