Abstract
Adherence of probiotics to dietary fibers present in the intestinal tract may affect adhesion to intestinal epithelial cells. The properties of the adhesion of bifidobacteria to mucin or epithelial cells have been well studied; however, adhesion of bifidobacteria to dietary fiber has not been investigated. The adhesion ratio of six Bifidobacterium strains to cellulose and chitin was examined; among the strains, Bifidobacterium animalis subsp. lactis JCM 10602 showed high adherence to both cellulose and chitin, and two strains showed high adherence to only chitin. The ratios of adhesion of B. animalis to cellulose and chitin were positively and negatively correlated with ionic strength, respectively. These data suggest that hydrophobic and electrostatic interactions are involved in the adhesion to cellulose and chitin, respectively. The adhesion ratios of the cells in the late logarithmic phase to cellulose and chitin decreased by approximately 40% and 70% of the cells in the early logarithmic phase, respectively. Furthermore, the adhesion ratio to cellulose decreased with increasing bile concentration regardless of the culture phase of the cells. On the other hand, the adhesion ratio to chitin of cells in the early logarithmic phase decreased with increasing bile concentration; however, that of cells in the late logarithmic phase increased slightly, suggesting that adhesins differ depending on the culture phase. Our results indicated the importance of considering adhesion to both dietary fibers and the intestinal mucosa when using bifidobacteria as probiotics.
Keywords: Bifidobacterium, cellulose, chitin, adhesion, bile, growth phase
INTRODUCTION
A large number of diverse microorganisms form the intestinal microbiota and exert multiple physiological effects on the host. In particular, bifidobacteria and lactic acid bacteria have beneficial effects on the host, such as regulation of intestinal function [1, 2] and the immune system [3, 4]. These bacteria are defined as probiotics, “living microorganisms, which when administered in adequate amounts confer a health benefit on the host” [5]. These health benefits occur due to interactions between the probiotics and the host; thus, it is important to consider the phenomenon of adhesion of probiotics to the intestinal tract.
Intestinal bacteria adhere to the intestinal tract by recognizing carbohydrate moieties of mucin, which is the main component of the mucus layer covering the intestinal epithelial cells. Many studies have shown that specific cell-surface proteins act as adhesion factors, known as adhesins [6]. Several bacterial adhesins have been identified in bifidobacteria, including type IVb tight adherence pili of Bifidobacterium breve UCC2003 [7], transaldolase of Bifidobacterium bifidum A8 [8], DnaK of Bifidobacterium animalis subsp. lactis Bi-07 [9], BopA (lipoprotein) of B. bifidum MIMBb75 [10], and exopolysaccharides (EPSs) of B. animalis IPLA-R1 [11]. Lactic acid bacteria express specific adhesins, including of Lactobacillus reuteri 1063 [12], Spa pili of Lactobacillus rhamnosus GG [13], and GroEL of Lactobacillus johnsonii NCC53 [14]. In particular, proteins that were primarily identified as metabolic enzymes and chaperones (e.g., transaldolase, GroEL and DnaK) are called moonlighting proteins because they act as adhesins on the cell surface.
In the human intestine, there are also dietary fibers from the diet. Dietary fibers are classified into two groups: soluble dietary fibers such as galactooligosaccharides and insoluble dietary fibers such as cellulose. The majority of soluble dietary fibers are metabolized by bacteria in the large intestine to short-chain fatty acids such as butyric acid and propionic acid [15], which can be used as energy sources for colonic epithelial cells and also inhibit the growth of pathogens [16]. Insoluble dietary fibers are excreted out of the body by intestinal peristalsis, which is activated to promote fecal excretion; additionally, bile acid that has flowed into the large intestine without being reabsorbed is adsorbed by insoluble dietary fiber and excreted outside the body [17, 18].
Considering that both dietary fiber and mucin have sugar chains as basic structures, the adhesion of probiotics to dietary fiber may affect whether or not it settles in the intestinal tract. Fernando et al. mentioned the possibility that dietary fiber can be a vehicle that carries lactic acid bacteria and bifidobacteria, which adhere to dietary fiber, to the intestinal tract [19]. We previously reported that cytoplasmic proteins such as DnaK and glyceraldehyde-3-phosphate dehydrogenase are present on the cell surface of Lactococcus lactis IL 1403 and that they also have an affinity for mannan [20]. Subsequent studies have elucidated that several lactic acid bacteria strains adhere to cellulose, a typical insoluble dietary fiber (unpublished data).
The aim of this study was to understand the properties of the adhesion of bifidobacteria abundant in the intestinal tract to the typical dietary fibers cellulose and chitin. We examined whether several bifidobacteria strains derived from the human intestinal tract, feces, and milk products adhered to dietary fiber and then analyzed the effects of pH and bile on the adhesion.
MATERIALS AND METHODS
Bacterial strains and culture conditions
Six Bifidobacterium strains were purchased from the Japan Collection of Microorganisms (JCM) of RIKEN BioResource Research Center (Table 1). All strains were cultured anaerobically at 37°C in a de Man, Rogosa, Sharpe (MRS) broth (10.0 g Bacto peptone, Becton, Dickinson and Company [BD], Tokyo, Japan; 10.0 g Bacto beef extract, BD; 5.0 g Bacto yeast extract, BD; 20.0 g glucose; 1.0 g polyoxyethylene (20) sorbitan monooleate; 2.0 g triammonium citrate; 5.0 g sodium acetate; 0.2 g magnesium sulfate heptahydrate; 0.08 g manganese(II) sulfate pentahydrate; and 2.0 g dipotassium hydrogenphosphate per liter) containing 0.05% cysteine, using an AnaeroPack system (Mitsubishi Gas Chemical Company, Tokyo, Japan).
Table 1. Bifidobacterium strains used in this study.
| Strains | Origin |
|---|---|
| Bifidobacterium animalis subsp. lactis JCM 10602 | Milk product |
| Bifidobacterium longum subsp. infantis JCM 1222 | Intestine of infant |
| Bifidobacterium longum subsp. longum JCM 1217 | Intestine of adult |
| Bifidobacterium breve JCM 1192 | Intestine of infant |
| Bifidobacterium pseudocatenulatum JCM 1200 | Feces of infant |
| Bifidobacterium angulatum JCM 7096 | Feces of adult |
Adhesion assay
The adhesion ratio of bifidobacteria cells to dietary fiber powder was calculated using the difference in sedimentation rate between the cells and the powder. Since dietary fiber powder settles faster than cells, the percentage of cells that adhered and co-settled with dietary fiber powder was calculated.
Cellulose (Nacalai Tesque, Kyoto, Japan) and chitin (Fujifilm Wako Pure Chemical, Osaka, Japan) powders were used as insoluble dietary fibers. Powders with a diameter of 38–100 μm were selected by passing them through sieves and suspended at 30 mg/mL in PC buffer (5.2 mM citrate, 5.8 mM sodium phosphate, 150 mM NaCl) adjusted to pH 5.0 or 7.0 with NaOH. Then, decantation was performed three times to remove fine particles which did not settle naturally. When changing the ionic strength, PC buffer was diluted 1, 3, 9, and 30 times, resulting in ionic strengths of 193, 64, 21, and 6.4 mmol/L, respectively. When investigating the effects of bile on the adhesion of bacteria to fiber, bile (Fujifilm Wako Pure Chemical, Osaka, Japan) was adjusted to 2 g/L with PC buffer and diluted 10 to 1,000 times. The cells were washed twice with PC buffer (9,500 × g, 5 min, 4°C) and then suspended at an optical density at 660 nm (OD660) of 2.
Equal volumes (0.5 mL each) of the cell suspension and the cellulose or chitin powder suspension were mixed in a microtube and incubated at 37°C for 10 min with rotation at 6 rpm. After centrifugation (500 × g, 10 sec), the mixture was left for 5 min. A 0.5 mL supernatant sample was collected from the top, and the OD660 was measured by using a Ratio Beam Spectrophotometer U-5100 (Hitachi, Tokyo, Japan). To calculate the adhesion ratio, which was the percentage of cells that adhered to the dietary fiber powder, the OD660 was compared with the OD660 of two controls containing (i) bacteria but no dietary fiber powder and (ii) dietary fiber powder but no bacteria. The adhesion ratio of bifidobacteria to dietary fiber was defined as
where A represents the OD660 of the supernatant from the microtube containing a dietary fiber powder plus cells, B represents the OD660 of the supernatant from a control tube containing a dietary fiber powder but no cells, and C represents the OD660 from a control tube containing cells but no dietary fiber powder.
Statistical analysis
All data are expressed as the mean ± standard error (SE). The level of statistical significance was set at p<0.05 based on Student’s t-test or Tukey’s test.
RESULTS
Adhesion of Bifidobacterium strains to dietary fiber
First, the adhesion ratios of six human intestine Bifidobacterium strains to cellulose and chitin were examined at pH 7.0 (Fig. 1). B. animalis subsp. lactis JCM 10602 showed high adhesion ratios to both dietary fibers under conditions in which the adhesion sites of these dietary fiber powders were not saturated (Supplementary Fig. 1). Bifidobacterium pseudocatenulatum JCM 1200 and Bifidobacterium longum subsp. longum JCM 1217 also exhibited high adhesion ratios to chitin; however, all of the tested strains, except B. animalis, showed low adhesion to cellulose ratio of less than 10%. Each Bifidobacterium exhibited different properties of adhesion to dietary fiber, and our further studies of the phenomenon of adhesion of bifidobacteria to dietary fiber were performed using B. animalis subsp. lactis JCM 10602, which exhibited a high adhesion ratio to both cellulose and chitin.
Fig. 1.
Adhesion ratios of Bifidobacterium strains to dietary fiber. Filled columns, cellulose; open columns, chitin. Adhesion ratios (%) are presented as means ± standard error of triplicate samples. Bars with the same letters are not significantly different from each other (p<0.05) by Tukey’s test.
Effect of pH on the adhesion of B. animalis to dietary fiber
When bifidobacteria produce organic acids such as acetic acid in the intestine, the pH is considered to be locally decreased. Therefore, the adhesion ratio of B. animalis to dietary fiber was examined not only at the average human intestinal pH of 7.0 but also at pH 5.0. The adhesion ratio to cellulose at pH 7.0 was 82%, whereas at pH 5.0, the ratio was 27%. On the other hand, the adhesion ratio to chitin was 39% at pH 7.0 but increased to 94% at pH 5.0 (Fig. 2). Therefore, the data suggested that the mechanisms of adhesion of B. animalis to cellulose or chitin were different.
Fig. 2.
Effect of pH on adhesion of B. animalis to dietary fiber. **p<0.01 by Student’s t-test. Filled columns, pH 7.0; open columns, pH 5.0.
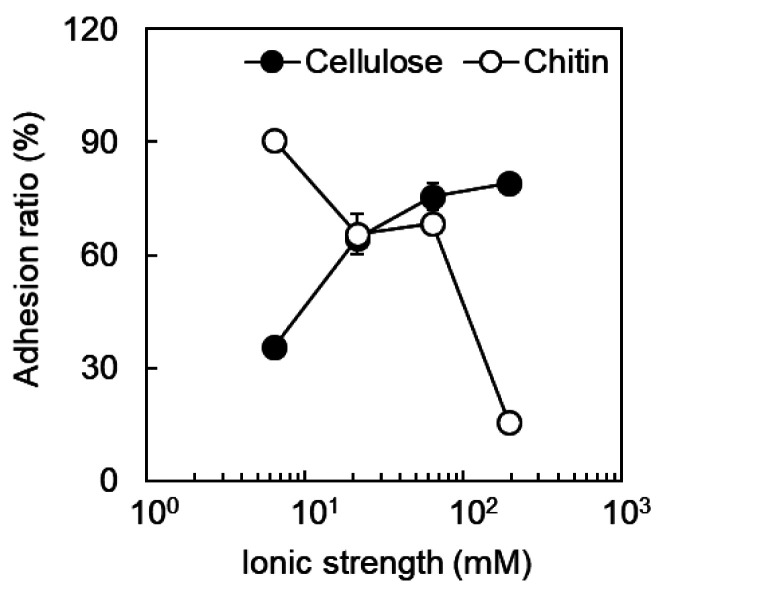
Effect of ionic strength on the adhesion of B. animalis to dietary fiber
In order to estimate what interactions are involved in the adhesion of B. animalis to dietary fiber, the effects of ionic strength on the adhesion ratio were investigated. The adhesion ratio to cellulose decreased with decreasing ionic strength (Fig. 3). It was confirmed that glucose, sucrose, and lactose (10 g/L) do not inhibit the adhesion of B. animalis to cellulose (data not shown). Considering that crystalline cellulose has a hydrophobic region [21] and that the hydrophobic interaction becomes stronger with increasing ionic strength, the results suggested that a hydrophobic interaction occurs between the bacterial cell and cellulose. On the other hand, the adhesion ratio to chitin increased with decreasing ionic strength. It has been reported that part of the acetamide group of chitin is converted to an amino group and is positively charged [22]. The surface of Gram-positive bacteria is negatively charged due to the presence of phosphate groups contained in lipoteichoic acid (LTA), which possess glycolipids anchored in the cell membrane and a repeating structure of glycerol phosphate that crosses the cell wall [23]. Therefore, it was suggested that the Coulomb force acts between B. animalis and chitin and that the difference in the adhesion ratio to chitin among other bifidobacteria depends on the structure or degree of surface exposure of LTA.
Fig. 3.
Effect of ionic strength on adhesion of B. animalis to dietary fiber (pH 7.0). Closed circles, cellulose; open circles, chitin.
Effects of growth phase on the adhesion of B. animalis to dietary fiber
The effects of the cell culture phase on the adhesion of B. animalis to dietary fiber were evaluated. Various components, such as sortase-dependent pili and surface-associated EPSs, are exposed on the cell wall of bifidobacteria [24]. It is also known that sialidase, which utilizes sialylated mucin and acts as an adhesion factor for mucin, is embedded in peptidoglycan as the culture time progresses [25]. Therefore, based on the growth curve of B. animalis, the effects of culture phase on the adhesion ratio to dietary fibers were investigated using cells in the early logarithm (8 hr), middle (12 hr), late (20 hr), and stationary phases (36 hr) (Fig. 4). The adhesion ratio to dietary fiber decreased as the culture progressed (Fig. 5). This result suggested that the state of the cell surface involved in the adhesion to dietary fiber of B. animalis also changed as the culture progressed.
Fig. 4.
Time course of the growth of B. animalis. Circles, triangles, and squares indicate the values of OD660 of the culture broth in different runs.
Fig. 5.
Effect of culture time on adhesion of B. animalis to dietary fiber (pH 7.0). Closed circles, cellulose; open circles, chitin.
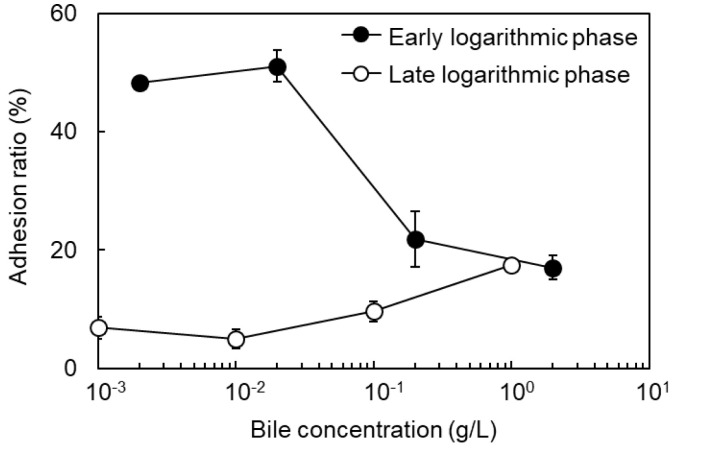
Effects of bile on the adhesion of B. animalis to dietary fiber
Based on the possibility that hydrophobic interactions were involved in the adhesion of B. animalis to dietary fibers (Fig. 3), the effects of bile on the adhesion ratio of the cells in the early and late logarithmic phase were investigated. Bile is mainly composed of bile acids such as cholic acid and chenodeoxycholic acid and forms micelles with diet-derived fat to promote absorption of lipophilic components. If the adhesion of cells to dietary fibers was due to hydrophobic interaction, it would be assumed that bile inhibits the adhesion. The adhesion ratio to cellulose decreased with increasing bile concentration, regardless of the culture phase (Fig. 6). Therefore, it was confirmed that hydrophobic interaction is dominant in the adhesion of B. animalis to cellulose. On the other hand, the adhesion ratio to chitin was reduced with increasing bile concentration in early logarithmic phase cells and was slightly increased in late logarithmic phase cells (Fig. 7). Therefore, it was considered that hydrophobic interaction and electrostatic interaction were dominant in the adhesion of the cells to chitin in the early logarithm and late logarithm phases, respectively.
Fig. 6.
Effect of growth phase and bile on adhesion of B. animalis to cellulose (pH 7.0). Closed circles, early logarithmic phase cells; open circles, late logarithmic phase cells.
Fig. 7.
Effect of growth phase and bile on adhesion of B. animalis to chitin (pH 7.0). Closed circles, early logarithmic phase cells; open circles, late logarithmic phase cells.
DISCUSSION
There have been several studies on the adhesion of bifidobacteria to intestinal epithelial cells and mucin [26,27,28,29]. Since bacteria recognize sugar chains of mucin and adhere to the intestinal tract, the effect of soluble dietary fiber with sugar chain structures such as oligosaccharides on adhesion has been investigated [30, 31]. When bacteria adhere to insoluble dietary fiber, they may not be able to adhere to the intestinal tract; however, there are no reports referring to the adhesion of probiotics to dietary fibers from that perspective. Therefore, in this study, we examined the properties of the adhesion of bifidobacteria to insoluble dietary fiber, as well as the factors that affect them.
The six Bifidobacterium strains showed different properties of adhesion to cellulose or chitin. In particular, B. animalis subsp. lactis JCM 10602 exhibited a high adhesion ratio to both cellulose and chitin. As various adhesins involved in intestinal epithelial cells and mucins have been reported in several bifidobacteria [7,8,9,10,11], it was suggested that several adhesins were involved in the adhesion to dietary fiber and that the adhesins involved depend on each Bifidobacterium. We also showed that the adhesion of B. animalis to cellulose involved a hydrophobic interaction between the hydrophobic region of cellulose and the bacterial cells. It has been reported that the adhesion of bifidobacteria to intestinal epithelial cells is correlated with the hydrophobicity of the bacterial cell surface [32,33,34], suggesting that these adhesins may contribute to the adhesion to dietary fiber.
Furthermore, the adhesion ratio of B. animalis to dietary fiber decreased as the culture time progressed. It has been reported that the adhesion ratio of B. bifidum S17 to Caco-2 cells is more than 2-fold lower in the stationary phase cells compared with the log phase cells and that the RNA expression levels of proteins such as pili decreased [35]. It has also been reported that LTA of Lactobacillus gasseri JCM 1131 is embedded in the cell wall during the logarithmic growth phase; however, it is exposed outside the cells because of cell wall degradation during the stationary and death phases [36]. Therefore, even if the same bacteria are used, the cell surface structure may change during the growth process; it is necessary to consider these contributions when considering adhesion of bifidobacteria to dietary fibers. Incidentally, it is thought that the attachment of bifidobacteria to these insoluble dietary fibers is not a beneficial phenomenon for bifidobacteria. This is because bifidobacteria cannot be assimilated even when attached to insoluble dietary fiber; they are excreted from the intestinal tract, which is rich in substrates. Therefore, it is possible that the bifidobacteria unintentionally adhere to dietary fibers as a result of increased adherence to the intestinal tract.
Bile is made in the liver, secreted into the duodenum, reabsorbed from the small intestine, and shunted back to the liver, while unabsorbed bile flows to the large intestine [37]. Therefore, bile as a surfactant is expected to prevent hydrophobic adhesion of bifidobacteria to cellulose. Our results revealed that the adhesion ratio of B. animalis to cellulose decreased to less than 10% with the addition of 1–2 g/L bile. Although the total bile concentration in the intestine is 1–10 g/L, the free bile concentration varies depending on the region of the intestinal tract and the amount of ingested lipid. It is thought that when the amount of bile secretion increases and the free bile concentration increases, bile inhibits the adhesion of bifidobacteria to insoluble dietary fibers. Conversely, the free bile concentration is low in the large intestine after the bile is reabsorbed, so it is hypothesized that bifidobacteria easily adhere to insoluble dietary fiber. Therefore, bile is one of the important factors affecting the adhesion of bifidobacteria to dietary fibers. It is also necessary to understand the effect of bile on the adhesion of bifidobacteria to intestinal epithelial cells.
In addition, since bile acids also show bactericidal effects, stress responses and resistance mechanisms against them are induced in the cells and on the cell surfaces of live intestinal bacteria [38,39,40]. Therefore, many studies have been conducted on the correlation between the bile acid resistance of bifidobacteria and adhesion to the intestinal tract. For example, bifidobacteria were exposed to bile acids to confer bile acid resistance, and this resulted in a 1.4- to 4-fold increase in the adhesion ratio to mucin [41]. In addition, the acquisition of bile acid resistance by bifidobacteria changed the expression levels of substances that can be adhesins [42]. On the other hand, for bifidobacteria, the bile resistance phenotype was not related to improvement of in vitro adhesion capability after gastrointestinal tract transit [43]. In the future, it will be necessary to consider the effects of resistance to bile acids on the adhesion of bifidobacteria to dietary fiber.
In conclusion, this study elucidated that the adhesion of B. animalis subsp. lactis JCM 10602 to dietary fiber involves hydrophobic interaction and the Coulomb force and that it depends on the growth phase and bile concentration. By clarifying the adhesion of B. animalis to dietary fiber, we showed the importance of considering adhesion to dietary fiber as well as adhesion to the intestinal mucosa when using Bifidobacterium as a probiotic.
Supplementary Material
Acknowledgments
We gratefully acknowledge the Japan Society for the Promotion of Science (JSPS) KAKENHI grants received (Nos. 16K18302 and 18K04857 to S.Y.Y. and 23656534 to Y.K.) for support of this study.
REFERENCES
- 1.Dicks LMT, Botes M. 2010. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes 1: 11–29. [DOI] [PubMed] [Google Scholar]
- 2.O’Callaghan A, van Sinderen D. 2016. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7: 925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perdigón G, Maldonado Galdeano C, Valdez JC, Medici M. 2002. Interaction of lactic acid bacteria with the gut immune system. Eur J Clin Nutr 56Suppl 4: S21–S26. [DOI] [PubMed] [Google Scholar]
- 4.Ruiz L, Delgado S, Ruas-Madiedo P, Sánchez B, Margolles A. 2017. Bifidobacteria and their molecular communication with the immune system. Front Microbiol 8: 2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FAO/WHO. 2002. Guidelines for the evaluation of probiotics in food. Food and agriculture organization of the United Nations and World Health OrganizationWorking Group Report.
- 6.Kainulainen V, Korhonen TK. 2014. Dancing to another tune-adhesive moonlighting proteins in bacteria. Biology (Basel) 3: 178–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connell Motherway M, Zomer A, Leahy SC, Reunanen J, Bottacini F, Claesson MJ, O’Brien F, Flynn K, Casey PG, Munoz JA, Kearney B, Houston AM, O’Mahony C, Higgins DG, Shanahan F, Palva A, de Vos WM, Fitzgerald GF, Ventura M, O’Toole PW, van Sinderen D. 2011. Functional genome analysis of Bifidobacterium breve UCC2003 reveals type IVb tight adherence (Tad) pili as an essential and conserved host-colonization factor. Proc Natl Acad Sci USA 108: 11217–11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.González-Rodríguez I, Sánchez B, Ruiz L, Turroni F, Ventura M, Ruas-Madiedo P, Gueimonde M, Margolles A. 2012. Role of extracellular transaldolase from Bifidobacterium bifidum in mucin adhesion and aggregation. Appl Environ Microbiol 78: 3992–3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Candela M, Centanni M, Fiori J, Biagi E, Turroni S, Orrico C, Bergmann S, Hammerschmidt S, Brigidi P. 2010. DnaK from Bifidobacterium animalis subsp. lactis is a surface-exposed human plasminogen receptor upregulated in response to bile salts. Microbiology (Reading) 156: 1609–1618. [DOI] [PubMed] [Google Scholar]
- 10.Guglielmetti S, Tamagnini I, Mora D, Minuzzo M, Scarafoni A, Arioli S, Hellman J, Karp M, Parini C. 2008. Implication of an outer surface lipoprotein in adhesion of Bifidobacterium bifidum to Caco-2 cells. Appl Environ Microbiol 74: 4695–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ruas-Madiedo P, Gueimonde M, Margolles A, de los Reyes-Gavilán CG, Salminen S. 2006. Exopolysaccharides produced by probiotic strains modify the adhesion of probiotics and enteropathogens to human intestinal mucus. J Food Prot 69: 2011–2015. [DOI] [PubMed] [Google Scholar]
- 12.Roos S, Jonsson H. 2002. A high-molecular-mass cell-surface protein from Lactobacillus reuteri 1063 adheres to mucus components. Microbiology (Reading) 148: 433–442. [DOI] [PubMed] [Google Scholar]
- 13.Nishiyama K, Ueno S, Sugiyama M, Yamamoto Y, Mukai T. 2016. Lactobacillus rhamnosus GG SpaC pilin subunit binds to the carbohydrate moieties of intestinal glycoconjugates. Anim Sci J 87: 809–815. [DOI] [PubMed] [Google Scholar]
- 14.Bergonzelli GE, Granato D, Pridmore RD, Marvin-Guy LF, Donnicola D, Corthésy-Theulaz IE. 2006. GroEL of Lactobacillus johnsonii La1 (NCC 533) is cell surface associated: potential role in interactions with the host and the gastric pathogen Helicobacter pylori. Infect Immun 74: 425–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macfarlane GT, Gibson GR. 1995. Microbiological aspects of the production of short-chain fatty acids in the large bowel. In Physiological and Clinical Aspects of Short-Chain Fatty Acids, Cummings JH, Rombeau JL and Sakata T (eds), Cambridge University Press, Cambridge pp. 87–105. [Google Scholar]
- 16.Topping DL, Clifton PM. 2001. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol Rev 81: 1031–1064. [DOI] [PubMed] [Google Scholar]
- 17.El-Salhy M, Ystad SO, Mazzawi T, Gundersen D. 2017. Dietary fiber in irritable bowel syndrome (Review). Int J Mol Med 40: 607–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Story JA, Furumoto EJ, Buhman KK. 1997. Dietary fiber and bile acid metabolism—an update. Adv Exp Med Biol 427: 259–266. [DOI] [PubMed] [Google Scholar]
- 19.Fernando WM, Flint S, Brennan CS, Ranaweera KK, Bamunuarachchi A. 2012. The influence of environmental factors on the adhesion of combinations of probiotics to rice fibre fractions. World J Microbiol Biotechnol 28: 2293–2302. [DOI] [PubMed] [Google Scholar]
- 20.Katakura Y, Sano R, Hashimoto T, Ninomiya K, Shioya S. 2010. Lactic acid bacteria display on the cell surface cytosolic proteins that recognize yeast mannan. Appl Microbiol Biotechnol 86: 319–326. [DOI] [PubMed] [Google Scholar]
- 21.Bergenstråhle M, Wohlert J, Himmel ME, Brady JW. 2010. Simulation studies of the insolubility of cellulose. Carbohydr Res 345: 2060–2066. [DOI] [PubMed] [Google Scholar]
- 22.Younes I, Rinaudo M. 2015. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar Drugs 13: 1133–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuhaus FC, Baddiley J. 2003. A continuum of anionic charge: structures and functions of D-alanyl-teichoic acids in gram-positive bacteria. Microbiol Mol Biol Rev 67: 686–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turroni F, Ventura M, Buttó LF, Duranti S, O’Toole PW, Motherway MO, van Sinderen D. 2014. Molecular dialogue between the human gut microbiota and the host: a Lactobacillus and Bifidobacterium perspective. Cell Mol Life Sci 71: 183–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishiyama K, Yamamoto Y, Sugiyama M, Takaki T, Urashima T, Fukiya S, Yokota A, Okada N, Mukai T. 2017. Bifidobacterium bifidum extracellular sialidase enhances adhesion to the mucosal surface and supports carbohydrate assimilation. MBio 8: e00928–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turroni F, Serafini F, Foroni E, Duranti S, O’Connell Motherway M, Taverniti V, Mangifesta M, Milani C, Viappiani A, Roversi T, Sánchez B, Santoni A, Gioiosa L, Ferrarini A, Delledonne M, Margolles A, Piazza L, Palanza P, Bolchi A, Guglielmetti S, van Sinderen D, Ventura M. 2013. Role of sortase-dependent pili of Bifidobacterium bifidum PRL2010 in modulating bacterium-host interactions. Proc Natl Acad Sci USA 110: 11151–11156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang LQ, Zhao F, Liu F, Meng XC. 2013. Live/dead state is not the factor influencing adhesion ability of Bifidobacterium animalis KLDS2.0603. J Microbiol 51: 584–589. [DOI] [PubMed] [Google Scholar]
- 28.Nishiyama K, Kawanabe A, Miyauchi H, Abe F, Tsubokawa D, Ishihara K, Yamamoto Y, Mukai T. 2014. Evaluation of bifidobacterial adhesion to acidic sugar chains of porcine colonic mucins. Biosci Biotechnol Biochem 78: 1444–1451. [DOI] [PubMed] [Google Scholar]
- 29.Andriantsoanirina V, Teolis AC, Xin LX, Butel MJ, Aires J. 2014. Bifidobacterium longum and Bifidobacterium breve isolates from preterm and full term neonates: comparison of cell surface properties. Anaerobe 28: 212–215. [DOI] [PubMed] [Google Scholar]
- 30.Koh JH, Kim N, Hwang D, Lim YH. 2013. Effect of water-soluble fraction of cherry tomatoes on the adhesion of probiotics and Salmonella to intestinal epithelial cells. J Sci Food Agric 93: 3897–3900. [DOI] [PubMed] [Google Scholar]
- 31.Altamimi M, Abdelhay O, Rastall RA. 2016. Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe 39: 136–142. [DOI] [PubMed] [Google Scholar]
- 32.Pérez PF, Minnaard Y, Disalvo EA, De Antoni GL. 1998. Surface properties of bifidobacterial strains of human origin. Appl Environ Microbiol 64: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Del Re B, Sgorbati B, Miglioli M, Palenzona D. 2000. Adhesion, autoaggregation and hydrophobicity of 13 strains of Bifidobacterium longum. Lett Appl Microbiol 31: 438–442. [DOI] [PubMed] [Google Scholar]
- 34.Pan WH, Li PL, Liu Z. 2006. The correlation between surface hydrophobicity and adherence of Bifidobacterium strains from centenarians’ faeces. Anaerobe 12: 148–152. [DOI] [PubMed] [Google Scholar]
- 35.Westermann C, Zhurina DS, Baur A, Shang W, Yuan J, Riedel CU. 2012. Exploring the genome sequence of Bifidobacterium bifidum S17 for potential players in host-microbe interactions. Symbiosis 58: 191–200. [Google Scholar]
- 36.Shiraishi T, Yokota S, Sato Y, Ito T, Fukiya S, Yamamoto S, Sato T, Yokota A. 2018. Lipoteichoic acids are embedded in cell walls during logarithmic phase, but exposed on membrane vesicles in Lactobacillus gasseri JCM 1131T. Benef Microbes 9: 653–662. [DOI] [PubMed] [Google Scholar]
- 37.Hundt M, Basit H, John S. 2019. Physiology, Bile Secretion. StatPearls. http://knowledge.statpearls.com/chapter/0/18272/ (accessed 2020-7-20). [PubMed]
- 38.Sánchez B, Champomier-Vergès MC, Anglade P, Baraige F, de Los Reyes-Gavilán CG, Margolles A, Zagorec M. 2005. Proteomic analysis of global changes in protein expression during bile salt exposure of Bifidobacterium longum NCIMB 8809. J Bacteriol 187: 5799–5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kato S, Tobe H, Matsubara H, Sawada M, Sasaki Y, Fukiya S, Morita N, Yokota A. 2019. The membrane phospholipid cardiolipin plays a pivotal role in bile acid adaptation by Lactobacillus gasseri JCM1131T. Biochim Biophys Acta Mol Cell Biol Lipids 1864: 403–412. [DOI] [PubMed] [Google Scholar]
- 40.Ruas-Madiedo P, Gueimonde M, Arigoni F, de los Reyes-Gavilán CG, Margolles A. 2009. Bile affects the synthesis of exopolysaccharides by Bifidobacterium animalis. Appl Environ Microbiol 75: 1204–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gueimonde M, Noriega L, Margolles A, de los Reyes-Gavilan CG, Salminen S. 2005. Ability of Bifidobacterium strains with acquired resistance to bile to adhere to human intestinal mucus. Int J Food Microbiol 101: 341–346. [DOI] [PubMed] [Google Scholar]
- 42.An H, Douillard FP, Wang G, Zhai Z, Yang J, Song S, Cui J, Ren F, Luo Y, Zhang B, Hao Y. 2014. Integrated transcriptomic and proteomic analysis of the bile stress response in a centenarian-originated probiotic Bifidobacterium longum BBMN68. Mol Cell Proteomics 13: 2558–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.de los Reyes-Gavilán CG, Suárez A, Fernández-García M, Margolles A, Gueimonde M, Ruas-Madiedo P. 2011. Adhesion of bile-adapted Bifidobacterium strains to the HT29-MTX cell line is modified after sequential gastrointestinal challenge simulated in vitro using human gastric and duodenal juices. Res Microbiol 162: 514–519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.