Abstract
Objective
The aim of this study was to compare the survival rates of Korean females aged 40 to 49 years with breast cancer detected by supplemental screening ultrasound (US) or screening mammography alone.
Materials and Methods
This single-institution retrospective study included 240 patients with breast cancer (mean age, 45.1 ± 2.8 years) detected by US or mammography who had undergone breast surgery between 2003 and 2008. Medical records were reviewed for clinicopathologic characteristics and detection methods. Disease-free survival (DFS) and overall survival (OS) were compared between patients with breast cancer in the US and mammography groups using the log-rank test. Multivariable cox regression analysis was used to identify independent variables associated with DFS and OS.
Results
Among the 240 cases of breast cancer, 43 were detected by supplemental screening US and 197 by screening mammography (mean follow-up: 7.4 years, 93.3% with dense breasts). There were 19 recurrences and 16 deaths, all occurring in the mammography group. While the US group did not differ from the mammography group in tumor stage, the patients in this group were more likely to undergo breast-conserving surgery and radiation therapy than the mammography group. The US group also showed better DFS (p = 0.016); however, OS did not differ between the two groups (p = 0.058). In the multivariable analysis, the US group showed a lower risk of recurrence (hazard ratio, 0.097; 95% confidence interval, 0.001–0.705) compared to the mammography group.
Conclusion
Our study found that Korean females aged 40–49 years with US-detected breast cancer showed better DFS than those with mammography-detected breast cancer. However, there were no statistically significant differences in OS.
Keywords: Breast cancer, Mammography, Ultrasound, Screening, Survival rate
INTRODUCTION
Mammography is recommended as a screening tool for the early detection of breast cancer by various internationally recognized guidelines such as those presented by the American Cancer Society (ACS) and the U.S. Preventive Service Task Force (USPSTF) (1,2). In 2009, the USPSTF updated its guidelines by raising the recommended age for screening mammography to 50 years and older (3) and in 2015, the ACS also changed its guidelines to women over 45 years (4). This was due to continuing controversies regarding the high false positive and false negative rates seen with screening mammography in younger women in their 40s who have dense breasts.
However, in eastern Asian countries such as Korea and Japan, the incidence of breast cancer peaks in women in their late 40s (5), which is considerably earlier than that of western countries, peaking between 60 and 70 years of age (6). Therefore, concerns have been raised as to whether the USPSTF or ACS guidelines can be directly applied to patients in these regions (7). In addition, over 80–90% of Korean women in their 40s have heterogeneously dense or extremely dense breasts (8,9), with a low sensitivity on mammography (10). Ultrasound (US) has been widely used as a supplemental screening tool in Korea to overcome the low sensitivity of mammography in dense breasts. Previous studies have shown the increased detection of breast cancer by supplemental US in women with mammography-negative dense breasts (11,12) with one study showing the excellent diagnostic performance for US in patients with Breast Imaging-Reporting and Data System (BI-RADS) category 0 mammography (13). Although a previous study has shown excellent survival outcomes (five-year overall survival [OS], 100%; disease-free survival [DFS], 98%) for women with breast cancers detected by screening US (14), there have been no publications regarding the benefits of screening US on OS and DFS in breast cancer, compared to screening mammography. Some states in the U.S. mandate reports on mammogram density and recommend supplemental screening tests, such as US, for women with dense breasts through breast density notification laws. However, in Korea, US is performed in consultation between doctors and patients with dense breasts. There are currently no guidelines or legislations on the use of screening US in women with dense breasts as to date, there is not enough evidence to support its use in Korean women aged between 40 and 49 years.
Therefore, the objective of our study was to compare the survival rates of Korean women aged 40 to 49 years with breast cancer detected by supplemental screening US to those with breast cancer detected by screening mammography alone.
MATERIALS AND METHODS
This retrospective study was approved by the Institutional Review Board of Severance Hospital. The requirement for informed consent was waived due to the retrospective nature of the study.
Patients
We collected the data from 915 females from the breast cancer surgery database who had undergone surgery for breast cancer between the ages of 40 and 49 at Severance Hospital between January 2003 and November 2008. The methods used to detect breast lesions were assessed through retrospective image reviews, medical records, and questionnaires from patients who visited our hospital for the first time. Among the initial 915 subjects, patients with self-reported symptoms, such as palpable mass or nipple discharge, at the time of diagnosis (n = 481) and a single patient with cancer detected by positron emission tomography (PET) scan (n = 1) were excluded. Patients whose detection modality could not be determined as they did not undergo mammography or if they did not have mammographic images available at the time of review were also excluded (n = 77). Finally, patients with a previous history of breast cancer surgery (n = 74), bilateral breast cancer (n = 39), or lose who were lost during follow-up immediately after surgery (n = 3) were excluded. In the initial 240 patients, a total of 240 females were included in the study population.
Group Classification according to Detection Modality
Based on medical records, radiologic reports, and retrospective image reviews, the site of the lesion detected by mammogram or US and the actual lesion site proven to be a malignancy were correlated and patients were classified into the US or mammography group according to their detection modality. In our study, detection modality was evaluated only for the index tumor. The mean time interval between mammography and US was 7.5 days (median: 0, interquartile range [IQR]: 0–6). If an asymptomatic patient had suspicious findings for malignancy on screening mammography and was diagnosed pathologically with breast cancer afterwards, the patient was included in the mammography group. If a patient had negative results on screening mammography but had breast cancer detected on subsequent supplemental screening US, the patient was included in the US group. Two radiologists (with 1 and 15 years of experience) independently determined the detection modality when the mammography category was not 1 or 2. In cases of disagreement, the detection modality was decided by a consensus.
Data and Statistical Analyses
Demographic and clinical data were collected from our breast cancer database. Patient histories of high-risk breast lesions, such as atypical ductal hyperplasia, lobular carcinoma in situ, radial scar, and atypical papilloma, was also reviewed. Patients were considered to have a family history of breast cancer if they had a first-degree relative with breast cancer. In our study, females at a high risk for breast cancer were defined as patients with a history of high-risk breast lesions or a family history of breast cancer. Breast parenchymal density on mammograms was retrospectively reviewed according to the BI-RADS® lexicon by radiologist with 15 years of experience (15). Patient histories of neoadjuvant chemotherapy, adjuvant chemotherapy, endocrine therapy, and radiation therapy were also reviewed.
The type of surgery each patient underwent was reviewed. Breast cancer pathology was determined as invasive cancer or ductal carcinoma in situ (DCIS). Cancer stage was assessed according to the American Joint Committee on Cancer staging system (16). Histologic grade was divided into two groups (high grade [grade 3] and not high [grades 1 and 2]). Tumor size based on the invasive component was reviewed. Hormone receptor positivity was defined as estrogen receptor and/or progesterone receptor positivity (≥ 10% nuclear staining) (17). Human epidermal growth factor receptor 2 (HER2) positivity was defined as an immunohistochemical (IHC) HER2 score of 3+ or with gene amplification by fluorescence in situ hybridization in tumors with an IHC HER2 score of 2+ (18). Ki-67 was scored by counting the number of cells with positively stained nuclei and expressing this value as a percentage of the total tumor cells. Recurrence was defined as a relapse of breast cancer after initial treatment and was categorized into two groups, local recurrence and systemic recurrence. Sites of local recurrence included the ipsilateral remnant breast, chest wall, lymph nodes inside the breast, axillary, supraclavicular fossa, or internal mammary regions. Systemic recurrence was defined as any recurrence away from the breast, axillary, or internal mammary nodes regions. Local recurrence was pathologically confirmed while systemic recurrence was diagnosed through biopsy or imaging studies such as PET/CT, CT or MRI.
Characteristics were compared between the detection modality groups and analyzed using the chi-square test, Fisher's exact test for categorical variables, and the independent two-sample test for continuous variables. DFS was calculated by subtracting the date of surgery from the date of first recurrence or death. For patients with no recurrence, the disease-free interval was calculated by subtracting the date of surgery from the date of the last follow-up. DFS and OS were compared between the detection modality groups using the log-rank test and Kaplan-Meier curves were obtained. Univariate cox regression analysis was used to identify factors affecting DFS and OS. Multivariable cox regression analysis was used to identify independent variables associated with DFS and OS. For rare events, Firth's penalized likelihood logistic regression was used. Differences were considered statistically significant if the p value was less than 0.05. All statistical analyses were performed with SPSS statistics version 25.0 (IBM Corp.) or R version 3.5.1 (http://www.r-project.org).
RESULTS
Among the 240 patients with breast cancer, 197 were classified into the mammography group and 43 into the US group. As for breast density on mammograms, 93.3% (224 of 240) of all patients had heterogeneously (n = 189) or extremely dense breasts (n = 35) and the remaining 6.7% (16 of 240) had scattered fibroglandular density, all of which were in the mammography group. In the US group, all patients had heterogeneously (n = 34) or extremely dense breasts (n = 9). The mean follow-up period was 7.4 years (range, 1.3–12.3 years). The mean follow-up period for patients without recurrence was 7.7 years and the mean follow-up period for patients with recurrence was 5.6 years. There were neither recurrences nor deaths among the 43 subjects in the US group, while there were 19 recurrences (median, 964 days; IQR, 373–1469 days) and 16 deaths (median, 2026 days; IQR, 921–3270 days) among the 197 subjects in the mammography group. For the 19 recurrences, there were 3 patients with locoregional recurrence (remnant breast, supraclavicular lymph node, and internal mammary lymph node), 15 patients with systemic recurrence (lung [n = 6], brain [n = 1], liver [n = 7], bone [n = 11], adrenal gland [n = 1], and kidney [n = 1]) and 1 patient with both local and systemic recurrence (chest wall and mediastinal lymph node).
US vs. Mammography: Characteristics and Survival
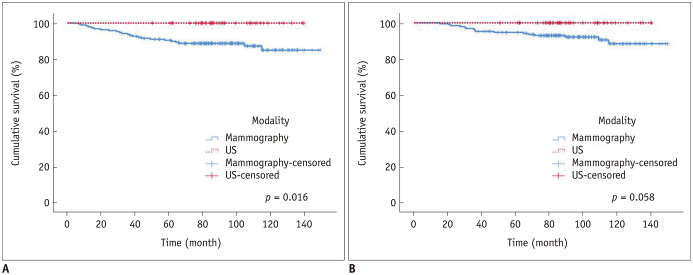
There were no statistically significant differences in T and N staging between the US and mammography groups. The US group had a higher proportion of patients with breast-conserving surgery (32 of 43, 74.4%), radiation therapy (35 of 43, 81.4%), and a family history of breast cancer (5 of 43, 11.6%) compared to the mammography group (37.1%, p < 0.001; 46.2%, p < 0.001; 3.6%, p = 0.045; respectively) (Table 1, Supplementary Table 1). The US group showed a significantly higher DFS rate compared to the mammography group (5-year DFS: 100%, 90.0%, respectively, p = 0.016) (Fig. 1A). The US group tended to have a higher OS rate compared to the mammography group but without statistical significance (p = 0.058) (Fig. 1B). In our study, only 6.7% of all subjects (16 of 240) had non-dense breasts. In individuals with dense breasts, the US group tended to show a higher DFS rate compared to the mammography group, with statistical significance (p = 0.017) and superior OS compared to the mammography group but without statistical significance (p = 0.056).
Table 1. Demographic Characteristics of the US and Mammography Group.
| Characteristics | Mammography (n = 197) | US (n = 43) | P |
|---|---|---|---|
| Age (years) | 45.2 ± 2.8 | 45.1 ± 3.1 | 0.832 |
| Breast disease with high risk for breast cancer | 0.497 | ||
| Absent | 185 (93.9) | 39 (90.7) | |
| Present | 12 (6.1) | 4 (9.3) | |
| Family history of breast cancer | 0.045 | ||
| Absent | 188 (95.4) | 38 (81.4) | |
| Present | 7 (3.6) | 5 (11.6) | |
| N/A | 2 (1.0) | 0 (0) | |
| Breast density | 0.084 | ||
| Non-dense breast | 16 (8.1) | 0 (0) | |
| Dense breast | 181 (91.9) | 43 (100) | |
| Mammography finding | < 0.001 | ||
| Mass only | 101 (51.3) | 0 (0) | |
| Calcification with/without mass | 96 (48.7) | 0 (0) | |
| Negative | 0 (0) | 43 (100) |
N/A = not available, US = ultrasound
Fig. 1. DFS and OS (US vs. mammography).
DFS (A) and OS (B) between the US and mammography group (p = 0.016 and 0.058, respectively). DFS = disease-free survival, OS = overall survival, US = ultrasound
Factors Associated with DFS
In the univariable analysis, the US group had a lower risk of recurrence (hazard ratio [HR], 0.086, p = 0.007) compared to the mammography group and patients with a larger tumor size and high histologic grade had a higher risk of recurrence (HR, 1.577, p = 0.012; HR, 2.789, p = 0.012, respectively) compared with those with smaller size and not-high histologic grade (Table 2). In the multivariable analysis, the US group had a lower risk of recurrence (HR, 0.097; 95% confidence interval [CI], 0.001–0.705) compared with the mammography group (Table 2). Patients with a high histologic grade had a higher risk of recurrence (HR, 2.457; 95% CI, 1.108–5.451) compared to those with not-high histologic grade (Table 2).
Table 2. Univariate and Multivariable Analysis of Variables Independently Associated with Disease-Free Survival.
| Characteristics | No. of Patients | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| Hazard Ratio | P | Hazard Ratio | P | ||
| Age (years) | 1.071 (0.925–1.240) | 0.361 | |||
| Detection modality | |||||
| Mammography (ref.) | 197 | ||||
| US | 43 | 0.086 (0.001–0.613) | 0.007 | 0.097 (0.001–0.705) | 0.014 |
| Breast disease with high risk for breast cancer | |||||
| Absent (ref.) | 224 | ||||
| Present | 16 | 0.259 (0.002–1.852) | 0.231 | ||
| Family history of breast cancer | |||||
| Absent (ref.) | 226 | ||||
| Present | 12 | 0.832 (0.112–6.173) | 0.857 | ||
| Surgery type | |||||
| Breast-conserving surgery (ref.) | 105 | ||||
| Total mastectomy | 135 | 0.887 (0.396–1.984) | 0.770 | ||
| Invasiveness | |||||
| Non-invasive (ref.) | 51 | ||||
| Invasive | 189 | 1.916 (0.571–6.423) | 0.292 | ||
| Tumor size (cm) | 240 | 1.577 (1.107–2.246) | 0.012 | 0.975 (0.637–1.443) | 0.902 |
| T stage | |||||
| T0 (ref.) | 51 | ||||
| T1 | 141 | 1.468 (0.414–5.201) | 0.552 | ||
| T2 | 48 | 3.234 (0.875–11.950) | 0.078 | ||
| T3 | 0 | ||||
| T4 | 0 | ||||
| N stage | |||||
| Nx | |||||
| N0 (ref.) | 183 | ||||
| N1 | 36 | 1.285 (0.426–3.872) | 0.656 | ||
| N2 | 14 | 2.947 (0.853–10.187) | 0.088 | ||
| N3 | 7 | 3.958 (0.902–17.365) | 0.068 | ||
| Histology grade | |||||
| Not-high (ref.) | 173 | ||||
| High | 67 | 2.789 (1.252–6.210) | < 0.012 | 2.457 (1.108–5.451) | 0.027 |
| Chemotherapy | |||||
| Absent (ref.) | 113 | ||||
| Present | 127 | 2.102 (0.870–5.077) | 0.099 | ||
| Endocrine therapy | |||||
| Absent (ref.) | 66 | ||||
| Present | 173 | 0.913 (0.377–2.213) | 0.840 | ||
| Radiation therapy | |||||
| Absent (ref.) | 114 | ||||
| Present | 126 | 1.870 (0.799–4.377) | 0.149 | ||
| Estrogen receptor | |||||
| Negative (ref.) | 66 | ||||
| Positive | 168 | 0.973 (0.402–2.353) | 0.951 | ||
| Progesterone receptor | |||||
| Negative (ref.) | 69 | ||||
| Positive | 165 | 0.858 (0.366–2.011) | 0.724 | ||
| HER2 receptor | |||||
| Negative (ref.) | 154 | ||||
| Positive | 47 | 1.025 (0.375–2.799) | 0.961 | ||
| Unknown | 33 | 0.796 (0.232–2.732) | 0.717 | ||
HER2 = human epidermal growth factor receptor 2, ref. = reference
Factors Associated with OS
In the univariable analysis, detection modality, tumor size, N stage, histology grade, and a history of chemotherapy were associated with OS (HR, 0.134 [US], p = 0.049; HR, 1.646, p = 0.020; HR, 6.362 [N3], p = 0.019; HR, 2.689, p = 0.048; HR, 3.637, p = 0.044, respectively) (Table 3). In the multivariable analysis, patients with a high histologic grade had a higher risk of death (HR, 2.820; 95% CI, 1.056–7.524, p = 0.039) compared to those with not-high histologic grade. The US group had a lower tendency of death but without statistical significance (HR, 0.155, p = 0.077).
Table 3. Univariate and Multivariable Analysis of Variables Independently Associated with Overall Survival.
| Characteristics | No. of Patients | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|---|
| Hazard Ratio | P | Hazard Ratio | P | ||
| Age (years) | 1.074 (0.896–1.288) | 0.439 | |||
| Detection modality | |||||
| Mammography (ref.) | 197 | 0.049 | 0.155 (0.001–1.162) | 0.077 | |
| US | 43 | 0.134 (0.001–0.992) | |||
| Breast disease with high risk for breast cancer | |||||
| Absent (ref.) | 224 | ||||
| Present | 16 | 0.376 (0.003–2.775) | 0.423 | ||
| Family history of breast cancer | |||||
| Absent (ref.) | 226 | ||||
| Present | 12 | 1.304 (0.171–9.919) | 0.798 | ||
| Surgery type | |||||
| Breast-conserving surgery (ref.) | 105 | ||||
| Total mastectomy | 135 | 0.936 (0.347–2.525) | 0.896 | ||
| Invasiveness | |||||
| Non-invasive (ref.) | 51 | ||||
| Invasive | 189 | 1.931 (0.439–8.495) | 0.384 | ||
| Tumor size (cm) | 240 | 1.646 (1.081–2.505) | 0.020 | 1.040 (0.641–1.641) | 0.868 |
| T stage | |||||
| T0 (ref.) | 51 | ||||
| T1 | 141 | 1.311 (0.272–6.310) | 0.736 | ||
| T2 | 48 | 3.666 (0.761–17.657) | 0.105 | ||
| T3 | 0 | ||||
| T4 | 0 | ||||
| N stage | |||||
| N0 (ref.) | 183 | ||||
| N1 | 36 | 1.548 (0.419–5.723) | 0.512 | 0.832 (0.196–2.946) | 0.767 |
| N2 | 14 | 2.993 (0.645–13.885) | 0.161 | 1.594 (0.290–6.300) | 0.549 |
| N3 | 7 | 6.362 (1.364–29.670) | 0.019 | 3.393 (0.618–13.456) | 0.142 |
| Histology grade | |||||
| Not-high (ref.) | 173 | ||||
| High | 67 | 2.689 (1.008–7.170) | 0.048 | 2.820 (1.056–7.524) | 0.039 |
| Chemotherapy | |||||
| Absent (ref.) | 113 | ||||
| Present | 127 | 3.637 (1.034–12.791) | 0.044 | 3.523 (0.987–15.020) | 0.053 |
| Endocrine therapy | |||||
| Absent (ref.) | 66 | ||||
| Present | 173 | 0.864 (0.298–2.506) | 0.787 | ||
| Radiation therapy | |||||
| Absent (ref.) | 114 | ||||
| Present | 126 | 2.100 (0.728–6.063) | 0.170 | ||
| Estrogen receptor | |||||
| Negative (ref.) | 66 | ||||
| Positive | 168 | 0.680 (0.246–1.880) | 0.458 | ||
| Progesterone receptor | |||||
| Negative (ref.) | 69 | ||||
| Positive | 165 | 0.979 (0.338–2.830) | 0.968 | ||
| HER2 receptor | |||||
| Negative (ref.) | 154 | ||||
| Positive | 47 | 0.528 (0.118–2.360) | 0.403 | ||
| Unknown | 33 | 0.737 (0.165–3.292) | 0.689 | ||
DISCUSSION
Due to the lower sensitivity of screening mammography in dense breasts, the need for an adjuvant screening tool has been recognized and US has been discussed as a promising adjunctive screening modality (19). Recently, the Japan Strategic Anti-cancer Randomized Trial showed a lower incidence of interval cancer when supplemental US was performed compared to when only screening mammography was performed (20). However, there have been very few studies on the survival benefits of screening US for breast cancer, despite US being widely used as a supportive screening tool in Asia (21,22). To the best of our knowledge, our study is the first to evaluate the survival benefit of detecting breast cancers by adjunctive screening US in comparison to mammography alone and we were able to show improved DFS with screening US (p = 0.016) compared to screening mammography. A previous study suggested that avoiding local recurrence for 5 years is important in improving patient survival and that doing so is of comparable relevance to 15-year breast cancer mortality (23). In our study, despite the excellent 5-year DFS seen in the US group, OS did not significantly differ between the two groups (p = 0.058). However, considering previous research (23), we can speculate that patients who did not develop a recurrence within 5 years of the original breast cancer will potentially benefit in OS in a future study with more than 15 years of follow-up.
The sensitivity of screening mammography is dependent on breast density (24) and nearly 80% of Korean females in their 40s have either heterogeneously or extremely dense breasts (25). In our study, 93.3% of all subjects had dense breasts on mammography, which is a higher proportion than in the general population. The reason for the high proportion of dense breasts was that the study was made up of only patients with breast cancer. A previous matched study in Korea showed that patients with breast cancer were 10% more likely to have dense breasts than a matched healthy subject (26). Breast cancer has been found to peak in Korean females in their late 40s, which is considerably earlier than in western females (5). Patients diagnosed with breast cancer before the age of 49 have shown higher death rates from breast cancer compared to patients diagnosed after age of 49 (27). Therefore, any limitations mammography has related to dense breasts can be magnified or more critical in Asian females of this age group due to the prevalence of dense breasts in this population.
In our study, 78–86% of breast cancer cases were T0 or T1 in size in both groups, consistent with the screened group in a previous study (28). Although there was no difference in T and N stage between the two groups, the US group tended to have a higher proportion of tumors with not-high histologic grade than the mammography group. Differences in histologic grade between the two groups may affect survival. Our study found that patients aged 40–49 years who were diagnosed with breast cancer by supplemental screening US had higher rates of breast-conserving surgery than patients diagnosed by screening mammography, although cancer stages were not significantly different between the two groups. This may be due to the presence of calcifications. In our study, almost half (48.7%) of the patients in the mammography group had calcifications. Since calcifications are a representative finding of DCIS, breast cancers with calcifications on mammography are likely to have an additional DCIS component, which is not included in tumor size. Considering that breast cancer detected by mammography are more likely to have calcifications and that calcifications are likely to indicate additional DCIS components, there is a high chance of total mastectomy. The frequency of radiation therapy was higher in the US group than in the mammography group due to patients in the US group undergoing breast-conserving surgery more frequently.
Our study has a few limitations. Firstly, as this is a retrospective and single center study, there were limitations to the statistical power and to the inclusion and exclusion criteria, which may have resulted in selection bias. Secondly, although not analyzed for, the early diagnosis of breast cancer may have led to seemingly prolonged survival (lead time bias). However, our study shows no significant difference in T and N stage and tumor size between the two groups. Thirdly, our study did not perform follow-ups that went on longer than 10 years. Finally, although survival analysis that matched patients of the two groups could have shown more reliable results, this was not possible due to the lack of recurrence or death in the US group. A larger study will be needed in the future. In addition, although overdiagnosis and overtreatment caused by false-positive results in screening mammography and US have become major concerns, our study does not address this issue in depth.
In conclusion, our results from a single institution found that Korean females between 40 and 49 years of age with breast cancer detected by breast US showed excellent survival with neither recurrences nor deaths during follow-up and showed increased survival rates than females with breast cancer detected by screening mammography. To obtain more reliable results on the survival benefit of breast US for the detection of breast cancer, a multi-center trial with a larger sample size is required.
Footnotes
This study was supported by the Basic Science Research Program of the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning, Republic of Korea (grant 2017R1A2B4010407). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Materials
The Data Supplement is available with this article at https://doi.org/10.3348/kjr.2019.0588.
Therapeutic and Pathologic Characteristics of the US and Mammography Group
References
- 1.Smith RA, Brooks D, Cokkinides V, Saslow D, Brawley OW. Cancer screening in the United States, 2013: a review of current American Cancer Society guidelines, current issues in cancer screening, and new guidance on cervical cancer screening and lung cancer screening. CA Cancer J Clin. 2013;63:87–105. doi: 10.3322/caac.21174. [DOI] [PubMed] [Google Scholar]
- 2.Humphrey LL, Helfand M, Chan BK, Woolf SH. Breast cancer screening: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137:347–360. doi: 10.7326/0003-4819-137-5_part_1-200209030-00012. [DOI] [PubMed] [Google Scholar]
- 3.US Preventive Services Task Force. Screening for breast cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2009;151:716–726. doi: 10.7326/0003-4819-151-10-200911170-00008. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Fontham ET, Etzioni R, Herzig A, Michaelson JS, Shih YCT, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314:1599–1614. doi: 10.1001/jama.2015.12783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leong SP, Shen ZZ, Liu TJ, Agarwal G, Tajima T, Paik NS, et al. Is breast cancer the same disease in Asian and Western countries? World J Surg. 2010;34:2308–2324. doi: 10.1007/s00268-010-0683-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Youlden DR, Cramb SM, Yip CH, Baade PD. Incidence and mortality of female breast cancer in the Asia-Pacific region. Cancer Biol Med. 2014;11:101–115. doi: 10.7497/j.issn.2095-3941.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang JY, Han BK, Ko EY, Shin JH, Hahn SY, Nam MY. Screening ultrasound in women with negative mammography: outcome analysis. Yonsei Med J. 2015;56:1352–1358. doi: 10.3349/ymj.2015.56.5.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Youn I, Choi S, Kook SH, Choi YJ. Mammographic breast density evaluation in Korean women using fully automated volumetric assessment. J Korean Med Sci. 2016;31:457–462. doi: 10.3346/jkms.2016.31.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolb TM, Lichy J, Newhouse JH. Comparison of the performance of screening mammography, physical examination, and breast US and evaluation of factors that influence them: an analysis of 27,825 patient evaluations. Radiology. 2002;225:165–175. doi: 10.1148/radiol.2251011667. [DOI] [PubMed] [Google Scholar]
- 11.Rebolj M, Assi V, Brentnall A, Parmar D, Duffy SW. Addition of ultrasound to mammography in the case of dense breast tissue: systematic review and meta-analysis. Br J Cancer. 2018;118:1559–1570. doi: 10.1038/s41416-018-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tagliafico AS, Calabrese M, Mariscotti G, Durando M, Tosto S, Monetti F, et al. Adjunct screening with tomosynthesis or ultrasound in women with mammography-negative dense breasts: interim report of a prospective comparative trial. J Clin Oncol. 2016;34:1882–1888. doi: 10.1200/JCO.2015.63.4147. [DOI] [PubMed] [Google Scholar]
- 13.Zanello PA, Robim AFC, de Oliveira TMG, Elias Junior J, de Andrade JM, Monteiro CR, et al. Breast ultrasound diagnostic performance and outcomes for mass lesions using Breast Imaging Reporting and Data System category 0 mammogram. Clinics (Sao Paulo) 2011;66:443–448. doi: 10.1590/S1807-59322011000300014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SY, Han BK, Kim EK, Choi WJ, Choi Y, Kim HH, et al. Breast cancer detected at screening US: survival rates and clinical-pathologic and imaging factors associated with recurrence. Radiology. 2017;284:354–364. doi: 10.1148/radiol.2017162348. [DOI] [PubMed] [Google Scholar]
- 15.Sickles EA, D'Oris CJ, Bassett LW, et al. ACR BI-RADS® mammography. In: D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA, editors. ACR BI-RADS® atlas, breast imaging reporting and data system. 5th ed. Reston: American College of Radiology; 2013. pp. 123–132. [Google Scholar]
- 16.Greene FL, Balch CM, Fleming ID, Fritz A, Haller DG, Morrow M, et al. AJCC cancer staging handbook: TNM classification of malignant tumors. 6th ed. New York: Springer Science & Business Media; 2002. pp. 221–240. [Google Scholar]
- 17.Allred DC, Bustamante MA, Daniel CO, Gaskill HV, Cruz AB., Jr Immunocytochemical analysis of estrogen receptors in human breast carcinomas: evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg. 1990;125:107–113. doi: 10.1001/archsurg.1990.01410130113018. [DOI] [PubMed] [Google Scholar]
- 18.Wolff AC, Hammond MEH, Hicks DG, Dowsett M, McShane LM, Allison KH, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. Arch Pathol Lab Med. 2014;138:241–256. doi: 10.5858/arpa.2013-0953-SA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corsetti V, Houssami N, Ferrari A, Ghirardi M, Bellarosa S, Angelini O, et al. Breast screening with ultrasound in women with mammography-negative dense breasts: evidence on incremental cancer detection and false positives, and associated cost. Eur J Cancer. 2008;44:539–544. doi: 10.1016/j.ejca.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Ohuchi N, Suzuki A, Sobue T, Kawai M, Yamamoto S, Zheng YF, et al. Sensitivity and specificity of mammography and adjunctive ultrasonography to screen for breast cancer in the Japan Strategic Anti-cancer Randomized Trial (J-START): a randomised controlled trial. Lancet. 2016;387:341–348. doi: 10.1016/S0140-6736(15)00774-6. [DOI] [PubMed] [Google Scholar]
- 21.Moon HJ, Jung I, Park SJ, Kim MJ, Youk JH, Kim EK. Comparison of cancer yields and diagnostic performance of screening mammography vs. supplemental screening ultrasound in 4394 women with average risk for breast cancer. Ultraschall Med. 2015;36:255–263. doi: 10.1055/s-0034-1366288. [DOI] [PubMed] [Google Scholar]
- 22.Chang JM, Koo HR, Moon WK. Radiologist-performed hand-held ultrasound screening at average risk of breast cancer: results from a single health screening center. Acta Radiol. 2015;56:652–658. doi: 10.1177/0284185114538252. [DOI] [PubMed] [Google Scholar]
- 23.Early Breast Cancer Trialists' Collaborative Group (EBCTCG) Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366:2087–2106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 24.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med. 2003;138:168–175. doi: 10.7326/0003-4819-138-3-200302040-00008. [DOI] [PubMed] [Google Scholar]
- 25.Jeon JH, Kang JH, Kim Y, Lee HY, Choi KS, Jun JK, et al. Reproductive and hormonal factors associated with fatty or dense breast patterns among Korean women. Cancer Res Treat. 2011;43:42–48. doi: 10.4143/crt.2011.43.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park B, Cho HM, Lee EH, Song S, Suh M, Choi KS, et al. Does breast density measured through population-based screening independently increase breast cancer risk in Asian females? Clin Epidemiol. 2018;10:61–70. doi: 10.2147/CLEP.S144918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Webb ML, Cady B, Michaelson JS, Bush DM, Calvillo KZ, Kopans DB, et al. A failure analysis of invasive breast cancer: most deaths from disease occur in women not regularly screened. Cancer. 2014;120:2839–2846. doi: 10.1002/cncr.28199. [DOI] [PubMed] [Google Scholar]
- 28.James TA, Wade JE, Sprague BL. The impact of mammographic screening on the surgical management of breast cancer. J Surg Oncol. 2016;113:496–500. doi: 10.1002/jso.24184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Therapeutic and Pathologic Characteristics of the US and Mammography Group