Abstract
Objective
Clinical outcomes of patients who undergo transarterial chemoembolization (TACE) for single small hepatocellular carcinoma (HCC) are not consistent, and may differ based on certain imaging findings. This retrospective study was aimed at determining the efficacy of pre-TACE CT or MR imaging findings in predicting survival outcomes in patients with small HCC upon being treated with TACE. Besides, the study proposed to build a risk prediction model for these patients.
Materials and Methods
Altogether, 750 patients with functionally good hepatic reserve who received TACE as the first-line treatment for single small HCC between 2004 and 2014 were included in the study. These patients were randomly assigned into training (n = 525) and validation (n = 225) sets.
Results
According to the results of a multivariable Cox analysis, three pre-TACE imaging findings (tumor margin, tumor location, enhancement pattern) and two clinical factors (age, serum albumin level) were selected and scored to create predictive models for overall, local tumor progression (LTP)-free, and progression-free survival in the training set. The median overall survival time in the validation set were 137.5 months, 76.1 months, and 44.0 months for low-, intermediate-, and high-risk groups, respectively (p < 0.001). Time-dependent receiver operating characteristic curves of the predictive models for overall, LTP-free, and progression-free survival applied to the validation cohort showed acceptable areas under the curve values (0.734, 0.802, and 0.775 for overall survival; 0.738, 0.789, and 0.791 for LTP-free survival; and 0.671, 0.733, and 0.694 for progression-free survival at 3, 5, and 10 years, respectively).
Conclusion
Pre-TACE CT or MR imaging findings could predict survival outcomes in patients with small HCC upon treatment with TACE. Our predictive models including three imaging predictors could be helpful in prognostication, identification, and selection of suitable candidates for TACE in patients with single small HCC.
Keywords: Hepatocellular carcinoma, Transarterial chemoembolization, Computed tomography imaging, Magnetic resonance imaging, Survival
INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the fourth leading cause of cancer-related deaths worldwide (1). The improved surveillance of patients with chronic liver disease and advances in imaging have led to more patients being diagnosed at an early stage (2). According to the Barcelona Clinic Liver Cancer (BCLC) staging system, the treatments of choice for single small (≤ 3 cm) HCC (very-early/early stage; BCLC stage 0/A) are generally curative treatments such as surgical resection, liver transplantation, and radiofrequency ablation (3,4,5). The lack of liver donors is a major limitation for liver transplantation (6), thereby making surgical resection and radiofrequency ablation the most feasible treatment options against small (≤ 3 cm) HCC (7,8,9).
However, there are distinct scenarios in which surgical resection or radiofrequency ablation might not be feasible such as, in individuals with insufficient liver function or combined comorbidities, or a tumor location that is risky or not accessible for ablation (10). In such cases, where the three established treatment choices are contraindicated, transarterial chemoembolization (TACE) can be an alternative treatment against single small HCC (10). A previous study reported that TACE is an effective and safe treatment for single small (≤ 3 cm) HCC, achieving long-term survival rates comparable to those of hepatic resection and radiofrequency ablation when the underlying liver dysfunction is similar (11). However, a high recurrence rate after TACE remains a challenging issue (11). We hypothesized that the clinical outcomes of TACE against single small HCC are not consistent, and may differ based on imaging findings that are not clearly understood. Therefore, in this study we aimed to determine the effectiveness of pre-TACE CT and MR imaging findings in predicting survival outcomes in patients with small (≤ 3 cm) HCC, upon treatment with TACE. Furthermore, the study has generated and validated predictive models for overall, local tumor progression (LTP)-free, and progression-free survival using imaging and clinical parameters obtained from a large patient cohort.
MATERIALS AND METHODS
Patient Eligibility
Our Institutional Review Board approved this study and waived the requirement for informed patient consent because of the retrospective nature of the review. HCC was diagnosed according to the American Association for the Study of Liver Diseases or European Association for the Study of the Liver criteria (3,5).
Patients who met the following criteria were included in this study: 1) underlying liver cirrhosis and diagnosis of HCC between January 2004 and February 2014; 2) Child-Pugh class A liver function; 3) single-nodule HCC smaller than or equal to 3 cm without vascular invasion; and 4) TACE as the first-line treatment (treatment-naïve patient). Patients were excluded from this study if they had Child-Pugh class B or C liver function, a previous or current malignancy other than HCC, or if the patients could not be followed-up after TACE. Since the survival of patients who have HCC with Child-Pugh class B or worse liver function is usually determined by hepatic failure, it might be impossible to discern any effect of TACE on the cancer. Thus, we considered it justifiable to exclude such patients from this study (12). Altogether, 750 patients treated between January 2004 and February 2014 conformed to our inclusion and exclusion criteria and were randomly assigned to training and validation data sets according to a 7/3 split.
Transarterial Chemoembolization
The TACE procedure performed in our institution has been described previously (13,14). Cisplatin-based TACE was performed using a cisplatin dose of 2 mg/kg body weight. Using a microcatheter, an emulsion of iodized oil (Lipiodol®, Guerbet) and cisplatin at a 1:1 ratio was infused into the segmental, subsegmental, or more peripheral-level feeding artery, followed by embolization with Gelfoam slurry (Upjohn) until arterial flow stasis was achieved (13,14).
Technical success after TACE was evaluated and defined as successful catheter placement into a tumor vascular supply and transarterial therapy (selected chemotherapeutic and embolic agents) administration according to an investigator-designated plan (15).
Data Collection and Image Analysis
Demographic data (age at the time of diagnosis, sex, and etiology of the liver cirrhosis), clinical data (overall survival time, LTP-free survival time, and progression-free survival time), and other quantitative data (initial α-fetoprotein, initial serum albumin level, and maximum tumor diameter) were obtained from our anonymized database. Pre-TACE CT or MR images were reviewed with the consensus of three radiologists. All observers were aware of the HCC diagnosis, but were blinded to clinical, laboratory, histopathologic, and follow-up results.
The following qualitative imaging parameters were evaluated on the pre-TACE CT or MR imaging: 1) tumor margin (a smooth tumor margin or irregular tumor margin defined as a non-smooth margin with budding portion at the tumor periphery on transverse and coronal images) (16); 2) enhancement pattern (a typical enhancement pattern defined as arterial hyperenhancement with washout on the portal or delayed phase, or an atypical enhancement pattern defined as arterial and persistent enhancement, gradual enhancement, no or minimal enhancement, or the presence of irregular ring-like areas of enhancement with central hypoattenuation/intense areas in the arterial phase [targetoid appearance]) (17,18); and 3) whether the tumor location was central, defined as a tumor location within 0.5 cm of the first or second branches of the portal vein, or a tumor located at least 3 cm away from the liver capsule (19,20).
The efficacy of TACE was first analyzed by evaluating the tumor response on dynamic CT or MR imaging, 1 month after the initial TACE, according to the modified Response Evaluation Criteria In Solid Tumors (21) for HCC. After the initial CT or MR imaging scan, subsequent follow-up contrast-enhanced CT or MR imaging scans were repeated every 2–3 months during the first 2 years or until recurrence of HCC, and thereafter every 3–6 months until recurrence of HCC. When tumor growth or a residual new tumor was found, an additional local therapy consisting of TACE, radiofrequency ablation, or stereotactic body radiation therapy was initiated (9). Some patients underwent surgical resection or liver transplantation during the follow-up period after the initial or repeated TACE.
Statistical Analysis
Overall survival time was measured in months from the time of the initial TACE to a patient's death. Progression-free survival time was measured in months from the time of the initial TACE to the earliest signs of HCC progression (LTP, intrahepatic distant recurrence, gross vascular invasion, or extrahepatic distant metastasis) as determined by CT or MR imaging using the modified RECIST criteria, or death from any cause (22,23). LTP-free survival time was measured in months from the time of the initial TACE to LTP or death from any cause. Survival calculations were censored when the patients received surgical resection or liver transplantation after TACE, as such changes may significantly influence patient survival, making it impossible to properly evaluate whether certain imaging parameters can predict survival outcomes after TACE. Patients who were alive at the end of this study (April 2019) were also censored for the survival rate calculations.
Using the training cohort, the imaging-parameter-based prediction model was developed as follows. First, a univariable Cox proportional hazard model was used to identify the potential risk factors. Potential risk factors were considered as those predictors with a p value of less than 0.05. Second, these identified potential risk factors were included in a multivariable analysis. A risk score was then derived using each predictor weighted according to its estimated β regression coefficient in the multivariable Cox regression analyses, and the performance of our prediction model was evaluated according to this risk score. We then applied this point-scoring algorithm to patients in the validation cohort and derived the risk score as a summation of the points for the corresponding predictors. Time-dependent receiver operating characteristic (ROC) curves and calibration curves were used to determine the accuracy of the risk score as a marker for predicting overall, LTP-free and progression-free survival in the validation dataset (24). Time-dependent ROC curves for overall, LTP-free and progression-free survival were constructed for 3, 5, and 10 years after TACE. Statistical analyses were performed using R (version 3.6.1, R Development Core Team), and two-sided p values of < 0.05 were considered statistically significant.
RESULTS
Study Population
Of the 750 patients with single small HCC, 525 were randomly allocated to the training cohort and 225 to the testing cohort. The patient demographics and baseline characteristics were well balanced between the two cohorts (Table 1).
Table 1. Baseline Characteristics of the Patients.
| Variables | Training Data Set | Testing Data Set | P |
|---|---|---|---|
| Patients | 525 | 225 | |
| Age, mean ± SD (years) | 57.4 ± 9.9 | 58.3 ± 9.4 | 0.238 |
| Sex (%) | 0.742 | ||
| Male | 430 (81.9) | 182 (80.9) | |
| Female | 95 (18.1) | 43 (19.1) | |
| Etiology (%) | 0.359 | ||
| HBV | 419 (79.8) | 176 (78.2) | |
| HCV | 55 (10.5) | 31 (13.8) | |
| Others | 51 (9.7) | 18 (8.0) | |
| Tumor size (cm) | 1.90 ± 0.63 | 1.88 ± 0.64 | 0.668 |
| Albumin (g/dL), mean ± SD | 3.73 ± 0.47 | 3.79 ± 0.45 | 0.134 |
| Bilirubin (mg/dL), mean ± SD | 1.09 ± 0.49 | 1.06 ± 0.38 | 0.426 |
| AFP (ng/mL), median (IQR) | 14.3 (5.90–86.50) | 14.3 (5.70–39.50) | 0.492 |
| Tumor margin (%) | 0.189 | ||
| Smooth | 377 (71.8) | 172 (76.4) | |
| Irregular | 148 (28.2) | 53 (23.6) | |
| Tumor enhancement (%) | 0.368 | ||
| Typical | 439 (83.6) | 194 (86.2) | |
| Atypical | 86 (16.4) | 31 (13.8) | |
| Tumor location (%) | 0.115 | ||
| Central | 93 (17.7) | 51 (22.7) | |
| Peripheral | 432 (82.3) | 174 (77.3) |
AFP = α-fetoprotein, HBV = hepatitis B virus, HCV = hepatitis C virus, IQR = interquartile range, SD = standard deviation
Factors Predicting Overall Survival, LTP-Free Survival and Progression-Free Survival in Patients with Small HCC Treated with TACE
The median follow-up time for all the 750 study patients was 75 months (interquartile range, 43.3–108.5 months). During the follow-up period, 368 of the study patients died, 59 were censored at the time of surgical resection or liver transplantation, and 323 patients were alive. The median overall survival time after TACE in all 750 study patients was 100 months (95% confidence interval [CI], 90.6–109.4 months). The cumulative overall survival rates at 1, 3, 5, 10, and 15 years were 99.0%, 85.5%, 69.8%, 42.4%, and 30.6%, respectively. The overall survival rates were not significantly different between the training (median survival: 100.5 months) and testing cohorts (median survival: 94.4 months, p = 0.237).
The results of the univariable and multivariable Cox-proportional hazard models applied to the training cohort to evaluate the factors associated with overall survival in HCC patients treated with TACE are summarized in Table 2. In the multivariable analysis, irregular tumor margin (p = 0.001; adjusted hazard ratio = 1.821; 95% CI, 1.288–2.573), central tumor location (p = 0.018; adjusted hazard ratio = 1.465; 95% CI, 1.069–2.008), old age (≥ 65 years, p < 0.001; adjusted hazard ratio = 1.882; 95% CI, 1.407–2.518), and serum albumin level (≤ 3.7 g/dL, p < 0.001; adjusted hazard ratio = 1.682; 95% CI, 1.292–2.190) were statistically significant predictors of overall survival in patients with small HCC treated with TACE (Figs. 1, 2). Atypical tumor enhancement was marginally significant (p = 0.074; adjusted hazard ratio = 1.459; 95% CI, 0.964–2.208).
Table 2. Results of the Univariable and Multivariable Cox-Proportional Hazard Model for Evaluating the Factors Associated with Overall Survival in the Training Cohort (n = 525).
| Variable | Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| Irregular tumor margin | 2.347 | 1.806–3.049 | < 0.001 | 1.821 | 1.288–2.573 | 0.001 |
| Atypical tumor enhancement | 2.216 | 1.622–3.029 | < 0.001 | 1.459 | 0.964–2.208 | 0.074 |
| Central tumor location | 1.417 | 1.036–1.937 | 0.029 | 1.465 | 1.069–2.008 | 0.018 |
| Age (≥ 65 years, n = 126) | 2.311 | 1.774–3.012 | < 0.001 | 1.882 | 1.407–2.518 | < 0.001 |
| Male sex | 0.871 | 0.637–1.191 | 0.386 | NA | NA | NA |
| Etiology | ||||||
| HBV | 1.000 | < 0.001 | 1.000 | 0.091 | ||
| HCV | 2.007 | 1.406–2.866 | < 0.001 | 1.426 | 0.973–2.072 | 0.067 |
| Others | 1.817 | 1.252–2.639 | 0.002 | 1.376 | 0.943–2.058 | 0.109 |
| Tumor size (≤ 2 cm, n = 321) | 0.880 | 0.777–0.997 | 0.045 | 0.943 | 0.830–1.072 | 0.369 |
| Total bilirubin | 1.094 | 0.821–1.456 | 0.541 | NA | NA | NA |
| Albumin (≤ 3.7, n = 272) | 1.918 | 1.482–2.482 | < 0.001 | 1.682 | 1.292–2.190 | < 0.001 |
| AFP (≥ 14.3 ng/mL, n = 263) | 1.085 | 0.845–1.393 | 0.521 | NA | NA | NA |
CI = confidence interval, HR = hazard ratio, n = number, NA = non-applicable
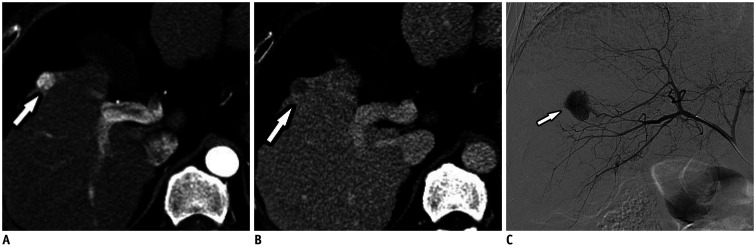
Fig. 1. A 53-year-old man with favorable imaging parameters.
A, B. Contrast-enhanced CT images in arterial and venous phases show a 1.3–cm tumor (arrows) with a typical enhancement pattern with arterial enhancement and venous washout, as well as, a smooth margin and peripheral tumor location. C. Hepatic angiographic image showing hypervascular tumor staining (arrow) in the right hemiliver.
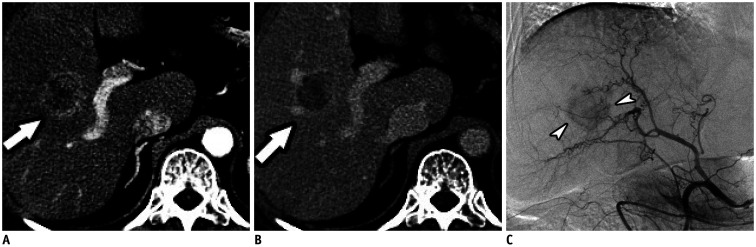
Fig. 2. Pathologically confirmed hepatocellular carcinoma in 63-year-old woman with unfavorable imaging parameters.
A, B. Contrast-enhanced CT images in arterial and venous phases show a 2.7–cm tumor (arrows) with irregular peripheral arterial enhancement and relatively hypovascular central portions, as well as, a central tumor location. C. Hepatic angiographic image showing hypovascular tumor staining (arrowheads) in the right hemiliver.
The median LTP-free and progression-free survival time after TACE in all 750 study patients were 42.3 months (95% CI, 35.9–48.7 months) and 25.0 months (95% CI, 21.9–28.1 months), respectively. The LTP-free and progression-free survival rates did not significantly differ between the training and testing cohorts (p = 0.189 for LTP-free survival; p = 0.07 for progression-free survival).
In the multivariable analysis, the statistically significant predictors of LTP- free survival were those that also predicted overall survival in patients with small HCC treated with TACE, with these being an irregular tumor margin (p < 0.001; adjusted hazard ratio = 2.353; 95% CI, 1.738–3.185), atypical tumor enhancement (p = 0.029; adjusted hazard ratio = 1.484; 95% CI, 1.041–2.115), a central tumor location (p < 0.001; adjusted hazard ratio = 1.895; 95% CI, 1.445–2.485), patient age (≥ 65 years, p = 0.001; adjusted hazard ratio = 1.487; 95% CI, 1.169–1.890), and serum albumin level (p = 0.001; adjusted hazard ratio = 1.428; 95% CI, 1.147–1.778) (Table 3).
Table 3. Results of the Univariable and Multivariable Cox-Proportional Hazard Model for Evaluating the Factors Associated with Local Tumor Progression-Free Survival in the Training Cohort (n = 525).
| Variable | Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| Irregular tumor margin | 2.862 | 2.277–3.597 | < 0.001 | 2.353 | 1.738–3.185 | < 0.001 |
| Atypical tumor enhancement | 2.789 | 2.133–3.646 | < 0.001 | 1.484 | 1.041–2.115 | 0.029 |
| Central tumor location | 1.783 | 1.364–2.330 | < 0.001 | 1.895 | 1.445–2.485 | < 0.001 |
| Age (≥ 65 years, n = 126) | 1.595 | 1.262–2.015 | < 0.001 | 1.487 | 1.169–1.890 | 0.001 |
| Male sex | 0.792 | 0.607–1.034 | 0.087 | NA | NA | NA |
| Etiology | ||||||
| HBV | 1.000 | 0.008 | 1.000 | 0.136 | ||
| HCV | 1.650 | 1.197–2.275 | 0.002 | 1.415 | 1.007–1.990 | 0.046 |
| Others | 1.304 | 0.930–1.828 | 0.124 | 1.092 | 0.765–1.558 | 0.629 |
| Tumor size (≤ 2 cm, n = 321) | 0.930 | 0.750–1.155 | 0.515 | NA | NA | NA |
| Total bilirubin | 1.050 | 0.833–1.324 | 0.680 | NA | NA | NA |
| Albumin (≤ 3.7, n = 272) | 1.469 | 1.183–1.822 | < 0.001 | 1.428 | 1.147–1.778 | 0.001 |
| AFP (≥ 14.3 ng/mL, n = 263) | 1.074 | 0.867–1.329 | 0.514 | NA | NA | NA |
The statistically significant predictors of progression-free survival in the multivariable analysis were irregular tumor margin (p < 0.001; adjusted hazard ratio = 2.134; 95% CI, 1.600–2.847), central tumor location (p < 0.001; adjusted hazard ratio = 1.664; 95% CI, 1.286–2.151), old age (≥ 65 years, p = 0.005; adjusted hazard ratio = 1.401; 95% CI, 1.110–1.769), and serum albumin level (≤ 3.7 g/dL, p = 0.001; adjusted hazard ratio = 1.439; 95% CI, 1.170–1.771) (Table 4). Atypical tumor enhancement was marginally significant (p = 0.079; adjusted hazard ratio = 1.357; 95% CI, 0.965–1.907).
Table 4. Results of the Univariable and Multivariable Cox-Proportional Hazard Model for Evaluating the Factors Associated with Progression-Free Survival in the Training Cohort (n = 525).
| Variable | Univariable Cox Regression Analysis | Multivariable Cox Regression Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | Adjusted HR | 95% CI | P | |
| Irregular tumor margin | 2.534 | 2.039–3.149 | < 0.001 | 2.134 | 1.600–2.847 | < 0.001 |
| Atypical tumor enhancement | 2.418 | 1.871–3.127 | < 0.001 | 1.357 | 0.965–1.907 | 0.079 |
| Central tumor location | 1.618 | 1.255–2.087 | < 0.001 | 1.664 | 1.286–2.151 | < 0.001 |
| Age (≥ 65 years, n = 126) | 1.586 | 1.268–1.984 | < 0.001 | 1.401 | 1.110–1.769 | 0.005 |
| Male sex | 0.902 | 0.699–1.163 | 0.426 | NA | NA | NA |
| Etiology | ||||||
| HBV | 1.000 | 0.277 | NA | - | NA | |
| HCV | 1.306 | 0.951–1.792 | 0.099 | NA | NA | NA |
| Others | 1.009 | 0.722–1.409 | 0.959 | NA | NA | NA |
| Tumor size (≤ 2 cm, n = 321) | 0.792 | 0.647–0.969 | 0.024 | 0.966 | 0.871–1.071 | 0.512 |
| Total bilirubin | 1.010 | 0.815–1.253 | 0.925 | NA | NA | NA |
| Albumin (≤ 3.7, n = 272) | 1.509 | 1.233–1.847 | < 0.001 | 1.439 | 1.170–1.771 | 0.001 |
| AFP (≥ 14.3 ng/mL, n = 263) | 1.120 | 0.917–1.368 | 0.267 | NA | NA | NA |
Imaging-Parameter-Based Prediction Model for Overall Survival
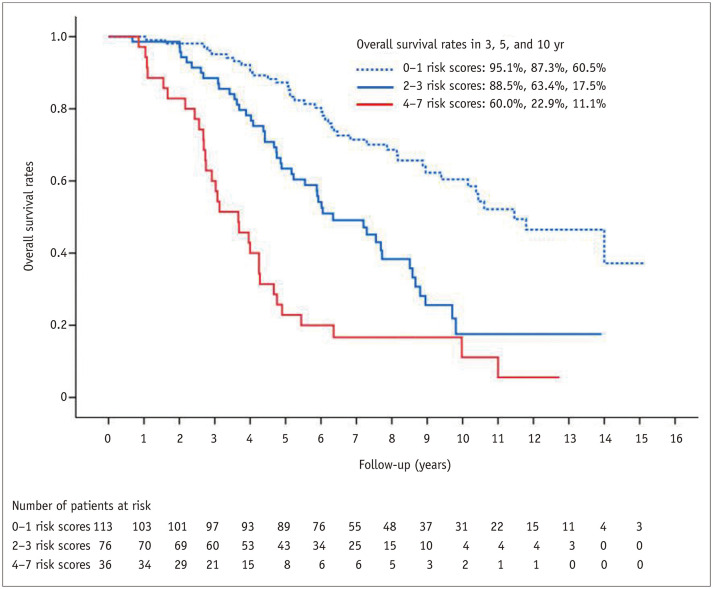
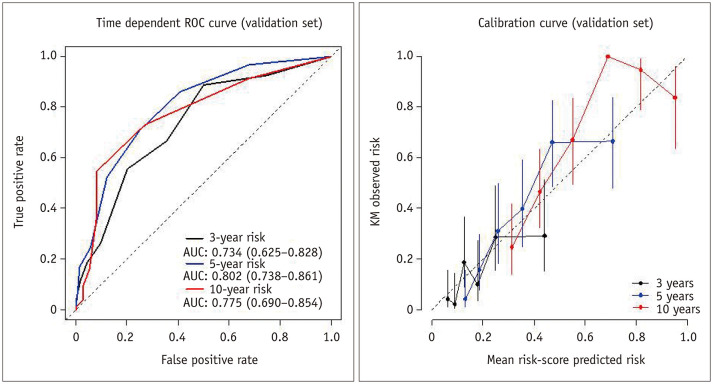
On the basis of the results of the multivariable Cox regression analysis in the training cohort, risk points were assigned to each predictor as follows: 2 when the tumor had an irregular margin, 1 when the tumor showed an atypical enhancement pattern, 1 when the tumor was located in a central area, 2 when the patient's age was 65 years or more, and 1 when the serum albumin level was 3.7 g/dL or less. Although the p value of atypical tumor enhancement in the multivariable analysis was marginal (p = 0.074), we decided to include it in the prediction model because it was a significant factor in the analysis for LTP-free survival, and it is also a clinically important imaging parameter. All patients in the validation set received risk scores that were the sum of these corresponding risk points and were classified into three groups according to the risk score: low- (score 0–1), intermediate- (score 2–3), and high-risk (score 4–7) groups. The median overall survival time in the validation cohort were 137.5 months (95% CI, 109.3–165.7) for the low-risk group, 76.1 months (95% CI, 57.2–95.0) for the intermediate-risk group, and 44.0 months (95% CI, 31.0–56.9) for the high-risk group (p < 0.001) (Fig. 3). The predictive model applied to the validation cohort demonstrated areas under the curve (AUCs) of 0.734 (95% CI: 0.625–0.828), 0.802 (95% CI: 0.738–0.861), and 0.775 (95% CI: 0.690–0.854) at 3, 5, and 10 years, respectively (Fig. 4).
Fig. 3. Kaplan-Meier curve for overall survival in the validation set.
Fig. 4. Time-dependent ROC and calibration curves for overall survival in the validation cohort.
AUC = areas under the curve, ROC = receiver operating characteristic, KM = Kaplan-Meier
Imaging-Parameter-Based Prediction Model for LTP-Free Survival
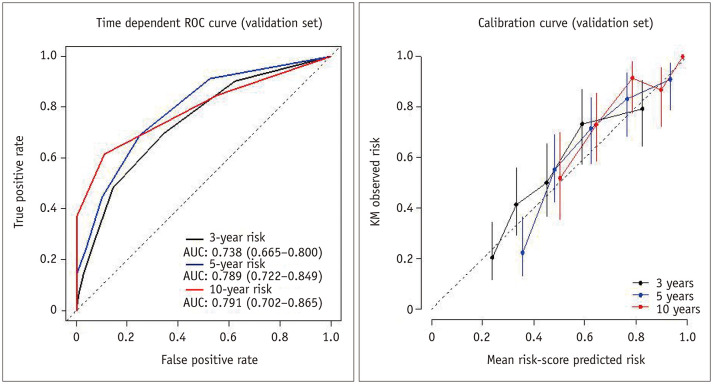
On the basis of the results of the multivariable Cox regression analysis in the training cohort, risk points were assigned to each predictor as follows: 2 when the tumor had an irregular margin, 1 when the tumor showed an atypical enhancement pattern, 2 when the tumor was located in a central area, 1 when the patient's age was 65 years or more, and 1 when the serum albumin level was 3.7 g/dL or less. The median LTP-free survival time in the validation cohort according to the risk score were 87.5 months (95% CI, 63.8–111.2) for the low-risk (score 0–1) group, 26.0 months (95% CI, 16.2–35.8) for the intermediate-risk (score 2–3) group, and 13.0 months (95% CI, 8.3–17.7) for the high-risk (score 4–7) group (p < 0.001). The predictive model applied to the validation cohort demonstrated AUCs of 0.738 (95% CI: 0.665–0.800), 0.789 (95% CI: 0.722–0.849), and 0.791 (95% CI: 0.702–0.865) at 3, 5, and 10 years, respectively (Fig. 5).
Fig. 5. Time-dependent ROC and calibration curves for local tumor progression-free survival in the validation cohort.
Imaging-Parameter-Based Prediction Model for Progression-Free Survival
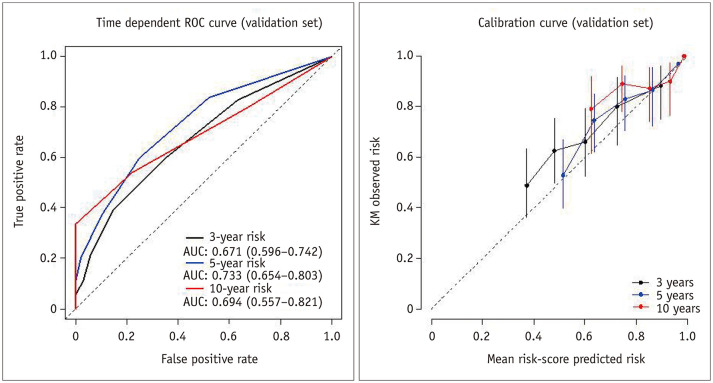
On the basis of the results of the multivariable Cox regression analysis in the training cohort, risk points were assigned to each predictor as follows: 2 when the tumor had an irregular margin, 1 when the tumor showed an atypical enhancement pattern, 2 when the tumor was located in a central area, 1 when the patient's age was 65 years or more, and 1 when the serum albumin level was 3.7 g/dL or less. The median progression-free survival time in the validation cohort according to the risk score were 32.2 months (95% CI, 23.7–40.7) for the low-risk (score 0–1) group, 21.5 months (95% CI, 19.2–23.8) for the intermediate-risk (score 2–3) group, and 10.7 months (95% CI, 5.7–15.6) for the high-risk (score 4–7) group (p < 0.001). The predictive model applied to the validation cohort demonstrated AUCs of 0.671 (95% CI: 0.596–0.742), 0.733 (95% CI: 0.654–0.803), and 0.694 (95% CI: 0.557–0.821) at 3, 5, and 10 years, respectively (Fig. 6).
Fig. 6. Time-dependent ROC and calibration curves for progression-free survival in the validation cohort.
Tumor Response after TACE
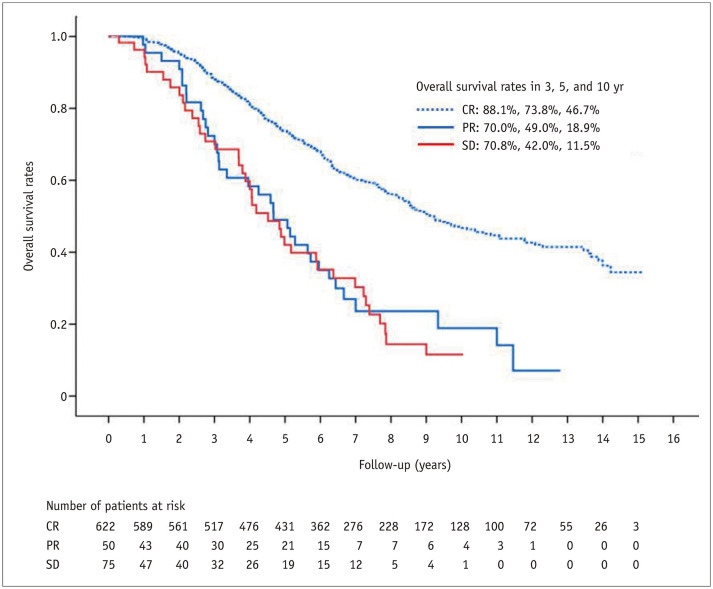
TACE was technically successful in all patients. At 1 month after TACE, 622 patients, out of the entire 750 patients (82.9%), showed a complete response, and 50 patients (6.7%) a partial response, while three patients (0.4%) showed progression of disease and 75 patients (10.0%) had stable disease. The Kaplan-Meier analysis of overall survival according to the tumor response 1 month after TACE is shown in Figure 7. The median overall survival times were 110.7 months (95% CI, 97.2–124.2) for the complete response group, 56.1 months (95% CI, 42.4–69.8) for the partial response group, and 54.2 months (95% CI, 41.3–67.1) for the stable disease group (p < 0.001) (Fig. 7).The rate of objective tumor regression (complete or partial response) 1 month after TACE was 89.6%.
Fig. 7. Kaplan-Meier curve for overall survival according to the tumor response 1 month after transarterial chemoembolization in the whole cohort.
CR = complete response, PR = partial response, SD = stable disease
DISCUSSION
The aim of this study was to gauge the potential of the pre-TACE CT and MR imaging findings in predicting the survival outcomes in patients with small (≤ 3 cm) HCC treated with TACE. In the multivariable Cox regression analyses, pre-TACE imaging findings (irregular tumor margin and central tumor location) and clinical factors (patient's age [≥ 65 years] and serum albumin level [≤ 3.7 g/dL]) were statistically significant predictors of overall survival, LTP-free survival, and progression-free survival in patients with small HCC treated with TACE. Atypical tumor enhancement was statistically significant in the LTP-free survival analysis, but marginally significant in the overall survival (p = 0.074) and progression-free survival (p = 0.079) analyses. We created and validated models using these parameters to predict the overall, LTP-free, and progression-free survival. When the time-dependent ROC curves of the predictive models were applied to the validation cohort to predict overall, LTP-free, and progression-free survival, acceptable AUC values (0.734, 0.802, and 0.775 for overall survival, 0.738, 0.789, and 0.791 for LTP-free survival, and 0.671, 0.733, and 0.694 for progression-free survival at 3, 5, and 10 years, respectively) were obtained.
In this study, we identified three prognostic imaging findings of HCC in a pre-TACE imaging study and evaluated the associations between these and survival outcomes in patients with small HCC treated with TACE. First, an irregular tumor margin is known to be associated with a poor prognosis in HCC. Chou et al. (25) reported that irregular tumor margins correlated with the histopathologic presence and location of microvascular invasion, and Lee et al. (26) reported that an irregular tumor margin was one of the three significant MR imaging findings for predicting microvascular invasion, which is a major prognostic factor of HCC after surgical resection or liver transplantation (27). Choi et al. (16) reported that an irregular tumor margin was one of the four significant MR imaging findings for predicting CK19-positive HCC, which has been linked to poorer prognosis, higher rate of recurrence, and lymph node metastasis.
Secondly, a central tumor location of HCC is a risk factor for recurrence after TACE. In a study involving 133 HCC patients treated with TACE, Murakami et al. (19) reported that a central location of HCC was the only significant risk factor for recurrence within the Milan criteria. Centrally located HCC is often fed by multiple fine arteries arising from the proximal portion of the hepatic artery. As these fine tumor feeders are too small to be catheterized, centrally located HCC is typically treated from the proximal portion of the hepatic artery. Under these circumstances, embolization of the HCC might be insufficient to cause complete necrosis, while avoiding massive non-target embolization of the normal liver parenchyma. This could be a potential factor contributing to the higher post-TACE recurrence rate in centrally located lesions.
Finally, atypical enhancement of HCC is associated with poor prognosis for HCC. The typical enhancement pattern of HCC is known as arterial hyperenhancement with a washout appearance on the portal or delayed phase. In this study, we defined atypical enhancement as arterial and persistent enhancement, gradual enhancement, no or minimal enhancement, or the presence of irregular ring-like areas of enhancement with central hypoattenuation/intense areas in the arterial phase (targetoid appearance). Weak or no arterial hyperenhancement and/or a washout appearance means non-hypervascular HCC. Non-vascular HCCs tend to respond poorly to TACE because of the limited amount of chemotherapeutic and embolic agents being delivered to the tumor. The targetoid appearance, another atypical enhancement pattern of HCC, reflects peripheral hypercellularity and central ischemia or fibrosis within the tumor (17). Central ischemia may result in a targetoid appearance in biologically aggressive tumors such as poorly differentiated HCC or sarcomatoid HCC (28,29). HCCs with more fibrotic components also frequently express many hepatic progenitor cell markers, which are important factors in indicating a poor prognosis for HCC (30).
According to our model, the low-risk group for predicting overall survival had significantly better overall survival than the intermediate- and high-risk groups. The 5-year survival rates of the low-risk groups in the validation set was 87.3%, which is similar to the rates reported after surgical resection for single small (≤ 3 cm) HCC (74.4–85.6%) (11,31,32,33). Thus, following a pretreatment risk evaluation, TACE can be recommended as an option as effective as surgical resection if patients belong to the low-risk group. This low-risk group usually has favorable imaging parameters (smooth tumor margin, typical enhancement pattern, peripheral tumor location). In future, it would be interesting to compare TACE and surgical resection for single small HCC with favorable imaging parameters. From our results, we suggest that overall survival would be similar between TACE and surgical resection for single small HCCs with favorable imaging parameters; however, comparative studies are required to validate our suggestion.
In the high-risk group, overall survival was discouraging as the 5-year survival rates in the validation cohort was 22.9%. High-risk patients typically show unfavorable imaging parameters (irregular tumor margin, atypical enhancement pattern, central location). Thus, treatment options other than TACE, such as radioembolization, ablative therapy, or stereotactic body radiation, should be actively considered for this high-risk group.
This study's limitations include its retrospective nature, which makes it vulnerable to a variety of potential biases (10). However, for the evaluation of survival outcomes in patients with a single small (≤ 3 cm, BCLC 0/A stage) HCC, it may be difficult to perform a prospective study, because it would probably require a prohibitively large sample size due to the longer survival time of these patients compared to those with other BCLC-stage (B, C, D) HCC (10). Moreover, we believe that we have compensated for this limitation by applying our model, derived from the training cohort, to a separate validation cohort to increase its reliability (18). Another limitation is that this is a single-center study, which may limit the generalizability of the results. Thus, it remains possible that our results may not be pertinent for patients with HCC in other countries, owing to differences in demographics and underlying causes of liver disease (10).
In conclusion, pre-TACE CT or MR imaging findings can predict survival outcomes in patients with small (≤ 3 cm) HCC treated with TACE. Our predictive models including three imaging predictors could help determine the appropriate prognosis and identify good candidates for TACE in patients with a single small (≤ 3 cm) HCC.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61–74. doi: 10.1055/s-0030-1247133. [DOI] [PubMed] [Google Scholar]
- 5.Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018;68:723–750. doi: 10.1002/hep.29913. [DOI] [PubMed] [Google Scholar]
- 6.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 7.Farinati F, Sergio A, Baldan A, Giacomin A, Di Nolfo MA, Del Poggio P, et al. Early and very early hepatocellular carcinoma: when and how much do staging and choice of treatment really matter? A multi-center study. BMC Cancer. 2009;9:33. doi: 10.1186/1471-2407-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong RJ, Devaki P, Nguyen L, Cheung R, Nguyen MH. Ethnic disparities and liver transplantation rates in hepatocellular carcinoma patients in the recent era: results from the surveillance, epidemiology, and end results registry. Liver Transpl. 2014;20:528–535. doi: 10.1002/lt.23820. [DOI] [PubMed] [Google Scholar]
- 9.Chu HH, Kim JH, Yoon HK, Ko HK, Gwon DI, Kim PN, et al. Chemoembolization combined with radiofrequency ablation for medium-sized hepatocellular carcinoma: a propensity-score analysis. J Vasc Interv Radiol. 2019;30:1533–1543. doi: 10.1016/j.jvir.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 10.Chu HH, Kim JH, Kim PN, Kim SY, Lim YS, Park SH, et al. Surgical resection versus radiofrequency ablation very early-stage HCC (≤ 2 cm Single HCC): a propensity score analysis. Liver Int. 2019;39:2397–2407. doi: 10.1111/liv.14258. [DOI] [PubMed] [Google Scholar]
- 11.Yang HJ, Lee JH, Lee DH, Yu SJ, Kim YJ, Yoon JH, et al. Small single-nodule hepatocellular carcinoma: comparison of transarterial chemoembolization, radiofrequency ablation, and hepatic resection by using inverse probability weighting. Radiology. 2014;271:909–918. doi: 10.1148/radiol.13131760. [DOI] [PubMed] [Google Scholar]
- 12.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim JH, Shim JH, Yoon HK, Ko HK, Kim JW, Gwon DI. Chemoembolization related to good survival for selected patients with hepatocellular carcinoma invading segmental portal vein. Liver Int. 2018;38:1646–1654. doi: 10.1111/liv.13719. [DOI] [PubMed] [Google Scholar]
- 14.Shim JH, Lee HC, Kim SO, Shin YM, Kim KM, Lim YS, et al. Which response criteria best help predict survival of patients with hepatocellular carcinoma following chemoembolization? A validation study of old and new models. Radiology. 2012;262:708–718. doi: 10.1148/radiol.11110282. [DOI] [PubMed] [Google Scholar]
- 15.Gaba RC, Lokken RP, Hickey RM, Lipnik AJ, Lewandowski RJ, Salem R, et al. Quality improvement guidelines for transarterial chemoembolization and embolization of hepatic malignancy. J Vasc Interv Radiol. 2017;28:1210–1223.e3. doi: 10.1016/j.jvir.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Choi SY, Kim SH, Park CK, Min JH, Lee JE, Choi YH, et al. Imaging features of gadoxetic acid-enhanced and diffusion-weighted MR imaging for identifying cytokeratin 19-positive hepatocellular carcinoma: a Retrospective observational study. Radiology. 2018;286:897–908. doi: 10.1148/radiol.2017162846. [DOI] [PubMed] [Google Scholar]
- 17.CT/MRI LI-RADS® v2018. American College of Radiology Web site. [Updated July, 2018]. [Accessed November 1, 2019]. https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/CT-MRI-LI-RADS-v2018.
- 18.An C, Kim DW, Park YN, Chung YE, Rhee H, Kim MJ. Single hepatocellular carcinoma: preoperative MR imaging to predict early recurrence after curative resection. Radiology. 2015;276:433–443. doi: 10.1148/radiol.15142394. [DOI] [PubMed] [Google Scholar]
- 19.Murakami T, Ishimaru H, Sakamoto I, Uetani M, Matsuoka Y, Daikoku M, et al. Percutaneous radiofrequency ablation and transcatheter arterial chemoembolization for hypervascular hepatocellular carcinoma: rate and risk factors for local recurrence. Cardiovasc Intervent Radiol. 2007;30:696–704. doi: 10.1007/s00270-007-9003-z. [DOI] [PubMed] [Google Scholar]
- 20.Peng ZW, Lin XJ, Zhang YJ, Liang HH, Guo RP, Shi M, et al. Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology. 2012;262:1022–1033. doi: 10.1148/radiol.11110817. [DOI] [PubMed] [Google Scholar]
- 21.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 22.Saad ED, Katz A. Progression-free survival and time to progression as primary end points in advanced breast cancer: often used, sometimes loosely defined. Ann Oncol. 2009;20:460–464. doi: 10.1093/annonc/mdn670. [DOI] [PubMed] [Google Scholar]
- 23.Lee M, Chung JW, Lee KH, Won JY, Chun HJ, Lee HC, et al. Korean multicenter registry of transcatheter arterial chemoembolization with drug-eluting embolic agents for nodular hepatocellular carcinomas: six-month outcome analysis. J Vasc Interv Radiol. 2017;28:502–512. doi: 10.1016/j.jvir.2016.08.017. [DOI] [PubMed] [Google Scholar]
- 24.Kamarudin AN, Cox T, Kolamunnage-Dona R. Time-dependent ROC curve analysis in medical research: current methods and applications. BMC Med Res Methodol. 2017;17:53. doi: 10.1186/s12874-017-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chou CT, Chen RC, Lin WC, Ko CJ, Chen CB, Chen YL. Prediction of microvascular invasion of hepatocellular carcinoma: preoperative CT and histopathologic correlation. AJR Am J Roentgenol. 2014;203:W253–W259. doi: 10.2214/AJR.13.10595. [DOI] [PubMed] [Google Scholar]
- 26.Lee S, Kim SH, Lee JE, Sinn DH, Park CK. Preoperative gadoxetic acid-enhanced MRI for predicting microvascular invasion in patients with single hepatocellular carcinoma. J Hepatol. 2017;67:526–534. doi: 10.1016/j.jhep.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Sumie S, Kuromatsu R, Okuda K, Ando E, Takata A, Fukushima N, et al. Microvascular invasion in patients with hepatocellular carcinoma and its predictable clinicopathological factors. Ann Surg Oncol. 2008;15:1375–1382. doi: 10.1245/s10434-008-9846-9. [DOI] [PubMed] [Google Scholar]
- 28.Asayama Y, Yoshimitsu K, Nishihara Y, Irie H, Aishima S, Taketomi A, et al. Arterial blood supply of hepatocellular carcinoma and histologic grading: radiologic-pathologic correlation. AJR Am J Roentgenol. 2008;190:W28–W34. doi: 10.2214/AJR.07.2117. [DOI] [PubMed] [Google Scholar]
- 29.Gu KW, Kim YK, Min JH, Ha SY, Jeong WK. Imaging features of hepatic sarcomatous carcinoma on computed tomography and gadoxetic acid-enhanced magnetic resonance imaging. Abdom Radiol (NY) 2017;42:1424–1433. doi: 10.1007/s00261-016-1038-7. [DOI] [PubMed] [Google Scholar]
- 30.Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, Yoo JE, et al. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology. 2012;55:1776–1786. doi: 10.1002/hep.25570. [DOI] [PubMed] [Google Scholar]
- 31.Hung HH, Chiou YY, Hsia CY, Su CW, Chou YH, Chiang JH, et al. Survival rates are comparable after radiofrequency ablation or surgery in patients with small hepatocellular carcinomas. Clin Gastroenterol Hepatol. 2011;9:79–86. doi: 10.1016/j.cgh.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 32.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤ 3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Wang JH, Wang CC, Hung CH, Chen CL, Lu SN. Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol. 2012;56:412–418. doi: 10.1016/j.jhep.2011.05.020. [DOI] [PubMed] [Google Scholar]