Abstract
Expression of long non-coding RNA KIAA0125 has been incorporated in various gene expression signatures for prognostic prediction in acute myeloid leukemia (AML) patients, yet its functions and clinical significance remain unclear. This study aimed to investigate the clinical and biological characteristics of AML bearing different levels of KIAA0125. We profiled KIAA0125 expression levels in bone marrow cells from 347 de novo AML patients and found higher KIAA0125 expression was closely associated with RUNX1 mutation, but inversely correlated with t(8;21) and t(15;17) karyotypes. Among the 227 patients who received standard chemotherapy, those with higher KIAA0125 expression had a lower complete remission rate, shorter overall survival (OS) and disease-free survival (DFS) than those with lower expression. The prognostic significance was validated in both TCGA and GSE12417 cohorts. Subgroup analyses showed that higher KIAA0125 expression also predicted shorter DFS and OS in patients with normal karyotype or non-M3 AML. In multivariable analysis, higher KIAA0125 expression remained an adverse risk factor independent of age, WBC counts, karyotypes, and mutation patterns. Bioinformatics analyses revealed that higher KIAA0125 expression was associated with hematopoietic and leukemic stem cell signatures and ATP-binding cassette transporters, two predisposing factors for chemoresistance.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00277-020-04358-y.
Keywords: Long non-coding RNA, KIAA0125, Acute myeloid leukemia, Chemoresistance, Leukemic stem cell signatures
Introduction
Long non-coding RNAs (lncRNAs) are non-protein coding RNAs that are longer than 200 nucleotides. Comparing to other classes of ncRNAs, lncRNAs exhibit a wide range of structures and functions [1]. Recently, lncRNAs have emerged as important regulators for gene expression via remodeling nuclear architecture, modulating mRNA stability and translation, and post-translational modifications [1–4]. Besides, some lncRNAs are dysregulated and harbor prognostic relevance in several types of cancers [5–8]. However, the roles of lncRNAs in tumorigenesis are still largely unknown.
In recent years, research on lncRNAs has increased drastically, and the results are robust. Although the functions of lncRNAs have not been elusive, recent studies suggested the expressions of lncRNAs could be used as prognostic factors, predictors of response, and potential therapeutic targets in acute leukemia [9–18]. Moreover, several gene expression-based prognostic scores have been developed for better risk stratification of acute myeloid leukemia (AML) patients [19–24]. Among those high-risk genes, lncRNA gene KIAA0125 (also named as FAM30A), a hematopoietic stem cell gene localized on chromosome 14, is unique because it is the only non-coding gene and is expressed in humans but not in mice (From the UniProt database, https://www.uniprot.org/uniprot/Q9NZY2). Additionally, KIAA0125 expression was integrated into a recently proposed 17-gene stemness score, which could predict outcomes in AML patients [19].
This study aimed to investigate the association of KIAA0125 expression with clinical and biological characteristics in AML patients. We first profiled the expression levels of KIAA0125 in bone marrow (BM) cells from AML patients and normal controls and demonstrated that AML patients had higher KIAA0125 expression than normal controls. Higher expression of KIAA0125 was associated with distinct clinical and biological characteristics and served as an independent poor prognostic biomarker for AML patients in ours and two other publicly annotated cohorts. Further bioinformatics analyses showed that higher expression of KIAA0125 in AML was closely associated with hematopoietic stem cell (HSC) and leukemic stem cell (LSC) signatures and several important ATP-binding cassette transporters (ABC transporters); these factors are regarded responsible for chemoresistance in AML. Further functional studies are needed to unravel its underlying mechanism and pathogenetic role in AML.
Materials and methods
Patients
We recruited 347 adult patients with de novo AML diagnosed in the National Taiwan University Hospital (NTUH) from 1996 to 2011 who had enough cryopreserved BM cells for tests. The diagnoses were based on the French–American–British (FAB) and the 2016 World Health Organization classifications [25, 26]. Among them, 227 patients received standard chemotherapy. Non M3 (acute promyelocytic leukemia, APL) patients received idarubicin 12 mg/m2 per day days 1–3 and cytarabine 100 mg/m2 per day days 1–7, and then consolidation chemotherapy with 2–4 courses of high-dose cytarabine 2000 mg/m2 q12h for total 8 doses, with or without an anthracycline (Idarubicin or Mitoxantrone), after achieving complete remission (CR) as described previously [27]. APL patients received concurrent all-trans retinoic acid and chemotherapy. The remaining 120 patients received supportive care and/or reduced-intensity anti-leukemia therapy due to underlying comorbidities or based on the decision of the physicians or patients. BM samples from 30 healthy donors of hematopoietic stem cell transplantation (HSCT) were collected as normal controls. This study was approved by the Research Ethics Committee of NTUH with informed consent obtained from all participants.
Microarray and genetic alteration analysis
We profiled the global gene expression of BM mononuclear cells from 347 AML patients and 30 healthy transplant donors by Affymetrix GeneChip Human Transcriptome Array 2.0 as described previously [21, 28, 29]. The raw and normalized microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE68469 and GSE71014) [21, 28, 29]. For external validation, we analyzed two publicly annotated datasets, the microarray dataset of GSE12417-GPL96 cohort, which includes the gene expression profile of 163 patients with cytogenetically normal AML, and the RNAseq dataset of the TCGA cohort (n = 186) [20, 30]. Cytogenetic analyses were performed and interpreted as described previously [31]. We also analyzed the mutation statuses of 17 myeloid-relevant genes, including ASXL1, IDH1, IDH2, TET2, DNMT3A, FLT3-ITD, FLT3-TKD, KIT, NRAS, KRAS, RUNX1, MLL/PTD, CEBPA, NPM1, PTPN11, TP53, and WT1 by Sanger sequencing as previously described [27, 28, 31–34].
Analysis of gene expression in next-generation sequencing datasets
We analyzed gene expression data of 141 AML samples profiled with Illumina Genome Analyzer RNA Sequencing in the TCGA database [30] to investigate the absolute gene expression levels.
Gene set enrichment analysis
The preranked Gene Set Enrichment Analysis (GSEA) implemented by R package clusterProfiler was performed using the stem cell-related gene sets from the MSigDB databases. The genes were ranked based on the Spearman’s correlation coefficient between the given gene and KIAA0125.
Statistical analysis
We used the Mann-Whitney U test and ANOVA test, where appropriate, to compare continuous variables and medians/means of distributions. The Fisher exact test or the χ2 test was performed to examine the difference in discrete variables, including gender, cytogenetic changes, and genetic alterations between patients with lower and higher KIAA0125 expression. Overall survival (OS) was the duration from the date of initial diagnosis to the time of last follow-up or death from any cause, whichever occurred first. Disease-free survival (DFS) was the duration from the date of attaining a leukemia-free state until the date of AML relapse or death from any cause, whichever occurred first. The survival prediction power of KIAA0125 expression was evaluated by both the log-rank test and the univariate Cox proportional hazards model. We plotted the survival curves with Kaplan-Meier analysis and calculated the statistical significance with the log-rank test. To find the optimal cutoff for separating patient groups, we used maximally selected rank statistics implemented in the maxstat R package. The Cox proportional hazards model was used in multivariable regression analysis. P values < 0.05 were considered statistically significant. All statistical analyses were performed with BRB-ArrayTools (version 4.5.1; Biometric Research Branch, National Cancer Institute, Rockville, MD), and IBM SPSS Statistics 23 for Windows.
Results
The median age of the 347 AML patients was 57 years. Among the 331 patients who had cytogenetic data at diagnosis, 165 (49.8%) had clonal chromosomal abnormalities. Sixty patients (18.1%) had favorable cytogenetics; 223 (67.2%), intermediate-risk cytogenetics; and 14.8% unfavorable cytogenetics (Supplement Table 1) based on the refined British Medical Research Council (MRC) classification [35]. The clinical and laboratory characteristics of these patients at diagnosis are summarized in Table 1.
Table 1.
Comparison of clinical and laboratory features between AML patients with lower and higher BM KIAA0125 expression
| Clinical characters | Total (N = 347) | High KIAA0125 (n = 174) | Low KIAA0125 (n = 173) | P value |
|---|---|---|---|---|
| Sex | 0.174 | |||
| Male | 196 | 92 | 104 | |
| Female | 151 | 82 | 69 | |
| Age* | 57 (15–91) | 58 (18–90) | 0.830 | |
| Laboratory data* | ||||
| WBC, X 109 /L | 21.9 (0.38–423) | 21.4 (0.38–417.5) | 22.38 (0.65–423.0) | 0.872 |
| Hb, g/dL | 8.1 (3.3–16.2) | 8.1 (3.3–13.2) | 8.1 (3.7–16.2) | 0.959 |
| Platelet, X 109 /L | 45 (2–655) | 54 (6–455) | 41 (2–655) | 0.060 |
| Blast, X 109 /L | 9.1 (0–369.1) | 12.3 (0–345.9) | 5.7 (0–369.1) | 0.021 |
| LDH (U/L) | 917 (202–13,130) | 892.5 (242–7734) | 925 (202–13,130) | 0.787 |
| Risk groups | ||||
| t(8;21) | 24 | 0 (0) | 24 (14.3) | < 0.001 |
| t(15;17) | 27 | 3 (1.8) | 24 (14.3) | < 0.001 |
| inv(16) | 9 | 6 (3.7) | 3 (1.8) | 0.332 |
| CEBPAdouble | 27 | 13 (48.1) | 14 (51.9) | 0.829 |
| NPM1+/FLT3-ITD- | 57 | 32 (18.4) | 25 (14.5) | 0.385 |
| NPM1-/FLT3-ITD+ | 19 | 3 (1.7) | 16 (9.2) | 0.002 |
| RUNX1 | 50 | 32 (64) | 18 (36) | 0.034 |
| ASXL1 | 52 | 26 (50) | 26 (50) | 0.982 |
| Unfavorable karyotypes†‡ | 49 | 30 (18.3) | 19 (11.3) | 0.089 |
| Induction response, n (%) | 227 | 116 | 111 | |
| CR | 165 (72.7) | 71 (61.2) | 94 (84.7) | < 0.001 |
| PR | 5 (2.2) | 4 (3.4) | 1 (0.9) | 0.191 |
| Refractory | 42 (18.5) | 33 (28.4) | 9 (8.1) | < 0.001 |
| Induction death | 15 (6.6) | 8 (6.9) | 7 (6.3) | 0.858 |
| Relapse (%) | 72 (31.7) | 42 (36.2) | 30 (27.0) | 0.137 |
Abbreviations: CR complete remission, Hb hemoglobin, HSCT allogeneic hematopoietic stem cell transplantation, LDH lactate dehydrogenase, PR partial remission
*Median (range)
†Cytogenetic data at diagnosis were available in 332 patients, including 168 with lower KIAA0125 expression and 164 with higher KIAA0125 expression
‡Based on the refined Medical research Council (MRC) classification
Comparison of clinical characteristics and genetic alterations between patients with higher and lower KIAA0125 expression
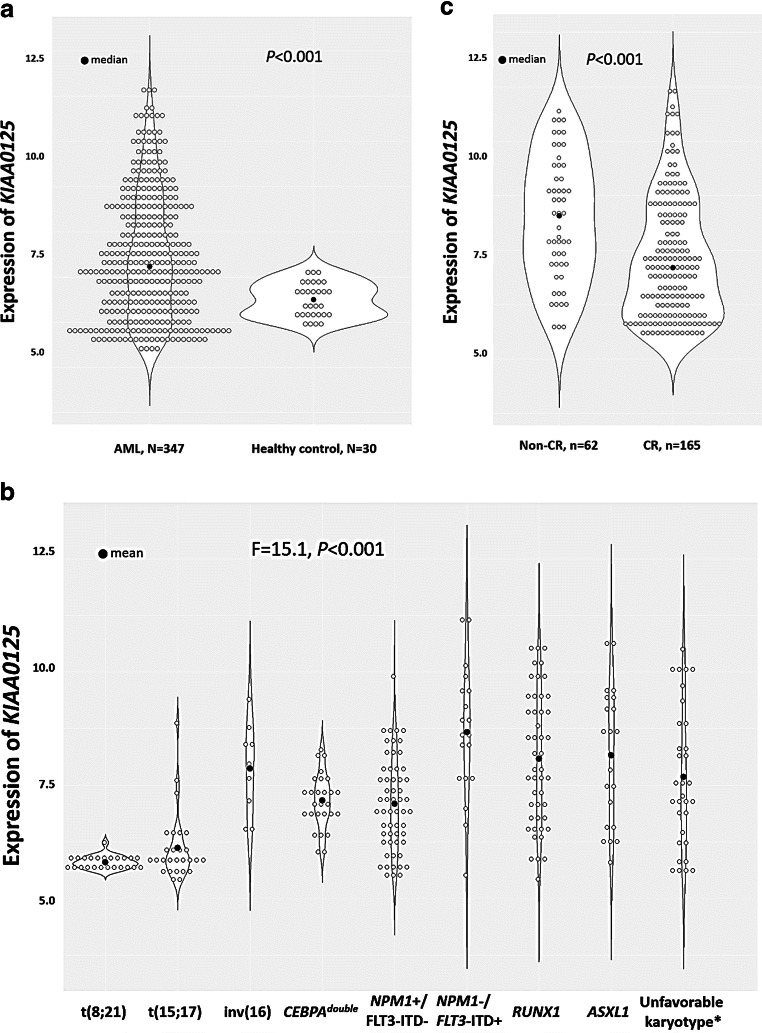
The distribution of KIAA0125 expression of 347 AML patients is shown with dot plots in Supplement Fig. 1. We first compared the BM KIAA0125 expression between the 30 healthy controls and 347 AML patients. The expression of KIAA0125 was significantly higher in AML samples than healthy controls (p < 0.001, Fig. 1a). Then, the 347 AML patients were divided into two groups by the median value of the KIAA0125 expression. The comparison of clinical and laboratory features between the two groups is shown in Table 1. The higher-KIAA0125 group had higher circulating blasts at diagnosis (p = 0.021) and higher incidence of FLT3-ITD in the absence of NPM1 mutation (NPM1-/FLT3-ITD+) (p = 0.002) and RUNX1 mutation (p = 0.034), but lower incidence of t(8;21) and t(15;17) (both p < 0.001), compared with the lower-KIAA0125 group (Table 1). From another perspective, patients with t(8;21) or t(15;17) had lower KIAA0125 expression, whereas those with RUNX1 mutation, ASXL1 mutation, NPM1-/FLT3-ITD+, or unfavorable karyotypes had higher expression of KIAA0125 (F = 15.124, p < 0.001, Fig. 1b, Supplement Table 1 and Supplement Table 2). Furthermore, the association of higher-KIAA0125 with lower frequencies of t(8;21) and t(15;17) was observed in both the NTUH cohort (both p < 0.001, Supplement Table 3) and TCGA cohort (p = 0.006 and p < 0.001, respectively, Supplement Table 3). The higher-KIAA0125 patients more frequently had FLT3-ITD (p = 0.048) and mutations in DNMT3A (p = 0.015) and RUNX1 (p = 0.034) (Supplement Table 4). Compatible with this finding, patients with DNMT3A or RUNX1 mutation had higher KIAA0125 expression than those without the mutation (p = 0.019 and 0.045, respectively, Supplement Fig. 2). Similarly, there was close association between higher KIAA0125 expression and DNMT3A (p = 0.001) and RUNX1 mutations (p = 0.017) in the TCGA cohort (Supplement Table 5). Among the 227 patients who received standard chemotherapy, 165 (72.7%) patients attained a complete remission (CR), while 42 (18.5%) patients had primary refractory diseases. Notably, the patients with higher KIAA0125 expression had a lower CR rate (61.2% vs. 84.7%, p < 0.001) than those with lower expression. In accordance with this finding, the patients who achieved CR after induction chemotherapy had lower expression of BM KIAA0125 at diagnosis than those who did not (p < 0.001, Fig. 1c).
Fig. 1.
Dot plots depicting expression levels of KIAA0125 in healthy controls and various AML subgroups. a Patients with AML had significantly higher expression of KIAA0125 than healthy controls; b patients with karyotypes of t(8;21) or t(15;17) had significantly lower expression of KIAA0125 than any other subgroups while patients with NPM1-/FLT3-ITD+, RUNX1, ASXL1, or unfavorable karyotypes had highest expression among all subgroups; and c patients who achieved CR after induction chemotherapy had lower expression of BM KIAA0125 at diagnosis than those who did not. *Based on the refined Medical research Council (MRC) classification
The impacts of the KIAA0125 expression on OS and DFS
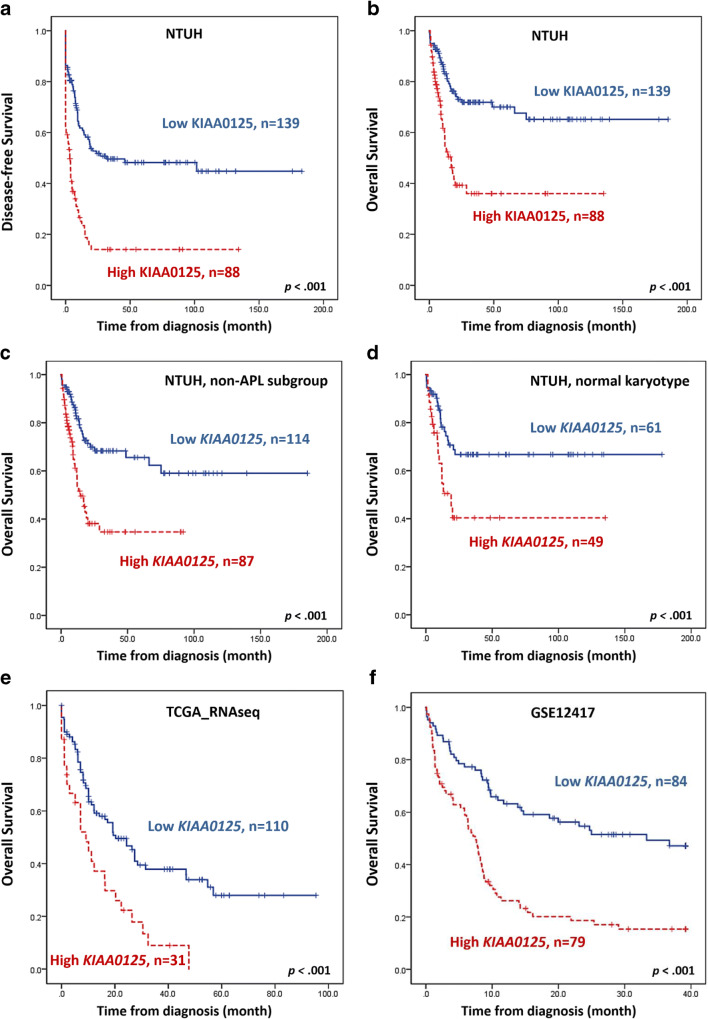
Next, we divided patients into two groups with high and low KIAA0125 expression with cut points determined by the maximally selected rank statistics (7.72 in the NTUH cohort, 8.56 in the TCGA cohort, and 9.71 in GSE12417 cohort, respectively, Supplement Fig. 3). As expected, patients with higher KIAA0125 expression had an inferior DFS and OS than those with lower expression, no matter whether the survival was censored on the day of hematopoietic stem cell transplantation (HSCT) (median, 3.2 months vs. 31.7 months, p < 0.001; and 17 months vs. not reached (NR), p < 0.001, respectively; Fig. 2a and b) or not (p < 0.001 and p < 0.001, respectively; Supplement Fig. 4a and 4b). Subgroup analyses showed that the prognostic significance of KIAA0125 expression for DFS and OS remained valid in both non-APL and normal karyotype patients (Figs. 2c and d).
Fig. 2.
Kaplan-Meier survival curves stratified by expression of KIAA0125. DFS a and OS b of the 227 AML patients receiving standard chemotherapy in the NTUH cohort; OS of 201 non-APL patients c and 110 cytogenetically normal AML patients d who received standard treatment in the NTUH cohort; and OS of 141 patients in the TCGA cohort e and GSE12417-GPL96 cohort f. Patients with higher KIAA0125 expression had worse clinical outcomes than those with lower expression
In multivariable analysis, we included clinically relevant parameters and variables with a p value < 0.05 in univariate Cox regression analysis (Supplement Table 4) as covariates, including age, white blood cell counts at diagnosis, karyotypes, mutation statuses of NPM1/FLT3-ITD, CEBPAdouble mutations, RUNX1, MLL-PTD, and TP53, and KIAA0125 expression. Higher KIAA0125 expression, either divided by the selected cut-point (Table 2) or calculated as continuous values (Supplement Table 5), was an independent adverse prognostic factor for DFS (p < 0.001 and p < 0.001, respectively) and OS (p = 0.003 and p = 0.001, respectively). To verify the prognostication power of the KIAA0125 expression, we analyzed the expression of KIAA0125 and its prognostic significance in the TCGA cohort and the GSE12417-GPL96 cohort. Consistent with the findings in the NTUH cohort, patients with higher KIAA0125 expressions had a significantly shorter OS (9.2 months vs. 20.3 months, p < 0.001, and 7.4 months vs. 33.3 months, p < 0.001, respectively, Figs. 2e and f) than those with lower KIAA0125 expression in the two external validation cohorts.
Table 2.
Multivariable analysis for DFS and OS in 227 AML patients who received standard intensive chemotherapy
| DFS | OS | |||||||
|---|---|---|---|---|---|---|---|---|
| 95% CI | 95% CI | |||||||
| Variable | HR | Lower | Upper | P | HR | Lower | Upper | P |
| Age* | 1.007 | 0.995 | 1.019 | 0.253 | 1.030 | 1.014 | 1.047 | < 0.001 |
| WBC* | 1.004 | 1.002 | 1.007 | 0.001 | 1.005 | 1.001 | 1.008 | 0.012 |
| Karyotype† | 1.610 | 1.201 | 2.160 | 0.001 | 1.706 | 1.158 | 2.513 | 0.007 |
| NPM1/FLT3-ITD‡ | 0.601 | 0.332 | 1.089 | 0.093 | 0.895 | 0.443 | 1.808 | 0.757 |
| CEBPAdouble | 0.598 | 0.286 | 1.252 | 0.173 | 0.451 | 0.137 | 1.488 | 0.191 |
| RUNX1 | 1.532 | 0.875 | 2.683 | 0.136 | 1.432 | 0.726 | 2.821 | 0.300 |
| MLL-PTD | 2.706 | 1.263 | 5.799 | 0.010 | 2.882 | 1.077 | 7.710 | 0.035 |
| TP53 | 1.918 | 0.697 | 5.283 | 0.207 | 3.030 | 0.956 | 9.608 | 0.060 |
| Higher KIAA0125 expression§ | 2.300 | 1.569 | 3.371 | <0.001 | 2.188 | 1.317 | 3.636 | 0.003 |
p values < .05 are considered statistically significant
Abbreviations: HR, hazard ratios; CI, confidence interval
*As continuous variable
†Unfavorable cytogenetics versus others. The classification of favorable, intermediate and unfavorable cytogenetics is based on the refined Medical Research Council (MRC) classification [27]. Favorable: t(15;17)(q22;q21), t(8;21)(q22;q22), and inv.(16)(p13q22)/t(16;16)(p13;q22); unfavorable: abn(3q) (excluding t(3;5)(q25;q34)), inv.(3)(q21q26)/t(3;3)(q21;q26), add(5q)/del(5q), −5, −7, add(7q)/del(7q), t(6;11)(q27;q23), t(10;11)(p1113;q23), other t(11q23) (excluding t(9;11)(p21 ~ 22;q23) and t(11;19)(q23;p13)), t(9;22)(q34;q11), −17, and abn(17p); and intermediate: entities not classified as favorable or adverse. Seven patients without chromosome data were not included in the analysis
‡NPM1+/FLT3-ITD- versus other subtypes
§High vs. low expression of KIAA0125
Biological impacts of KIAA0125 in AML
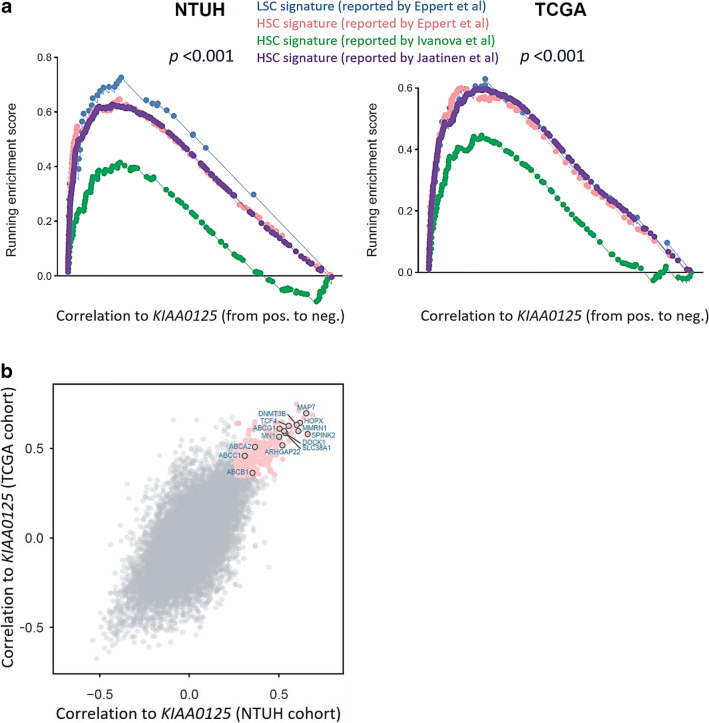
To gain biological insights into the underlying mechanism of unfavorable prognosis related to KIAA0125 overexpression, we investigated the genes whose expression is strongly correlated with that of KIAA0125. Since KIAA0125 was reported as an LSC marker [19], we curated several published HSC and LSC signatures from different studies [36–38]. GSEA showed HSC and LSC signatures were all significantly enriched in the patients with higher KIAA0125 expression in both the NTUH and TCGA cohorts (both p < 0.001, Fig. 3a). We next checked the leading-edge genes whose expression levels were most positively correlated to KIAA0125 expression in both NTUH and TCGA cohorts. Among them, SPINK2, MAP7, HOPX, MMRN1, DNMT3B, TCF4, SLC38A1, DOCK1, ARHGAP22, MN1, and 4 genes in the ATP-binding cassette (ABC) superfamily (ABCG1, ABCA2, ABCB1, and ABCC1) have been reported to be associated with poor prognosis or chemoresistance in AML (Fig. 3b and Table 3) [19, 39–58].
Fig. 3.
GSEA enrichment plots of HSC and LSC signatures and scatter plot of genes positively associated with higher KIAA0125 expression. a GSEA enrichment plots show positive association of higher KIAA0125 expression with HSC and LSC signatures curated from several published reports in both the NTUH and TCGA cohorts; b the scatter plot reveals the genes strongly correlated to KIAA0125 expression in both the NTUH and TCGA cohorts (pink). The correlation measurement is based on the Spearman’s correlation coefficient between the given gene and KIAA0125. The strongly correlated genes are defined as their correlation values at top 5% of all genes in both cohorts
Table 3.
Summary of the biological functions of the KIAA0125-associated genes that have been reported to be associated with prognosis or drug resistance in AML patients and their correlation values with KIAA0125 in ours and the TCGA cohorts
| Genes | Correlation coefficient (p value) | Association with leukemia | |||
|---|---|---|---|---|---|
| NTUH | TCGA | ||||
| SPINK2 | 0.661 | (3.4E-45) | 0.5798 | (6.2E-15) | Serine Peptidase Inhibitor; upregulation is associated with poor outcomes in adult patients with AML [30]; integrated into a 6-gene LSC score to identifies high risk pediatric AML [31] |
| MAP7 | 0.653 | 1.0E-43 | 0.696 | (<E-45) | Microtubule-associated proteins, overexpressed in cytogenetically normal AML patients with dismal outcomes [32] |
| HOPX | 0.619 | (2.6E-38) | 0.643 | (<E-45) | The smallest homeodomain protein; higher expression predicts poor prognosis in de novo AML [33] |
| MMRN1 | 0.609 | (9.7E-37) | 0.597 | (<E-45) | A member of the elastin microfibrillar interface protein; an adverse marker in both pediatric and adult AML [34] |
| DNMT3B | 0.599 | (1.7E-35) | 0.631 | (<E-45) | DNA methyltransferases; an important LSC marker [35–37] |
| TCF4 | 0.556 | (1.1E-29) | 0.626 | (<E-45) | A transcription factor; predict outcome in RUNX1 mutated and translocated AML [38, 39] |
| SLC38A1 | 0.536 | (2.3E-27) | 0.585 | (<E-45) | A glutamine amino acid transporter, overexpressed in AML patients with adverse clinical outcomes [40] |
| DOCK1 | 0.530 | (1.1E-26) | 0.597 | (5.9E-16) | A novel class of guanine nucleotide exchange factors; high expression confers poor prognosis in AML [41] |
| ARHGAP22 | 0.519 | (1.5E-25) | 0.518 | (<E-45) | Rho GTPase activating protein, incorporated in the 17-gene LSC score which predicts treatment response in AML [9] |
| MN1 | 0.502 | (1.1E-23) | 0.565 | (<E-45) | A transcriptional coactivator, overexpression could induce AML in mice and predict ATRA resistance in human AML patients [42, 43] |
| ABCG1 | 0.504 | (6.7E-24) | 0.610 | (<E-45) | Belongs to ATP-binding cassette (ABC) superfamily; responsible for important chemoresistance mechanism in AML [44–49] |
| ABCA2 | 0.367 | (1.5E-12) | 0.507 | (2.3E-11) | Belongs to ATP-binding cassette (ABC) superfamily; a strong prognostic biomarker for multidrug resistance in pediatric acute lymphoblastic leukemia [44–49] |
| ABCB1 | 0.353 | (1.2E-11) | 0.364 | (5.2E-6) | Belongs to ATP-binding cassette (ABC) superfamily; responsible for important chemoresistance mechanism in AML [44–49] |
| ABCC1 | 0.310 | (3.2E-9) | 0.458 | (5.2E-9) | Belongs to ATP-binding cassette (ABC) superfamily; responsible for important chemoresistance mechanism in AML [44–49] |
Discussion
AML cells have abnormal genetic background, either mutations or aberrant expression of specific genes. In recent years, several gene expression scores have been proposed for prognostic prediction of AML patients. We previously developed a 11-gene mRNA expression signature, including AIF1L, CXCR7, DNTT, GPR56, H1F0, IFITM3, KIAA0125, MX1, STAB1, TM4SF1, and TNS3, for prognostication in AML patients [21]. Another group built a six-gene leukemia stem cell (LSC) score with the incorporation of DNMT3B, GPR56, CD34, SOCS2, SPINK2, and KIAA0125 expressions for pediatric AML [40]. Recently, Ng et al. proposed a 17-gene LSC score that incorporated expressions of 17 stemness-related genes, including KIAA0125, and showed the scoring system was powerful to predict prognosis in AML patients [19]. Among these prognostic-relevant genes, KIAA0125 is the only non-coding gene and expressed only in the Homo sapiens, but not in mice.
KIAA0125 is located on chromosome 14 of the human genome. It was reported to be upregulated in ameloblastoma but shown as a tumor suppressor gene in colorectal cancer [59, 60]. Nonetheless, the clinical relevancy and biological role of KIAA0125 in tumorigenesis were still largely unclear.
In this study, we found that the expression level of KIAA0125 in BM was significantly higher in AML patients than normal HSC transplant donors. The expression of KIAA0125 was lower in patients with t(8;21) and t(15;17) which are associated with more differentiated AML subtypes, but higher in patients with RUNX1, ASXL1 mutations, NPM1-/FLT3-ITD+ or poor-risk karyotypes. It is interesting that the expression of KIAA0125 was high in patients with RUNX1 mutation but modest in those with RUNX1/RUNX1T1 fusion consisting with the fact that AML patients with a RUNX1 mutation usually had poor outcomes while those with RUNX1/RUNX1T1 fusion had favorable prognosis. Recently, Hornung et al. identified that expression of CD109, HOPX, and KIAA0125 genes might be responsible for inferior survival in AML patients with RUNX1 mutations but, on the other hand, better outcome in RUNX1/RUNX1T1 fusion through a newly proposed statistical tool “mediation analysis.” The three genes’ expression levels were significantly higher in patients with RUNX1 mutant but lower in those with RUNX1/RUNX1T1 fusion [61]. Intriguingly, though there has been no study showing direct evidence that RUNX1 binds to KIAA0125 till now in the literature, RUNX1 has been reported to bind to TGTGG core sequences as a heterodimer of RUNX1 and CBFβ [62]. We downloaded and retrieved the DNA sequence of KIAA0125 from the UCSC Genome Browser (https://genome.ucsc.edu/) and found several sequences of TGTGG (Supplement Table 8) within the 3000 bp upstream sequence, which might be the potential binding sites of RUNX1. Further studies are needed to explore the effect of the possible interaction between RUNX1 domain and KIAA0125.
Bioinformatics of the present study showed highly significant association of KIAA0125 expression with stem cell signatures, either HSC or LSC. We found that expressions of SPINK2, MAP7, HOPX, MMRN1, DNMT3B, TCF4, SLC38A1, DOCK1, ARHGAP22, MN1, and 4 genes in the ATP-binding cassette (ABC) superfamily (ABCG1, ABCA2, ABCB1, and ABCC1), which have been reported to be associated with poor prognosis or chemoresistance in AML, were positively correlated to higher expression of KIAA0125 (Fig. 3b and Table 3). HOPX, DOCK1, DNMT3B, MMRN1, and ARHGAP22 genes were reported as important leukemia stem cell markers [19, 42, 43, 45, 50, 63]. Higher SPINK2 expression was associated with poor prognosis in adult and pediatric AML [39, 40]. TCF4 expression could predict outcome in RUNX1-mutated and translocated AML [47, 48]. MN1 overexpression could induce AML in mice and predict ATRA resistance in human AML patients [51, 52]. Current knowledge about the association between theses KIAA0125-correlated genes and AML is summarized in Table 3.
Interestingly, the expression levels of several ABC transporter genes, including ABCA2, ABCB1, ABCC1, and ABCG1, were also significantly higher in AML patients with higher KIAA0125 expression. The ABC transporter family consists of 48 proteins in subfamilies designated A to G and some of them are known to be associated with multidrug resistance via ATP-dependent drug efflux [53, 54, 57]. ABCB1, ABCC1, and ABCG1 were reported to be responsible for chemoresistance in AML [53, 56]. The translational expression of ABCA2 was shown to be a prognostic marker for drug resistance in pediatric acute lymphoblastic leukemia [55, 58]. The underlying mechanistic basis of the high correlation of these 4 genes to the expression of KIAA0125 warrants further studies.
This study’s limitations lie in its retrospective nature and, crucially, the unsorted BM sample, as many cells in BM may be differentiated cells of myeloid and erythroid lineages. The study could have been more informative if we could profile KIAA0125 expression of healthy CD34 + CD38- HSCs and more mature progenitors (CD34 + CD38- and CD34-CD117+, respectively) and compare those with leukemia blasts. Moreover, the putative oncogenic role of KIAA0125 could be more strengthened were the expressions of KIAA0125 investigated in AML stem cells and bulk. Despite the limitations mentioned, to the best of our knowledge, this is by far the first study specifically addressing the expression of lncRNA KIAA0125 and its clinical and biological associations in AML patients. We found that higher KIAA0125 expression was closely associated with RUNX1 and DNMT3A1 mutations in both the NTUH and TCGA cohorts. Patients with higher KIAA0125 expression were more refractory to chemotherapy with a lower CR rate and higher refractory rate (Table 1). They had shorter OS and DFS among the total cohort and subgroups of patients with non-APL and those with normal karyotype. Based on its crucial clinical significance, further experimental studies are necessary to delineate how KIAA0125 participates in the stem cell biology of hematopoietic lineages and its role in the pathogenesis in AML.
Supplementary Information
(DOCX 680 kb)
Acknowledgements
We would like to acknowledge the service provided by Department of Laboratory Medicine, Department of Medical Research, and Division of Hematology, Department of Internal Medicine, National Taiwan University Hospital.
Authorship contributions
YHW and CCL contribute equally to this study. YHW and CCL were responsible for data collection and management, statistical analysis and interpretation, literature research, and manuscript writing; SYH and CYY were responsible for data management and statistical analysis; CLH assisted in statistical analysis; SHL, CHT, and HAH were responsible for data collection and management; and WCC and HFT planned, designed, and coordinated the study over the entire period and wrote the manuscript.
Funding
The study was supported by grants from Ministry of Health and Welfare, Taiwan (project number: MOHW107-TDU-B-211-114009) and Ministry of Science and Technology, Taiwan (project number: MOST 107–2314-B-002-013 and MOST 108–2314-B-002-011).
Data availability statement
The raw and normalized microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE68469 and GSE71014).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no relevant competing financial interests.
Informed consent
This study was approved by the Research Ethics Committee of NTUH with informed consent obtained from all individual participants included in the study.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yu-Hung Wang and Chien-Chin Lin contributed equally to this work.
Contributor Information
Wen-Chien Chou, Email: wchou@ntu.edu.tw.
Hwei-Fang Tien, Email: hftien@ntu.edu.tw.
References
- 1.Fatica A, Bozzoni I. Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 2.Wilusz JE, Sunwoo H, Spector DL. Long noncoding RNAs: functional surprises from the RNA world. Genes Dev. 2009;23(13):1494–1504. doi: 10.1101/gad.1800909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kung JT, Colognori D, Lee JT. Long noncoding RNAs: past, present, and future. Genetics. 2013;193(3):651–669. doi: 10.1534/genetics.112.146704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 5.Lin C, Yang L. Long noncoding RNA in Cancer: wiring signaling circuitry. Trends Cell Biol. 2018;28(4):287–301. doi: 10.1016/j.tcb.2017.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer discovery. 2011;1(5):391–407. doi: 10.1158/2159-8290.Cd-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huarte M. The emerging role of lncRNAs in cancer. Nat Med. 2015;21(11):1253–1261. doi: 10.1038/nm.3981. [DOI] [PubMed] [Google Scholar]
- 8.Cheetham SW, Gruhl F, Mattick JS, Dinger ME. Long noncoding RNAs and the genetics of cancer. Br J Cancer. 2013;108(12):2419–2425. doi: 10.1038/bjc.2013.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alvarez-Dominguez JR, Lodish HF. Emerging mechanisms of long noncoding RNA function during normal and malignant hematopoiesis. Blood. 2017;130(18):1965–1975. doi: 10.1182/blood-2017-06-788695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Clara E, Gourvest M, Ma H, Vergez F, Tosolini M, Dejean S, et al. Long non-coding RNA expression profile in cytogenetically normal acute myeloid leukemia identifies a distinct signature and a new biomarker in NPM1-mutated patients. Haematologica. 2017;102(10):1718–1726. doi: 10.3324/haematol.2017.171645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papaioannou D, Nicolet D, Volinia S, Mrózek K, Yan P, Bundschuh R, Carroll AJ, Kohlschmidt J, Blum W, Powell BL, Uy GL, Kolitz JE, Wang ES, Eisfeld AK, Orwick SJ, Lucas DM, Caligiuri MA, Stone RM, Byrd JC, Garzon R, Bloomfield CD. Prognostic and biologic significance of long non-coding RNA profiling in younger adults with cytogenetically normal acute myeloid leukemia. Haematologica. 2017;102(8):1391–1400. doi: 10.3324/haematol.2017.166215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schwarzer A, Emmrich S, Schmidt F, Beck D, Ng M, Reimer C, Adams FF, Grasedieck S, Witte D, Käbler S, Wong JWH, Shah A, Huang Y, Jammal R, Maroz A, Jongen-Lavrencic M, Schambach A, Kuchenbauer F, Pimanda JE, Reinhardt D, Heckl D, Klusmann JH. The non-coding RNA landscape of human hematopoiesis and leukemia. Nat Commun. 2017;8(1):218. doi: 10.1038/s41467-017-00212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzon R, Volinia S, Papaioannou D, Nicolet D, Kohlschmidt J, Yan PS, Mrózek K, Bucci D, Carroll AJ, Baer MR, Wetzler M, Carter TH, Powell BL, Kolitz JE, Moore JO, Eisfeld AK, Blachly JS, Blum W, Caligiuri MA, Stone RM, Marcucci G, Croce CM, Byrd JC, Bloomfield CD. Expression and prognostic impact of lncRNAs in acute myeloid leukemia. Proc Natl Acad Sci U S A. 2014;111(52):18679–18684. doi: 10.1073/pnas.1422050112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JL, Ries RE, Farrar JE, Alonzo TA, Gerbing RB, Leonti AR, et al. Long non-coding RNAs (lncRNAs) are highly associated with disease characteristics and outcome in pediatric acute myeloid leukemia - a COG and Tpaml study. Blood. 2019;134(Supplement_1):2741. doi: 10.1182/blood-2019-127318. [DOI] [Google Scholar]
- 15.Pashaiefar H, Izadifard M, Yaghmaie M, Montazeri M, Gheisari E, Ahmadvand M, Momeny M, Ghaffari SH, Kasaeian A, Alimoghaddam K, Ghavamzadeh A. Low expression of long noncoding RNA IRAIN is associated with poor prognosis in non-M3 acute myeloid leukemia patients. Genet Test Mol Biomarkers. 2018;22(5):288–294. doi: 10.1089/gtmb.2017.0281. [DOI] [PubMed] [Google Scholar]
- 16.Fernando TR, Contreras JR, Zampini M, Rodriguez-Malave NI, Alberti MO, Anguiano J, Tran TM, Palanichamy JK, Gajeton J, Ung NM, Aros CJ, Waters EV, Casero D, Basso G, Pigazzi M, Rao DS. The lncRNA CASC15 regulates SOX4 expression in RUNX1-rearranged acute leukemia. Mol Cancer. 2017;16(1):126. doi: 10.1186/s12943-017-0692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salehi M, Sharifi M, Bagheri M. Knockdown of long noncoding RNA Plasmacytoma variant translocation 1 with antisense locked nucleic acid GapmeRs exerts tumor-suppressive functions in human acute Erythroleukemia cells through Downregulation of C-MYC expression. Cancer Biother Radiopharm. 2019;34(6):371–379. doi: 10.1089/cbr.2018.2510. [DOI] [PubMed] [Google Scholar]
- 18.El-Khazragy N, Elayat W, Matbouly S, Seliman S, Sami A, Safwat G, et al. The prognostic significance of the long non-coding RNAs "CCAT1, PVT1" in t(8;21) associated acute myeloid leukemia. Gene. 2019;707:172–177. doi: 10.1016/j.gene.2019.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016;540(7633):433–437. doi: 10.1038/nature20598. [DOI] [PubMed] [Google Scholar]
- 20.Metzeler KH, Hummel M, Bloomfield CD, Spiekermann K, Braess J, Sauerland MC, Heinecke A, Radmacher M, Marcucci G, Whitman SP, Maharry K, Paschka P, Larson RA, Berdel WE, Büchner T, Wörmann B, Mansmann U, Hiddemann W, Bohlander SK, Buske C, Cancer and Leukemia Group B. German AML Cooperative Group An 86-probe-set gene-expression signature predicts survival in cytogenetically normal acute myeloid leukemia. Blood. 2008;112(10):4193–4201. doi: 10.1182/blood-2008-02-134411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chuang MK, Chiu YC, Chou WC, Hou HA, Tseng MH, Kuo YY, et al. An mRNA expression signature for prognostication in de novo acute myeloid leukemia patients with normal karyotype. Oncotarget. 2015;6(36):39098–39110. doi: 10.18632/oncotarget.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilop S, Chou WC, Jost E, Crysandt M, Panse J, Chuang MK, Brümmendorf TH, Wagner W, Tien HF, Kharabi Masouleh B. A three-gene expression-based risk score can refine the European LeukemiaNet AML classification. J Hematol Oncol. 2016;9(1):78. doi: 10.1186/s13045-016-0308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marcucci G, Yan P, Maharry K, Frankhouser D, Nicolet D, Metzeler KH, Kohlschmidt J, Mrózek K, Wu YZ, Bucci D, Curfman JP, Whitman SP, Eisfeld AK, Mendler JH, Schwind S, Becker H, Bär C, Carroll AJ, Baer MR, Wetzler M, Carter TH, Powell BL, Kolitz JE, Byrd JC, Plass C, Garzon R, Caligiuri MA, Stone RM, Volinia S, Bundschuh R, Bloomfield CD. Epigenetics meets genetics in acute myeloid leukemia: clinical impact of a novel seven-gene score. J Clin Oncol. 2014;32(6):548–556. doi: 10.1200/jco.2013.50.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Herold T, He C, Valk PJ, Chen P, Jurinovic V, et al. Identification of a 24-gene prognostic signature that improves the European LeukemiaNet risk classification of acute myeloid leukemia: an international collaborative study. J Clin Oncol. 2013;31(9):1172–1181. doi: 10.1200/JCO.2012.44.3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127(20):2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 26.Bennett JM, Catovsky D, Daniel MT, Flandrin G, Galton DA, Gralnick HR, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) co-operative group. Br J Haematol. 1976;33(4):451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 27.Tang JL, Hou HA, Chen CY, Liu CY, Chou WC, Tseng MH, Huang CF, Lee FY, Liu MC, Yao M, Huang SY, Ko BS, Hsu SC, Wu SJ, Tsay W, Chen YC, Lin LI, Tien HF. AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood. 2009;114(26):5352–5361. doi: 10.1182/blood-2009-05-223784. [DOI] [PubMed] [Google Scholar]
- 28.Chiu YC, Tsai MH, Chou WC, Liu YC, Kuo YY, Hou HA, Lu TP, Lai LC, Chen Y, Tien HF, Chuang EY. Prognostic significance of NPM1 mutation-modulated microRNA-mRNA regulation in acute myeloid leukemia. Leukemia. 2016;30(2):274–284. doi: 10.1038/leu.2015.253. [DOI] [PubMed] [Google Scholar]
- 29.Chuang MK, Chiu YC, Chou WC, Hou HA, Chuang EY, Tien HF. A 3-microRNA scoring system for prognostication in de novo acute myeloid leukemia patients. Leukemia. 2015;29(5):1051–1059. doi: 10.1038/leu.2014.333. [DOI] [PubMed] [Google Scholar]
- 30.Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson A, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368(22):2059–2074. doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou WC, Chou SC, Liu CY, Chen CY, Hou HA, Kuo YY, Lee MC, Ko BS, Tang JL, Yao M, Tsay W, Wu SJ, Huang SY, Hsu SC, Chen YC, Chang YC, Kuo YY, Kuo KT, Lee FY, Liu MC, Liu CW, Tseng MH, Huang CF, Tien HF. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood. 2011;118(14):3803–3810. doi: 10.1182/blood-2011-02-339747. [DOI] [PubMed] [Google Scholar]
- 32.Chou WC, Hou HA, Chen CY, Tang JL, Yao M, Tsay W, Ko BS, Wu SJ, Huang SY, Hsu SC, Chen YC, Huang YN, Chang YC, Lee FY, Liu MC, Liu CW, Tseng MH, Huang CF, Tien HF. Distinct clinical and biologic characteristics in adult acute myeloid leukemia bearing the isocitrate dehydrogenase 1 mutation. Blood. 2010;115(14):2749–2754. doi: 10.1182/blood-2009-11-253070. [DOI] [PubMed] [Google Scholar]
- 33.Chou WC, Huang HH, Hou HA, Chen CY, Tang JL, Yao M, Tsay W, Ko BS, Wu SJ, Huang SY, Hsu SC, Chen YC, Huang YN, Chang YC, Lee FY, Liu MC, Liu CW, Tseng MH, Huang CF, Tien HF. Distinct clinical and biological features of de novo acute myeloid leukemia with additional sex comb-like 1 (ASXL1) mutations. Blood. 2010;116(20):4086–4094. doi: 10.1182/blood-2010-05-283291. [DOI] [PubMed] [Google Scholar]
- 34.Hou HA, Lin CC, Chou WC, Liu CY, Chen CY, Tang JL, Lai YJ, Tseng MH, Huang CF, Chiang YC, Lee FY, Kuo YY, Lee MC, Liu MC, Liu CW, Lin LI, Yao M, Huang SY, Ko BS, Hsu SC, Wu SJ, Tsay W, Chen YC, Tien HF. Integration of cytogenetic and molecular alterations in risk stratification of 318 patients with de novo non-M3 acute myeloid leukemia. Leukemia. 2014;28(1):50–58. doi: 10.1038/leu.2013.236. [DOI] [PubMed] [Google Scholar]
- 35.Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ, Burnett AK, on behalf of the National Cancer Research Institute Adult Leukaemia Working Group Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood. 2010;116(3):354–365. doi: 10.1182/blood-2009-11-254441. [DOI] [PubMed] [Google Scholar]
- 36.Eppert K, Takenaka K, Lechman ER, Waldron L, Nilsson B, van Galen P, Metzeler KH, Poeppl A, Ling V, Beyene J, Canty AJ, Danska JS, Bohlander SK, Buske C, Minden MD, Golub TR, Jurisica I, Ebert BL, Dick JE. Stem cell gene expression programs influence clinical outcome in human leukemia. Nat Med. 2011;17(9):1086–1093. doi: 10.1038/nm.2415. [DOI] [PubMed] [Google Scholar]
- 37.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 38.Jaatinen T, Hemmoranta H, Hautaniemi S, Niemi J, Nicorici D, Laine J, Yli-Harja O, Partanen J. Global gene expression profile of human cord blood-derived CD133+ cells. Stem Cells. 2006;24(3):631–641. doi: 10.1634/stemcells.2005-0185. [DOI] [PubMed] [Google Scholar]
- 39.Xue C, Zhang J, Zhang G, Xue Y, Zhang G, Wu X. Elevated SPINK2 gene expression is a predictor of poor prognosis in acute myeloid leukemia. Oncol Lett. 2019;18(3):2877–2884. doi: 10.3892/ol.2019.10665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elsayed AH, Rafiee R, Cao X, Raimondi S, Downing JR, Ribeiro R, Fan Y, Gruber TA, Baker S, Klco J, Rubnitz JE, Pounds S, Lamba JK. A six-gene leukemic stem cell score identifies high risk pediatric acute myeloid leukemia. Leukemia. 2020;34(3):735–745. doi: 10.1038/s41375-019-0604-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu L, Fu H, Zhou L, Xu K, Pang Y, Hu K, Wang J, Tian L, Liu Y, Wang J, Jing H, Huang W, Ke X, Shi J. High expression of MAP7 predicts adverse prognosis in young patients with cytogenetically normal acute myeloid leukemia. Sci Rep. 2016;6:34546. doi: 10.1038/srep34546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin CC, Hsu YC, Li YH, Kuo YY, Hou HA, Lan KH, Chen TC, Tzeng YS, Kuo YY, Kao CJ, Chuang PH, Tseng MH, Chiu YC, Chou WC, Tien HF. Higher HOPX expression is associated with distinct clinical and biological features and predicts poor prognosis in de novo acute myeloid leukemia. Haematologica. 2017;102(6):1044–1053. doi: 10.3324/haematol.2016.161257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laszlo GS, Alonzo TA, Gudgeon CJ, Harrington KH, Gerbing RB, Wang YC, Ries RE, Raimondi SC, Hirsch BA, Gamis AS, Meshinchi S, Walter RB. Multimerin-1 (MMRN1) as novel adverse marker in pediatric acute myeloid leukemia: a report from the Children’s oncology group. Clin Cancer Res. 2015;21(14):3187–3195. doi: 10.1158/1078-0432.Ccr-14-2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayette S, Thomas X, Jallades L, Chabane K, Charlot C, Tigaud I, Gazzo S, Morisset S, Cornillet-Lefebvre P, Plesa A, Huet S, Renneville A, Salles G, Nicolini FE, Magaud JP, Michallet M. High DNA methyltransferase DNMT3B levels: a poor prognostic marker in acute myeloid leukemia. PLoS One. 2012;7(12):e51527. doi: 10.1371/journal.pone.0051527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niederwieser C, Kohlschmidt J, Volinia S, Whitman SP, Metzeler KH, Eisfeld AK, Maharry K, Yan P, Frankhouser D, Becker H, Schwind S, Carroll AJ, Nicolet D, Mendler JH, Curfman JP, Wu YZ, Baer MR, Powell BL, Kolitz JE, Moore JO, Carter TH, Bundschuh R, Larson RA, Stone RM, Mrózek K, Marcucci G, Bloomfield CD. Prognostic and biologic significance of DNMT3B expression in older patients with cytogenetically normal primary acute myeloid leukemia. Leukemia. 2015;29(3):567–575. doi: 10.1038/leu.2014.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wong KK, Lawrie CH, Green TM. Oncogenic roles and inhibitors of DNMT1, DNMT3A, and DNMT3B in acute myeloid Leukaemia. Biomark Insights. 2019;14:1177271919846454. doi: 10.1177/1177271919846454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gerritsen M, Yi G, Tijchon E, Kuster J, Schuringa JJ, Martens JHA, Vellenga E. RUNX1 mutations enhance self-renewal and block granulocytic differentiation in human in vitro models and primary AMLs. Blood Adv. 2019;3(3):320–332. doi: 10.1182/bloodadvances.2018024422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.In't Hout FEM, Gerritsen M, Bullinger L, van der Reijden BA, Huls G, Vellenga E, et al. Transcription factor 4 (TCF4) expression predicts clinical outcome in RUNX1 mutated and translocated acute myeloid leukemia. Haematologica. 2019;105:e454–e457. doi: 10.3324/haematol.2019.232827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Y, Shao H, Da Z, Pan J, Fu B. High expression of SLC38A1 predicts poor prognosis in patients with de novo acute myeloid leukemia. J Cell Physiol. 2019;234(11):20322–20328. doi: 10.1002/jcp.28632. [DOI] [PubMed] [Google Scholar]
- 50.Lee SH, Chiu YC, Li YH, Lin CC, Hou HA, Chou WC, et al. High expression of dedicator of cytokinesis 1 (DOCK1) confers poor prognosis in acute myeloid leukemia. Oncotarget. 2017;8(42):72250–72259. doi: 10.18632/oncotarget.19706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heuser M, Argiropoulos B, Kuchenbauer F, Yung E, Piper J, Fung S, Schlenk RF, Dohner K, Hinrichsen T, Rudolph C, Schambach A, Baum C, Schlegelberger B, Dohner H, Ganser A, Humphries RK. MN1 overexpression induces acute myeloid leukemia in mice and predicts ATRA resistance in patients with AML. Blood. 2007;110(5):1639–1647. doi: 10.1182/blood-2007-03-080523. [DOI] [PubMed] [Google Scholar]
- 52.Heuser M, Yun H, Berg T, Yung E, Argiropoulos B, Kuchenbauer F, Park G, Hamwi I, Palmqvist L, Lai CK, Leung M, Lin G, Chaturvedi A, Thakur BK, Iwasaki M, Bilenky M, Thiessen N, Robertson G, Hirst M, Kent D, Wilson NK, Göttgens B, Eaves C, Cleary ML, Marra M, Ganser A, Humphries RK. Cell of origin in AML: susceptibility to MN1-induced transformation is regulated by the MEIS1/AbdB-like HOX protein complex. Cancer Cell. 2011;20(1):39–52. doi: 10.1016/j.ccr.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi YH, Yu AM. ABC transporters in multidrug resistance and pharmacokinetics, and strategies for drug development. Curr Pharm Des. 2014;20(5):793–807. doi: 10.2174/138161282005140214165212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dean M. The genetics of ATP-binding cassette transporters. Methods Enzymol. 2005;400:409–429. doi: 10.1016/s0076-6879(05)00024-8. [DOI] [PubMed] [Google Scholar]
- 55.Efferth T, Gillet JP, Sauerbrey A, Zintl F, Bertholet V, de Longueville F, Remacle J, Steinbach D. Expression profiling of ATP-binding cassette transporters in childhood T-cell acute lymphoblastic leukemia. Mol Cancer Ther. 2006;5(8):1986–1994. doi: 10.1158/1535-7163.Mct-06-0086. [DOI] [PubMed] [Google Scholar]
- 56.Marzac C, Garrido E, Tang R, Fava F, Hirsch P, De Benedictis C, et al. ATP binding cassette transporters associated with chemoresistance: transcriptional profiling in extreme cohorts and their prognostic impact in a cohort of 281 acute myeloid leukemia patients. Haematologica. 2011;96(9):1293–1301. doi: 10.3324/haematol.2010.031823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vasiliou V, Vasiliou K, Nebert DW. Human ATP-binding cassette (ABC) transporter family. Hum Genomics. 2009;3(3):281–290. doi: 10.1186/1479-7364-3-3-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aberuyi N, Rahgozar S, Khosravi Dehaghi Z, Moafi A, Masotti A, Paolini A. The translational expression of ABCA2 and ABCA3 is a strong prognostic biomarker for multidrug resistance in pediatric acute lymphoblastic leukemia. Onco Targets Ther. 2017;10:3373–3380. doi: 10.2147/ott.S140488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diniz MG, Franca JA, Vilas-Boas FAS, de Souza FTA, Calin GA, Gomez RS, et al. The long noncoding RNA KIAA0125 is upregulated in ameloblastomas. Pathol Res Pract. 2019;215(3):466–469. doi: 10.1016/j.prp.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 60.Yang Y, Zhao Y, Hu N, Zhao J, Bai Y. lncRNA KIAA0125 functions as a tumor suppressor modulating growth and metastasis of colorectal cancer via Wnt/beta-catenin pathway. Cell Biol Int. 2019;43:1463–1470. doi: 10.1002/cbin.11196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hornung R, Jurinovic V, Batcha AMN, Bamopoulos SA, Rothenberg-Thurley M, Amler S, Sauerland MC, Berdel WE, Wörmann BJ, Bohlander SK, Braess J, Hiddemann W, Lehmann S, Mareschal S, Spiekermann K, Metzeler KH, Herold T, Boulesteix AL. Mediation analysis reveals common mechanisms of RUNX1 point mutations and RUNX1/RUNX1T1 fusions influencing survival of patients with acute myeloid leukemia. Sci Rep. 2018;8(1):11293. doi: 10.1038/s41598-018-29593-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bowers SR, Calero-Nieto FJ, Valeaux S, Fernandez-Fuentes N, Cockerill PN. Runx1 binds as a dimeric complex to overlapping Runx1 sites within a palindromic element in the human GM-CSF enhancer. Nucleic Acids Res. 2010;38(18):6124–6134. doi: 10.1093/nar/gkq356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin C-C, Yao C-Y, Hsu Y-C, Hou H-A, Yuan C-T, Li Y-H, Kao CJ, Chuang PH, Chiu YC, Chen Y, Chou WC, Tien HF. Knock-out of Hopx disrupts stemness and quiescence of hematopoietic stem cells in mice. Oncogene. 2020;39(28):5112–5123. doi: 10.1038/s41388-020-1340-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 680 kb)
Data Availability Statement
The raw and normalized microarray data reported in this article have been deposited in the Gene Expression Omnibus database (accession number GSE68469 and GSE71014).