Abstract
Objective
Muscle depletion in patients undergoing liver transplantation affects the recipients' prognosis and therefore cannot be overlooked. We aimed to evaluate whether changes in muscle and fat mass during the preoperative period are associated with prognosis after deceased donor liver transplantation (DDLT).
Materials and Methods
This study included 72 patients who underwent DDLT and serial computed tomography (CT) scans. Skeletal muscle index (SMI) and fat mass index (FMI) were calculated using the muscle and fat area in CT performed 1 year prior to surgery (1 yr Pre-LT), just before surgery (Pre-LT), and after transplantation (Post-LT). Simple aspects of serial changes in muscle and fat mass were analyzed during three measurement time points. The rate of preoperative changes in body composition parameters were calculated (preoperative ΔSMI [%] = [SMI at Pre-LT − SMI at 1 yr Pre-LT] / SMI at Pre-LT × 100; preoperative ΔFMI [%] = [FMI at Pre-LT − FMI at 1 yr Pre-LT] / FMI at Pre-LT × 100) and assessed for correlation with patient survival.
Results
SMI significantly decreased during the preoperative period (mean preoperative ΔSMI, −13.04%, p < 0.001). In the multivariable analysis, preoperative ΔSMI (p = 0.016) and model for end-stage liver disease score (p = 0.011) were independent prognostic factors for overall survival. The mean survival time for patients with a threshold decrease in the preoperative ΔSMI (≤ −30%) was significantly shorter than for other patients (p = 0.007). Preoperative ΔFMI was not a prognostic factor but FMI increased during the postoperative period (p = 0.009) in all patients.
Conclusion
A large reduction in preoperative SMI was significantly associated with reduced survival after DDLT. Therefore, changes in muscle mass during the preoperative period can be considered as a prognostic factor for survival after DDLT.
Keywords: Liver transplantation, Sarcopenia, Computed tomography, Preoperative period, Survival
INTRODUCTION
Sarcopenia, defined as severe depletion of skeletal muscle mass and function (1), is one of the most common complications of cirrhosis and is related to adverse clinical outcomes (2,3,4,5). Multiple factors can induce sarcopenia in patients with cirrhosis including decreases in the substance for producing muscle caused by poor oral intake, malabsorption, depleted glycogen stores, and impaired protein synthesis (3,6). In particular, muscle depletion can be aggravated immediately after liver transplantation due to malnutrition during the perioperative period, surgical stress, postsurgical complications, immunosuppressive treatment, and postoperative impairment of protein metabolism (7).
The model for end-stage liver disease (MELD) score is the most common way to prioritize liver transplantation among patients with chronic liver disease (8). Despite its strong predictive value, it is unable to reflect a patient's nutritional status, which is a serious limitation of this score (9,10). Although many efforts have been made to complement the original MELD score system, the integration of nutritional status into existing prognostic models is still limited and ineffective (11). Recently, cross-sectional imaging techniques, typically abdominal computed tomography (CT), have been considered as standard references for measuring muscle mass to evaluate sarcopenia in candidates for liver transplantation. Moreover, CT scans are one of the routine examinations for preoperative evaluation and postoperative management in most liver transplant candidates. The association between image-diagnosed sarcopenia before liver transplantation and survival rate has been reported from many transplantation centers (12,13,14,15,16,17), as well as in a recent meta-analysis study where sarcopenia was significantly associated with post-transplantation mortality (18).
However, from a longitudinal perspective, it is more advantageous to investigate changes in muscle mass in the preoperative period as overcoming the limitations of image-diagnosed sarcopenia from the single cross-sectional evaluation is possible (19,20). Moreover, muscle wasting can be progressive in the patients with advanced, decompensated liver cirrhosis and is associated with mortality (21,22). Thus, we hypothesized that evaluating serial changes in the muscle mass of patients on the waiting list for transplantation would be more helpful in differentiating progressive sarcopenia from stationary muscle depletion and predicting the survival of patients after transplantation, compared to a single measurement of muscle mass just before surgery. Therefore, the present study aimed to evaluate whether changes in muscle and fat mass (FM) during the preoperative period are associated with prognosis after deceased donor liver transplantation (DDLT).
MATERIALS AND METHODS
Patients
The Institutional Review Board of our institution approved this retrospective study (IRB No. 2015-10-191) and the informed consent requirement could be waived.
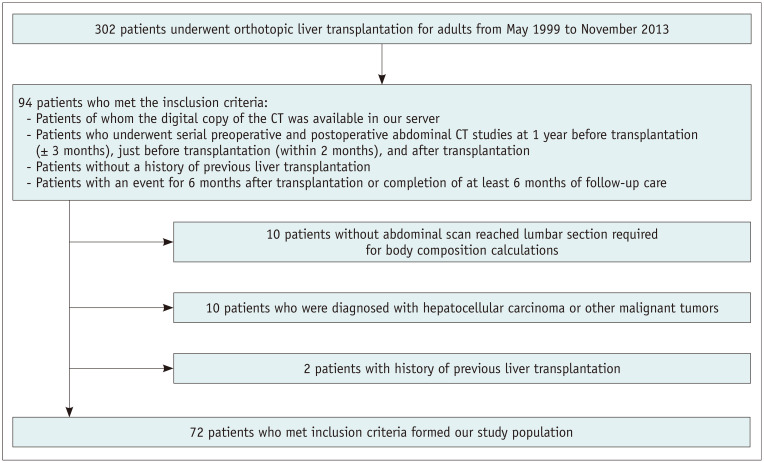
A total of 302 adults underwent DDLT from May 1999 to November 2013 at Samsung Medical Center (Seoul, Republic of Korea). Among these patients, the study population was selected according to the following inclusion criteria: 1) patients of whom a digital copy of the CT was available on our server; 2) patients who underwent serial preoperative and postoperative abdominal CT studies at least 1 year before transplantation (1 year ± 3 months; 1 yr Pre-LT), just before transplantation (within 2 months; Pre-LT), and after transplantation (Post-LT); and 3) patients who had an event less than 6 months after transplantation or completed at least 6 months of follow-up after transplantation. Exclusion criteria were as follows: 1) the abdominal CT scans were inadequate for analysis due to lack of lumbar section; 2) the patients were preoperatively diagnosed with hepatocellular carcinoma or other malignant tumors; or 3) the patients had a history of previous liver transplantation. Among the patients who met the inclusion criteria (n = 94), a total of 72 patients (mean age, 53.2 years ± 8.7; range, 41–70 years), including 52 males (mean age, 52.5 years ± 8.8; range, 41–70 years) and 20 females (mean age, 53.0 years ± 7.6; range, 41–66 years), were enrolled as our study population (Fig. 1).
Fig. 1. Flow chart showing the inclusion and exclusion criteria of the study.
CT = computed tomography
Clinical and Laboratory Assessments
Variables of interest were obtained from electronic medical records including age, sex, weight, height, body mass index (BMI), Child-Pugh and MELD scores, serum albumin, bilirubin, international normalized ratio, encephalopathy, and etiology of cirrhosis. The ethnicity of all subjects was Asian. Clinical and laboratory data for analyses were acquired within 7 days before DDLT surgery.
CT Scans
Preoperative and postoperative abdominal CT scans (16- to 256-row multidetector CT scanners [Brilliance 40, Philips Healthcare; Lightspeed QX/I, Lightspeed Ultra, Lightspeed VCT XT, and Discovery 750 HD, GE Healthcare; Aquilion 64, Canon Medical System; Somatom Definition Flash, Siemens Healthineers]: 120 or 130 kV, 90–320 mA, field of view 290–460 mm, matrix 256–512, reconstructed slice thickness 3–5 mm) were retrieved from the picture archiving and communication system for image analysis.
Preoperative CT scans classed as 1 yr Pre-LT were performed at a median of 12 months (range, 9–15 months) before DDLT, while preoperative CT scans classed as Pre-LT were performed at a median of 17.5 days (range, 1–60 days) before DDLT. Post-LT CT scans were performed at a median of 13 months (range, 1–63 months) after DDLT.
Image Analyses
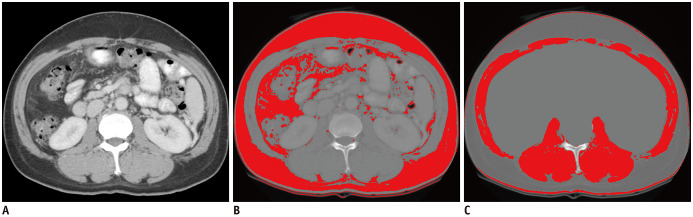
A radiologist (6 years of experience in abdominal imaging) measured the CT imaging data using ImageJ (National Institutes of Health). The third lumbar vertebra (L3), described as the first axial image that clearly demonstrates the spinous process, was selected as the standard landmark for the muscle groups consisting of the psoas, quadratus lumborum, erector spinae, external and internal obliques, transversus abdominis, and rectus abdominis. It has been reported that the assessment of an abdominal CT image at the L3 level can yield reliable results in the measurement of whole-body skeletal muscle mass (23). Muscle area was normalized to height and is reported as the skeletal muscle index (SMI) (cm2/m2). Semi-automated demarcation of specific regions was conducted to measure the cross-sectional muscle and fat areas using the following Hounsfield unit thresholds on portal venous phase images: 8 to 100 (skeletal muscle) and −300 to −50 (visceral and subcutaneous fat), as published previously (Fig. 2) (24).
Fig. 2. An example of body composition analysis using a CT scan.
Semi-automated demarcation of a specific region was conducted to measure the cross-sectional muscle and fat areas using the following HU thresholds: 8 to 100 (skeletal muscle) and −300 to −50 (visceral and subcutaneous fat). Initially, segmentation of fat was automatically performed, and the area of fat was measured. The line was manually drawn along the abdominal cavity to remove the internal area in which a substance with HU similar to skeletal muscle, such as a solid organ, exists (not shown). Then, segmentation of the skeletal muscle area was also automatically performed and the area of skeletal muscle was measured.
A. Cross-sectional CT images at the L3 transverse section. B. Total fat area. C. Skeletal muscle area. HU = Hounsfield unit
The total FM was acquired by the regression equations below, and the FM was normalized to height and expressed as FM index (FMI) (kg/m2), as reported elsewhere (25,26):
| Total body FM (kg) = 0.042 × (total adipose tissue at L3 [cm2]) + 11.2 |
| FMI (kg/m2) = Total body FM / (height × height [m2]) |
SMI and FMI were calculated with the muscle and fat area in the CT images at 1 yr Pre-LT, Pre-LT, and Post-LT. Serial changes in muscle and FM were analyzed during three measurement time points.
The rate of preoperative changes in body composition parameters were also calculated using CT images for correlation with patient survival:
| Preoperative ΔSMI (%) = ([SMI at Pre-LT − SMI at 1 yr Pre-LT] / SMI at Pre-LT) × 100 |
| Preoperative ΔFMI (%) = ([FMI at Pre-LT − FMI at 1 yr Pre-LT] / FMI at Pre-LT) × 100 |
Evaluation of Overall Survival
Overall survival time was calculated from the date of transplantation to the date of death due to disease-related cause. Likewise, for patients who were alive during the last assessment period, overall survival time was calculated from the date of transplantation to the date of the last visit of our clinic.
Statistical Analysis
Percentage changes in serial preoperative and postoperative SMI and FMI were computed. To compare percentage changes in SMI and FMI, paired t tests were performed for both the preoperative and postoperative periods. Repeated measures ANOVA was used to analyze the within-subject effect of parameters at 1 yr Pre-LT, Pre-LT, and Post-LT. Subgroup analysis was performed to evaluate the between-subject effect between patients who died and those who survived.
Univariable and multivariable Cox proportional hazard regression analyses were performed to determine prognostic factors for overall survival including the following characteristics: age, sex, BMI, encephalopathy, Child-Pugh score, preoperative SMI, preoperative FMI, preoperative ΔSMI, and preoperative ΔFMI.
To select the optimal cutoff values for statistically significant parameters that classify the poor prognostic group after DDLT, the Youden index of the receiver operating characteristic curve was calculated (27).
Overall survival rates of the two groups based on preoperative ΔSMI (a group with a reduction greater than the threshold value and another group with a reduction less than the threshold value) were estimated using the Kaplan-Meier method, and differences in survival between the two groups were compared using the log-rank test.
All statistical analyses were performed using SAS version 9.4 (SAS Institute) and MedCalc version 18 (MedCalc Software). The significance level was set as a p value less than 0.05.
RESULTS
Patient Characteristics
A summary of clinical, demographic, and biochemical characteristics of patients is given in Table 1. There were 56 patients (77.8%; 43 males [82.7%] and 13 females [65%]) who were sarcopenic at 1 yr Pre-LT, and this number increased to 71 patients (98.6%; 52 males [100%] and 19 females [95%]) at Pre-LT. All patients were sarcopenic after DDLT. The median follow-up period for all patients was 37.7 months (range, 6.4–151.3 months). Fifty-five (76.4%) of the 72 patients were alive (median follow-up period, 68.0 months; range, 15.1–151.3 months) and 17 (23.6%) of the 72 patients died (median follow-up period, 14.5 months; range, 6.4–69.5 months).
Table 1. Baseline Characteristics of Patients.
| Characteristics | Number of Patients (n = 72) |
|---|---|
| Age (years)* | 53.2 ± 8.7 |
| Sex | |
| Male | 52 (72) |
| Female | 20 (28) |
| Weight (kg)* | 69.1 ± 17.0 |
| Height (cm)* | 165.6 ± 8.6 |
| BMI (kg/m2)* | 25.1 ± 5.3 |
| MELD score* | 21.1 ± 9.0 |
| Child-Pugh score | |
| A | 2 (3) |
| B | 32 (44) |
| C | 38 (53) |
| Albumin (g/L)* | 2.9 ± 0.4 |
| Bilirubin (μmol/L)* | 10.0 ± 12.8 |
| INR* | 1.9 ± 1.0 |
| Encephalopathy | 15 (21) |
| Etiology of cirrhosis | |
| HBV | 52 (72) |
| HCV | 9 (13) |
| HBV + HCV | 1 (1) |
| Alcohol | 2 (3) |
| Others† | 8 (11) |
Unless otherwise specified, data are number of patients with percentage in parentheses. *Data are mean ± SD, †Includes autoimmune liver diseases, primary sclerosing cholangitis, and Budd-Chiari syndrome. BMI = body mass index, HBV = hepatitis B virus, HCV = hepatitis C virus, INR = international normalized ratio, MELD = model for end-stage liver disease, SD = standard deviation
Serial Changes in Body Composition Parameters during the 3 Measurement Time Points
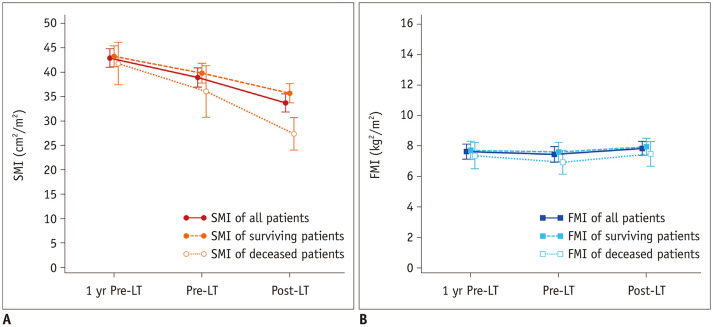
Both the SMI and FMI showed significant changes over time (p < 0.001) (Fig. 3A). Serial changes in the body composition parameters before and after DDLT are summarized in Table 2. During the preoperative period, the change in the SMI was −4.0 ± 5.5 cm2/m2 (p < 0.001) and −1.6 ± 2.2 cm2/m2 during the postoperative period (p < 0.001). In subgroup analysis, the SMI declined more in the deceased patients compared to the surviving patients during both the preoperative and postoperative periods (preoperative −5.7 ± 8.4 cm2/m2 [p = 0.013] vs. −3.5 ± 4.2 cm2/m2 [p < 0.001]) and postoperative −8.7 ± 11.6 cm2/m2 [p = 0.007] vs. −4.1 ± 5.2 cm2/m2 [p < 0.001], respectively) (Fig. 3B). There was also a decrease in the FMI during the preoperative period, but the result was not statistically significant (−0.1 ± 0.9 kg/m2, p = 0.089). There was however, a significant increase in the FMI during the postoperative period in all patients (0.4 ± 1.2 kg/m2, p = 0.009). The mean preoperative ΔSMI was −13.04% (range, −62.02–11.35%) and preoperative ΔFMI was −13.86% (range, −91.25–63.24%).
Fig. 3. Serial changes in body composition parameters of patients.
Patients underwent preoperative and postoperative abdominal CT studies at 1 yr Pre-LT (± 3 months), Pre-LT (within 2 months), and Post-LT with subgroup analysis between deceased and surviving patients.
A. Serial changes in SMI. B. Serial changes in FMI. FMI = fat mass index, Post-LT = after transplantation, Pre-LT = just before transplantation, SMI = skeletal mass index, 1 yr Pre-LT = 1 year before transplantation
Table 2. Serial Changes in Body Composition Parameters.
| Group | Parameters | Mean (SD) | Change (SD) | 95% CI | P |
|---|---|---|---|---|---|
| SMI (cm2/m2) | |||||
| All (n = 72) | 1 yr Pre-LT | 42.9 (8.0) | −4.0 (5.5)* −5.2 (7.4)† |
−5.28– −2.69* −6.95– −3.47† |
< 0.001* < 0.001† |
| Pre-LT | 38.9 (8.3) | ||||
| Post-LT | 33.7 (7.9) | ||||
| Surviving (n = 55) | 1 yr Pre-LT | 43.3 (7.9) | −3.5 (4.2)* −4.1 (5.2)† |
−4.58– −2.30* −5.54– −2.73† |
< 0.001* < 0.001† |
| Pre-LT | 39.8 (7.5) | ||||
| Post-LT | 35.7 (7.3) | ||||
| Deceased (n = 17) | 1 yr Pre-LT | 41.8 (8.5) | −5.7 (8.4)* −8.7 (11.6)† |
−10.06– −1.41* −14.66– −2.73† |
0.013* 0.007† |
| Pre-LT | 36.1 (10.3) | ||||
| Post-LT | 27.4 (6.5) | ||||
| FMI (kg/m2) | |||||
| All (n = 72) | 1 yr Pre-LT | 7.6 (2.1) | −0.1 (0.9)* 0.4 (1.2)† |
−0.40–0.03* 0.10–0.68† |
0.089* 0.009† |
| Pre-LT | 7.5 (2.2) | ||||
| Post-LT | 7.9 (2.0) | ||||
| Surviving (n = 55) | 1 yr Pre-LT | 7.7 (2.2) | −0.1 (0.9)* 0.3 (1.3)† |
−0.34–0.12* −0.01–0.70† |
0.346* 0.050† |
| Pre-LT | 7.6 (2.3) | ||||
| Post-LT | 7.9 (2.1) | ||||
| Deceased (n = 17) | 1 yr Pre-LT | 7.4 (1.7) | −0.5 (1.1)* 0.6 (1.1)† |
−0.97–0.12* −0.01–1.09† |
0.117* 0.052† |
| Pre-LT | 6.9 (1.5) | ||||
| Post-LT | 7.5 (1.6) |
*Pre-LT − 1 yr Pre-LT, †Post-LT − Pre-LT. CI = confidence interval, FMI = fat mass index, Post-LT = after transplantation, Pre-LT = just before transplantation, SMI = skeletal muscle index, 1 yr Pre-LT = 1 year before transplantation
Predicting Factors Associated with Overall Survival after DDLT
Univariable Cox analysis showed that MELD score (p = 0.006) and preoperative ΔSMI (p = 0.006) were associated with overall survival after DDLT. Additional assessment through multivariable analysis using the Cox proportional hazard model showed that the preoperative ΔSMI (p = 0.016) and MELD score (p = 0.011) were independent prognostic factors for overall survival after DDLT (Table 3).
Table 3. Univariable and Multivariable Analyses for the Prediction of Overall Survival after Liver Transplantation.
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio | 95% CI | P | Hazard Ratio | 95% CI | P | |
| Age | 0.973 | 0.923–1.025 | 0.298 | |||
| Sex | 0.957 | 0.337–2.718 | 0.935 | |||
| BMI | 0.937 | 0.812–1.081 | 0.372 | |||
| MELD score | 1.064 | 1.018–1.112 | 0.006 | 1.062 | 1.014–1.114 | 0.011 |
| Encephalopathy | 1.456 | 0.593–3.572 | 0.413 | |||
| Child-Pugh score | 1.776 | 0.687–4.591 | 0.236 | |||
| Preoperative SMI* | 0.951 | 0.893–1.012 | 0.115 | |||
| Preoperative FMI* | 0.896 | 0.692–1.160 | 0.381 | |||
| Preoperative ΔSMI† | 0.242 | 0.088–0.664 | 0.006 | 0.284 | 0.102–0.789 | 0.016 |
| Preoperative ΔFMI† | 0.090 | 0.002–4.480 | 0.236 | |||
*Pre-LT, †Pre-LT − 1 yr Pre-LT.
Overall Survival Rates after DDLT according to the Cutoff Value of the Preoperative ΔSMI
Using the Youden index, 30% of the preoperative ΔSMI was determined as the optimal cutoff value. The mean survival times for patients with a large decrease in the preoperative ΔSMI (preoperative ΔSMI ≤ −30%; n = 7) and a small decrease or increase in the preoperative ΔSMI (preoperative ΔSMI > −30%; n = 65) were 47.7 ± 17.7 months (range, 2.2–101.7 months) and 121.6 ± 7.3 months (range, 1.4–151.3 months), respectively (p = 0.007). The 1- and 3-year overall survival rates after DDLT were 57.1% and 42.9% for patients with a large decrease in the preoperative ΔSMI, and 89.2% and 80.2% for patients with a small decrease or increase in the preoperative ΔSMI, respectively (Fig. 4).
Fig. 4. Comparison of survival curves in relation to the preoperative ΔSMI in patients with deceased donor liver transplantation.
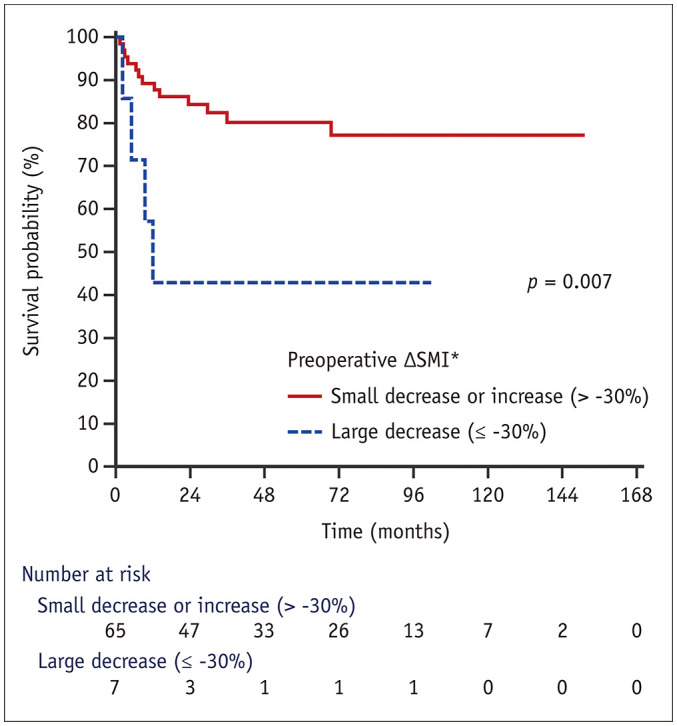
The overall survival rates of the patients with a large decrease in the preoperative ΔSMI was significantly lower than those with a small decrease or increase in the preoperative ΔSMI.
*Pre-LT − 1yr Pre-LT.
DISCUSSION
In this study, we investigated the changes in skeletal muscle and fat during the pre- and postoperative periods of DDLT and how these preoperative changes affect the post-transplantation outcomes. We determined that the SMI of patients who underwent transplantation significantly decreased during the preoperative and postoperative periods. In the analysis of our study, the SMI before and after transplantation were reduced more in the deceased patients than the surviving patients. In multivariable analysis, the preoperative ΔSMI was an independent prognostic factor for overall survival and the mean survival time for patients with a threshold decrease in the preoperative ΔSMI (≤ −30%) was significantly shorter than for other patients. This suggests that a large decrease in the SMI has a negative impact on the postoperative outcome. Therefore, it is plausible that the severity of sarcopenia is closely related to a patient's poor postoperative prognosis, which supports our hypothesis. It is therefore important for clinicians to examine serial changes in muscle mass during the preoperative period.
As a result of our study, the MELD score was identified as a statistically significant prognostic factor for overall survival after DDLT, which is well known (28). However, the drawback of the MELD score is the lack of an objective parameter assessing the physical and nutritional status of a patient. Studies have attempted to overcome this limitation by showing that the modified MELD score, which includes sarcopenia, is superior to predicting mortality than the MELD score itself in cirrhotic patients (29,30). Since our results show that the preoperative ΔSMI is an independent prognostic factor for recipients of DDLT, it is expected that the preoperative ΔSMI could play a role in liver allocation, in conjunction with the MELD score, in the further studies.
The results of our study could support the need for active treatment to prevent muscle mass depletion prior to transplantation. Some studies have reported that increased muscle mass contributes to postoperative survival in patients with cirrhosis (31). If a patient with cirrhosis has serious sarcopenia, it is necessary to treat this in advance of the surgery and to revolve the transplantation and intensive postoperative care around the sarcopenia.
Interestingly, our results showed that the FMI significantly increased during the postoperative period, but there was no correlation with mortality after transplantation. A few studies have reported that postoperative weight gain is due to increased fat tissue while muscle mass decreases (32,33,34). The etiology of altering postoperative body composition is uncertain, but several possible mechanisms can be inferred. One potential cause is that there is a steady increase in resting energy expenditure after liver transplantation in cirrhotic patients, which may contribute to post-transplant sarcopenia. Immunosuppressants interfere with the recovery of skeletal muscle after transplantation as they increase the risk of infection and change skeletal muscle protein metabolism. Corticosteroids compromise protein synthesis and are responsible for proteolysis and therefore play an important role in FM increase, by increasing appetite and fat accumulation and reducing fat oxidation. Calcineurin inhibitors can affect muscle mass and energy metabolism (35,36). Therefore, it can be assumed that these various contributors cause a decline in muscle mass and increment in FM in the short term after liver transplantation.
There are some limitations in this study. Firstly, this was a retrospective, single center study. The period of follow up for the CT scans was not exactly the same in all patients and the size of the sample was reduced by selecting a case that matched the condition. Although the CT scan time was not fixed and showed severe temporal deviation in the postoperative period, it was not used to analyze prognostic factors, but only to assess the trends in the changes of muscle and FM. Secondly, as our study evaluated the association between skeletal muscle mass and the prognosis of patients undergoing liver transplantation without considering muscle function, we are only able to report on “skeletal muscle mass” rather than sarcopenia, since the definition of sarcopenia is not only a reduction of the skeletal muscle mass, but also muscle function. Future studies should include measurements of muscle function as well as muscle mass. As in recent studies, the method of assessing muscle quality using sonoelastography could be one approach (37). Thirdly, we emphasized the importance of serial changes in muscle mass and presented a cutoff value, but this may not have relevance to other medical centers and populations. Ideally, clinical trials should investigate a range of cutoff values that can be applied to various populations of age, sex, ethnicities, and diseases. However, since these studies are difficult to carry out, meta-analysis, including subsequent large-scale observational studies reported in various regions, can be used as an alternative to identify a more accurate cutoff value.
In conclusion, muscle mass was significantly and continuously decreased on preoperative and postoperative CT scans in patients who underwent DDLT. A large reduction in the preoperative ΔSMI, in other words, a more severe decrease of muscle mass, was significantly associated with a reduction in overall survival after DDLT. Therefore, changes in muscle mass during the preoperative period can be considered a prognostic factor for outcomes of DDLT.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasarathy S. Consilience in sarcopenia of cirrhosis. J Cachexia Sarcopenia Muscle. 2012;3:225–237. doi: 10.1007/s13539-012-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montano-Loza AJ, Meza-Junco J, Prado CM, Lieffers JR, Baracos VE, Bain VG, et al. Muscle wasting is associated with mortality in patients with cirrhosis. Clin Gastroenterol Hepatol. 2012;10:166–173.e1. doi: 10.1016/j.cgh.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 4.Periyalwar P, Dasarathy S. Malnutrition in cirrhosis: contribution and consequences of sarcopenia on metabolic and clinical responses. Clin Liver Dis. 2012;16:95–131. doi: 10.1016/j.cld.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gu DH, Kim MY, Seo YS, Kim SG, Lee HA, Kim TH, et al. Clinical usefulness of psoas muscle thickness for the diagnosis of sarcopenia in patients with liver cirrhosis. Clin Mol Hepatol. 2018;24:319–330. doi: 10.3350/cmh.2017.0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dasarathy S, Merli M. Sarcopenia from mechanism to diagnosis and treatment in liver disease. J Hepatol. 2016;65:1232–1244. doi: 10.1016/j.jhep.2016.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Merli M, Lucidi C, Giannelli V, Giusto M, Riggio O, Falcone M, et al. Cirrhotic patients are at risk for health care-associated bacterial infections. Clin Gastroenterol Hepatol. 2010;8:979–985. doi: 10.1016/j.cgh.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 8.Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: strengths and weaknesses. J Hepatol. 2011;54:1297–1306. doi: 10.1016/j.jhep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Myers RP, Shaheen AA, Faris P, Aspinall AI, Burak KW. Revision of MELD to include serum albumin improves prediction of mortality on the liver transplant waiting list. PLoS One. 2013;8:e51926. doi: 10.1371/journal.pone.0051926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montano-Loza AJ. Clinical relevance of sarcopenia in patients with cirrhosis. World J Gastroenterol. 2014;20:8061–8071. doi: 10.3748/wjg.v20.i25.8061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014;18:179–190. doi: 10.1016/j.cld.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, et al. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–1556. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 13.Krell RW, Kaul DR, Martin AR, Englesbe MJ, Sonnenday CJ, Cai S, et al. Association between sarcopenia and the risk of serious infection among adults undergoing liver transplantation. Liver Transpl. 2013;19:1396–1402. doi: 10.1002/lt.23752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Masuda T, Shirabe K, Ikegami T, Harimoto N, Yoshizumi T, Soejima Y, et al. Sarcopenia is a prognostic factor in living donor liver transplantation. Liver Transpl. 2014;20:401–407. doi: 10.1002/lt.23811. [DOI] [PubMed] [Google Scholar]
- 15.Montano-Loza AJ, Meza-Junco J, Baracos VE, Prado CM, Ma M, Meeberg G, et al. Severe muscle depletion predicts postoperative length of stay but is not associated with survival after liver transplantation. Liver Transpl. 2014;20:640–648. doi: 10.1002/lt.23863. [DOI] [PubMed] [Google Scholar]
- 16.Valero V, 3rd, Amini N, Spolverato G, Weiss MJ, Hirose K, Dagher NN, et al. Sarcopenia adversely impacts postoperative complications following resection or transplantation in patients with primary liver tumors. J Gastrointest Surg. 2015;19:272–281. doi: 10.1007/s11605-014-2680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uchiyama H. Sarcopenia in liver transplant recipients: its relevance to peritransplant morbidity and mortality. Hepatobiliary Surg Nutr. 2017;6:196–199. doi: 10.21037/hbsn.2017.03.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Vugt JL, Levolger S, de Bruin RW, van Rosmalen J, Metselaar HJ, IJzermans JN. Systematic review and meta-analysis of the impact of computed tomography-assessed skeletal muscle mass on outcome in patients awaiting or undergoing liver transplantation. Am J Transplant. 2016;16:2277–2292. doi: 10.1111/ajt.13732. [DOI] [PubMed] [Google Scholar]
- 19.Kim G, Kang SH, Kim MY, Baik SK. Prognostic value of sarcopenia in patients with liver cirrhosis: a systematic review and meta-analysis. PLoS One. 2017;12:e0186990. doi: 10.1371/journal.pone.0186990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levolger S, van Vugt JL, de Bruin RW, IJzermans JN. Systematic review of sarcopenia in patients operated on for gastrointestinal and hepatopancreatobiliary malignancies. Br J Surg. 2015;102:1448–1458. doi: 10.1002/bjs.9893. [DOI] [PubMed] [Google Scholar]
- 21.Praktiknjo M, Book M, Luetkens J, Pohlmann A, Meyer C, Thomas D, et al. Fat-free muscle mass in magnetic resonance imaging predicts acute-on-chronic liver failure and survival in decompensated cirrhosis. Hepatology. 2018;67:1014–1026. doi: 10.1002/hep.29602. [DOI] [PubMed] [Google Scholar]
- 22.Kalafateli M, Konstantakis C, Thomopoulos K, Triantos C. Impact of muscle wasting on survival in patients with liver cirrhosis. World J Gastroenterol. 2015;21:7357–7361. doi: 10.3748/wjg.v21.i24.7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shen W, Punyanitya M, Wang Z, Gallagher D, St-Onge MP, Albu J, et al. Total body skeletal muscle and adipose tissue volumes: estimation from a single abdominal cross-sectional image. J Appl Physiol (1985) 2004;97:2333–2338. doi: 10.1152/japplphysiol.00744.2004. [DOI] [PubMed] [Google Scholar]
- 24.Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord. 1996;20:570–573. [PubMed] [Google Scholar]
- 25.Mourtzakis M, Prado CM, Lieffers JR, Reiman T, McCargar LJ, Baracos VE. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl Physiol Nutr Metab. 2008;33:997–1006. doi: 10.1139/H08-075. [DOI] [PubMed] [Google Scholar]
- 26.Yip C, Goh V, Davies A, Gossage J, Mitchell-Hay R, Hynes O, et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 27.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 28.Kim HJ, Lee HW. Important predictor of mortality in patients with end-stage liver disease. Clin Mol Hepatol. 2013;19:105–115. doi: 10.3350/cmh.2013.19.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Vugt JLA, Alferink LJM, Buettner S, Gaspersz MP, Bot D, Darwish Murad S, et al. A model including sarcopenia surpasses the MELD score in predicting waiting list mortality in cirrhotic liver transplant candidates: a competing risk analysis in a national cohort. J Hepatol. 2018;68:707–714. doi: 10.1016/j.jhep.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 30.Montano-Loza AJ, Duarte-Rojo A, Meza-Junco J, Baracos VE, Sawyer MB, Pang JXQ, et al. Inclusion of sarcopenia within MELD (MELD-Sarcopenia) and the prediction of mortality in patients with cirrhosis. Clin Transl Gastroenterol. 2015;6:e102. doi: 10.1038/ctg.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsien C, Garber A, Narayanan A, Shah SN, Barnes D, Eghtesad B, et al. Post-liver transplantation sarcopenia in cirrhosis: a prospective evaluation. J Gastroenterol Hepatol. 2014;29:1250–1257. doi: 10.1111/jgh.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giusto M, Lattanzi B, Di Gregorio V, Giannelli V, Lucidi C, Merli M. Changes in nutritional status after liver transplantation. World J Gastroenterol. 2014;20:10682–10690. doi: 10.3748/wjg.v20.i31.10682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jeon JY, Wang HJ, Ock SY, Xu W, Lee JD, Lee JH, et al. Newly Developed sarcopenia as a prognostic factor for survival in patients who underwent liver transplantation. PLoS One. 2015;10:e0143966. doi: 10.1371/journal.pone.0143966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaido T, Tamai Y, Hamaguchi Y, Okumura S, Kobayashi A, Shirai H, et al. Effects of pretransplant sarcopenia and sequential changes in sarcopenic parameters after living donor liver transplantation. Nutrition. 2017;33:195–198. doi: 10.1016/j.nut.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 35.van den Ham EC, Kooman JP, Christiaans MH, Leunissen KM, van Hooff JP. Posttransplantation weight gain is predominantly due to an increase in body fat mass. Transplantation. 2000;70:241–242. [PubMed] [Google Scholar]
- 36.Sakuma K, Yamaguchi A. The functional role of calcineurin in hypertrophy, regeneration, and disorders of skeletal muscle. J Biomed Biotechnol. 2010;2010:721219. doi: 10.1155/2010/721219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klauser AS, Miyamoto H, Bellmann-Weiler R, Feuchtner GM, Wick MC, Jaschke WR. Sonoelastography: musculoskeletal applications. Radiology. 2014;272:622–633. doi: 10.1148/radiol.14121765. [DOI] [PubMed] [Google Scholar]