Abstract
Background and aim
Maclura cochinchinensis (MC) (Lour.) heartwood extract have been used traditionally to treat diabetes in Thailand, but their mechanism of action has not been elucidated.
Experimental procedure
This study investigated the effects of an aqueous heartwood extract of MC on α-glucosidase and α-amylase activities. Moreover, its antidiabetic effect was evaluated using an oral glucose tolerance test (OGTT) and a 28-day administration to streptozotocin (STZ)-nicotinamide (NA)-induced type 2 diabetic mice.
Results
In both OGTT and the daily oral administration for 28 days in STZ-NA-induced type 2 diabetic mice models, the extract (1,000 mg/kg) significantly decreased fasting blood glucose. This hypoglycemic effect was explained by increased insulin levels and α-glucosidase inhibition (IC50:1.53 ± 0.03 μg/mL).
Conclusion
This first study on the hypoglycemic activity of MC heartwood extract indicates that it could be a potential natural remedy for type 2 diabetes.
Keywords: Hypoglycemia, α-Glucosidase, α-Amylase, Oral glucose tolerance test (OGTT), Diabetic
Abbreviations: CMC-Na, carboxymethylcellulose; DNSA, 3, 5-dinitrosalicylic acid; FBG, fasting blood glucose; NA, nicotinamide; OGTT, oral glucose tolerance test; pNPG, p-nitrophenyl α-d-glucopyranoside; STZ, streptozotocin
Graphical abstract
Highlights of the findings and novelties
-
•
Maclura cochinchinensis (MC) extract exhibits hypoglycemic activity in STZ-NA-induced diabetic mice.
-
•
TMorin is the major active compound found in the MC heartwood extract.
-
•
The antidiabetic properties of MC extract may be due to the inhibition of α-glucosidase and enhance plasma insulin level.
1. Introduction
Maclura cochinchinensis (Lour.) Corner, a scrambling branched or woody climber, belongs to the Moraceae family. It is widely distributed in many countries in Asia, such as Southern China, Japan, Korea, Taiwan, India, and Thailand.1 The heartwood extract of M. cochinchinensis (MC) has listed in the Thai National List of Essential Medicines as a herbal remedy for the postnatal period.2 MC extract contains a variety of naturally occurring substances (e.g., flavonoids, xanthones, triterpenes, steroids, and benzenoids) but morin is known as a major component which possesses antioxidant, anti-inflammatory, and antidiabetic activities.3,4 MC heartwood is listed in polyherbal preparation prepared by boiling with other medicinal plants and recommended as alternative therapy for diabetes patients.1 However, pharmacological mechanisms to support its clinical effects have not been reported so far. The present study was therefore conducted to provide pharmacological insights into the hypoglycemic effect of an aqueous MC heartwood extract. Its inhibition of α-glucosidase and α-amylase activities was quantitatively analyzed in vitro, and the modes of its inhibition against these enzymes were determined. The effect of the extract on fasting glucose levels was examined in a streptozotocin (STZ)-nicotinamide (NA)-induced diabetic mouse model using an oral glucose tolerance test (OGTT). The hypoglycemic effect of the extract after its daily oral administration for 28 days was also investigated in STZ-NA-induced type 2 diabetic mice.
2. Materials and methods
2.1. Identification of plant materials
The heartwoods of M. cochinchinensis (MC) were purchased from a local supplier in Bangkok in August 2016. The samples were identified by comparison with an authentic sample by the authors. The voucher specimens (SCFC-0816-02) were deposited at the Department of Food Chemistry, Faculty of Pharmacy, Mahidol University, Bangkok, Thailand.
2.2. Chemicals
Acarbose, α-amylase from porcine pancreas, α-glucosidase from S. cerevisiae, p-nitrophenyl, morin, streptozotocin, nicotinamide, were purchased from Sigma Aldrich Chemical Co. (St. Louis, MO, USA). Glibenclamide (Daonil®) was obtained from Sanofi-Aventis (Indonesia). All other chemical reagents were of analytical grade and used without further purification.
2.3. Preparation of crude extract
The heartwoods of M. cochinchinensis were dried, ground to coarse powder, and sieved through a mesh #18 sieve. To obtain the extract, dried heartwood powder (50 g) was boiled with distilled water (1,000 mL) for 15 min, then, the liquid part was filtered through a Whatman filter paper no.4. and repeated for 3 times. The filtrate was collected and added with maltodextrin (20%), as a drying aids5 and further treated to dry by using a spray dryer (Büchi mini spray dryer B-290, Switzerland) under the following conditions; inlet air temperature, 150 °C; aspirator rate, 100%; pump rate, 15%; and nozzle cleaning. The percentage yield was calculated, and the prepared MC heartwood extract was stored in an airtight container at −20 °C until use.
2.4. Quantitative phytochemical analysis
The content of morin, as the major bioactive phytochemicals, was determined by a high-performance thin-layer chromatography (HPTLC) following the previous study.6 Briefly, the HPTLC separation was performed on F254 silica gel HPTLC plates (20 × 10 cm, 0.2 mm thickness; Merck, Germany). The plate was developed at room temperature in a CAMAG twin trough chamber, pre-saturated with a mobile phase consisting of toluene: ethyl acetate: formic acid (36: 12: 7, v/v/v). Morin standard solution and MC heartwood extract dissolved in methanol were applied to the HPTLC plate. Morin content in MC heartwood extract was calculated and expressed as % w/w.
2.5. In vivo hypoglycemic effect
2.5.1. Animals
Male ICR mice (Mus musculus) were purchased from the National Laboratory Animal Center (NLAC), Mahidol University. Before starting the experiment, mice were acclimatized for 7 days under a constant 12 h light-dark cycle at controlled temperature (23 ± 2 °C) and humidity (40–70%). Mice had free access to water and a standard pellet diet. The animal protocol for this study was issued by the Institutional Animal Care and Use Committee (MU-PY-IACUC), Mahidol University, with the permission numbers PYT005/2560 and PYSP 006/2018.
2.5.2. Induction of type 2 diabetes
Diabetes was induced in 12 h-fasted mice by pretreatment with a single intraperitoneal injection (i.p.) of 120 mg/kg nicotinamide (NA) dissolved in normal saline, followed 15 min later by a single i. p. injection of 150 mg/kg streptozotocin (STZ) prepared freshly in 0.1 M citrate buffer (pH 4.5).7 To prevent mortality from STZ-induced hypoglycemia, a 10% glucose solution was supplied to all mice for 12 h. Twenty-one days after the induction, the diabetic mice were identified by measurement of fasting blood glucose (FBG). Blood was withdrawn from the tail vein and the glucose level was measured immediately using a glucose meter (Accu-chek®, Roche, Thailand). Mice with FBG >180 mg/dL were considered to be diabetic and were included in this study. The STZ-NA-induced diabetic mice were randomly divided into subgroups (5–6 mice/group).
2.5.3. Oral glucose tolerance test (OGTT) in normal and diabetic mice
To determine the hypoglycemic activity of MC heartwood extract in vivo, the extract was orally administered to normal mice and STZ-NA-induced diabetic mice, with a glucose loading in the OGGT study. The normal mice were divided into 3 groups treated with water (as a control) and with 500 and 1,000 mg/kg MC heartwood extract, respectively. The STZ-NA-induced diabetic mice were separated into 4 groups treated with water (as a negative control), with 5 mg/kg glibenclamide (as a positive control), and with 500 and 1,000 mg/kg MC heartwood extract, respectively.
All groups were fasted overnight for 12 h before starting the experiment. Each treatment was administered 30 min before glucose loading. Blood samples were collected from the tail vein, and the glucose level was measured (time −30 min). After the mice were fed with 2 g/kg glucose solution, the blood glucose was measured again (time 0 min), and at 30, 60, 90, and 120 min, after glucose administration. At the end of the study, mice were sacrificed using CO2 inhalation. Oral glucose tolerance curves for normal and diabetic mice were determined by plotting fasting blood glucose concentrations (mg/dL) against time after glucose administration (0–120 min). Area under the curve for glucose (AUC at 0-120) over time was calculated by a linear trapezoidal method as shown in Equation (1).8
| (1) |
where C0, C30, C60, C90, and C120 are fasting blood glucose concentration (mg/dL) at 0, 30, 60, 90 and 120 min after glucose administration, respectively, and t0, t30, t60, t90, and t120 are time (min) after glucose administration, respectively,.
2.5.4. Hypoglycemic effect in STZ-NA-induced diabetic mice after continuous treatment by MC heartwood extract
Hypoglycemic effect of MC heartwood extract was also determined after daily oral administration of remedies for 28 days to five groups of mice. Normoglycemic mice were given with water as a normal control. STZ-NA-induced diabetic mice were separated into 4 groups treated with water (as a negative control), 5 mg/kg glibenclamide (as a positive control), and 500 and 1,000 mg/kg MC heartwood extract, respectively.
Body weights and average amount of food consumption were examined for each experiment group. Blood samples were collected from the tail vein of the fasted mice at the onset (day 0), day 14, and day 28 after initiating the experiment, and blood glucose was measured using a glucometer (Accu-Chek®, Roche, Thailand). At the end of the study, mice were sacrificed by anesthesia with CO2 inhalation, and whole blood was collected by cardiac puncture into a heparinized tube. After centrifuging the blood samples at 3,000 g for 10 min, plasma was obtained and stored at −20 °C for analysis.
2.5.5. Measurement of insulin levels by enzyme-linked immunosorbent assay (ELISA)
The amount of insulin in the plasma of the mice was analyzed using a mouse insulin ELISA kit according to the manufacturer’s instructions (FUJIFILM Wako Shibayagi Corporation, Japan).
2.6. Effect on α-glucosidase activity
α-Glucosidase inhibition with MC heartwood extract was determined using a previously published method9 with some modifications. Briefly, 10 μL of either MC heartwood extract or acarbose (as a positive control) was gently combined with 40 μL of α-glucosidase enzyme (0.1 U/mL) in 0.1 M phosphate buffer (pH 6.9). After pre-incubation at 37 °C for 10 min, the reaction was initiated by the addition of 50 μL of 2 mM p-nitrophenyl α-d-glucopyranoside (pNPG) and the solution was further incubated at 37 °C for 20 min. The reaction was terminated by adding 100 μL of 1 M Na2CO3. Released p-nitrophenol was measured in triplicate at 405 nm using a microplate reader (Tecan, Switzerland). Inhibitory percentage and the half maximal inhibitory concentration (IC50)were calculated.
2.7. Enzyme kinetics of α-glucosidase inhibition
The mode of α-glucosidase inhibition by MC heartwood extract was calculated by Lineweaver-Burk plot analysis, using three concentrations of MC heartwood extract (0.5, 1.0, and 2.0 μg/mL) and five different concentrations of pNPG substrate (0.25, 0.5, 1.0, 2.0, and 4.0 mM). The enzyme reaction was performed as described above, and double reciprocal plots (1/v vs. 1/[S]) were prepared. Maximum velocity (Vmax), the substrate concentration which yields a half-maximal velocity (Km), and the inhibition constant (Ki) were determined from the plots by a non-least squares regression of the observed data as shown in equation (2) to calculate v, using Solver Add-in equipped with Microsoft Excel 2010.
| (2) |
where v and vmax represent the initial and maximum velocities, respectively (mmol/min), Km represents the Michaelis constant, S, and I represent the substrate (μM) and inhibitor concentrations (μg/mL), respectively.
2.8. Effect on α-amylase activity
α-Amylase inhibition with MC heartwood extract was determined using the 3, 5-dinitrosalicylic acid (DNSA) method.10,11 The herbal extract and enzyme were prepared in a reaction buffer of 20 mM sodium phosphate, 6.7 mM sodium chloride (pH 6.9), and 200 μL of sample solution. Then, 200 μL of α-amylase solution (2 U/mL) were pre-incubated at 30 °C for 10 min, before the addition of 200 μL of a 1% w/v starch solution and a further 3 min incubation. The reaction was terminated by the addition of 200 μL of DNSA reagent (25 mL of 96 mM DNSA and 8 mL of 5.31 M sodium potassium tartrate in 2 M sodium hydroxide) and boiling at 85–90 °C for 10 min. After cooling to room temperature, 200 μL of the mixture was transferred to a 96-well plate and the absorbance was measured in triplicate at 540 nm using a microplate reader (Tecan, Switzerland). An enzyme-free blank reaction was prepared to eliminate the background. Acarbose was used as a positive control at concentrations of 5, 10, 12.5, 20, 40, and 50 μg/mL.
2.9. Enzyme kinetics of α-amylase inhibition
The mode of α-amylase inhibition by MC heartwood extract was determined using Lineweaver-Burk plot analysis. Three concentrations of the extract (0.75, 1.5, and 3 mg/mL) were assessed, using five different concentrations of starch solution as the substrate (2.5, 5, 10, 20, and 40 mg/mL). Enzyme kinetic parameters (Km, Ki, and Vmax) were calculated according to 2.7.
2.10. Statistical analysis
Data are expressed as the mean ± standard error of the mean (SEM). For comparison of the mean values among the multiple groups, data were analyzed using one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test, using SPSS statistics version 18 (IBM Corporation, USA). Differences between the mean values were considered to be statistically significant at p < 0.05.
3. Results and discussion
3.1. Phytochemical analysis
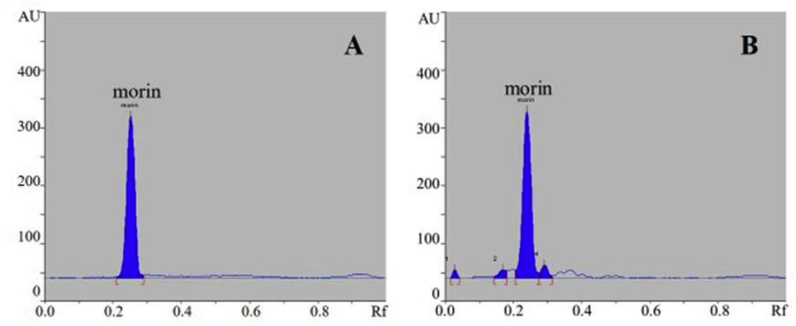
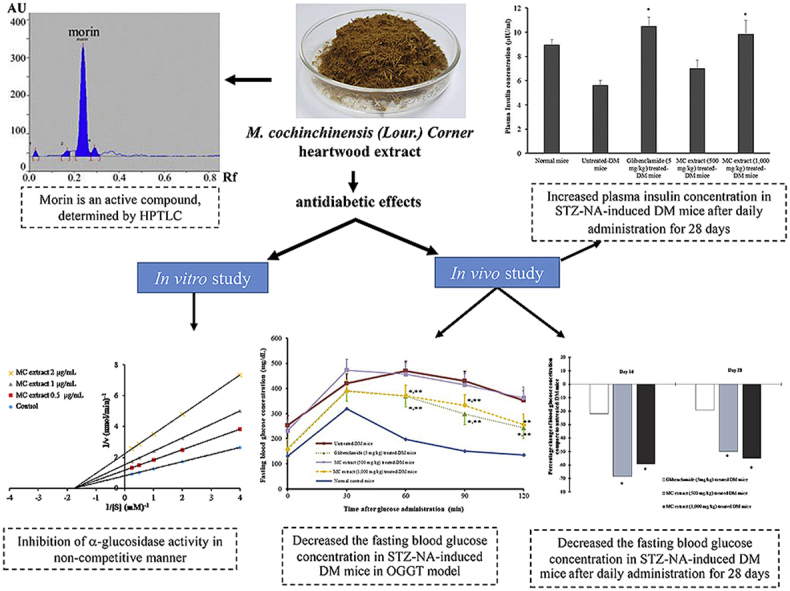
The obtained powder of MC heartwood extract showed a yellowish-brown color, and the extraction yield was 18.99 ± 0.65% w/w. In the present study, morin was found to be a major component of MC heartwood extract and quantified by HPTLC (Fig. 1). Amount of morin in the extract was calculated to be 7.35 ± 0.16% w/w.
Fig. 1.
Densitogram of morin as a reference standard (A) and the aqueous MC heartwood extract (B) examined by HPTLC method. Rf is the retention factor.
3.2. In vivo antidiabetic activity
3.2.1. OGTT in normal and diabetic mice
STZ is an antibiotic derived from Streptomyces achromogenes, which is particularly toxic to insulin-producing β-cells of the pancreas in mammals. It increases the activity of poly(ADP-ribose) polymerase (PARP-1) to repair DNA, depletes the intracellular NAD+ and ATP, and induces the necrosis of insulin-secreting cells. NA is administered intraperitoneally 15 min before administration of STZ, in order to inhibit the PARP-1 activity and prevent the depletion of NAD+ and ATP in the cells exposed to STZ.12 STZ-NA-induction of type 2 diabetes mellitus is characterized by moderate hyperglycemia associated with 40–60% loss of β-cell function,13 and has been widely used in the study of diabetes and development of antidiabetic agents.
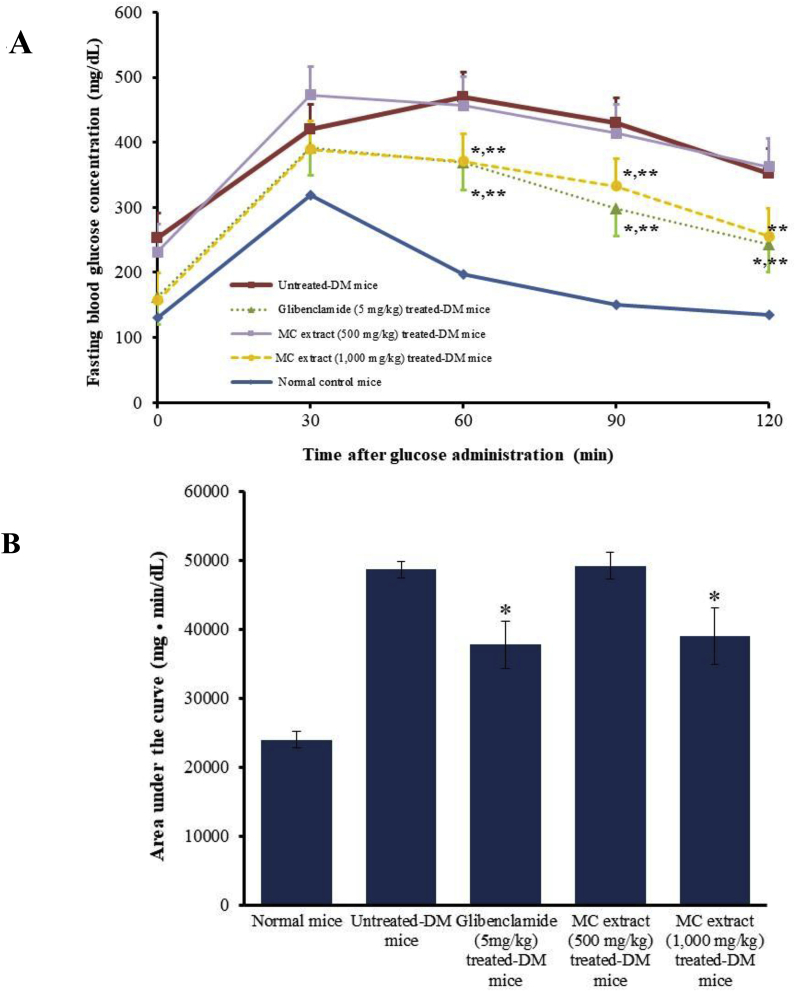
In normal mice, an oral administration of the glucose solution (2 g/kg) produced a rapid increase in the blood glucose level. Fasting blood glucose levels and the areas under the blood glucose-time curves (AUCglucose) in MC heartwood extract-treated normal groups (500 and 1,000 mg/kg) were not significantly different from those of normal control group. These results indicate that MC heartwood extract does not have a noticeable hypoglycemic effect in normal mice. The diabetic mice developed hyperglycemia after STZ injection (150 mg/kg i. p.), and exhibited sign of diabetic such as weight loss, increased food consumption and polyuria, indicating the successes of DM induction. After glibenclamide administration, blood glucose levels at 60, 90, and 120 min (Fig. 2A) and AUCglucose (Fig. 2B) were significantly lower than those seen in the untreated diabetic group (p < 0.05). Oral treatment with 500 mg/kg MC heartwood extract did not produce any marked changes in the blood glucose level; however, treatment with 1,000 mg/kg MC heartwood extract significantly decreased the blood glucose level 60–90 min as well as AUCglucose after the glucose loading (p < 0.05) and its effect was comparable to that seen in the positive control (glibenclamide-treated group). These results indicate that MC heartwood extract exhibits hypoglycemic effects in STZ-NA-induced type 2 diabetic mice, but not in normal mice.
Fig. 2.
The effect of MC heartwood extract on fasting blood glucose concentration (A) and area under the curve (AUCglucose) plotting between glucose concentration and time profile of glucose during the oral glucose tolerance test (OGTT) of STZ-NA-induced diabetic mice (B). Data are given as mean ± SEM (n = 5–6). * and ** represent p < 0.05 and p < 0.01, compared to untreated STZ-NA-induced diabetic group, respectively.
3.2.2. Hypoglycemic effect in STZ-NA-induced diabetic mice after continuous treatment by MC heartwood extract
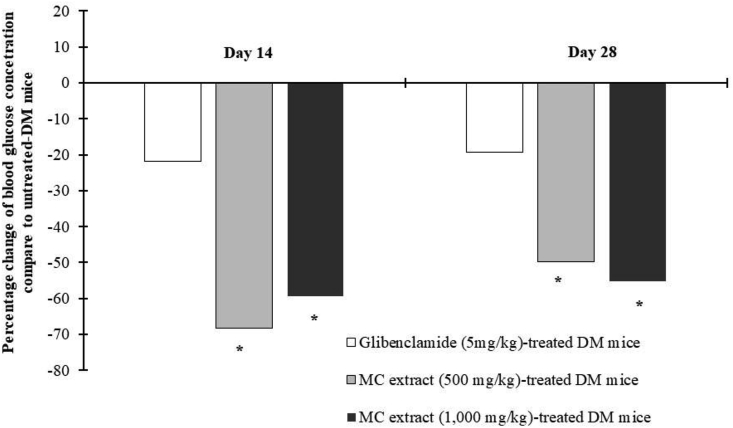
After daily administration of with 500 and 1,000 mg/kg MC heartwood extract for 14 and 28 days, the fasting blood glucose concentration of diabetic mice was markedly decreased, by approximately 50–60% (p < 0.05) compared with that of the untreated-diabetic mice (Fig. 3). The hypoglycemic effect of MC heartwood extract was more potent than that of glibenclamide which reduced blood glucose by approximately 20% after 14 and 28 days.
Fig. 3.
The percentage changes of fasting blood glucose concentration as compared with untreated STZ-NA-induced diabetic mice after daily administration for 28 days. Data are given as mean ± SEM (n = 5–6). * indicates statistically significant difference at p < 0.05, when compared to untreated STZ-NA-induced diabetic group.
3.2.3. Effect on plasma insulin concentration
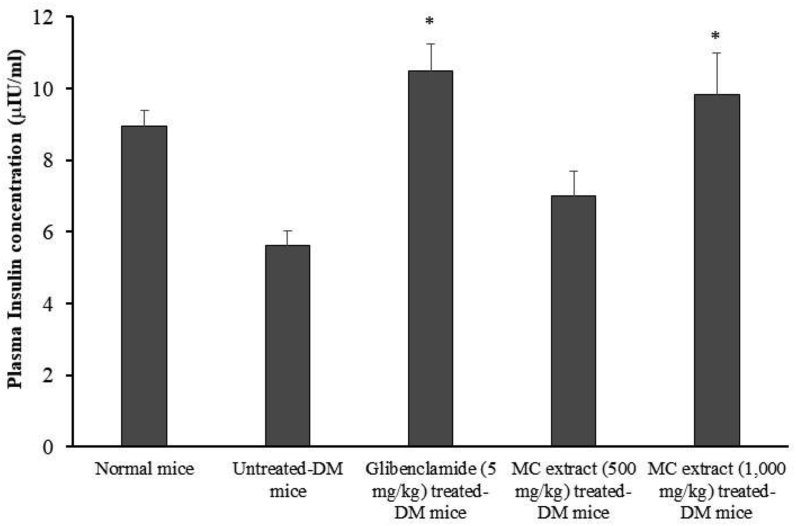
Fig. 4 shows the effect of MC heartwood extract on plasma insulin concentration after 28 days of daily administration. In the untreated-DM mice, the plasma insulin concentration was significantly lower than that of the normal mice. Continuous daily oral administration of glibenclamide and MC heartwood extract (1,000 mg/kg) for 28 days significantly increased the plasma insulin level, compared with that of the untreated-diabetic mice (p < 0.05), suggesting that the mechanism of its hypoglycemic effect may be via stimulation of insulin level or enhanced transport of blood glucose in the peripheral tissue. Antidiabetic effects of herbal extracts have been demonstrated in association with increased insulin levels, improved HbA1c levels, and histology of islet of Langerhans of pancreatic cells in STZ-NA-induced diabetic mice.13,14 The mechanism of the regulation of insulin release by MC extract, observed in this study, might be explained by combination effects of antidiabetic flavonoids in the extract (such as morin and quercetin), which may prevent the progressive impairment of pancreatic α-cell function due to oxidative stress and may thus reduce the occurrence of type 2 diabetes.15,16
Fig. 4.
Plasma insulin concentration after daily administered with MC heartwood extract for 28 days. Data are given as mean ± SEM (n = 5–6). * indicates statistically significant difference at p < 0.05, when compared to the untreated- STZ-NA-induced diabetic group.
3.3. Inhibitory effect on α-glucosidase and α-amylase activity
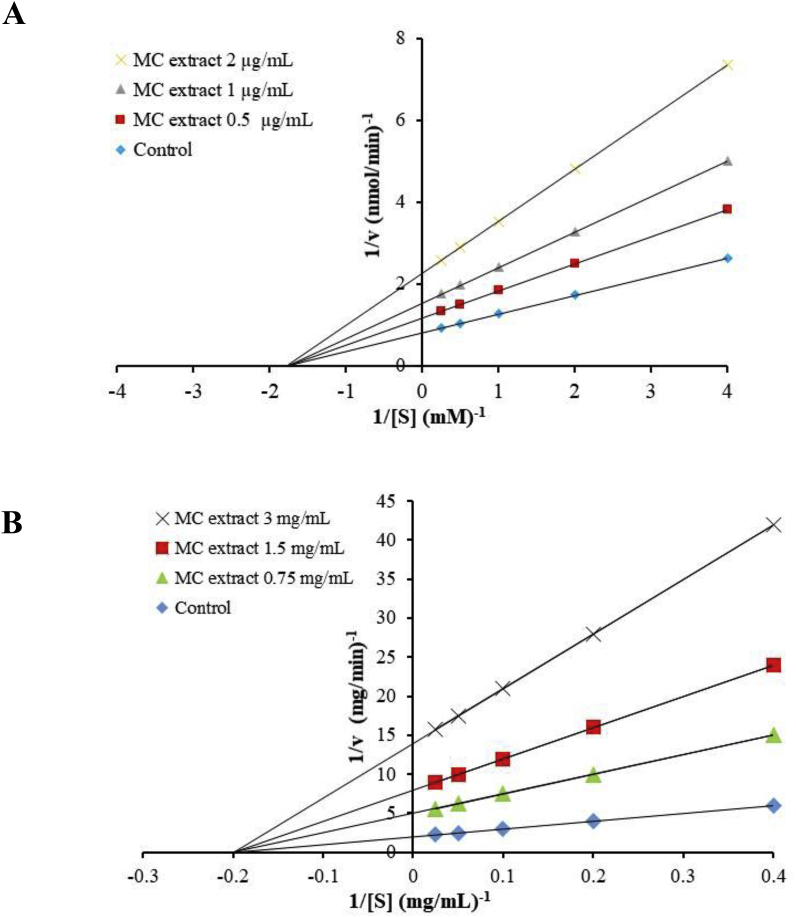
MC heartwood extract markedly inhibited α-glucosidase in a concentration-dependent manner. IC50 value of the herbal extract was 1.53 ± 0.03 μg/mL, which was approximately 300 times more potent than that of acarbose (IC50, 513 ± 39 μg/mL) (p < 0.05). Lineweaver-Burk plot (Fig. 5A) demonstrated that MC heartwood extract inhibited α-glucosidase in a non-competitive manner, in contrast to acarbose which competitively inhibits α-glucosidase.17 The kinetic parameters, Vmax, Km, and Ki, were calculated to be 1.23 nmol/min, 0.57 mM, and 1.13 μg/mL, respectively.
Fig. 5.
Lineweaver-Burk plot for the kinetic analysis of α-glucosidase (A) and α-amylase (B) inhibitory activities of MC heartwood extract.
[V] and [S] are the reaction velocity and the concentration of substrate, respectively.
IC50 values for α-amylase inhibition with MC heartwood and acarbose were found to be 1.63 ± 0.38 mg/mL and 32.62 ± 2.83 μg/mL, respectively, demonstrating that the inhibition of α-amylase with the herbal extract is significantly lower than that of acarbose (p < 0.05). As shown in Fig. 5B, the Lineweaver-Burk plot for determination of kinetic parameters demonstrated that the mode of α-amylase inhibition by MC heartwood extract was non-competitive manner, and Vmax, Km, and Ki were calculated to be 0.163 mg/min, 3.60 mg/mL, and 1.29 mg/mL, respectively.
α-Glucosidase and α-amylase are two key enzymes that are involved in carbohydrate hydrolysis, resulting in a postprandial glucose surge that is related to the progression of diabetes. Inhibition of these enzymes helps to delay glucose absorption and reduce postprandial hyperglycemia.17 The α-glucosidase inhibition have delayed glucose absorption and reduced postprandial hyperglycemia which may largely contribute to the hypoglycemic effect of MC heartwood extract in the diabetic mice in OGTT model. The α-glucosidase inhibitory activity of MC heartwood extract may be due to the synergistic effects of various phenolic compounds, especially morin, which exhibits α-glucosidase inhibitory activity in a mixed-type manner, with an IC50 value of 1.35 ± 0.01 μg/mL.18 Studies of the structure-activity relationship of morin indicated that the hydroxyl groups at the C-3 and C-5 positions, and additional C2=C3 double bond in the C-ring, are advantageous for α-glucosidase inhibitory activity. Morin contains many hydroxyl groups, and the hydroxylation in ring C is able to weaken the binding affinity between morin and α-amylase.19 These structural data supports the results observed in this study that MC heartwood extract, a major component of which is morin, potently inhibited α-glucosidase, but it was less active against α-amylase.
It is now known that hyperglycemic conditions in cells are associated with enhanced levels of reactive oxygen species (ROS) which plays an important role in the development of vascular complications, apoptosis, and impair the pancreatic beta-cell function, resulting in insulin resistance, particularly in type 2 diabetes.20 Antioxidants and free radical scavengers are promising for the treatment of diabetes. Our previous study found that an aqueous extract of MC heartwoods contains high total phenolic and flavonoid phytochemicals, and thus exhibited an antioxidant activity determined in vitro.3 However, in vivo antioxidant effect of the herbal extract should also be assessed in future, preferably in human, to provide a proof of concept for MC extract aimed at the treatment of diabetes and its complication.
In this study, any toxicities were not observed in mice dosed with either 500 or 1,000 mg/kg aqueous MC heartwood extract, suggesting that it is not toxic. This is in accordance with a previous study,1 in which MC extract exhibited no acute toxicity in mice even at very high doses of 16 g/kg administered via gastric intubation and 10 g/kg after subcutaneous routes.
4. Conclusion
The results of the present study indicate for the first time that an aqueous extract of MC heartwoods exhibited significant hypoglycemic activity in STZ-NA-induced type 2 diabetic mice by inhibiting α-glucosidase and increasing plasma insulin levels, but it does not exert hypoglycemic activity in normal mice. The α-glucosidase inhibition may delay glucose absorption and reduce postprandial hyperglycemia in the diabetic mice, and the increased insulin levels may contribute to the hypoglycemic effect observed after 14 and 28 days of administration. Therefore, an extract of MC heartwoods could be a potential natural remedy for type 2 diabetes, and further clinical study is warranted.
Declaration of competing interest
We declare that we do not have any conflicts of interest related to this work.
Acknowledgements
This work was financially supported by Mahidol University, Thailand. We would like to thank to Miss Ruksinee Khammanit, Mr. Mana Thaploha, Miss Chalichittra Tangtontrakun, and Mr. Chayanun Stamitr, Faculty of Pharmacy, Mahidol University for assisting with the in vivo studies.
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.10.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Mokkhasmit M., Ngarmwathana W., Sawasdimongkol K., Permphiphat U. Pharmacological evaluation of Thai medicinal plants. J Med Assoc Thail. 1971;54(7):490–503. [PubMed] [Google Scholar]
- 2.Ministry of Public Health . 2013. National List of Essential Medicines (List of Herbal Medicinal Products) Thailand. [Google Scholar]
- 3.Sato V.H., Chewchinda S., Parichatikanond W., Vongsak B. In vitro and in vivo evidence of hypouricemic and anti-inflammatory activities of Maclura cochinchinensis (Lour.) Corner heartwood extract. J Tradit Complement Med. 2019 doi: 10.1016/j.jtcme.2019.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kongkiatpaiboon S., Tungsukruthai P., Sriyakool K., Pansuksan K., Tunsirikongkon A., Pandith H. Determination of morin in Maclura cochinchinensis heartwood by HPLC. J Chromatogr Sci. 2017;55(3):346–350. doi: 10.1093/chromsci/bmw191. [DOI] [PubMed] [Google Scholar]
- 5.Mishra P., Mishra S., Mahanta C.L. Effect of maltodextrin concentration and inlet temperature during spray drying on physicochemical and antioxidant properties of amla (Emblica officinalis) juice powder. Food Bioprod Process. 2014;92(3):252–258. [Google Scholar]
- 6.Leakaya N., Sato V.H., Chewchinda S. Antioxidant activity, total phenolic, total flavonoid content and HPTLC analysis of morin in Maclura cochinchinensis heartwood extract. TJPS. 2018;5 [Google Scholar]
- 7.Ghasemi A., Khalifi S., Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review) Acta Physiol Hung. 2014;101(4):408–420. doi: 10.1556/APhysiol.101.2014.4.2. [DOI] [PubMed] [Google Scholar]
- 8.Sakaguchi K., Takeda K., Maeda M. Glucose area under the curve during oral glucose tolerance test as an index of glucose intolerance. Diabetol Int. 2016;7(1):53–58. doi: 10.1007/s13340-015-0212-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Supasuteekul C., Nonthitipong W., Tadtong S., Likhitwitayawuid K., Tengamnuay P., Sritularak B. Antioxidant, DNA damage protective, neuroprotective, and α-glucosidase inhibitory activities of a flavonoid glycoside from leaves of Garcinia gracilis. Rev Bras Farmacogn. 2016;26(3):312–320. [Google Scholar]
- 10.Tan Y., Chang S.K.C., Zhang Y. Comparison of α-amylase, α-glucosidase and lipase inhibitory activity of the phenolic substances in two black legumes of different genera. Food Chem. 2017;214:259–268. doi: 10.1016/j.foodchem.2016.06.100. [DOI] [PubMed] [Google Scholar]
- 11.Meng Y., Su A., Yuan S. Evaluation of total flavonoids, myricetin, and quercetin from Hovenia dulcis Thunb as inhibitors of α-amylase and α-glucosidase. Plant Foods Hum Nutr. 2016;71(4):444–449. doi: 10.1007/s11130-016-0581-2. [DOI] [PubMed] [Google Scholar]
- 12.Ghasemi A., Khalifi S., Jedi S. Streptozotocin-nicotinamide-induced rat model of type 2 diabetes (review) Acta Physiol Hung. 2014;101(4):408–420. doi: 10.1556/APhysiol.101.2014.4.2. [DOI] [PubMed] [Google Scholar]
- 13.Petchi R.R., Vijaya C., Parasuraman S. Antidiabetic activity of polyherbal formulation in streptozotocin–nicotinamide induced diabetic Wistar rats. J Tradit Complement Med. 2014;4(2):108–117. doi: 10.4103/2225-4110.126174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jaiswal Y.S., Tatke P.A., Gabhe S.Y., Vaidya A.B. Antidiabetic activity of extracts of Anacardium occidentale Linn. leaves on n-streptozotocin diabetic rats. J Tradit Complement Med. 2017;7(4):421–427. doi: 10.1016/j.jtcme.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vadivelan R., Krishnan G.R., Kannan R. Antidiabetic potential of Asparagus racemosus Willd leaf extracts through inhibition of α-amylase and α-glucosidase. J Tradit Complement Med. 2019;9(1):1–4. doi: 10.1016/j.jtcme.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hui H., Tang G., Go V.L.W. Hypoglycemic herbs and their action mechanisms. Chin Med. 2009;4(1):11. doi: 10.1186/1749-8546-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ratner R.E. Controlling postprandial hyperglycemia. Am J Cardiol. 2001;88(suppl):26H–31H. doi: 10.1016/s0002-9149(01)01834-3. [DOI] [PubMed] [Google Scholar]
- 18.Zeng L., Zhang G., Liao Y., Gong D. Inhibitory mechanism of morin on alpha-glucosidase and its anti-glycation properties. Food Funct. 2016;7(9):3953–3963. doi: 10.1039/c6fo00680a. [DOI] [PubMed] [Google Scholar]
- 19.Yuan E., Liu B., Wei Q., Yang J., Chen L., Li Q. Structure activity relationships of flavonoids as potent alpha-amylase inhibitors. Nat Prod Commun. 2014;9(8):1173–1176. [PubMed] [Google Scholar]
- 20.Afanas’ ev I. Signaling of reactive oxygen and nitrogen species in Diabetes mellitus. Oxid Med Cell Longev. 2010;3(6):361–373. doi: 10.4161/oxim.3.6.14415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.