Abstract
Objective
This study aimed to prospectively compare the efficacy, safety, and mid-term outcomes of dual-switching monopolar (DSM) radiofrequency ablation (RFA) to those of conventional single-switching monopolar (SSM) RFA in the treatment of hepatocellular carcinoma (HCC).
Materials and Methods
This single-center, two-arm, parallel-group, randomized controlled study was approved by the Institutional Review Board. Written informed consent was obtained from all patients upon enrollment. A total of 80 patients with 94 HCC nodules were randomized into either the DSM-RFA group or SSM-RFA group in a 1:1 ratio, using a blocked randomization method (block size 2). The primary endpoint was the minimum diameter of the ablation zone per unit time. The secondary endpoints included other technical parameters, complication rate, technique efficacy, and 2-year clinical outcomes.
Results
Significantly higher ablation energy per unit time was delivered to the DSM-RFA group than to the SSM-RFA group (1.7 ± 0.2 kcal/min vs. 1.2 ± 0.3 kcal/min; p < 0.001). However, no significant differences were observed between the two groups for the analyzed variables, including primary endpoint, regarding size of the ablation zone and ablation time. Major complication rates were 4.9% in the DSM-RFA group and 2.6% in the SSM-RFA group (p = 1.000). The 2-year local tumor progression (LTP) rates of the HCC nodules treated using DSM-RFA and SSM-RFA were 8.5% and 4.7%, respectively (p = 0.316). The 2-year LTP-free survival rates of patients in the DSM-RFA and SSM-RFA groups were 90.0% and 94.4%, respectively (p = 0.331), and the 2-year recurrence-free survival rates were 54.9% and 75.7%, respectively (p = 0.265).
Conclusion
Although DSM-RFA using a separable clustered electrode delivers higher ablation energy than SSM-RFA, its effectiveness failed to show superiority over SSM-RFA in the treatment of HCC.
Keywords: Hepatocellular carcinoma, Radiofrequency ablation, Randomized controlled trial
INTRODUCTION
Image-guided tumor ablation has been widely accepted as one of the most effective nonsurgical locoregional treatment options for hepatocellular carcinoma (HCC) (1,2,3). When compared to surgical resection, radiofrequency ablation (RFA) provides comparable clinical outcomes with lower complication rates and greater cost-effectiveness (4,5,6,7,8,9,10,11). Recent guidelines from Europe and North America recommend RFA as a curative-intent treatment for very early or early stage HCCs in patients who are not surgical candidates (12,13). However, a significant drawback of RFA is the higher rate of local tumor progression (LTP) than in cases of surgical resection due to insufficient ablation at the tumor margin (14). Previous studies have reported 5-year LTP rates of HCCs after RFA were up to 30%, whereas those after surgical resection were less than 5% (6,8,9,14). Therefore, a sufficient ablative margin has to be ensured to lower the LTP rate after ablation, for which investigators have widely adopted 5–10 mm as the threshold (14,15,16). Thus, various investigational approaches have been adopted to efficiently create an ablation volume large enough to achieve a sufficient ablative margin, and these include modern high-powered radiofrequency (RF) devices with multiple electrodes using switching monopolar (17,18) or multipolar RFA (19,20,21,22), microwave ablation systems (23,24,25), and combination with transarterial embolization or drugs (26,27,28,29).
Recently, dual-switching monopolar RFA (DSM-RFA) was developed to enhance RF energy delivery to the target tumor and improve efficiency of the single-switching monopolar RFA (SSM-RFA) in creating an ablation zone (30,31). In ex vivo and in vivo animal experiments, Yoon et al. (30,31) reported that DSM-RFA allowed significantly greater RF energy delivery to the target tissue per unit time, and created a significantly larger ablation zone, than did SSM-RFA in the liver. Thereafter, a retrospective comparative study on patients with HCC by Choi et al. (32) reported that DSM-RFA created a significantly larger ablation volume than did SSM-RFA while showing similar LTP rates. Nevertheless, whether the physical differences between SSM-RFA and DSM-RFA translate into better clinical outcomes remains an open question. Considering that the choice of equipment or energy delivery mode is an essential factor in planning image-guided ablations, we reasoned that a prospective comparison between DSM-RFA and SSM-RFA would help in improving the results of image-guided RFA.
Therefore, the purpose of this study was to prospectively compare the efficacy, safety, and mid-term outcomes of DSM-RFA to those of conventional SSM-RFA in the treatment of HCC.
MATERIALS AND METHODS
Study Design
This single-center, two-arm, parallel-group, randomized controlled study was approved by the Institutional Review Board of Seoul National University Hospital (#1402-073-557). Written informed consent was obtained from all patients upon enrollment. STARmed Co., Ltd. (Goyang, Korea) provided financial support for this project. The authors had complete control over the data and information from this study at all times. Patients were randomly assigned to either the DSM-RFA group or SSM-RFA group in a 1:1 ratio. Randomization was performed using a blocked randomization method (block size 2), based on a web-based allocation table generated ahead of the study and managed by our institution's medical research collaboration center, which was not involved in this study. As the length of an active tip of the RFA electrode influences the size of the ablation zone, randomization was stratified based on the length of the active tip (2 or 2.5 cm).
Patients
Among the patients referred to our department for RFA as the first-line treatment for HCC, those who satisfied the inclusion and exclusion criteria were selected for the study. We initially included candidates for the study based on the following criteria: 1) aged 20–80 years, 2) underlying liver cirrhosis, with HCC nodules (< 5 cm maximal diameter) visualized by computed tomography (CT) and/or magnetic resonance imaging (MRI) performed within 60 days prior to the scheduled RFA, and 3) no previous locoregional treatment for index tumors. The exclusion criteria were as follows: 1) more than three HCC nodules, 2) tumors abutting the central portal vein or hepatic vein with a diameter > 5 mm, 3) tumors with major vascular invasion, 4) extrahepatic metastasis (EM), 5) Child-Pugh class C, and 6) platelet cell count < 50000 cells/mm3 or international normalized ratio (INR) prolongation > 50%.
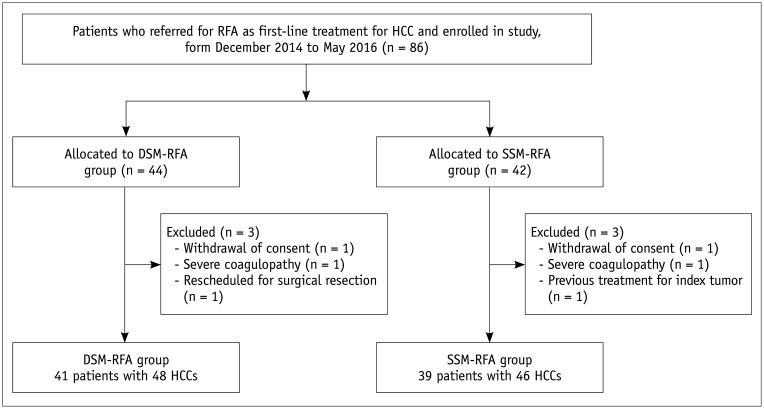
From December 2014 to April 2016, a total of 80 patients with 94 HCC nodules were enrolled and randomized into either the DSM-RFA or SSM-RFA group (Fig. 1). The baseline characteristics of the study patients are summarized in Table 1. No significant differences were observed in any of the baseline characteristics between the two groups.
Fig. 1. Flow chart of study population.
DSM = double-switching monopolar, HCC = hepatocellular carcinoma, RFA = radiofrequency ablation, SSM = single-switching monopolar
Table 1. Baseline Characteristics of Study Population.
| DSM-RFA (n = 41†) | SSM-RFA (n = 39†) | P* | |
|---|---|---|---|
| Active tip, % | 0.662 | ||
| 2 cm | 51.2 (21/41) | 46.2 (18/39) | |
| 2.5 cm | 48.8 (20/41) | 53.8 (21/39) | |
| Age (years), mean ± SD | 64.8 ± 7.9 | 62.5 ± 9.4 | 0.239 |
| Male, % | 63.4 (26/41) | 66.7 (26/39) | 0.817 |
| Single HCC, % | 87.8 (36/41) | 84.6 (33/39) | 0.753 |
| Size‡ (cm), mean ± SD | 1.9 ± 0.6 | 1.8 ± 0.6 | 0.492 |
| Subcapsular location‡, % | 37.5 (18/48) | 26.1 (12/46) | 0.273 |
| AFP (ng/mL), mean ± SD | 60.1 ± 192.4 | 54.2 ± 200.6 | 0.894 |
| Child-Pugh class, % | 0.111 | ||
| A | 100.0 (41/41) | 92.3 (36/39) | |
| B | 0.0 (0/41) | 7.7 (3/39) | |
| Albumin (g/dL), mean ± SD | 3.9 ± 0.5 | 3.9 ± 0.5 | 0.951 |
| Bilirubin (mg/dL), mean ± SD | 0.8 ± 0.5 | 0.8 ± 0.5 | 0.697 |
| PT INR, mean ± SD | 1.1 ± 0.1 | 1.1 ± 0.1 | 0.251 |
| Platelet (× 1000/mm3), mean ± SD | 125.8 ± 45.4 | 134.9 ± 52.4 | 0.405 |
*Categorical variables were compared by using Fisher's exact test or chi-squared test, and continuous variables were compared by using independent t test, †Number of patients, ‡Tumor size and frequency of subcapsular tumor were measured on per-nodule basis. AFP = alpha-fetoprotein, DSM = dual-switching monopolar, HCC = hepatocellular carcinoma, RFA = radiofrequency ablation, SD = standard deviation, SSM = single-switching monopolar, PT INR = prothrombin time international normalized ratio
RFA Procedures
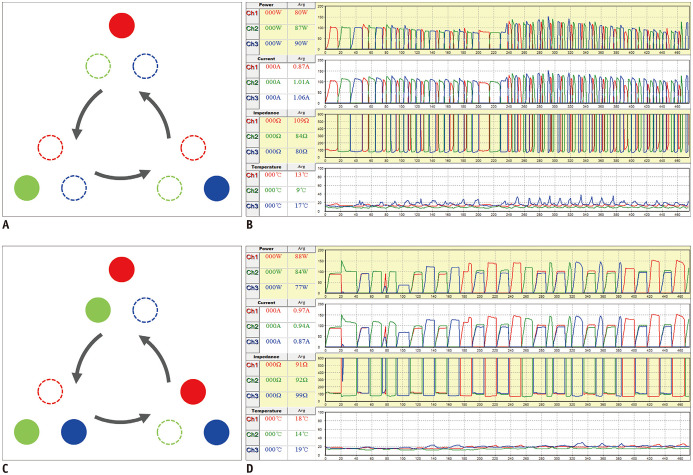
A single experienced radiologist with 20 years of experience in RFA performed all RFA procedures on an inpatient basis with the help of a clinical fellow or radiology resident. They used a separable clustered electrode (Octopus®, STARmed, Goyang, Korea) and a three-channel dual-generator unit (VIVA Multi®, STARmed). Intravenous conscious sedation was induced and the vital signs were monitored. The separable clustered electrode consisted of three internally cooled electrodes whose interelectrode distances could be adjusted by the operator (17,32). The dual-generator unit allowed independent and simultaneous control of the power of each RF amplifier, thus implementing both DSM and SSM modes (31,32). Each generator delivered a maximum power of 200 W. In the SSM mode, RF energy was delivered to one of three electrodes at a time and automatically switched to the adjacent electrode, whereas in the DSM mode, RF energy was initially delivered to a pair of electrodes and then switched to a single electrode or a pair of electrodes to prevent excessive increases in impedance (22,30). In both modes, the automatic alternation of active tips was done by thresholding both the consecutive delivery time (30 seconds) and the impedance increase (50 Ω above the baseline in SSM; 170% of the baseline in DSM) (Fig. 2) (17,31,32). The details of three-channel dual-generator RFA with the separable clustered electrode have been described in previous studies (31,32,33). Additionally, initial targeting of the index tumor and intraprocedural monitoring were aided by real-time fusion imaging between ultrasound (US) and preprocedural CT or MRI (34,35). Based on requirement, a 5% dextrose solution was injected into the perihepatic space for artificial ascites to enhance the sonic window or to prevent adjacent organ injury.
Fig. 2. SSM mode and DSM mode.
A, B. In SSM mode, RF energy is delivered to one of three electrodes and is switched to adjacent electrode based on impedance increase. C, D. In DSM mode, RF energy is delivered to one electrode or pair of electrodes at a time and switching mechanism is similar to that of SSM mode. RF = radiofrequency
Postprocedural Assessment and Follow-Up
Immediately after the RFA procedures, all patients underwent multiphasic contrast-enhanced CT studies for assessing technical parameters, complications, and technique efficacy based on the reporting criteria suggested by the International Working Group on Image-Guided Tumor Ablation (15). An area of non-enhancing hypoattenuation on the portal phase was considered as the ablation zone (15). As explained in a previous study (17), the ablation volume and effective ablation volume were calculated as follows:
where Dmax and Dmin are the longest and shortest diameters, respectively, of the ablation zone on the axial image with the largest ablation area, and Dv is the longest vertical diameter of the ablation zone on the coronal reconstructed image. In addition, ablation time, impedance, and amount of energy were recorded.
Postprocedural complications were assessed on the basis of the Society of Interventional Radiology classifications, wherein major complications were defined as events that increased the level of care or lengthened hospital stay (15,36).
Technical success was defined as complete coverage of the index tumor with an ablative margin ≥ 5 mm on immediate postprocedural CT, as per standardized terminology and reporting criteria proposed by the International Working Group on Image-Guided Tumor Ablation (15). Any irregular or nodular peripheral enhancement at the ablative margin was considered to reflect residual unablated tumor and treatment failure (37). If ablation was unsuccessful, additional ablation was performed within 24 hours. Technique efficacy was defined when follow-up CT or MRI performed 1 month after the procedure showed complete coverage of the index tumor and showed no nodular arterial enhancement at the ablation zone (17,32).
We only included patients in whom technique efficacy was achieved in further analysis. For a mean follow-up period of 23.9 ± 9.2 months, the patients were observed for LTP, intrahepatic distant recurrence (IDR), and EM, using contrast-enhanced CT or MRI performed every 3 months (15). LTP was defined as the appearance of tumor foci around the ablation zone, if at least one contrast-enhanced follow-up study documented adequate ablation and an absence of viable tissue in the target tumor and surrounding ablative margin by using pre-defined imaging criteria (15). Recurrence-free survival (RFS) time was defined as the duration of follow-up until the occurrence of any of the following: LTP, IDR, EM, or death.
Outcomes
The primary endpoint was Dmin per unit time. The secondary endpoints included Dmin, Dmax, Dv, ablation volume and ablation volume per unit time, average ablation time, average impedance, average energy and energy per unit time, complication rate, technical success, technique efficacy, and 2-year clinical outcomes.
Statistical Analysis
Technical parameters, ablation time, amount of energy, and LTP rates, were analyzed on a per-nodule basis. Technique efficacy and clinical outcomes were compared with per-patient data. Categorical variables were compared using Fisher's exact test. We compared continuous variables by using the independent t test for those that passed the Shapiro-Wilk normality test and the Mann-Whitney test for those that did not. Survival analysis was performed using the Kaplan-Meier method, and survival curves were compared using the log-rank test. All values are represented as mean ± standard deviation. Statistical analyses were performed using MedCalc Statistical Software version 17.6 (MedCalc Software bvba, Ostend, Belgium).
RESULTS
Technical Parameters
The primary endpoint, Dmin per unit time, did not significantly differ between the DSM-RFA and SSM-RFA groups. No significant differences were observed in ablation time and other variables related to the size of the ablation zone between the two groups. Significantly higher ablation energy per unit time (1.7 ± 0.2 kcal/min vs. 1.2 ± 0.3 kcal/min; p < 0.001) as well as higher total amount of energy (23.8 ± 12.1 kcal vs. 17.1 ± 8.4 kcal; p = 0.004) were delivered to the DSM-RFA group than to the SSM-RFA group. Moreover, the average impedance was higher in the DSM-RFA group than in the SSM-RFA group (93.9 ± 9.0 Ω vs. 73.7 ± 9.3 Ω; p < 0.001). A comparison of technical parameters between the two groups is shown in Table 2.
Table 2. Comparison of Technical Parameters between DSM-RFA and SSM-RFA Groups.
| DSM-RFA (n = 48†) | SSM-RFA (n = 46†) | P | |
|---|---|---|---|
| Dmin/time, mm/min | 2.9 ± 1.2 | 2.9 ± 1.5 | 0.849 |
| Dmin, cm | 3.5 ± 0.7 | 3.4 ± 0.6 | 0.806* |
| Dmax, cm | 4.9 ± 1.0 | 4.7 ± 0.8 | 0.174* |
| Dv, cm | 4.4 ± 1.1 | 4.5 ± 1.1 | 0.308 |
| Ablation time, min | 14.3 ± 6.7 | 14.1 ± 6.1 | 0.841 |
| Energy, kcal | 23.8 ± 12.1 | 17.1 ± 8.4 | 0.004 |
| Energy/time, kcal/min | 1.7 ± 0.2 | 1.2 ± 0.3 | < 0.001* |
| Average impedance, Ω | 93.9 ± 9.0 | 73.7 ± 9.3 | < 0.001* |
| Ablation volume, cm3 | 41.1 ± 20.9 | 39.6 ± 18.1 | 0.810 |
| Ablation volume/time, cm3/min | 3.3 ± 1.8 | 3.0 ± 1.2 | 0.976 |
| Effective ablation volume, cm3 | 24.5 ± 14.7 | 23.0 ± 12.1 | 0.764 |
| Effective ablation volume/time, cm3/min | 1.9 ± 1.2 | 1.8 ± 1.0 | 0.934 |
All data are mean ± SD. Variables that passed Shapiro-Wilk normality test were compared using independent t test (*) and others were compared using Mann-Whitney test. †Number of HCC nodules. Dmax, Dmin = the longest and shortest diameters, respectively, of the ablation zone on the axial image with the largest ablation area, Dv = longest vertical diameter of ablation zone on coronal plane
Procedure-Related Complications
No procedure-related death occurred, and major complications were observed in only 2 (4.9%) of 41 patients in the DSM-RFA group and 1 (2.6%) of 39 patients in the SSM-RFA group (p = 1.000). In the DSM-RFA group, one patient experienced intercostal arterial bleeding that required embolization, and the other developed pleural effusion with a small amount of suspected hemorrhage on CT; this necessitated further hospitalization for close observation but eventually resolved spontaneously. One patient in the SSM-RFA group underwent percutaneous drainage tube insertion for complicated pleural effusion supposedly associated with diaphragmatic ischemic injury.
Technical Success, Technique Efficacy, and Clinical Outcomes
The technical success rates of the DSM-RFA and SSM-RFA groups were 100.0% (41/41) and 97.4% (38/39), respectively (p = 0.490). Moreover, technique efficacy rates at the 1 month follow-up were 100.0% (41/41) in the DSM-RFA group and 94.9% (37/39) in the SSM-RFA group (p = 0.230). Thereafter, patients were observed for a mean follow-up period of 23.9 ± 9.2 months (median, 25.5 months).
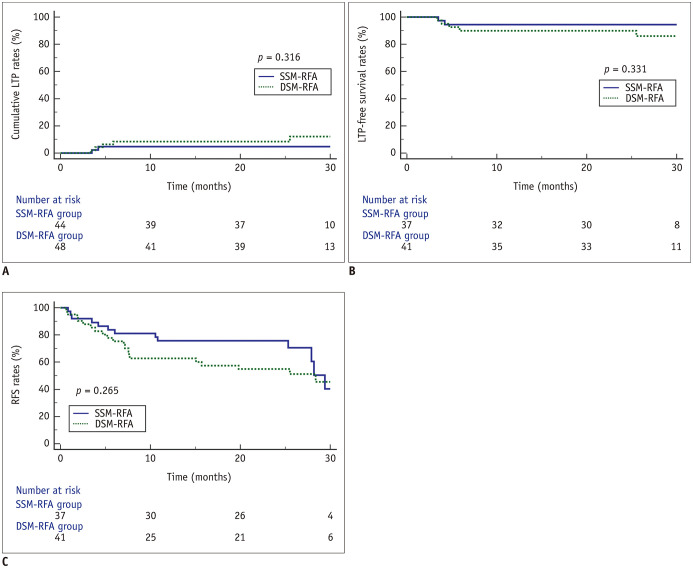
Out of 48 HCC nodules treated using DSM-RFA and 46 treated using SSM-RFA, LTP rates in the DSM-RFA and SSM-RFA groups were 8.5% and 4.7%, respectively, at 2 years (p = 0.316; Fig. 3A). The 2-year LTP-free survival rates of patients in the DSM-RFA and SSM-RFA groups were 90.0% and 94.4%, respectively (p = 0.331; Fig. 3B), and the 2-year RFS rates were 54.9% and 75.7%, respectively (p = 0.265; Fig. 3C). In patients with tumors ≥ 2 cm, the 2-year LTP-free survival rates in the DSM-RFA (n = 21) and the SSM-RFA (n = 18) groups were 85.0% and 100.0%, respectively (p = 0.092), and the 2-year RFS rates were 59.7% and 94.4%, respectively (p = 0.343). No patient deaths were recorded during the follow-up period in our study.
Fig. 3. Comparison of clinical outcomes between DSM-RFA and SSM-RFA groups.
Patients who initially achieved treatment success and effectiveness were observed for LTP, intrahepatic distant recurrence, and extrahepatic metastasis.
(A) LTP rates of HCC nodules. (B) LTP-free survival and (C) RFS in patients treated using DSM-RFA or SSM-RFA. LTP = local tumor progression, RFS = recurrence-free survival
DISCUSSION
Our results showed that DSM-RFA delivered a significantly higher amount of total ablation energy with significantly greater power than SSM-RFA. However, DSM-RFA failed to create a significantly larger ablation volume or to yield better LTP rates than SSM-RFA. The results of similar ablation volume between the two modes were discordant with those of previous experiments in animal models (30,31) and those of a retrospective comparative study (32) that showed a larger ablation volume with DSM-RFA than with SSM-RFA. We surmised that this could be due to smaller tumor sizes (< 2 cm) and longer ablation times (14 minutes) in both our study groups than in the previous retrospective study (32). Considering that the goal of RFA is usually to create at least a 5-mm safety margin around the target tumor, ablation volume could be closely related to the size of the target tumors. Therefore, the discrepancy between the current study and the previous retrospective study could be explained by the smaller tumor size (mean tumor size < 2 cm) wherein the RF energy delivered using SSM-RFA was potentially large enough to create an ablation zone covering the target tumor.
Furthermore, in our study, despite the high total RF energy delivered using DSM-RFA, no difference was observed in ablation time between the two groups. Considering that ablation time was determined by operators when the echogenic ablation zone was more than 5 mm from the tumor border in our study, we believe that a potential electrical interference between the electrodes in the DSM-RFA mode (Faraday cage effect) (38) could lower the efficiency of RF heat energy in the tissue. As RF electrodes are usually placed in the peripheral portion of the target tumor in multiple-electrode approaches, the interelectrode distance would be less than 1–1.5 cm in a small tumor (< 2 cm), and this may create interference between current flow (Faraday cage effect) and induce an increase in impedance (31). The higher average impedance in the DSM-RFA group (93.9 ± 9.0 Ω vs. 73.7 ± 9.3 Ω; p < 0.001) may reflect such an effect. Therefore, further optimization of the RFA procedure, including positioning of the grounding pads to lower the impedance, may be required. During the study, the grounding pads were applied to the thighs. However, we found that applying the pads to the back portion of the trunk significantly lowered impedance in our clinical practice; thus, it may be useful for shortening ablation time by further improving RF energy delivery in the DSM-RFA mode.
In addition, both the DSM-RFA and SSM-RFA groups showed good 2-year LTP-free survival rates of more than 90%. Our results were in good agreement with those of other studies on RFA using multiple electrodes (2-year LTP rate, 7–10%) (20,34,39,40). Similar ablation volumes between the two groups most likely accounted for the lack of significant differences in treatment performances, since a large ablation zone directly relates to a sufficient ablative margin (15,41,42,43). Further studies on HCCs with larger sizes (3–5 cm in diameter) are warranted to adequately evaluate the ablation capability as well as the potential therapeutic benefit of DSM-RFA in clinical settings. Moreover, the technical success rates in the DSM-RFA and SSM-RFA groups were 97.4% (38/39) and 100.0% (41/41), respectively, and technique efficacy rates at the 1 month follow-up were 100.0% (41/41) in the DSM-RFA group and 97.4% (37/38) in the SSM-RFA group. Several factors may account for the high technical effectiveness observed in both groups. First, the multiple-electrode approach, as used in both groups, is more efficient in creating large ablation zones than conventional RFA using a single electrode (44,45). Another benefit of the multiple-electrode approach is that it is technically less demanding than the single-electrode approach in targeting the index tumor and relocating electrodes during ablation, especially under US guidance (14). An additional factor is the use of a real-time fusion imaging technique employing both US and preprocedural CT or MRI for targeting the index tumor as well as for intraprocedural monitoring of the ablation zone (34,35). According to a recent study on real-time fusion imaging for RFA, fusion imaging improved tumor visibility, and fusion image-guided RFA showed a high technique effectiveness rate and lower LTP rate in patients with HCCs (46).
The major complication rate of DSM-RFA in our study was 4.9% (2 of 41 patients), which was comparable to those reported in previous studies on various RFA systems using single or multiple electrodes (2–7.2%) (8,20,39,47,48,49,50,51,52). Our results were also similar to those (4.1%) of a previous systematic review on mortality and complications rates of percutaneous ablative techniques (53). In theory, multiple-electrode approaches may have higher rates of procedure-related complications such as bleeding associated with the increasing number of electrode insertions or skin burn due to higher RF energy delivery (45). However, in our study, the use of real-time guidance using fusion imaging allowed safe and precise placement of electrodes while avoiding vital structures, such as the bile duct and major vessels; moreover, skin burn was prevented by the application of four large grounding pads.
Our study had some limitations. First, the study had only a small number of patients. Second, the tumor size was too small to compare the performance of DSM-RFA and SSM-RFA in creating ablation zones; however, our study was an exploratory one, comparing the two modes for treating HCCs. Third, only one radiologist, who had performed the largest number of procedures in routine clinical practice in our institute, participated in our study, mainly due to the difficulty of obtaining informed consent. Lastly, the follow-up period after RFA was relatively short, due to which only intermediate follow-up results were available. Further studies on larger groups of patients and with longer follow-up periods are therefore warranted.
In conclusion, although DSM-RFA using a separable clustered electrode delivers higher ablation energy than SSM-RFA, its effectiveness failed to show superiority over SSM-RFA in the treatment of HCC. Further optimization of DSM-RFA to overcome the Faraday cage effect seems necessary to achieve both technical efficiency and better clinical effectiveness than SSM-RFA for treating HCC.
Footnotes
This study was supported by a research grant from STARmed Co., Ltd. (06-2014-3930).
Conflicts of Interest: Jeong Min Lee received a research grant from STARmed Co., Ltd. Other authors have no potential conflicts of interest to disclose.
References
- 1.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49:453–459. doi: 10.1002/hep.22648. [DOI] [PubMed] [Google Scholar]
- 3.Lau WY, Lai EC. The current role of radiofrequency ablation in the management of hepatocellular carcinoma: a systematic review. Ann Surg. 2009;249:20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 4.Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013;59:89–97. doi: 10.1016/j.jhep.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.N'Kontchou G, Mahamoudi A, Aout M, Ganne-Carrié N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–1483. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 7.Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012;107:569–577. doi: 10.1038/ajg.2011.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee DH, Lee JM, Lee JY, Kim SH, Yoon JH, Kim YJ, et al. Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology. 2014;270:900–909. doi: 10.1148/radiol.13130940. [DOI] [PubMed] [Google Scholar]
- 9.Kim YS, Lim HK, Rhim H, Lee MW, Choi D, Lee WJ, et al. Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol. 2013;58:89–97. doi: 10.1016/j.jhep.2012.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, et al. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59:300–307. doi: 10.1016/j.jhep.2013.04.009. [DOI] [PubMed] [Google Scholar]
- 11.Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008;47:82–89. doi: 10.1002/hep.21933. [DOI] [PubMed] [Google Scholar]
- 12.European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 13.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67:358–380. doi: 10.1002/hep.29086. [DOI] [PubMed] [Google Scholar]
- 14.Lee DH, Lee JM. Recent advances in the image-guided tumor ablation of liver malignancies: radiofrequency ablation with multiple electrodes, real-time multimodality fusion imaging, and new energy sources. Korean J Radiol. 2018;19:545–559. doi: 10.3348/kjr.2018.19.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, William Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. Radiology. 2014;273:241–260. doi: 10.1148/radiol.14132958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015;16:1226–1239. doi: 10.3348/kjr.2015.16.6.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JM, Han JK, Kim HC, Choi YH, Kim SH, Choi JY, et al. Switching monopolar radiofrequency ablation technique using multiple, internally cooled electrodes and a multichannel generator: ex vivo and in vivo pilot study. Invest Radiol. 2007;42:163–171. doi: 10.1097/01.rli.0000252495.44818.b3. [DOI] [PubMed] [Google Scholar]
- 18.Laeseke PF, Frey TM, Brace CL, Sampson LA, Winter TC, 3rd, Ketzler JR, et al. Multiple-electrode radiofrequency ablation of hepatic malignancies: initial clinical experience. AJR Am J Roentgenol. 2007;188:1485–1494. doi: 10.2214/AJR.06.1004. [DOI] [PubMed] [Google Scholar]
- 19.Mulier S, Miao Y, Mulier P, Dupas B, Pereira P, de Baere T, et al. Electrodes and multiple electrode systems for radiofrequency ablation: a proposal for updated terminology. Eur Radiol. 2005;15:798–808. doi: 10.1007/s00330-004-2584-x. [DOI] [PubMed] [Google Scholar]
- 20.Hocquelet A, Aubé C, Rode A, Cartier V, Sutter O, Manichon AF, et al. Comparison of no-touch multi-bipolar vs. monopolar radiofrequency ablation for small HCC. J Hepatol. 2017;66:67–74. doi: 10.1016/j.jhep.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Seror O, Sutter O. RE: should we use a monopolar or bipolar mode for performing no-touch radiofrequency ablation of liver tumors? Clinical practice might have already resolved the matter once and for all. Korean J Radiol. 2017;18:749–752. doi: 10.3348/kjr.2017.18.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon JH, Lee JM, Woo S, Hwang EJ, Hwang I, Choi W, et al. Switching bipolar hepatic radiofrequency ablation using internally cooled wet electrodes: comparison with consecutive monopolar and switching monopolar modes. Br J Radiol. 2015;88:20140468. doi: 10.1259/bjr.20140468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin RC, Scoggins CR, McMasters KM. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol. 2010;17:171–178. doi: 10.1245/s10434-009-0686-z. [DOI] [PubMed] [Google Scholar]
- 24.Ding J, Jing X, Liu J, Wang Y, Wang F, Wang Y, et al. Comparison of two different thermal techniques for the treatment of hepatocellular carcinoma. Eur J Radiol. 2013;82:1379–1384. doi: 10.1016/j.ejrad.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 25.Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015;7:1054–1063. doi: 10.4254/wjh.v7.i8.1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamakado K, Nakatsuka A, Ohmori S, Shiraki K, Nakano T, Ikoma J, et al. Radiofrequency ablation combined with chemoembolization in hepatocellular carcinoma: treatment response based on tumor size and morphology. J Vasc Interv Radiol. 2002;13:1225–1232. doi: 10.1016/s1051-0443(07)61969-1. [DOI] [PubMed] [Google Scholar]
- 27.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–913. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 28.Feng X, Xu R, Du X, Dou K, Qin X, Xu J, et al. Combination therapy with sorafenib and radiofrequency ablation for BCLC Stage 0-B1 hepatocellular carcinoma: a multicenter retrospective cohort study. Am J Gastroenterol. 2014;109:1891–1899. doi: 10.1038/ajg.2014.343. [DOI] [PubMed] [Google Scholar]
- 29.Duffy AG, Ulahannan SV, Makorova-Rusher O, Rahma O, Wedemeyer H, Pratt D, et al. Tremelimumab in combination with ablation in patients with advanced hepatocellular carcinoma. J Hepatol. 2017;66:545–551. doi: 10.1016/j.jhep.2016.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014;15:235–244. doi: 10.3348/kjr.2014.15.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon JH, Lee JM, Han JK, Choi BI. Dual switching monopolar radiofrequency ablation using a separable clustered electrode: comparison with consecutive and switching monopolar modes in ex vivo bovine livers. Korean J Radiol. 2013;14:403–411. doi: 10.3348/kjr.2013.14.3.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi TW, Lee JM, Lee DH, Lee JH, Yu SJ, Kim YJ, et al. Percutaneous dual-switching monopolar radiofrequency ablation using a separable clustered electrode: a preliminary study. Korean J Radiol. 2017;18:799–808. doi: 10.3348/kjr.2017.18.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee ES, Lee JM, Kim WS, Choi SH, Joo I, Kim M, et al. Multiple-electrode radiofrequency ablations using Octopus® electrodes in an in vivo porcine liver model. Br J Radiol. 2012;85:e609–e615. doi: 10.1259/bjr/61619687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi JW, Lee JM, Lee DH, Yoon JH, Suh KS, Yoon JH, et al. Switching monopolar radiofrequency ablation using a separable cluster electrode in patients with hepatocellular carcinoma: a prospective study. PLoS One. 2016;11:e0161980. doi: 10.1371/journal.pone.0161980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Krücker J, Xu S, Venkatesan A, Locklin JK, Amalou H, Glossop N, et al. Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol. 2011;22:515–524. doi: 10.1016/j.jvir.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sacks D, McClenny TE, Cardella JF, Lewis CA. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2003;14:S199–S202. doi: 10.1097/01.rvi.0000094584.83406.3e. [DOI] [PubMed] [Google Scholar]
- 37.Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011;196:W205–W209. doi: 10.2214/AJR.10.4937. [DOI] [PubMed] [Google Scholar]
- 38.Lee FT, Jr, Haemmerich D, Wright AS, Mahvi DM, Sampson LA, Webster JG. Multiple probe radiofrequency ablation: pilot study in an animal model. J Vasc Interv Radiol. 2003;14:1437–1442. doi: 10.1097/01.rvi.0000096771.74047.c8. [DOI] [PubMed] [Google Scholar]
- 39.Lee J, Lee JM, Yoon JH, Lee JY, Kim SH, Lee JE, et al. Percutaneous radiofrequency ablation with multiple electrodes for medium-sized hepatocellular carcinomas. Korean J Radiol. 2012;13:34–43. doi: 10.3348/kjr.2012.13.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woo S, Lee JM, Yoon JH, Joo I, Kim SH, Lee JY, et al. Small- and medium-sized hepatocellular carcinomas: monopolar radiofrequency ablation with a multiple-electrode switching system-mid-term results. Radiology. 2013;268:589–600. doi: 10.1148/radiol.13121736. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010;195:758–765. doi: 10.2214/AJR.09.2954. [DOI] [PubMed] [Google Scholar]
- 42.Nakazawa T, Kokubu S, Shibuya A, Ono K, Watanabe M, Hidaka H, et al. Radiofrequency ablation of hepatocellular carcinoma: correlation between local tumor progression after ablation and ablative margin. AJR Am J Roentgenol. 2007;188:480–488. doi: 10.2214/AJR.05.2079. [DOI] [PubMed] [Google Scholar]
- 43.Zytoon AA, Ishii H, Murakami K, El-Kholy MR, Furuse J, El-Dorry A, et al. Recurrence-free survival after radiofrequency ablation of hepatocellular carcinoma. A registry report of the impact of risk factors on outcome. Jpn J Clin Oncol. 2007;37:658–672. doi: 10.1093/jjco/hym086. [DOI] [PubMed] [Google Scholar]
- 44.Lee JM, Han JK, Kim HC, Kim SH, Kim KW, Joo SM, et al. Multiple-electrode radiofrequency ablation of in vivo porcine liver: comparative studies of consecutive monopolar, switching monopolar versus multipolar modes. Invest Radiol. 2007;42:676–683. doi: 10.1097/RLI.0b013e3180661aad. [DOI] [PubMed] [Google Scholar]
- 45.Chang W, Lee JM, Lee DH, Yoon JH, Kim YJ, Yoon JH, et al. Comparison of switching bipolar ablation with multiple cooled wet electrodes and switching monopolar ablation with separable clustered electrode in treatment of small hepatocellular carcinoma: a randomized controlled trial. PLoS One. 2018;13:e0192173. doi: 10.1371/journal.pone.0192173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn SJ, Lee JM, Lee DH, Lee SM, Yoon JH, Kim YJ, et al. Real-time US-CT/MR fusion imaging for percutaneous radiofrequency ablation of hepatocellular carcinoma. J Hepatol. 2017;66:347–354. doi: 10.1016/j.jhep.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003;226:441–451. doi: 10.1148/radiol.2262012198. [DOI] [PubMed] [Google Scholar]
- 48.Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003;23:123–134. doi: 10.1148/rg.231025054. [DOI] [PubMed] [Google Scholar]
- 49.Kim JH, Kim PN, Won HJ, Shin YM. Percutaneous radiofrequency ablation using internally cooled wet electrodes for the treatment of hepatocellular carcinoma. AJR Am J Roentgenol. 2012;198:471–476. doi: 10.2214/AJR.11.6583. [DOI] [PubMed] [Google Scholar]
- 50.Seror O, N'Kontchou G, Tin-Tin-Htar M, Barrucand C, Ganne N, Coderc E, et al. Radiofrequency ablation with internally cooled versus perfused electrodes for the treatment of small hepatocellular carcinoma in patients with cirrhosis. J Vasc Interv Radiol. 2008;19:718–724. doi: 10.1016/j.jvir.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 51.Chen TM, Huang PT, Lin LF, Tung JN. Major complications of ultrasound-guided percutaneous radiofrequency ablations for liver malignancies: single center experience. J Gastroenterol Hepatol. 2008;23:e445–e450. doi: 10.1111/j.1440-1746.2007.05078.x. [DOI] [PubMed] [Google Scholar]
- 52.Tateishi R, Shiina S, Teratani T, Obi S, Sato S, Koike Y, et al. Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer. 2005;103:1201–1209. doi: 10.1002/cncr.20892. [DOI] [PubMed] [Google Scholar]
- 53.Bertot LC, Sato M, Tateishi R, Yoshida H, Koike K. Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol. 2011;21:2584–2596. doi: 10.1007/s00330-011-2222-3. [DOI] [PubMed] [Google Scholar]