Abstract
Background and aim
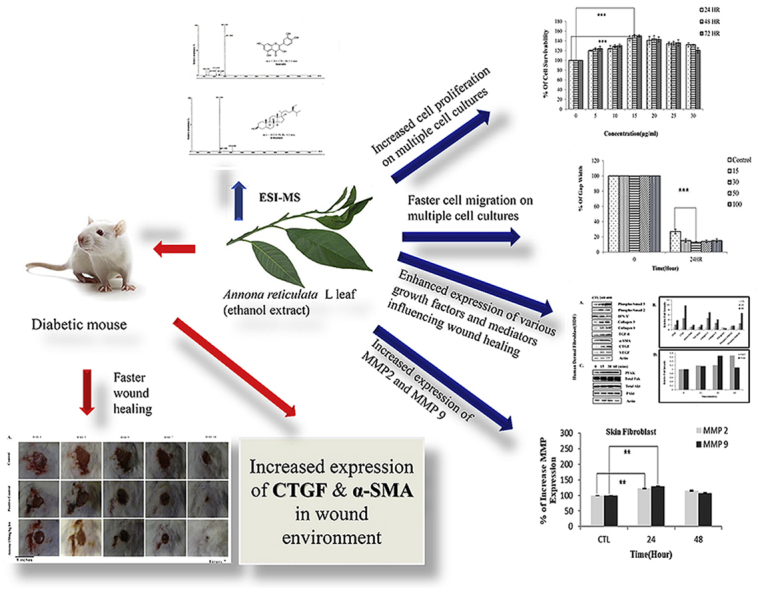
The leaves of AnnonareticulataLinn (牛心果niú x ınguǒ; Bullock’s heart), a member of Annonaceae family, have been used extensively in folk medicine; however, its wound healing potential is yet to be explored. Our aim was to investigate the wound healing ability of A. reticulataleaf extract in vitro and in streptozotocin induced diabetic mice model.
Material and methods
We observed the plant extract induced proliferation and migration of primary human dermal fibroblast (HDF), human skin fibroblast cell line (GM00637) and human keratinocyte cell line (HACAT). The expression of transforming growth factor beta (TGF-β), connective tissue growth factor (CTGF), vascular endothelial growth factor (VEGF), alpha smooth muscle actin (α-SMA), matrix metalloproteinases (MMP-2, MMP-9), collagen-1, collagen-3, focal adhesion kinase (FAK) were evaluated by Western blot and gelatin zymography. Excisional diabetic wound model was used for in vivo wound healing assay. Furthermore, we processed wound tissue for histological and immunohistochemical study.
Result
A. reticulata L. leaf extract stimulates proliferation and migration of HDF, skin fibroblast and keratinocyte significantly in a dose dependent manner; expression of TGF-β, CTGF, VEGF, α-SMA, MMP-2, MMP-9, collagen-1, collagen-3, FAK increased. Additionally, an enhanced expression of phospho-SMAD2, phospho-SMAD3 in the treated cells indicated the activation of TGF-β signal transduction pathway, similarly increased expression of phospho-AkT suggested activation of PI3/AkT pathway. Expression of CTGF and α-SMA was also increased significantly in wound tissue. Mass spectrometric analysis revealed that mainly two compounds to be present in the extract: quercetin and β-sitosterol.
Conclusion
Collective data suggest that A.reticulata leaf extract may have a stimulatory effect in diabetic wound healing.
Keywords: Annona reticulata L. leaf extract, Human fibroblast, Human keratinocyte, Diabetic mice, Wound healing
Graphical abstract
Highlights
-
•
While the leaf extract from other species of the Annonaceae family have been studied extensively for wound healing activity, Annona reticulata leaf has not been explored for the same.
-
•
The present study showed significantly increased proliferation and migration of both fibroblasts and keratinocytes in the treated group with enhanced expression of various growth factors critical to wound healing.Topical application accelerated wound closure in diabetic mice model compared to the positive control group.
-
•
Annona reticulata Linn leaf extract have stimulatory effect in diabetic wound healing.
List of abbreviation
- AkT
protein kinase B
- BSA
bovine serum albumin
- CTGF
connective tissue growth factor
- DMEM
Dulbecco’s modified eagle medium
- ECM
extracellular matrix
- FAK
focal adhesion kinase
- FBS
fetal Bovine Serum
- HDF
Human primary dermal fibroblast cells
- HPLC
High performance liquid chromatography
- HRP
Horseradish peroxidase
- IFN-γ
Interferon gama
- MEM
Minimal Essential Media
- MMP
matrix metalloproteinase
- MS
Mass spectrometry
- NEAA
non-essential amino acid
- p-AkT
phosphorylated protein kinase B
- PBS
phosphate buffer saline
- p-FAK
phosphorylated focal adhesion kinase
- p-SMAD
phosphorylated SMAD
- PVDF
Polyvinylidine difluoride
- SDS–PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SMAD
family of protein involved in TGF-β signaling pathway
- TBST
Tris buffered saline with tween
- TGF-β
transforming growth factor beta
- TIMP
tissue inhibitor of metalloproteinase
- VEGF
vascular endothelial growth factor
- α-SMA
alpha smooth muscle actin
1. Introduction
Wound healing is a highly coordinated dynamic process involves distinct phages that overlap in time and space.1 The medicinal properties inherited in different plants have been explored over the years which can accelerate the healing process by modifying the biological responses of the cells involving wound healing. Diverse biologically active molecules, cytokines, growth factors etc. determine the optimum healing response. Impairment of any of the responses may delay the healing as in case of chronic wound or diabetic wound. A diabetic wound is associated with connective tissue disorders like reduced angiogenesis, biosynthesis of collagens, and limited proliferation of different cells linked with healing. Other complications associated with diabetic wound are poor proliferation of fibroblasts, keratinocytes, and endothelial cells, diminished cell migration, and decreased production of growth factors.2
Among the growth factors and cytokines, TGF-β has the widest spectrum of actions in wound healing and has been found to be reduced in the experimental diabetic wound.3 TGF-β also organizes multiple cellular responses including re-epithelialization, neo-vascularization, fibroblast proliferation, deposition of ECM and granulation tissue formation.4 Recently it was reported that, Hesperidin, a plant flavonoid accelerated diabetic wound by up-regulation of TGF-β.5 Focal adhesion kinase (FAK) is another factor that contributes in healing by enhancing cell migration6 and it was found that FAK degradation by the protease calpain 1 is accelerated under diabetic condition.7 Additionally, connective tissue growth factor (CTGF)8 and vascular endothelial growth factor (VEGF)9 have plethora of actions in wound healing and their expression in chronic wounds is remarkably reduced or absent.10 Several plant products are reported to modulate angiogenesis and thereby diabetic wound.11 Besides degradation of extracellular matrix (ECM), metalloproteinases (MMP) releases the keratinocytes by disrupting its hemidesmosome attachments and helps in their migration into the wound site.12 Both MMP-2, MMP-9 has been shown to be expressed in keratinocytes at wound edge and linked to enhanced keratinocyte migration.13 However, a critical balance between matrix metalloproteinases (MMP) and tissue inhibitor of metalloproteinases (TIMP) is crucial for proper wound healing.12
Annona reticulata L (牛心果niú x ınguǒ; Bullock’s heart), commonly known as bullock’s heart or raamphal plant, is well distributed in tropical regions specially India.Traditionally, various parts of this plant have been used for diverse ailments.14 This plant has been used as anti-epileptic, antihelmintic, antibacterial, antispasmodic agent; it is also used in the treatment of lice infestation, dysentery, cardiac problems, constipation, haemorrhage, dysuria, fever, etc. Traditionally the crushed leaves in the form of paste have been applied to unhealthy ulcers.15In some parts of rural India the crushed leaves are applied to small wounds, cuts, and abrasions etc. even today. However, after extensive literature review, we spotted that unlike other two commonly known species (A. muricata, A. squamosa) of Annonaceae family,16,17 A.reticulata Linn leaf has not been evaluated for wound healing activity.Therefore, the present study aims to explore the effect of ethanol extract of A.reticulata L leafon wound healing, both in vitro and in streptozotocin induced diabetic mice. Varying percentages of ethanol was used for extraction. Using ethanol of different concentrations from 30% to 50%, we observed that the cell proliferation (MTT assay) in skin fibroblast cell line was maximal with 32% ethanol extract of A. reticulata leaf. Henceforth, we continued our investigation with 32% ethanol extract of A. reticulata. In particular, we noted the influence of the extract on proliferation and migration of human dermal fibroblasts, skin fibroblasts, and keratinocytes. Impact on the expression of various growth factors and proteins involved in the wound healing such as TGF-β, VEGF, CTGF, α-SMA, MMP-2, MMP-9 and FAKwas recorded. We also noted that the proliferative effect of A.reticulata leaf extract is mediated through PI3K dependent pathway with a rise in pAkt and pFAK. In vivo, we studied the effect of topical application of the crude extract in the excisional wound of diabetic mice both macroscopically, histologically, and immunohistochemically. Both high performance liquid chromatography (HPLC) and Mass spectrometry (MS) was performed to identify the active compound(s) in the extract.
2. Materials and method
2.1. Materials
Minimal Essential Media (MEM), Fibroblast Expansion Media and Fibroblast Growth factor, Fetal Bovine Serum (FBS) and l-glutamine, phosphate buffer saline (PBS) were purchased from HiMedia (Mumbai, India). Penicillin streptomycin, MEM NEAA, MEM amino acids, MEM vitamin solution were obtained from Life Technologies, USA. Antibody against collagen-1,collagen-3, CTGF, α-SMA, anti-mouse HRP linked secondary antibody, anti-rabbit HRP linked secondary antibody were purchased from Abcam (MA, USA). Antibodies against TGF-β, IFN-γ, VEGF, p-AkT, Actin were purchased from Santa Cruz Biotechnology (Dallas, Texas USA). Antibodies against p-SMAD2, p-SMAD3, p-FAK, FAK, AkT were purchased from Cell Signaling Technology (Danvers, MA, USA). Vectashield mounting medium was from Vector Laboratories, Inc. (CA, USA). MTT 3-(4, 5-dimethylthiazol-2-yl) −2, 5-diphenyltetrazolium bromide), Bovine serum albumin (BSA) were purchased from SRL India. Streptozotocin, Ketamine and Xylazine were purchased from Sigma-Aldrich, India.
2.2. Cell lines and culture conditions
Human primary dermal fibroblast cells (HDF) were maintained in fibroblast expansion media and fibroblast growth factor. The passage 3–6 was used for all experiments. GM00637 (SV40 transformed fibroblast) cells were cultured in minimal essential medium (MEM) supplemented with 10% FBS, 1% penicillin, 1% streptomycin, 1% l-glutamine, 1% MEM NEAA, 1% MEM amino acids, and 1% MEM vitamin solution. Human adult keratinocyte cell line (HACAT) was maintained in Ham’s F12 containing DMEM with 10% FBS, 1% penicillin, 1% streptomycin. All the cell lines were kept at 37 °C in a humidified atmosphere containing 5% CO2.
2.3. Collection and authentication of plant material
The fresh leaves of Annona reticulata L (the plant name has been checked with http://www.theplantlist.org) were procured from Pandua in the month of July 2017. The plant herbarium was authenticated by Mr.R.Gogoi, scientist, Central National Herbarium, Botanical Survey of India and a specimen voucher number (specimen no-SM-1; CNH/Tech.II/2018/24) was provided which is preserved in the Department of Life Science and Bio-Technology, Jadavpur University.
2.4. Extraction procedure
A. reticulata Linn leaves (30 g) were meticulously cleaned with autoclaved water. The leaves were then left to dry under shade. Then the leaves were smashed by a mixer grinder. The smashed leaves were macerated in 32% ethanol. The mixture was passed through a Whatman filter paper (No 1). The suspension thus produced was sterilized by filtration technique (membrane pore size 0.22 μm). The filtrate was then put on a lyophilizer to obtain the crude dry extract (4.56 g). Finally, the crude extract was stored at −20 °C. Fresh stock solutions were prepared from the crude dry extract every time during experiment.
2.5. Cell proliferation assay
Standard MTT assay using 24 well plates was executed with all the three cell lines separately. (Seeding density was of 5 x l04 cells per well; the cells allowed to adhere for 24 h before treatment.) At three different incubation periods, different concentrations of the plant extract were added to the test-wells whereas the control wells got only medium with 10% FBS. After 24 h, 48h and 72h of incubation, MTT solution (0.5 mg/ml) was added to each well and the plates were incubated for 3 h at 37 °C and 5% CO2. The absorbance of the formazon product was measured at 570 nm using a spectrophotometer. A preliminary screening was performed with A. reticulata leaf extract and MTT reagent in a 24 well culture plate without any cells to exclude any interaction between the plant extract and the MTT reagent.18
2.6. Cell migration assay
‘Scratch assay’ on the confluent monolayer of cells of different cell lines was performed after adding culture media with and without plant extract. Distances between one side of scratch and the other are then inspected microscopically. Images were captured by digital camera attached to the microscope and data were analyzed using Image J Software (NIH, USA). From each scratched area 3 photographs were taken to compare the cell migration. The distance between either sides of the gap (gap width) was calculated at 0 hand 24 hand plotted in chart.
2.7. Western blot
Standard protocol was followed using actin as the housekeeping protein (Detailed in supplement-1). Expression of various growth factors (TGF-β, VEGF, and CTGF), proteins (SMAD2, SMAD3, α-SMA), phospho-AkT, phospho-FAK in extract treated and saline treated human fibroblasts and keratinocytes was observed.
2.8. Gelatin zymography
Performed to detect secreted MMP-2, MMP-9 activity in conditioned media which was prepared from extract treated plate cells cultured in FBS. Gel running, gel washing and staining, and destaining were performed following manufacturer’s protocol with slight modification. (Abcam; detailed in supplement-2).
2.9. Wound healing assay: animal study
2.9.1. Experimental animals
Healthy adult Swiss albino male mice (6–8 week old mice, 25–30 g) were used in the study. All mouse experiments were conducted at and in accordance with the guidelines of the Institutional Animal Ethics Committee, Bose Institute, Kolkata, India (protocol number is IAEC/BI/33/2015); complying with the guidelines as established by the IACUC regarding the care and use of laboratory animals in scientific experiments.
2.9.2. Acute toxicity assay
Acute toxicity testing using the up-and-down (UDP) method described elsewhere19 showed no toxicity up to the dose of 2000 mg/kg.
2.9.3. Hyperglycemia induction & wound creation
After 1-week of adaption, the mice were kept in empty stomach overnight and hyperglycemia was induced by injecting low dose (40 mg/kg) streptozotocin intraperitoneally for consecutive 5 days using protocol described elsewhere.20 They were then anesthetized by administration of ketamine (40 mg/kg; Sigma), and the dorsal hairs were shaved and cleaned with 70% ethanol. One full thickness 5-mm diameter excisional skin wound was made on the side of the dorsal midline with the help of a biopsy punch forceps.
2.9.4. Experimental design
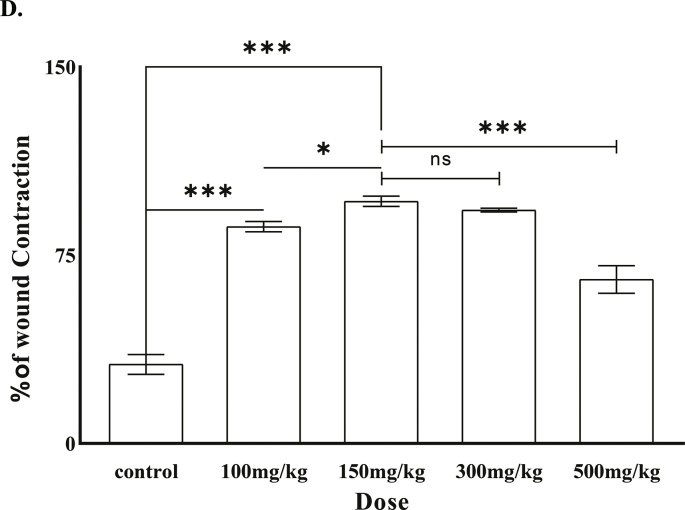
The animals (N = 18) were then randomly divided into 3 groups containing six animals each and each animal was kept individually in metallic cages containing autoclaved paper cuttings. The animals were grouped as follows: Group I: Mice treated with 0.9% NaCl (control). Group II: Mice treated with 10% povidone iodine ointment (positive control). Group III: Mice treated with 150 mg/kg body weight A. reticulata leaf extract as topical solution (Table 1). A primary screening of wound healing in vivo was done with increasing concentrations of A. reticulata L leaf extract and maximum healing efficiency was found at 150 mg/kg body weight (Supplement-3) and this concentration was taken for further experiments. The plant extract and povidone iodine ointment was applied topically once daily for 7 consecutive days. The control group of mice received normal saline (0.9% NaCl) for the same period.
Table 1.
Random distribution of the mice in 3 different treatment groups.
| Group | Name | Treatment received (topical application, once daily, for 7 consecutive days) |
|---|---|---|
| Group I | Control | with 0.9% normal saline |
| Group II | Positive Control | with 10% povidone iodine ointment |
| Group III | Extract treated | with 150 mg/kg body weight A. reticulata Linn leaf extract |
Single animal from each group was sacrificed on day 3, day 7, day 14 for histological study and immunohistochemistry.
2.9.5. Wound area measurement
The wound regions were digitally photographed daily up to 14th day. The wound area was calculated using Image J software, (version 1.52 J; NIH, USA) and plotted against the respective post wounding day. Contraction of wound area was calculated using a formula described elsewhere.21 For histological examination and immunohistochemistry, the mice were sacrificed at designated time points (day 3, day 7 and day 14) and tissue from the edge and center of the wound was collected and fixed in 10% formalin.
2.10. Haematoxylin and eosin staining
Paraffin block was prepared from the wound site tissue and multiple 4 μm sections were taken by a microtome. Deparaffinization was done by serial xylene wash. Tissue sections were rehydrated by serial baths in ethanol of decreasing strengths and water. The staining was performed following standard protocol. The slides were then observed under bright-field optical microscope.
2.11. Immunohistochemistry
The paraffin block of wound tissue was cut into 4 μm sections on clean, microscope slides. Immunostaining was started with overnight incubation of the slides with primary antibodies against CTGF (1:100; Abcam) and SMA (1:200; Abcam) at 4 °C and further proceedings were completed according to protocol (MP-7601; IHC Guide, Vector Laboratories). Bright field images were captured with a microscope.
3. HPLC followed by MS
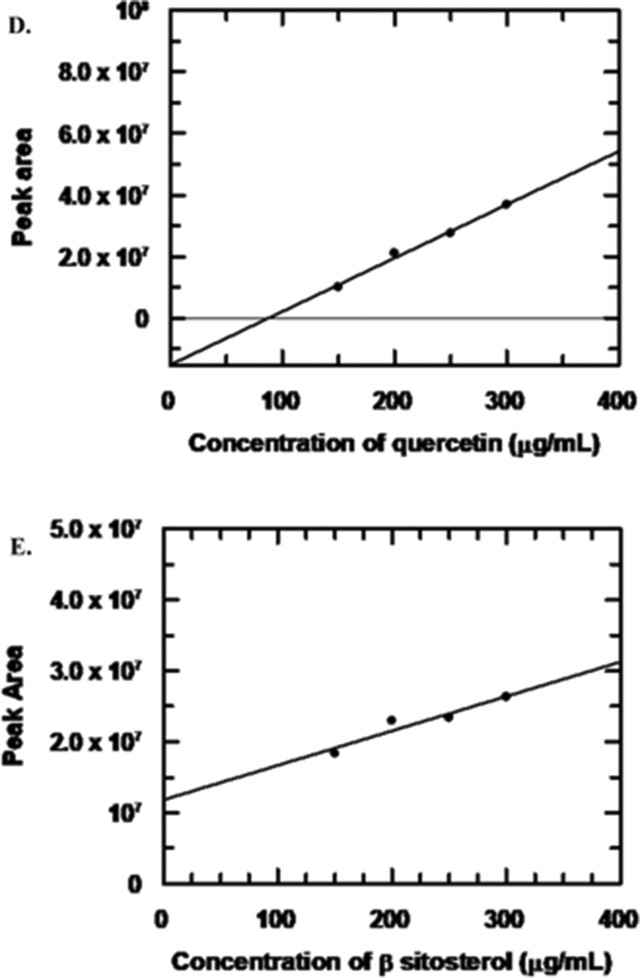
Reverse-phase HPLC analysis was performed on a Shimadzu HPLC system (Japan) using a C18 Luna 5u (250 × 4.6 mm, 5 μm) column. The mobile phase was methanol: water (85:15). The separation was performed with isocratic elution at 1 ml/min flow rate and 18 min run time. The injection volume was 20 μl and the chromatogram obtained at 220 nmwas used for analysis.Detector used in HPLC was SPD-M20A (Prominence Diode Array Detector). An ESI + mass spectrum was recorded after collecting the samples that eluted from the C18 column using a Micromass Q-Tofmicro™, Waters Corporation.The mass analyzer was set to scan in the range m/z 100–1100 at 30,000 resolutions in one polarity while the linear ion trap analyzer performed MS analysis on the most abundant ions in both polarities using an ion isolation window of ±2 m/z and relative collision energy of 35%. In order to determine the concentration of quercetin and β-sitosterol in the extract, we created standard curves for both substances by using known concentrations of each and performing HPLC under identical conditions as that used for the experimental extract (Mobile phase (methanol: water = 85:15, and C18 Luna 5u column). From standard curves concentration of each was determined and their percentage presence was calculated.
4. Statistical analysis
All data has been represented as mean ± SD. Variance among groups were evaluated using two factors ANOVA, single factor ANOVA, followed by post Hoc Tukey’s range test for multiple comparisons. P < 0.05 was accepted as statistically significant. P < 0.05, P < 0.01 and P < 0.001 are represented by *, **, and *** respectively. (Software: Graph pad Prism version-8; Excel 10).
5. Results
5.1. Ethanol extract of A. reticulata L. leaf stimulates proliferation and migration of cells associated with wound healing
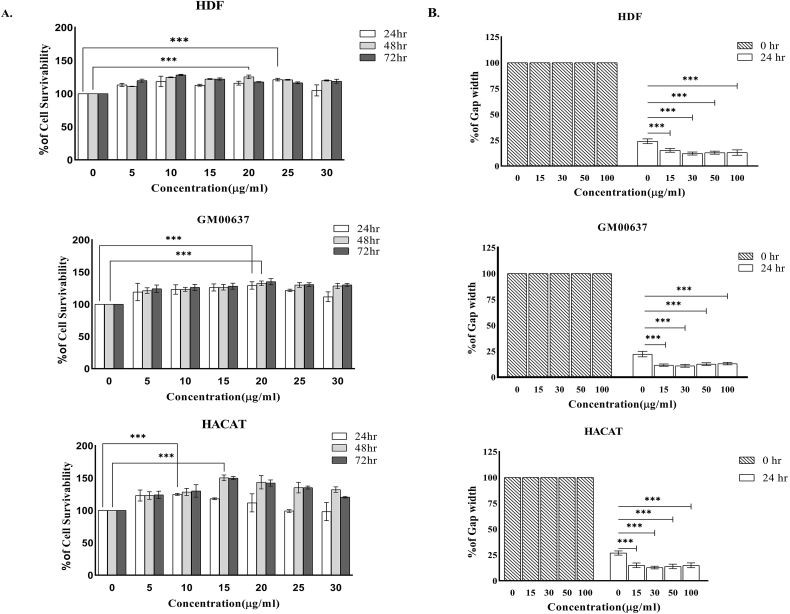
Proliferation of dermal fibroblasts (HDF) increased with increasing concentration of the extract (Fig. 1A). Maximum proliferation was seen at the concentration of 25 μg/ml (121.23 ± 2%) at 24 h; 20 μg/ml (127.023 ± 5.1%) at 48 h and 10 μg/ml (128.144 ± 3.72%) in 72 h. In case of skin fibroblasts (GM00637), proliferation was maximum at the concentration of 20 μg/ml (129.12 ± 3.12%, 132.7 ± 2.2%, 135 ± 4.12% in 24 h, 48 h, and 72 h respectively). Proliferation of keratinocytes (HACAT) also increased in a dose dependent manner with highest proliferation at the concentration of 10 μg/ml (145 ± 5.1%) in 24 h and at 15 μg/ml (150.67 ± 2.5%, 150.13 ± 2.4%) in 48 h, and 72 h respectively. On Scratch test, maximum migration of dermal fibroblasts, skin fibroblasts, and keratinocytes (Fig. 1B) into the gap area was observed with optimum concentration of 30 μg/ml after 24 h.
Fig. 1.
A Ethanol extract of A.reticulatastimulates proliferation of human dermal fibroblasts (HDF), human skin fibroblasts (GM00637), and keratinocyte (HACAT) cells.
Shows the effect of the increasing concentration of A.reticulataleaf extract on HDF, GM00637, and HACAT cells at24 h, 48 h, and 72husing MTT assay. The results expressed as mean ± S.D. of three independent experiments. The percentage of cell growth was calculated using untreated cells as 100%.* (P < 0.05),** (P < 0.01), *** (P < 0.001). Hr-hour.
B Ethanol extract of A.reticulatastimulates migration of human dermal fibroblasts (HDF), human skin fibroblasts (GM00637), and keratinocyte (HACAT) cells.
Fig. 1B represents the result of Scratch assay. The migration of the cells, 24 h after the scratch. The results expressed as mean ± S.D. of three independent experiments. Percentage of gap at 0 his taken as 100%; * (P < 0.05), ** (P < 0.01), *** (P < 0.001); hr-hour. Concentration of extract is expressed in μg/ml.
5.2. A. reticulata L. leaf extract up-regulated the expression of molecular mediators that regulate wound healing
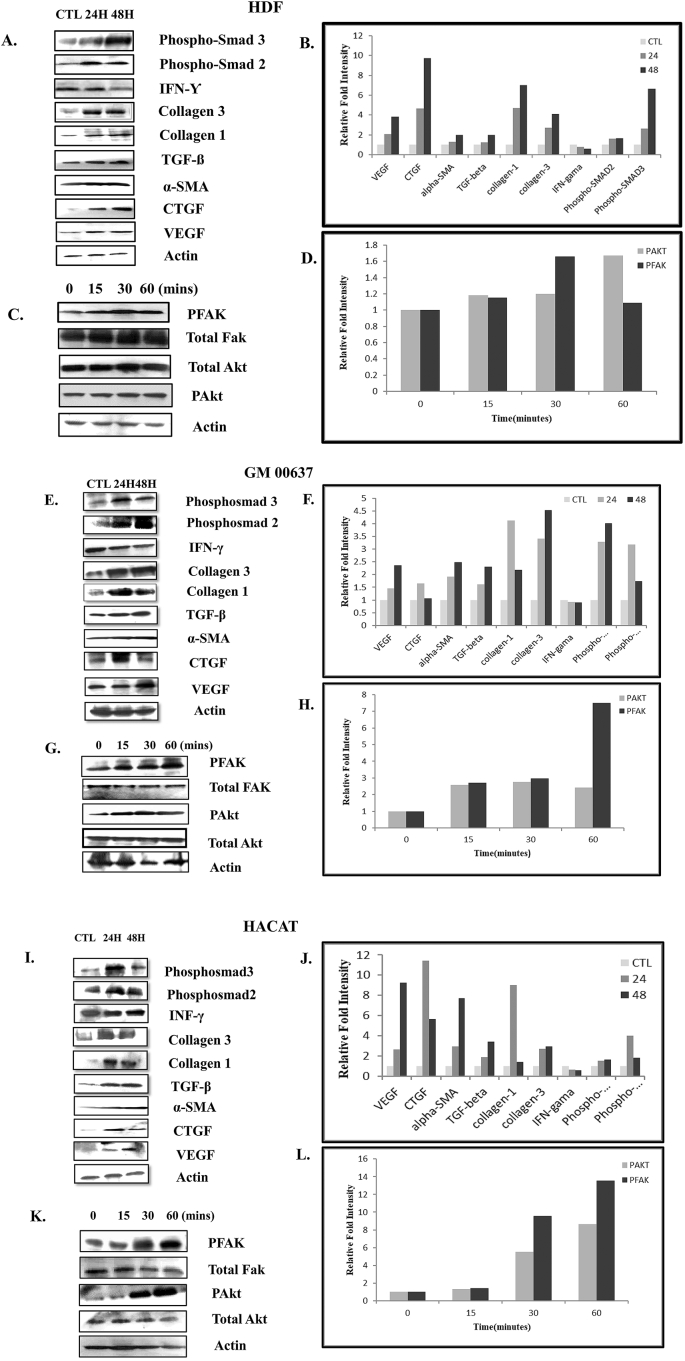
The significance of TGF-β in wound healing has been well established. Using Western blot analysis we observed that not only the expression of TGF-β but also phosphorylated-SMAD2 and phosphorylated-SMAD3 which are two important proteins in TGF-β signaling pathway, were increased compared to the control (Fig. 2A, 2B; 2E, 2F; 2I, 2J). We spotted increased expression of VEGF and CTGF in human fibroblasts and keratinocytes treated with plant extract compared to the mock-treated control. Expression of α-SMA, a marker of myofibroblasts responsible for wound contraction, was also seen to be enhanced (Fig. 2 A&B; E&F; I&J).
Fig. 2.
Shows the Western blot analysis on HDF, skin fibroblasts (GM00637) and keratinocytes (HACAT) cells.
HDF cell lysates from treated or mock treated cells were immunoblotted with different antibodies as indicated in Fig. 2A, 2C. Actin was used as loading control. The band intensity of the different proteins was quantified using ImageJ Software and the relative increase in expression of various proteins was plotted in Fig. 2B, 2D. The increase in the relative fold intensity was calculated considering untreated cells as 1 fold. Likewise, the result of Western blot analysis on GM00637 has been depicted in Fig. 2E to 2H and that of keratinocytes (HACAT) in Fig. 2I to 2L. Data from one of the three independent experiments with similar results is represented here. CTL- Control.
5.3. A. reticulata L. leaf extract augmented the expression of FAK with activation of AkT pathway
Studies have shown that high glucose in wound micro-environment inhibits phosphorylation of Focal Adhesion Kinase (FAK)22 and impairs the AkT pathway.23 Human fibroblast and keratinocytes treated with A. reticulata leaf extract showed an augmented level of p-FAK (Tyr 397) suggesting up-regulation of FAK expression in these cells; similarly, increased p-AkT (Ser 473) level indicated the activation of AkT pathway in the treated fibroblasts and keratinocytes (Fig. 2 C&D; G&H; K&L).
5.4. The extract increased the expression of collagen-3 and collagen-1 in human fibroblasts and keratinocytes along with up-regulation of MMP-2 and MMP-9
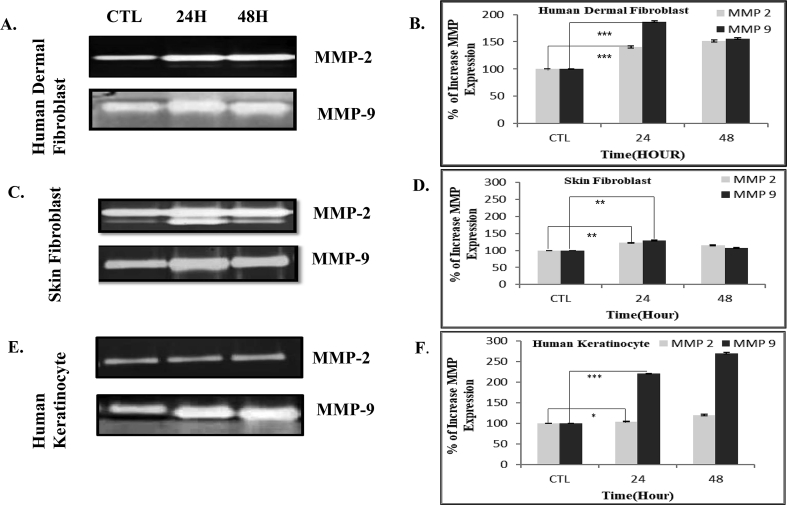
Collagen-3 and collagen-1 are the major components of scar tissue. Here, using immunoblotting in three different occasions, we observed that both collagen-3 and collagen-1 synthesis increased in extract treated fibroblasts and keratinocytes (Fig. 2 A&B; E&F; I&J).Both MMP-2 and MMP-9 have been linked to accelerate keratinocyte migration at the wound edge promoting re-epithelialization. We performed Gelatin Zymography to note that the expression of MMP-2 and MMP-9 was up-regulated significantly in the leaf extract treated human fibroblasts and keratinocytes compared to the control (Fig. 3).
Fig. 3.
A.reticulata leaf extract augments MMP-2 & MMP-9 expression.
Fig. 3A displays the expression of MMP-2 and MMP-9 by human dermal fibroblast, utilizing Gelatin Zymography. The band intensity of MMP-2 and MMP-9 was quantified using Image J Software and the relative increase in the expression of these two enzymes was plotted against time in Fig. 3B. Fig. 3C, 3D, 3E, 3F unveil the similar events in skin fibroblast cells (GM00637) and keratinocytes (HACAT) respectively. The results are expressed as mean ± S.D. of three independent experiments; * (P < 0.05), ** (P < 0.01) and *** (P < 0.001) in comparison to control (without extract). CTL- Control.
5.5. A. reticulata L. leaf extract accelerated wound healing in streptozotocin induced diabetic mice
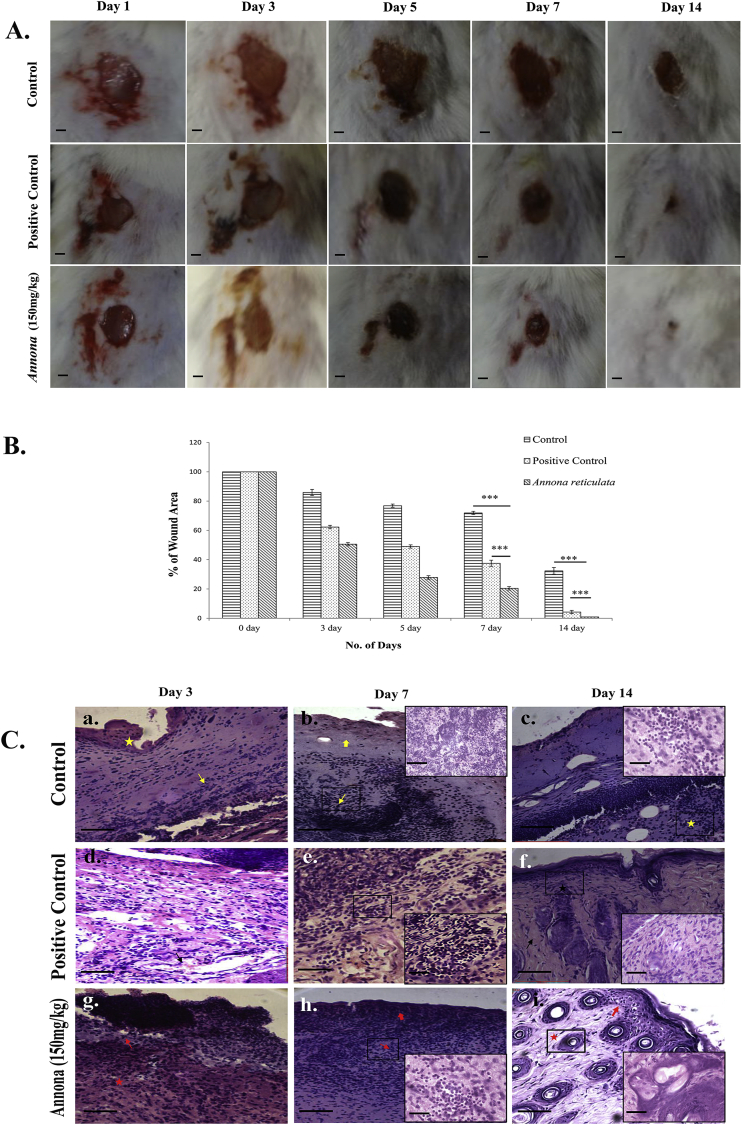
Animals treated with A. reticulata leaf extract showed accelerated healing compared to both placebo group and positive control group (mice treated with povidone iodine ointment) (Fig. 4A) Wound area was measured and plotted against the respective post wounding day (Fig. 4B). Percentage of wound contraction was also calculated (Table 2). As we can see from the Fig. 4A and 4B, in plant extract treated animals wound closure was almost complete (98.7 ± 0.7%) at 14th post wounding day; healing in the positive control group was also good (95.4 ± 1%). However significant amount of wound was still left in the placebo group (wound closure is 67.6 ± 1.1%).
Fig. 4.
A.reticulata leaf extract accelerates cutaneous wound healing.
(A) Shows full thickness excisional wounds from untreated/treated mice which were monitored on day 1, 3, 5, 7 and 14 post wounding and the corresponding images were taken with a digital camera (Sony Cyber-shot DSC-WX80. Scale bar = 4 mm. (B) The wound areas were calculated using ImageJ software and plotted against number of days post wounding. Results are expressed as mean ± SD; n = 6; * (P < 0.05), ** (P < 0.01), and *** (P < 0.001) in comparison to control. (C) Representative images of histological analysis of wound tissue by haematoxylin (H) and eosin (E) staining. Tissue sections from the wound milieu at different post wounding days (3, 7 and 14) in treated, un-treated and positive control (povidone iodine ointment) animals. Original magnification 100X, scale bar-50μm. Inset in higher magnification (400X and scale bar-10μm). Described in detail in the result section; sub-section −5.5.
Table 2.
Percentage of wound contraction on different day in different groups.
| Day | Control | Povidone Iodine | Extract Treated |
|---|---|---|---|
| Day 3 | 14.1 ± 4.7 | 40.9 ± 4.6* | 50.5 ± 2*a |
| Day 5 | 23.2 ± 1.8 | 53.5 ± 3* | 72.9 ± 1*a |
| Day 7 | 27.9 ± 0.9 | 63.5 ± 1.4* | 80.5 ± 1.4*a |
| Day 14 | 67.6 ± 1.1 | 95.4 ± 1* | 98.7 ± 0.7* |
All data are expressed as Mean ± SD (n = 6). (*) Signifies both plant extract and povidone Iodine caused statistically significant (p < 0.001) wound contraction compared to the control. (a) Signifies that the difference in wound contraction between povidone Iodine and plant extract was statistically significant. (p < 0.001).
Histological study supported our experimental findings. In the extract treated group, we noted accelerated bridging of the basal cell layer (red arrow) and early inflammatory granulation tissue (red asterisk) formation in the superficial dermis on day 3 (Fig. 4C,g). Section in the untreated group showed necrotic debris and fibrin (yellow asterisk) in the wound area with disrupted epidermis and few neutrophils and macrophages (yellow arrow) in the dermis (Fig. 4C,a). Ulcerated epidermis and edematous dermis with newly formed capillaries (black arrow) and fair number of neutrophil and macrophage infiltration were observed in the povidone iodine treated animals (Fig. 4C,b). At day 7, a regenerated epidermis (red broad arrow) with well-formed granulation tissue (red arrow) accompanied by fibroblastic proliferation in the dermis was noted in the treated group (Fig. 4C,h). However in the untreated animals, the regeneration of epidermis was incomplete (yellow broad arrow) and the granulation tissue was still in the developing phase. (Yellow arrow) (Fig. 4C,b). Povidone iodine treated animals showed a well-developed granulation tissue (Fig. 4C,e). On day 14 in the extract treated animals, the re-epithelialization of epidermis was complete (red arrow) and a well-healed dermis with newly formed skin adnexa (red asterisk) could be noted (Fig. 4C,i); positive control group animals showed healed dermis with marked fibrosis (black arrow) and skin adnexa in the early phase of formation (Fig. 4C,f); however granulation tissue (yellow asterisk) could still be seen in the dermis in the untreated group suggesting an incomplete healing in this group (Fig. 4C,c).
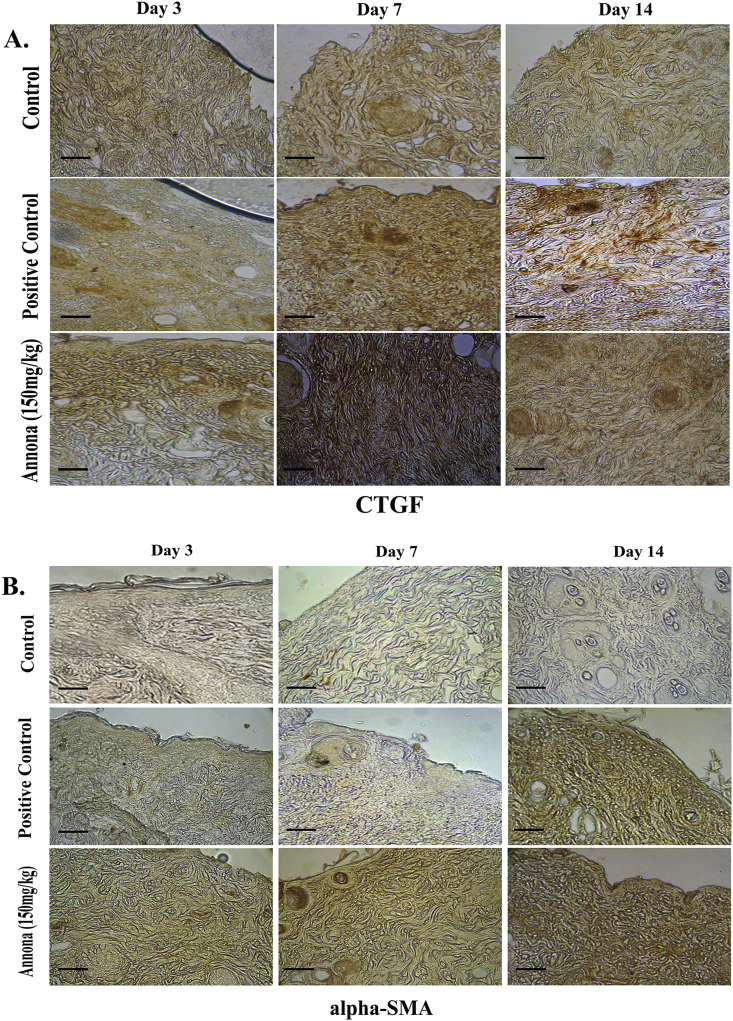
We then performed immunohistochemistry of wound tissue to determine the expression of CTGF and α-SMA proteins in the wound bed on day 3, day 7 and day 14. Expression of CTGF, seen as muddy brown discoloration, was more in both extract treated group and positive control group compared to the non-treated animals. Immunoreactivity was more at the wound edge. We also observed that CTGF expression enhanced in the early phase of healing (day 3, day 7) and then gradually diminished (day 14) (Fig. 5A). Similarly, immunohistochemical staining of α-SMA showed strong positivity in the extract treated animals implying increased expression. Expression of α-SMA both at and adjacent the wound edge indicated the uniform distribution of myofibroblasts in wound site. We noted that intensity of staining increased gradually starting from day 3 to day 14 suggesting the increasing population of myofibroblasts in the wound as it reaches its later phases. However, weakly positive staining for α-SMA was noted in untreated animals signifying delayed wound healing (Fig. 5B).
Fig. 5.
Immunohistochemical staining of wound tissue.
Immunostaining was performed with antibody against CTGF (A) and α-SMA(B)at different post wounding days (3, 7 and 14) in extract treated, un-treated and positive control (povidone iodine ointment) animals. In both the images immunoreactivity can be observed as brown discoloration. Magnification- 100 X in all the panels; scale bar- 50 μm.CTGF=Connective tissue growth factor; alpha-SMA = α-Smooth muscle actin.
5.6. Phytochemical composition ofA. reticulataleaf extract
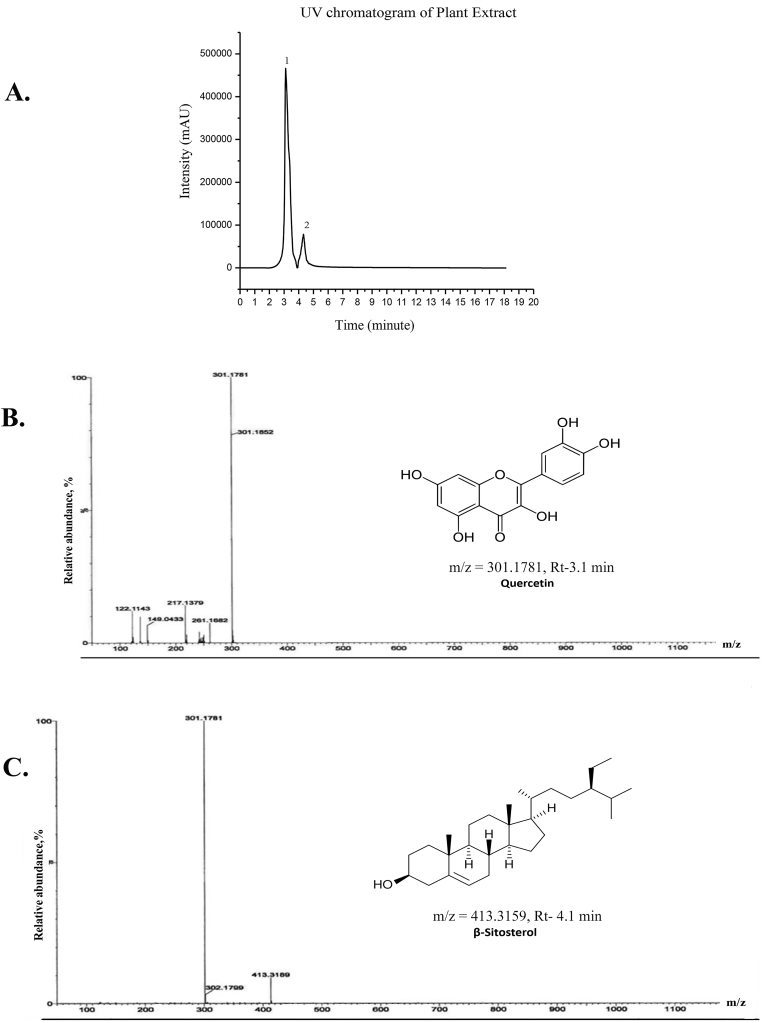
Samples collected from HPLC were marked as 1 and 2 (Fig. 6A) and were analyzed by electron spray ionization mass spectrometry. Compound marked 1 in Fig. 6A, eluting at retention time 3.1min, has a molecular ion peak at m/z = 301.1781 (Fig. 6B) being similar to that expected for quercetin (m/z = 302.238) whereas the compound marked 2 in Fig. 6A with a retention time 4.1min had the molecular ion peak at m/z = 413.3159 (Fig. 6C) being similar to β-sitosterol (m/z = 414.718).The concentration of quercetin in the experimental extract was found from the standard curve of quercetin (Fig. 6D; supplement-4) as 157.69 μg/mL.Similarly, using the standard curve, the concentration of β-sitosterol in the experimental extract was found to be as 30 μg/mL (Fig. 6E; supplement-4).Therefore the presence of quercetin and β-sitosterol in our extract was found to be 84.02% and 15.98% respectively.
Fig. 6.
HPLCfollowed by ESI-MS profile.
(A) HPLC chromatogram of A. reticulataleaf ethanol extract at 220 nm. The peaks are labeled according to the fractions collected and analyzed for ESI-MS (positive mode). (B) & (C) show the mass-spectrometric analysis of HPLC fractions with peaks corresponding to major phytochemicals present in 32% ethanol extract of A.reticulata Linn. leaf. Possible chemical structures of the two major components are shown.Rt- Retention time; m/z-mass to charge ratio.
6. Discussion
We noticed that study concerning the wound healing efficacy of A.reticulata L. leaf is inconspicuous and limited despite the fact that most of the Annona species have shown promising wound healing activity.17 In our study, we observed significantly faster wound closure after topical application of alcoholic extract of A.reticulata leaf compared to the untreated animals which was corroborated histologically by the early appearance of granulation tissue, rapid re-epithelialization and a well healed dermis with its adnexa.
CTGF is an extracellular matrix protein of the CCN family and its expression is distinctly up-regulated in fibroblasts present in the granulation tissue during wound repair.24 The increased expression of CTGF in the early phase of healing activates the signaling pathway that modulates all the phases of wound healing (from proliferative phase to remodeling phase) in a positive way.8 The enhanced expression of α-SMA during wound healing signifies accelerated wound contraction by myofibroblasts. In our Western blot analysis the extract treated HDF, GM00637 and HACAT cell line showed increased expression of CTGF and α-SMA, implying the wound healing capability ofA.reticulata L. leaf extract in hyperglycemic mice.
We investigated the efficacy of A. reticulata leaf extract in cell viability, cell proliferation and its ability to induce cell migration by MTT assay and Scratch assay respectively on HDF cells, skin fibroblasts (GM00637) and human keratinocytes (HACAT). A significant increment in fibroblast and keratinocyte migration rapidly covered the scratch wound.
Throughout the phases of wound healing, transforming growth factor beta (TGF-β) plays the most crucial role. In hemostasis and inflammation phase TGF-β recruits and activates inflammatory cells including neutrophils and macrophages whereas in proliferative phase it organizes multiple cellular responses including reepithelialization, angiogenesis, granulation tissue formation and extracellular matrix deposition.4,25It stimulates fibroblasts to proliferate and differentiate into myofibroblasts that partake in wound contraction in the remodeling phase.26 TGF-β exerts most of these actions via binding to a transmembrane receptor with serine/threonine kinase activity and subsequent phosphorylation of SMAD2, SMAD3 proteins.25 However, TGF-β mediated transition of fibroblast into myofibroblast occurs through activation of PI3K/AkT pathway.27 Malfunctioning of both the pathways leading to impaired phosphorylation of SMAD2, SMAD3, AkT (Protein Kinase B) has been observed in diabetic wound.23,28 Using Western blot technique we observed an enhanced expression of TGF-β as well as phospho-SMAD2, phospho-SMAD3, and phospho-AkT in extract treated fibroblasts and keratinocytes compared to the untreated cells, indicating wound healing capability of A. reticulataL leaf.
Another growth factor, VEGF has been investigated intensively in this context. It acts on its receptors, present on the vessels of the granulation tissue in a paracrine manner to cause basement membrane degradation, endothelial cell migration, proliferation, new capillary sprouts formation and new basement membrane formation.29 On the contrary, diminished expression or increased degradation of VEGF has been reported to be associated with defective healing. Despite an initial rise, a gradual decrease in the level of VEGF up to the point of undetectability has been noted in diabetic wounds30 and wounds of streptozotocin-induced diabetic mice.31 In this investigation, data from western Blot analysis unveils an upregulation in the expression of VEGF in keratinocyte and fibroblast cell line treated with A.reticulata leaf extract implying its wound healing attribute in vitro.
Focal adhesion kinase (FAK) is non-receptor, cytoplasmic tyrosine kinase involved in integrin dependent cytoskeleton mobilization and cell motility.6 Both migrating keratinocytes and fibroblasts show increased expression of FAK. It was observed that high glucose level inhibits cell migration by interrupting phosphorylation of FAK.22 Our data demonstrates enhanced phosphorylation of FAK both in keratinocytes and fibroblasts treated with A. reticulata L. leaf extract.
Both MMP-2 and MMP-9 are expressed by keratinocytes at the wound edge, conferring them the ability to dissociate from the basal lamina and migrate through the ECM and fibrin meshwork to complete the reepithelialization.12,13 Gelatin zymographic analysis in this study detected the presence of active form of MMP-2 and MMP-9 in the extract treated HACAT cell line suggesting an increased expression of MMP-2 and MMP-9 by keratinocytes.
Collagen, the cardinal component of connective tissue, is critical to wound healing as it provides the structural framework for tissue remodeling that contributes to wound strength. Schultz et al.reported that, During the early phase, type-3 collagen is more in the healing wound gradually replaced by type-1 collagen being the dominant collagen in adult scar.32 In our study, A reticulata treated fibroblasts produced a greater amount of collagen (both type-1 and type-3) compared to the control substantiating the efficacy of the extractin wound healing.
HPLC and mass spectrometry analysis of the crude ethanol (32%) extract of A.reticulata L. leaf unveiled the presence of two major compounds quercetin and β-sitosterolwhich is consistent with available literature15,33Sambucus ebulus L. is one of the best known medicinal herbs since ancient times and has been used in Turkish folk medicine. Bryophyllum pinnatum Lam. (crassulacea) is a perennial herb that grows in many parts of India including Karnataka. The leaves of both these herbs contain quercetin and have wound healing activity.34,35 The role of quercetin in augmentation of wound healing is well established in literature. It also increases the expression of VEGF and TGF-β which in turn potentiate wound healing.36,37 The other compound β-sitosterol aids re-epithelialization.38 It has been shown to possess angiogenic activity, anti-oxidant, and anti-diabetic effects all of which may contribute to a faster wound healing.39 Apart from these compounds, some investigators found acetogeninsin the leaf extract and that can promote wound healing.40,41 Terpenoids, another compound present in the leaves, may aid healing by producing free radicles in wound milieu.42
In conclusion, our data propounds that 32% ethanol extract of Annona reticulata Linn. leaf contains two major compounds that, either alone or in addition with the minor components present in the plant extract, can trigger the cellular events, critical to wound healing. However, this study is focused to establish the role of A.reticulata leaf extract in diabetic wound healing; therefore, future study and clinical trials need to be implemented for further elucidation.Nevertheless, to the best of our knowledge, this is probably the first study displaying the wound healing effect of Annona reticulata leaf extract.
Declaration of competing interest
Authors have none to declare.
Acknowledgements
This work was financially supported by DST Inspire fellowship program, Govt. of India and Department of Higher Education, Science & Technology and Biotechnology, Government of West Bengal (BT/ST/P/S&T/2G-13/2017).
Footnotes
Peer review under responsibility of The Center for Food and Biomolecules, National Taiwan University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jtcme.2019.12.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
References
- 1.Gosain A., DiPietro L.A. Aging and wound healing. World J Surg. 2004;28(3):321–326. doi: 10.1007/s00268-003-7397-6. [DOI] [PubMed] [Google Scholar]
- 2.Loots M.A.M. University of Amsterdam [Host]; Amsterdam: 2002. Wound Healing in Diabetic Ulcers. [Google Scholar]
- 3.Wetzler C., Kampfer H., Stallmeyer B., Pfeilschifter J., Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Investig Dermatol. 2000;115(2):245–253. doi: 10.1046/j.1523-1747.2000.00029.x. [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Qian Y., Jin R. Effects of TRAP-1-like protein (TLP) gene on collagen synthesis induced by TGF-β/smad signaling in human dermal fibroblasts. PLoS One. 2013;8(2) doi: 10.1371/journal.pone.0055899. Goumans MJ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li W., Kandhare A.D., Mukherjee A.A., Bodhankar S.L. Hesperidin, a plant flavonoid accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats: role of TGF-ß/Smads and Ang-1/Tie-2 signaling pathways. Excli J. 2018;17:399–419. doi: 10.17179/excli2018-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parsons J.T. Focal adhesion kinase: the first ten years. J Cell Sci. 2003;116(Pt 8):1409–1416. doi: 10.1242/jcs.00373. http://www.ncbi.nlm.nih.gov/pubmed/12640026 Accessed October 17, 2018. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Ma K., Kwon S.H. The abnormal architecture of healed diabetic ulcers is the result of FAK degradation by calpain 1. J Investig Dermatol. 2017;137(5):1155–1165. doi: 10.1016/j.jid.2016.11.039. [DOI] [PubMed] [Google Scholar]
- 8.Henshaw F.R., Boughton P., Lo L., McLennan S.V., Twigg S.M. Topically applied connective tissue growth factor/CCN2 improves diabetic preclinical cutaneous wound healing: potential role for CTGF in human diabetic foot ulcer healing. J Diabetes Res. 2015:1–10. doi: 10.1155/2015/236238. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stojadinovic O. A novel non-angiogenic mechanism of VEGF : stimulation of keratinocyte and fibroblast migration. Wound Repair Regen. 2007;15:A30. http://ci.nii.ac.jp/naid/20001385422/en/ Accessed October 5, 2018. [Google Scholar]
- 10.Elliott C.G., Forbes T.L., Leask A., Hamilton D.W. Inflammatory microenvironment and tumor necrosis factor alpha as modulators of periostin and CCN2 expression in human non-healing skin wounds and dermal fibroblasts. Matrix Biol. 2015;43:71–84. doi: 10.1016/j.matbio.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 11.Morgan C., Nigam Y. Naturally derived factors and their role in the promotion of angiogenesis for the healing of chronic wounds. Angiogenesis. 2013;16(3):493–502. doi: 10.1007/s10456-013-9341-1. [DOI] [PubMed] [Google Scholar]
- 12.Martins V.L., Caley M., O’Toole E.A. Matrix metalloproteinases and epidermal wound repair. Cell Tissue Res. 2013;351(2):255–268. doi: 10.1007/s00441-012-1410-z. [DOI] [PubMed] [Google Scholar]
- 13.Hattori N., Mochizuki S., Kishi K. MMP-13 plays a role in keratinocyte migration, angiogenesis, and contraction in mouse skin wound healing. Am J Pathol. 2009;175(2):533–546. doi: 10.2353/ajpath.2009.081080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jamkhande P.G., Wattamwar A.S. Annona reticulata Linn . (Bullock ’ s heart): plant profile , phytochemistry and pharmacological properties. J Tradit Complement Med. 2015;5(3):144–152. doi: 10.1016/j.jtcme.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak K., Zaman K. An overview on medicinally important plant - Annona reticulata linn. Int J Pharmacogn Phytochem Res. 2014;5(4):299–301. [Google Scholar]
- 16.Moghadamtousi S.Z., Rouhollahi E., Hajrezaie M., Karimian H., Abdulla M.A., Kadir H.A. Annona muricata leaves accelerate wound healing in rats via involvement of Hsp70 and antioxidant defence. Int J Surg. 2015;18:110–117. doi: 10.1016/j.ijsu.2015.03.026. [DOI] [PubMed] [Google Scholar]
- 17.Ponrasu T., Subamekala M.K., Ganeshkumar M., Suguna L. Role of Annona squamosa on antioxidants during wound healing in streptozotocinnicotinamide induced diabetic rats. J Pharmacogn Phytochem. 2013;2(4):77–84. [Google Scholar]
- 18.Bruggisser R., Von Daeniken K., Jundt G., Schaffner W., Tullberg-Reinert H. Interference of plant extracts, phytoestrogens and antioxidants with the MTT tetrazolium assay. Planta Med. 2002;68(5):445–448. doi: 10.1055/s-2002-32073. [DOI] [PubMed] [Google Scholar]
- 19.Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2(2):74. doi: 10.4103/0976-500X.81895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2015;70(1):5.47.1–5.47.20. doi: 10.1002/0471141755.ph0547s70. [DOI] [PubMed] [Google Scholar]
- 21.Ahanger A.A., Leo M.D., Gopal A., Kant V., Tandan S.K., Kumar D. Pro-healing effects of bilirubin in open excision wound model in rats. Int Wound J. 2016;13(3):398–402. doi: 10.1111/iwj.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura M., Osajima A., Nakayamada S. High glucose levels inhibit focal adhesion kinase-mediated wound healing of rat peritoneal mesothelial cells. Kidney Int. 2003;63(2):722–731. doi: 10.1046/j.1523-1755.2003.00772.x. [DOI] [PubMed] [Google Scholar]
- 23.Huang H., Cui W., Qiu W. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J Dermatol Sci. 2015;79(3):241–251. doi: 10.1016/j.jdermsci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 24.Frazier K., Williams S., Kothapalli D., Klapper H., Grotendorst G.R. Stimulation of fibroblast cell growth, matrix production, and granulation tissue formation by connective tissue growth factor. J Investig Dermatol. 1996;107(3):404–411. doi: 10.1111/1523-1747.ep12363389. http://www.ncbi.nlm.nih.gov/pubmed/8751978 Accessed October 17, 2018. [DOI] [PubMed] [Google Scholar]
- 25.Penn J.W., Grobbelaar A.O., Rolfe K.J. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma. 2012;2(1):18–28. http://www.ncbi.nlm.nih.gov/pubmed/22928164 Accessed October 17, 2018. [PMC free article] [PubMed] [Google Scholar]
- 26.Serrano I., McDonald P.C., Lock F.E., Dedhar S. Role of the integrin-linked kinase (ILK)/Rictor complex in TGFβ-1-induced epithelial–mesenchymal transition (EMT) Oncogene. 2013;32(1):50–60. doi: 10.1038/onc.2012.30. [DOI] [PubMed] [Google Scholar]
- 27.Li G., Li Y.-Y., Sun J.-E., Lin W., Zhou R. ILK–PI3K/AKT pathway participates in cutaneous wound contraction by regulating fibroblast migration and differentiation to myofibroblast. Lab Investig. 2016;96(7):741–751. doi: 10.1038/labinvest.2016.48. [DOI] [PubMed] [Google Scholar]
- 28.Kim B.-C., Kim H.T., Park S.H. Fibroblasts from chronic wounds show altered TGF-?-signaling and decreased TGF-? Type II Receptor expression. J Cell Physiol. 2003;195(3):331–336. doi: 10.1002/jcp.10301. [DOI] [PubMed] [Google Scholar]
- 29.Bao P., Kodra A., Tomic-Canic M., Golinko M.S., Ehrlich H.P., Brem H. The role of vascular endothelial growth factor in wound healing. J Surg Res. 2009;153(2):347–358. doi: 10.1016/j.jss.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lauer G., Sollberg S., Cole M. Expression and proteolysis of vascular endothelial growth factor is increased in chronic wounds. J Investig Dermatol. 2000;115(1):12–18. doi: 10.1046/j.1523-1747.2000.00036.x. [DOI] [PubMed] [Google Scholar]
- 31.Shukla A., Dubey M.P., Srivastava R., Srivastava B.S. Differential expression of proteins during healing of cutaneous wounds in experimental normal and chronic models. Biochem Biophys Res Commun. 1998;244(2):434–439. doi: 10.1006/bbrc.1998.8286. [DOI] [PubMed] [Google Scholar]
- 32.Schultz G.S., Davidson J.M., Kirsner R.S., Bornstein P., Herman I.M. Dynamic reciprocity in the wound microenvironment. Wound Repair Regen. 2011;19(2):134–148. doi: 10.1111/j.1524-475X.2011.00673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CISR . The Wealth of India. Revised Ed. 2003. Anonymous. Raw materials; pp. 279–294. New Delhi. [Google Scholar]
- 34.Süntar I.P., Akkol E.K., Yalçin F.N., Koca U., Keleş H., Yesilada E. Wound healing potential of Sambucus ebulus L. leaves and isolation of an active component, quercetin 3-O-glucoside. J Ethnopharmacol. 2010;129(1):106–114. doi: 10.1016/j.jep.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 35.Khan M., Patil P.A., Shobha J.C. Influence of Bryophyllum pinnatum (Lam.) leaf extract on wound healing in albino rats. J Nat Remedies. 2004;4(1):41–46. doi: 10.18311/jnr/2004/380. [DOI] [Google Scholar]
- 36.Ahmed O.M., Mohamed T., Moustafa H., Hamdy H., Ahmed R.R., Aboud E. Quercetin and low level laser therapy promote wound healing process in diabetic rats via structural reorganization and modulatory effects on inflammation and oxidative stress. Biomed Pharmacother. 2018;101:58–73. doi: 10.1016/j.biopha.2018.02.040. [DOI] [PubMed] [Google Scholar]
- 37.Gopalakrishnan A., Ram M., Kumawat S., Tandan S., Kumar D. Quercetin accelerated cutaneous wound healing in rats by increasing levels of {VEGF} and {TGF}-β1. Indian J Exp Biol. 2016;54(3):187–195. http://www.ncbi.nlm.nih.gov/pubmed/27145632 Accessed May 4, 2019. [PubMed] [Google Scholar]
- 38.Jewo P.I., Fadeyibi I.O., Babalola O.S. A comparative study of the wound healing properties of moist exposed burn ointment (MEBO) and silver sulphadiazine. Ann Burns Fire Disasters. 2009;22(2):79–82. http://www.ncbi.nlm.nih.gov/pubmed/21991159 Accessed May 4, 2019. [PMC free article] [PubMed] [Google Scholar]
- 39.Saeidnia S. The story of beta-sitosterol- A review. Eur J Med Plants. 2014;4(5):590–609. doi: 10.9734/EJMP/2014/7764. [DOI] [Google Scholar]
- 40.Padmaa M Paarakh1, Chansouria J.P.N.2, Khosa R.L.3. Wound healing activity of Annona muricata extract. J Pharm Res. 2015;2(3):404–406. [Google Scholar]
- 41.Moghadamtousi S.Z., Fadaeinasab M., Nikzad S., Mohan G., Ali H.M., Kadir H.A. Annona muricata (Annonaceae): a review of its traditional uses, isolated acetogenins and biological activities. Int J Mol Sci. 2015;16(7):15625–15658. doi: 10.3390/ijms160715625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agra L.C., Ferro J.N.S., Barbosa F.T., Barreto E. Triterpenes with healing activity: a systematic review. J Dermatol Treat. 2015;26(5):465–470. doi: 10.3109/09546634.2015.1021663. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.