Abstract
Somatic embryogenesis is an important and wonderful biotechnological tool used to develop whole plant from a single or a group of somatic cells. The differentiated somatic cells become totipotent stem cells by drastic reprogramming of a wide range of cellular activities, leading to the acquisition of embryogenic competence. After acquiring competence, the cells pass through globular, heart, torpedo and cotyledonary stages of embryo; however, all advanced embryos do not convert into full plant, produce adventive embryos or callus instead, thus reverses the programming. This is a big limitation in propagation of many plants. Understanding and unraveling the proteins at this ‘embryo to plantlet’ transition stage will help to get more numbers of plants. Thus, our study was aimed at an identification of differentially abundant proteins between two important advanced stages, i.e. cotyledonary—(T1) and maturation stage (T2) of somatic embryos in Catharanthus roseus. A total of 2949 and 3030 proteins were identified in cotyledonary and maturation stage, respectively. Of these, 1129 proteins were common to both. Several proteins were found to be differentially accumulated in two different embryo stages in which over 60 proteins were most accumulated during somatic embryo maturation time. More chlorophyll accumulation was noted at this time under the influence of gibberellic acid (GA3). Proteins like Mg-protoporphyrin IX chelatase, chlorophyll a–b-binding protein, photosystem I iron-sulfur center, photosystem II Psb, photosystem II subunit P-1, P-II domain-containing protein, RuBisCO large chain, RuBisCO small chain, RuBisCO activase, RuBisCO large subunit-binding proteins were synthesized. Some of the identified proteins are linked to chlorophyll synthesis, carbohydrate metabolism and stress. The identified proteins are categorized into different groups on the basis of their cellular location, role and other metabolic processes. Biochemical attributes like protein, sugar, proline, antioxidant enzyme (APX, SOD and CAT) activities were high in T2 as compared to T1. The proteins like peroxidases, pathogenesis-related proteins, the late-embryogenesis abundant proteins, argonaute, germin and others have been discussed in C. roseus somatic embryo maturation process.
Keywords: Somatic embryos, Cotyledonary stage, Stress protein, Pathogenesis-related proteins, Embryogenesis abundant proteins, Gibberellic acid, Shotgun, Gel-free proteomic method
Introduction
Somatic embryogenesis (SE) is an in vitro tool for plant development from a single or a group of somatic cells, studied extensively for last few decades (Guan et al. 2016). It has immense applications in plant biotechnology, especially in the area of threatened and endemic plant propagation, synthetic seed development, preservation of germplasm, crop improvement, and serves as a model for understanding plant growth but the mechanism underlying SE acquisition is still not understood at molecular level (Gulzar et al. 2020). For understanding the mechanism of SE process, in-depth studies have been made at various, i.e. morphological, physiological, biochemical and molecular level (Rupps et al. 2016; Orłowska et al. 2017; Gulzar et al. 2019) by exploiting genomics, transcriptomics and proteomics; however, the information is still found incomplete (Feher 2015).
As the somatic cell transforms into totipotent stem cell, i.e. to attain embryogenic competence, there is a drastic change in cell’s molecular networks (Guan et al. 2016). The reprogramming starts at genomic levels and in vitro stress conditions act as the main driver in switching on the expression of several genes (Jin et al. 2014). The prerequisite for SE initiation is the activation of DNA segments to transcriptional machinery, which was earlier silenced by DNA heterochromatinisation (Fehér et al. 2003; Altamura et al. 2016). The developmental plasticity shown by the plant cells in culture is due to varying expression levels of genes of different nature. A significant number of transcription factors and the genes or gene networks were reported to be active, but the intricate process of SE is still not elucidated well. One of the important things in the study of SE is to know when and how the SE occurs and to identify the embryogenic cells in culture. The identification of embryogenic cells from the mass of cells and the distinction from the non-embryogenic tissue is often difficult. To overcome such difficulties, there is a need to develop markers that can identify the embryogenic cells easily and one of the important strategies in this direction is to use protein markers that could easily identify the right tissues. Preparation of embryo-specific proteomic maps may help in identifying proteins, common to embryos of all investigated plant types. Proteomic study using LC–MS in plants like Araucaria angustifolia (Fraga et al. 2016), Coffea arabica (Campos et al. 2017), Gossypium hirsutum (Ge et al. 2015), Larix principis-rupprechtii (Zhao et al. 2015), Picea asperata (Jing et al. 2016), Picea balfouriana (Li et al. 2015), Saccharum spp. (Heringer et al. 2015), Catharanthus roseus (Gulzar et al. 2019) and Zea mays (Ge et al. 2017) is a step forward towards this direction. Heringer et al. (2015) recently proposed 41 proteins as potential markers for somatic embryos in sugarcane. The encoding proteins of various genes like SOMATIC EMBRYGENESIS RECEPTOR KINASE, SERK (Schmidt et al. 1997), LEAFY COTYLEDON, LEC (Curaba et al. 2014; Gazzarrini et al. 2004; Gaj et al. 2005), BABY BOOM, BBM (Boutilier et al. 2002), and WUSCHEL, WUS (Zuo et al. 2002), etc. were identified in promoting in vitro azygotic embryogenesis. Similarly, the chitinases, osmotin-like protein, and glucanase have also been among the important macromolecules noted to be linked with SE (Helleboid et al. 2000). In Picea asperata, partial desiccation treatment (PDT) stimulated embryo maturation, germination and conversion of somatic embryo where stress-related proteins were involved (Jing et al. 2016). Genome-wide transcriptomic study indicated that the embryogenesis is associated with higher rate of transcriptional activity (Radoeva et al. 2019).
The plant C. roseus was selected in our study because it is one of the model plants in alkaloid enrichment program; it also encourages in studying plant growth by exploiting unique SE incidence. The SE process composes of initiation, maturation and germination of somatic embryos and, in dicot, a few distinct stages of somatic embryos are noted (Lu et al. 2017). In this plant, the initiation and development of somatic embryo occur under the influence of auxin and cytokinin and the embryo maturation needs the amendment of GA3. In each stage, differential gene expression is accomplished and the proteins of diverse nature and functions appear in developing stages of embryos with varying abundances. The current study describes and presents the protein maps/profiles of two important advanced embryo stages, i.e. cotyledonary and maturation in C. roseus.
Materials and methods
Seed germination and establishment of culture
The C. roseus (L.) G. Don. seeds were collected from the herbal garden, Department of Botany, Jamia Hamdard (New Delhi). The seeds were soaked with 70% ethanol for two min, treated with 0.1% HgCl2 for 4 min and were washed thrice with sterilized distilled water. The seeds, after sterilization were inoculated on solidified 50 ml MS medium (Murashige and Skoog 1962) without plant growth regulator (PGR) in conical flask (Junaid et al. 2008; Mujib et al. 2014). The seeds were germinated and seedlings were allowed to grow for a few weeks and repeatedly transferred to new media until the seedling showed significant growth from where various explants were taken. Different explants (leaf, stem and hypocotyl) were inoculated in test tubes containing solidified 8.0 ml MS medium supplemented with 2 mg/l 2,4-dichlorophenoxyacetic acid (2,4-d) for callus induction (Junaid et al. 2008). After 5 weeks, sub-culturing of induced callus was made in MS without any change in PGR concentration. Somatic embryos were induced after application of 2.0 mg/l BAP (N6-benzyladenine) and 1.5 mg/l NAA (naphthalene acetic acid) in MS medium (Junaid et al. 2008). Within 2–3 weeks, only the hypocotyl-induced callus developed globular and other early stages of embryos. The calli derived from the other explants remained non-embryogenic. Embryos were proliferated by sub-culturing repeatedly. Cotyledonary embryos were obtained in significant numbers after twenty days of embryogenic culture. Some of the cotyledonary embryos were transferred to the MS medium added with 2.0 mg/l GA3 (gibberellic acid) for embryo maturation (Junaid et al. 2008). After 20 days of GA3 treatment, green mature embryos were observed in culture medium.
Protein extraction from tissues
The working samples of cotyledonary—and green matured embryos were drawn separately for the extraction of total protein. For the protein extraction, Isaacson et al. (2006) protocol (phenol method) was followed. 3 g each of cotyledonary and matured embryos (with three replicates) was crushed to fine powder using liquid nitrogen in pre-chilled mortar with a pestle. The fine powder obtained was transferred to Oak Ridge tubes where powder was suspended in 10 ml extraction buffer, a solution of pheny lmethane sulfonyl fluoride (PMSF), 4-(2-hydroxyethyl)-1-piperazine ethane sulfonic acid (HEPES), β-mercaptoethanol and sucrose. The solution was incubated for 2–3 min in ice and then vortexed for 1.5 min. This was again vortexed for another 2 min after addition of 15 ml of phenol. The solution was incubated for 30 min on an orbital shaker, in sealed Oak Ridge tubes embedded in ice tray. For 10 min, the solution was centrifuged at 5500 rpm and 4 °C. The phenolic phase (upper portion of solution) was carefully transferred to the separate tubes; and then 15 ml of ice-cold ammonium-acetate solution (0.1 M) was added and incubated at − 20 °C overnight for protein precipitation. The whitish pellets of proteins were obtained by centrifuging at 10,000 rpm for 15 min at 4 °C. The pellet was washed thrice, first by ice-cold methanol with an incubation period of 1 h. After centrifugation at 10,000 rpm for 10 min at 4 °C, the pellet was re-suspended in ice-cold acetone and then again incubated for 1 h. The protein was again pelleted at 10,000 rpm for 10 min at 4 °C and the same step was repeated third time. The whitish pellets were carefully solubilized in 6 M guanidium chloride, dissolved in 0.1 M Tris–HCl with pH 8.5 in fresh 2 ml Eppendorf tubes. For 10 min, the solution of 6 M guanidium chloride was boiled at 100 °C and then 200 µl solution was added to each 2 ml Eppendorf tube containing pellet. After vortexing for 2 min, the samples were boiled for 4–6 min. The solution was vortexed again for complete solubilization and finally the debris was pelleted out by centrifuging at 10,000 rpm for 10 min. The upper liquid phase (supernatant) was transferred to fresh tubes and stored at − 80 °C.
Sample preparation for LC–MS
For lysis, the sample was heated at 90 °C for 10 min. The sample was precipitated using trichloroacetic acid (TCA) acetone. The obtained precipitate was washed thrice with ice-cold acetone and then directly solubilized in 0.1% Rapigest (water) in 50 mM ammonium bicarbonate (ABC) (pH ~ 7). Reduction was carried out using 5 mM Tris (2-carboxyethyl) phosphine (TCEP) (sigma) in 25 mM ABC (pH ~ 7) at 37 °C for 30 min the alkylation of the samples was done at room temperature by 55 mM iodoacetamide (IAA) (Sigma) in 25 mM ABC (pH ~ 7) in dark for 30 min. The Trypsin (Promega) in 1:50 ratio was used for digestion and kept at 37 °C overnight. The sample was spun at 13,400 rpm for 10 min to remove the Rapigest. The supernatant was collected in a fresh tube (pellet discarded) for desalting column. Desalting of samples was done according to manufacturer’s protocol (https://assets.thermofisher.com/TFSAssets/LSG/manuals/MAN0011495_Pierce_C18_SpinCol_UG.pdf). After vacuum-drying by speedvac, the samples were finally reconstituted in 0.1% Formic Acid (FA). The column [PepMap RSLC C18 2 µm × 50 cm (Thermo scientific)] was loaded with ~ 3 µg amount of peptide and temperature of column was set at 40 °C. The A (water + 0.1% formic acid) and B (acetonitrile + 0.1% formic acid) were the mobile phases for two solvent gradient elution. All the solvents used were of LC–MS grade (Fischer Scientific). The internal calibration was done using Lock mass of 445.12003 Da. The Mass Spectroscopy, MS (Thermo Scientific Q Exactive Orbitrap), optimized run time was 0–123 min, with positive polarity and two as a default charge state. The MS (MS1MS2) optimized settings were used. For instance, microscan 1 for both, resolution was 70,000 and 17,500, respectively, for MS1 and MS2, AGC (Automatic gain control) 3e6 for MS1and 1e5 for MS2, maximum IT (ion transfer) time was 60 ms and 120 ms, respectively. The number of scan ranges was 1 with scan ranges of 350 to 2000 m/z and spectrum was obtained in profile mode. The loop count 10 and maximum number of precursor to be plexed in single event was 1. The isolation window 1.5 m/z and isolation offset 0.0 m/z were used. The fixed first mass 100 m/z and normalized collision energy 27 with the data dependent settings as minimum AGC target was 1.00e2. The charge exclusion was unassigned whereas 50.0 S was the dynamic exclusion time.
The C18 reverse-phase column was loaded by ~ 3 µg of sample and the raw data obtained were analyzed. The precursor mass tolerance was 10 ppm, fragment mass tolerance 0.05 Da, dynamic modification—oxidation of methionine and acetylation at N terminus of proteins, carbamido methylation was the static modification, target FDR (false discovery rate) 0.01 (for decoy database search) and validation were based on q value. The proteins were identified through UniProt Knowledgebase, UniProtKB (Swiss-Prot) downloaded on16 June, 2018. MASCOT tool (www.matrixscience.com) and following search criteria/parameters were used: Swissprot/ NCBIprot for database, Trypsin for enzyme, viridiplantae for taxonomy. The proteome data will be submitted to ProteomeXchange Consortium after scrutinizing important related information.
Biochemical analysis
Bradford (1976) method was followed for protein estimation. The sample 0.1 g of cotyledonary and mature embryos was separately homogenized in 2 ml phosphate buffer (0.1 M, pH 7.0) in ice-cold conditions, using pre-chilled mortar and pestle. The homogenate was centrifuged at 10,000 rpm for 10 min. The supernatant (1 ml) was taken and 1 ml of trichloroacetic acid (10%) was added, and centrifuged at 5000 rpm for 10 min. The pellets were washed with acetone 2–3 times and then dissolved by adding 1.0 ml of NaOH (0.1 N). The aliquot (1.0 ml) was taken in fresh tube with an addition of 1.0 ml of Bradford reagent, and finally the OD was measured at 595 nm. The concentration of proteins was expressed in mg/g fresh weight.
Bates et al. (1973) protocol with some modifications was followed for proline estimation. About 0.15 g of cotyledonary and mature embryos was separately homogenized in 2.0 ml of 3.0% aqueous sulphosalicylic acid under ice-cold conditions. After filtration of homogenate with Whatman filter paper (No. 1), 1.0 ml filtrate was transferred to fresh tubes and 1.0 ml of each ninhydrin and glacial acetic acid was added. For 1 h, reaction mixture was incubated at 100 °C and subsequently the reaction was terminated in ice. Finally, 2.0 ml toluene was added to the terminated reaction mixture and quantification of proline content in mg/g fresh weight (FW) was done by taking optical density (OD) at 520 nm.
For sugar estimation, Dey (1990) method was used. The cotyledonary and mature embryos of 0.1 g were separately taken in test tubes, 5 ml ethanol (90%) was added and incubated for 1 h at 60 °C. Final volume of extract was made up to 10 ml. An aliquot of 0.5 ml was mixed with 0.5 ml of phenol (5%) and added with 1.0 ml of concentrated sulphuric acid. The mixture was cooled in air and optical density was measured at 485 nm. The concentration of sugar was expressed mg/g fresh weight.
The cotyledonary and mature embryos of 0.2 g sample were separately homogenized in 2.0 ml of 0.1 M extraction buffer (0.5 mM EDTA, 0.1 M K-phosphate, 1.0 mM ascorbic acid) with pH 7.5. The solution was centrifuged at 10,000 rpm for 10 min. The supernatant was carefully transferred to the fresh tubes and used for enzyme analysis.
Aebi’s method (1984) was followed for determining catalase (CAT) activity by measuring a decrease in the absorbance at 240 nm of reaction mixture containing 1.0 ml of 0.5 M reaction phosphate buffer with pH 7.5, 0.1 ml EDTA, 0.2 ml enzyme extract and 0.1 mlH2O2. For 3 min, the reaction was run and one unit of enzyme is the amount necessary to decompose 1.0 μM of H2O2 per min. The CAT activity was expressed in EU mg−1 protein.
The ascorbate peroxidase (APX) activity was determined by following the method developed by Nakano and Asada (1981). A mixture of 0.1 ml of enzyme extract, 1.0 ml sodium phosphate buffer (0.1 M, pH 7.2) and 0.1 ml of EDTA was taken in a fresh test tube and 1.0 ml of 0.5 mM ascorbate was added and the reaction was run at 25 °C for 3 min. The activity of APX was measured from decrease in absorbance due to the breakdown of ascorbate by APX, which was expressed in EU mg−1 protein.
The method developed by Dhindsa et al. (1981) was followed to estimate the superoxide dismutase (SOD) activity with certain modifications. The cotyledonary and mature embryos of 0.1 g each were separately homogenised in 2.0 ml of extraction mixture. The extraction mixture was prepared by mixing the 0.5 M phosphate buffer (pH 7.3), 3.0 mM EDTA, 1.0% (w/v) polyvinylpyrollidone (PVP) and 1.0% (v/v) Triton X-100. The homogenate was centrifuged at 10,000 rpm for 10 min. The supernatant was carefully transferred to fresh tube and 0.1 ml aliquot taken with an addition of 1.5 ml reaction buffer followed by the 0.2 ml methionine, 0.1 ml each of 1.0 M NaCO3, 2.25 mM Nitro Blue Tetrazolium solution. The mixture was further added with the 3.0 mM EDTA, riboflavin, 1.0 ml of Millipore H2O and was incubated for 10 min under light at room temperature. The absorbance was checked at 560 nm and 50% reduction in colour is equal to 1.0 unit, and finally the SOD activity was expressed in EU mg−1 protein.
Statistical analysis
The experiments were kept under randomized block design and the data on the effect of PGRs on various stages of somatic embryogenesis were studied and represented as mean ± standard error. The experiments on biochemical attributes, such as protein, sugar, proline, antioxidant enzymes APX, SOD, CAT and other activities, were conducted in three replicates (test tubes/petriplates) and each set of experiment was replicated twice. Data were subject to one-way analysis of variance (ANOVA) and the mean values were separated using Duncan’s Multiple Range Tests (DMRT) at p = 0.05. The statistical analysis was made using SPSS Ver 16.0 (SPSS Inc, USA) software package. The LC–MS obtained protein raw data were analyzed through Proteome Discoverer User Guide Software Version 2.2, XCALI-97808, Revision A, June 2017 (ThermoScientific). A freely available online tool MetaboAnalyst was used to generate heat map, which represents protein expression level based on spot volume.
Results and discussion
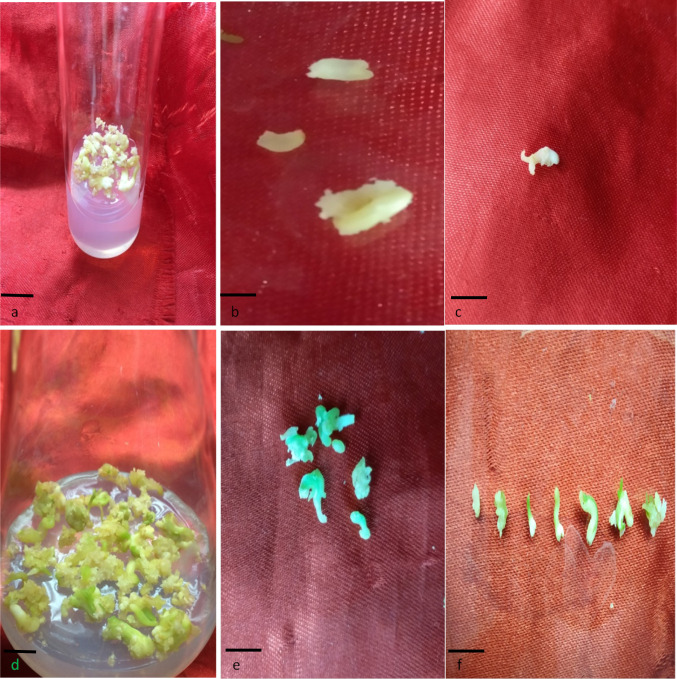
After 20 days of application of 2.0 mg/l BAP and 1.5 mg/l NAA to callus, the somatic embryos of different transitional stages were observed. The white, blister-like globular and spindle-shaped elongated embryos were noted; some of these embryos attained torpedo and cotyledonary stage (T1) after a few weeks (Fig. 1a–c). On treatment with 2.0 mg/l GA3, the cotyledonary embryos started to develop small minute green mature embryos (T2). These minute, green (mature) embryos were quite different from other induced embryo forms. After 24 days of treatment, the green mature embryos turned into complete bipolar structures having tapering brownish radicle end and green cotyledonary elongated plumule or shoot end (Fig. 1d–f). After 35 days of GA3 treatment, the tender plantlets were produced.
Fig. 1.
Cotyledonary and maturation stages of SE, a cotyledonary embryos in medium containing 2 mg/l BAP and 1.5 mg/l NAA; b, c cotyledonary embryos in magnified view d: mature green embryos in mediun containing 2.0 mg/l GA3, e, f mature green embryos in magnified view (Bars a 0.5 cm; b, c 1 cm; d 0.5 cm; e, f 1 cm)
In this present study, we tried to monitor and compare the proteomic maps/differences between cotyledonary and green mature embryos of C. roseus; and over 2949 and 3030 proteins were identified in T1 and T2, respectively. Of these, 1129 proteins were found to be common in both stages. Several proteins were present exclusively in T1 and T2, of which 141 and 128, respectively, were most important. Among the proteins found in both the stages of SE, more than 60 proteins were accumulated most in T2 with abundance of more than 2.5-fold in T2/T1 (Table 1). For better understanding and convenience, the proteins were grouped into different categories and are presented in Table 2.
Table 1.
Some of the differentially abundant proteins (accession numbers) with abundance ratios (T2/T1), most accumulated during SE maturation process
| Checked | Protein FDR confidence: combined | Accession | Protein name | Exp. q value: combined | # AAs | MW (kDa) | calc. pI | Abundance ratio: (T2)/(T1) |
|---|---|---|---|---|---|---|---|---|
| True | High | A0A1J7GJV4 | 3-Oxoacyl [acyl-carrier-protein] synthase | 0 | 467 | 49.5 | 7.94 | 100 |
| True | High | A0A1Q3BF12 | Peptidase_M24 domain-containing protein | 0 | 394 | 43.7 | 7.03 | 100 |
| True | High | A0A1Q3B1G0 | Peptidase_S8 domain-containing protein | 0 | 794 | 84.9 | 7.47 | 100 |
| True | High | A0A200QB76 | DapA-like | 0 | 357 | 39.1 | 6.9 | 100 |
| True | High | A0A218XUW7 | NmrA domain-containing protein | 0 | 308 | 33.8 | 5.6 | 12.246 |
| True | High | A0A1D1YNK4 | Peroxidase | 0 | 350 | 37.8 | 7.27 | 10.91 |
| True | High | A0A218XUJ8 | Malate dehydrogenase | 0 | 349 | 36.7 | 8.63 | 10.545 |
| True | High | A0A1Q3BBS1 | GST_C domain-containing protein/GST_N_3 domain-containing protein | 0 | 451 | 51.4 | 5.6 | 7.907 |
| True | High | A0A061EYD1 | Polygalacturonase inhibitor | 0 | 330 | 36.9 | 8.16 | 7.868 |
| True | High | A0A218VUG5 | Peroxidase | 0 | 371 | 40.3 | 5.6 | 7.767 |
| True | High | A0A0K9RAT7 | Malate dehydrogenase | 0 | 335 | 36.3 | 6.24 | 7.432 |
| True | High | A0A0K9QNV9 | Fructose-bisphosphate aldolase | 0 | 357 | 38.4 | 6.28 | 7.037 |
| True | High | A0A218W016 | Uncharacterized protein | 0 | 686 | 73.7 | 6 | 7.015 |
| True | High | A0A200Q9C9 | Alkyl hydroperoxide reductase subunit C/Thiol specific antioxidant | 0 | 248 | 27.6 | 6.8 | 6.752 |
| True | High | A0A1J3J622 | Isocitrate dehydrogenase (NAD) regulatory subunit 1, mitochondrial | 0 | 414 | 45.2 | 8.65 | 6.36 |
| True | High | A0A1Q3B2T7 | Snf7 domain-containing protein | 0 | 218 | 24.2 | 4.89 | 6.231 |
| True | High | A0A1R3HTG2 | Heme peroxidase, plant/fungal/bacterial | 0 | 225 | 24.7 | 6.68 | 6.181 |
| True | High | A0A1J7I946 | Fructose-bisphosphate aldolase | 0 | 358 | 38.6 | 6.99 | 6.16 |
| True | High | A0A1U8AQM2 | Peroxidase | 0 | 358 | 38.7 | 8.43 | 5.988 |
| True | High | A0A1D5VD11 | Fructose-bisphosphate aldolase | 0 | 358 | 38.9 | 6.79 | 5.686 |
| True | High | A0A218VUH2 | Fructose-bisphosphate aldolase | 0 | 358 | 38.5 | 8.28 | 5.225 |
| True | High | A0A1Q3CIP2 | Beta-amylase | 0 | 513 | 58.3 | 5.31 | 5.025 |
| True | High | A0A0K9RRM3 | Peptidyl-prolyl cis–trans isomerase | 0 | 260 | 28.4 | 7.59 | 4.826 |
| True | High | A0A1Q3B8N6 | Malate dehydrogenase | 0 | 332 | 35.6 | 6.64 | 4.659 |
| True | High | A0A0R0JF58 | Peptidyl-prolyl cis–trans isomerase | 0 | 172 | 18.2 | 8.27 | 4.554 |
| True | High | A0A200R5R3 | Adenosine kinase | 0 | 342 | 37.6 | 6.42 | 4.501 |
| True | High | A0A078HTW7 | BnaA01g32170D protein | 0 | 277 | 30.9 | 7.28 | 4.445 |
| True | High | A0A1Q3CMR6 | Fructose-bisphosphate aldolase | 0 | 357 | 38.5 | 7.64 | 4.404 |
| True | High | A0A1J3IYX1 | Arginase 1, mitochondrial (Fragment) | 0 | 382 | 41.9 | 6.64 | 4.21 |
| True | High | A0A218WGP4 | Uncharacterized protein | 0 | 249 | 26.9 | 8.47 | 4.041 |
| True | High | A0A1J0A0N7 | Elongation factor 1-gamma | 0 | 423 | 48.2 | 6.16 | 3.918 |
| True | High | A0A218WCG3 | Peroxidase | 0 | 322 | 34.8 | 9.39 | 3.616 |
| True | High | A0A0K9RE77 | Proteasome subunit alpha type | 0 | 237 | 26 | 4.82 | 3.551 |
| True | High | A0A1J7I0Z0 | DUF3700 domain-containing protein | 0 | 249 | 27.1 | 6.52 | 3.406 |
| True | High | AT5G18170.1 | Glutamate dehydrogenase 1 | 0 | 411 | 44.5 | 6.86 | 3.085 |
Table 2.
Different categories of proteins based on absence, presence and their abundance in T1 and T2
| Category | Presence | Abundance (T1/T2) | Abundance (T2/T1) | Numbers of proteins |
|---|---|---|---|---|
| 1 | Total common proteins | 1129 | ||
| 2 | Most accumulated common | ≥ 2.6 | 60 | |
| 3 | Accumulated common | 2.0–2.5 | 353 | |
| 4 | Accumulated common | 2.0–2.5 | 88 | |
| 5 | Common | 1.6–1.9 | 00 | 215 |
| 6 | Common | 00 | 1.6–1.9 | 305 |
| 7 | Least accumulated common | 00 | ≤ 1.5 | 48 |
| 8 | Least accumulated common | ≤ 1.5 | 00 | 57 |
| 9 | Exclusive (T1) | 141 | ||
| 10 | Exclusive (T2) | 128 |
Most accumulated: abundance ratio ≥ 2.6; Accumulated common: abundance ratio 2.0–2.5; common: abundance ratio 1.6–1.9; and least accumulated: abundance ratio ≤ 1.5
T1 cotyledonary, T2 maturation stage of embryo
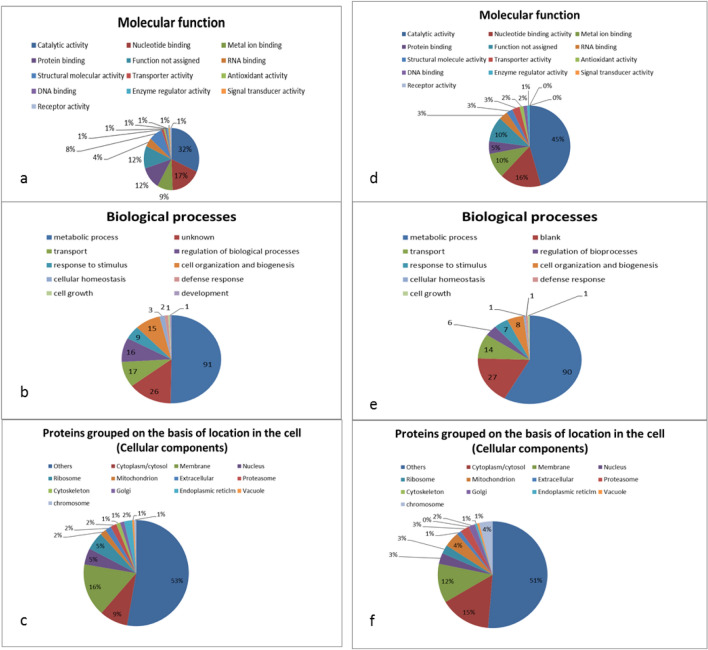
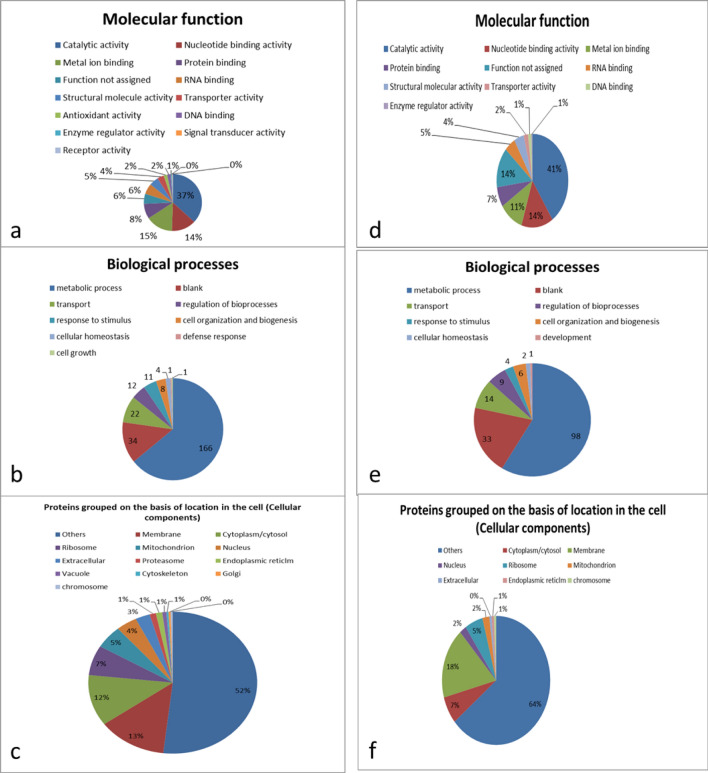
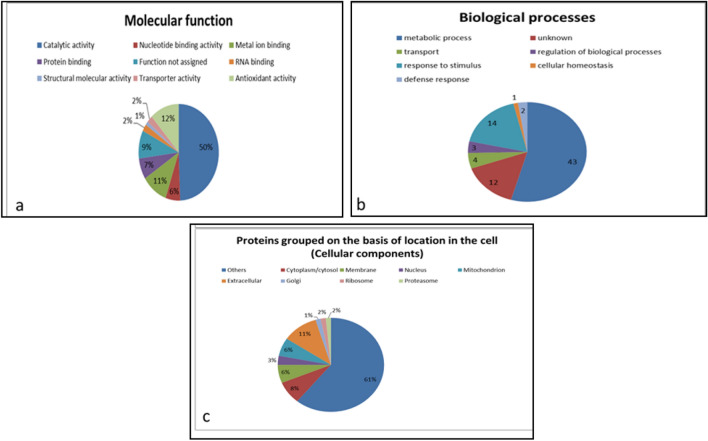
The identified proteins have different molecular functions, of which 32% of the category 3 proteins have catalytic activity, 17% bind to nucleotides, 9% to metal ions and the rest have other molecular functions (Fig. 2a). Similarly, 45%, 16% and 10% of the category 4 proteins have catalytic activity, bind to nucleotides, and attach to metal ions, respectively, and other molecular functions (Fig. 2d). Among the category 3 proteins, 91% take part in metabolic activities, 17% in transport, 16% in regulation of biological processes, and the rest in other processes (Fig. 2b). Similarly, 90, 14, 6% of category 4 proteins have roles in metabolic activities, transport, regulation of biological processes, respectively, and remaining proteins in other biological processes (Fig. 2e). Almost 9%, 16% and 5% of the category 3 proteins are present in cytoplasm/cytosol, membrane and nucleus, respectively, while remaining being associated with other cellular components of cell (Fig. 2c). For category 4 proteins, 15%, 12% and 3% are present in cytoplasm/cytosol, membrane and nucleus, respectively, while several proteins are associated with other cellular components (Fig. 2f). The proteins for the categories 5 and 6 are similarly classified and are presented in Fig. 3a–f as per procedure described above for categories 3 and 4. Zhen et al. (2015) identified 14 embryo-specific proteins while studying embryogenic and non-embryogenic callus of Liriodendron hybrid through matrix-assisted laser desorption ionization time-of-flight/time-of-flight. These identified proteins like eIF-5A, profilin were noted to play an important role in acquiring embryogenic competence, embryo development, in division and survival of cells.
Fig. 2.
Different categorization of proteins found exclusively in T1 (a–c) and T2 (d–f). Some of the proteins have more than one molecular function, cellular location or may be involved in more than one biological process
Fig. 3.
Different categorization of proteins found common but in low abundance in T1 (a–c) and T2 (d–f) with respect to each other. Some of the proteins have more than one molecular function, cellular location or may be involved in more than one biological process and several were unknown, hence the sum may not be equal to total number of proteins
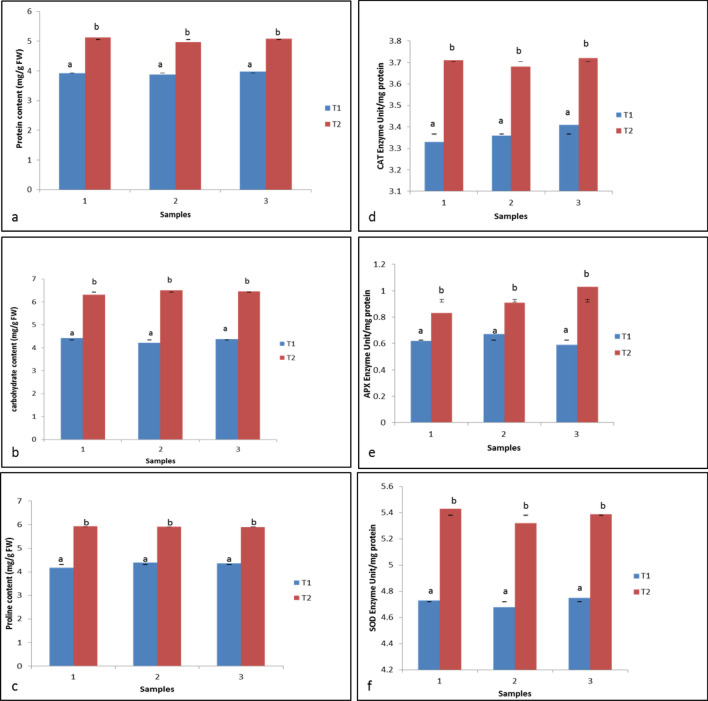
Biochemical analyses were carried out after 20 days of BAP + NAA and GA3 treatment to cotyledonary and mature green embryos, respectively. The total protein, carbohydrate and proline contents were found significantly high in T2 (5.05 mg/g FW, 6.42 mg/g of FW and 5.90 mg/g of FW, respectively) as compared to T1 (3.92 mg/g FW, 4.34 mg/g FW and 4.31 mg/g FW, respectively) (Fig. 4a–c). The enzymes (CAT, APX and SOD) combating physiological stress during cellular transition were checked after 20 days of treatments. Figure 4d–f shows that all the three enzyme activities were higher in T2 (0.923, 3.703 and 5.380 EU/mg protein, respectively) as compared to T1 (0.626, 3.366 and 4.720 EU/mg protein, respectively).
Fig. 4.
Biochemical attributes of T1 and T2: a–c protein, carbohydrate and proline content, respectively; d–f catalase, ascorbate peroxidase, and superoxide dismutase, respectively. Carbohydrate = glucose. Values are expressed as means ± standard errors of three replicates of two experiments. Means followed by a different letters, are significantly different at p ≤ 0.05 according Duncan’s multiple range test (DMRT)
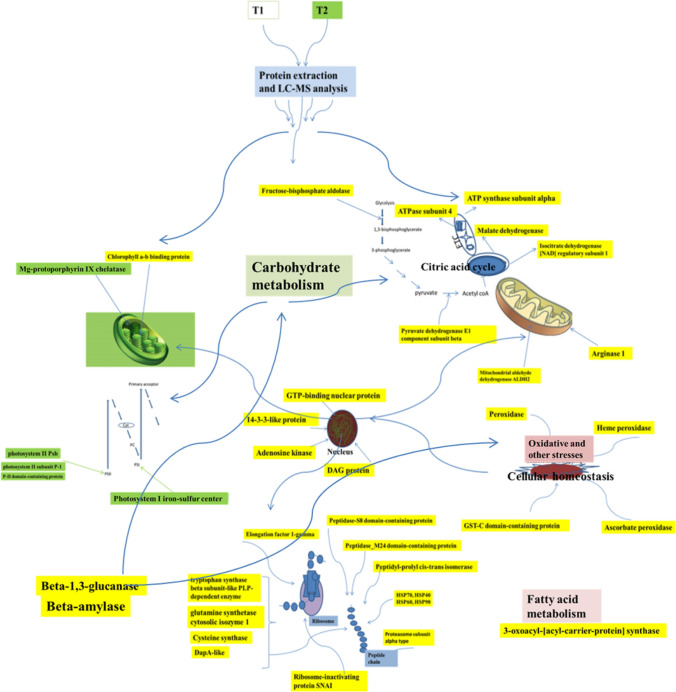
The proteomic map of cotyledonary and maturation stages of embryo was investigated in this C. roseus plant. The embryos started to turn green after 10 days of GA3 treatment as the T2 embryos synthesized higher level of chlorophyll. In our study of T2, we observed the presence of Mg-protoporphyrin IX chelatase (A0A072UYL1), an enzyme used in insertion of Mg2+ ion into protoporphyrin IX in chlorophyll biosynthesis. This protein was differentially expressed in T2 and absent in T1 which was whitish or watery/transparent in appearance and lacked green colour. We also observed the presence of Chlorophyll a–b-binding protein (A0A218W3L1) in significant abundance in T2. Hence, we may infer from the results that there was an enhancement in chlorophyll synthesis following GA3 treatment resulting in higher accumulation of chlorophyll in mature embryos. Cunha and Ferreira (1996) reported an enhanced accumulation of chlorophyll under zeatin influence in flax calli (Linum usitatissimum); in our study, however, we observed enhanced accumulation of chlorophyll during embryo maturation under the influence of GA3.
The most abundant 60 proteins in T2 are mostly associated with metabolic processes like energy metabolism and transport, indicating metabolically high active cells and rigorous transport of raw materials within and across the cells for the synthesis of new set of products essential for embryo maturation (Fig. 5). Among the differentially abundant proteins, several were related to the biosynthesis of different amino acids. For instance, dapA-like (dihydrodipicolinate synthase like) accession number A0A200QB76, is an important enzyme in the biosynthesis of lysine; cysteine synthase for synthesis of cysteine, CPSase synthase (carbamoyl phosphate synthase) accession number A0A1Q3AVL0, glutamine synthetasecytosolic isozyme 1, glutamine synthetase, tryptophan synthase beta subunit-like PLP-dependent enzyme (A0A200Q1Z5), amino transferase, accession number A0A200PUU4 (uniprot.org). This indicates the high demand of amino acids for protein synthesis during transition of one stage to other, i.e. T1–T2. Analysis of biochemical attributes like total protein content was found high in T2 as compared to T1 (Fig. 4a). This observation is consistent with the results of Cangahuala-Inocente et al. (2004) and Vale et al. (2014) where increase in protein concentration was observed during embryo maturation in Feijoa sellowiana and Carica papaya, respectively. Similarly, the proline content was also found maximum in T2 (Fig. 4c). The above-mentioned observations suggest that certain specific early synthesized (older) proteins are accumulated in high abundance to attain maturation stage of somatic embryos and some other proteins are also newly synthesized, such as the proteins exclusively found in T2. The high abundance of proteins related to protein synthesis, folding and degradation identified in our study corroborates the above view. Peptidyl-prolylcis-trans-isomerase (A0A0K9RRM3), for instance, which facilitates protein folding, plant growth and development was found in high abundance in T2. DnaJ domain-containing protein (A0A1Q3B6S6) is a member of J-protein family (also known as Heat Shock Protein40 or HSP40 family) by binding to HSP70, DnaJ and other chaperones stimulate ATPase activity and enhance stable interaction with polypeptide substrate. HSP70 mediates polypeptide translocation across membranes of organelles, targeting specific proteins/polypeptides for degradation, protein folding and stress responses (Park and Seo 2015). During the transition from one stage to the other in SE development pathway, the new sets of proteins need to be synthesized and older ones are to be disappeared or degraded (Heringer et al. 2018). Many of the newly synthesized proteins are uniquely folded by HSPs. An increase in abundance of DnaJ domain-containing protein (A0A1Q3B6S6) in T2 can be attributed to the increase in protein synthesis, and hence increase in demand of HSP70. This is also confirmed by the accumulation of translation factors or related proteins, e.g. elongation factor 1-gamma (A0A1J0A0N7), elongation factor 1-alpha, 40S ribosomal protein SA, elongation factor Tu, 40S ribosomal protein S12 (A0A0K9RUR2) and eukaryotic translation initiation factor 3 subunit E in T2. The heat map of total common proteins is presented in Fig. 6.
Fig. 5.
a–c Categorization of most accumulated proteins in T2 (T2/T1 ≥ 2.6). Some of the proteins have more than one molecular function, cellular location or may be involved in more than one biological process
Fig. 6.
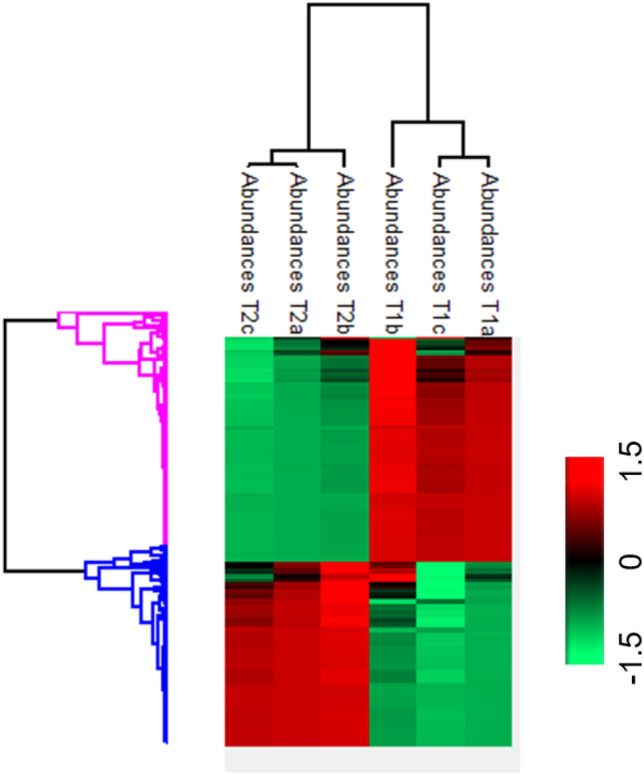
Heat map of total co-expressed proteins showing differential abundance in T1 and T2
Similarly, Chr5:6006172–6008248 (AT5G18170.1) is glutamate dehydrogenase 1 causing reversible deamination of its substrate and glutamate dehydrogenase (A0A1D5ZWQ6) showed accumulation in T2 probably because of the need of higher amination/deamination of its substrate as per the requirement and their utilization in other activities of the cell. These results indicate that during embryo maturation, there may be quite high demand of diverse molecules and building blocks in different parts of maturing embryo. Similarly, proteasome subunit alpha type (A0A0K9RE77), proteasome endopeptidase complex (A0A1J7FTA1), lon protease homolog (A0A1Q3CY01) are also found in our study, took part in degradation of proteins/polypeptides and renewal of amino acids, trafficked for other activities including the synthesis of new polypeptides/proteins. In banana, about twenty five different embryo-specific proteins were identified which played a central role in various cellular processes like growth, defense mechanisms and message signalling (Kumaravel et al. 2020). Current studies also indicated that proteins like HSPs, late-embryogenesis protein and others which appeared during adverse cellular situations protect cells from damages, improve cellular tolerance, induce stress-mediated embryo from somatic cells and regulate other important biological functions (Gao and Lan 2015; Kumaravel et al. 2019).
Peroxidase (A0A1D1YNK4, A0A1D1YNK4, A0A1U8AQM2, A0A218VUG5, A0A218WCG3) catalyses a number of oxidative reactions using H2O2 as an electron acceptor. Ascorbate peroxidase, alkyl hydroperoxy dereductase subunit C/thiol-specific antioxidant (A0A200Q9C9), GST-C domain-containing protein (A0A1Q3BBS1), carbonic anhydrase were accumulated in this present study and Tau class glutathione S-transferase was exclusively present in T2. These enzymes arose due to the presence of high stress under which embryo maturation occurs, under the influence of GA3 regulation. In papaya, several stress-induced proteins like enolase, esterase and others were also synthesized and played a central role in embryo maturation process (Vale et al. 2014). The results of biochemical analysis also showed the increased activity of APX, CAT, SOD and higher proline content in T2 as compared to the T1 (Fig. 4). The obtained results are in agreement with similar observation where authors noted stress induced differentially abundant proteins in embryo maturation process in Carica papaya (Vale et al. 2014).
Thaumatin-like protein is an antifungal low-molecular-weight defense protein (24.2 kD) showed a high accumulation in T2. This is a member of Pathogen Related-5 (PR-5) protein which shows antifungal activity, forms trans-membrane pores in fungi plasma membrane, affecting osmo-regulation process. Earlier, similar osmotin-like protein was identified in maturing somatic embryos in Cichorium (Helleboid et al. 2000). Different proteins related to carbohydrate metabolism were found in both the embryo samples but some of the proteins were more accumulated in T2. These include fructose-bisphosphate aldolase (A0A1Q3CMR6, A0A1J7I946, A0A218VUH2, A0A0K9QNV9, A0A1D5VD11), isocitrate dehydrogenase [NAD] regulatory subunit 1 (A0A1J3J622), malate dehydrogenase (A0A1Q3B8N6, A0A218XUJ8, A0A0K9RAT7), pyruvate dehydrogenase E1 component sub-unit beta. These observations suggest better carbohydrate metabolism in T2 to meet energy and other requirement to accomplish the task of embryo maturation and preparation of subsequent germination. Our results show that there is an accumulation of chlorophyll in T2 (as mentioned earlier) which suggests better photosynthesis ability of that specific tissue. Many of the photosynthesis-related proteins were present exclusively in T2, e.g., photosystem I iron–sulfur center (A0A059P4I1), photosystem II Psb (A0A200R7D0), photosystem II subunit P-1 (A0A0F7CZ97), P-II domain-containing protein (A0A1Q3B7X9), ribulosebisphosphate carboxylase large chain (A0A068LAV1, A0A223A946), ribulosebisphosphate carboxylase small chain, rubisco activase (A0A078BTE9), ruBisCO large subunit-binding protein (I3SKN7), RuBisCO large subunit-binding protein subunit alpha (B6SXW8) and many others. Further, biochemical analysis revealed that the glucose concentration was high in T2 (6.42 mg/g FW) as compared to T1 (4.34 mg/g FW) which confirmed that the carbohydrate metabolism was better in T2 as compared to T1.
In T1, argonaute 9 (A0A0A1WCV1), late-embryogenesis abundant protein (A0A072UGZ8) and germin (A0A200QSS9) were identified. The synthesis of these types of proteins indicates that the tissues witnessed stress in culture, induced altered genetic reprogramming and physiological modulation of cells (Smulders and Klerk 2011; Feher 2015). Amara et al. (2014) reported that the late-embryogenesis abundant (LEA)—a class of hydrophilic protein is directly associated with stress-related functions, tolerance to desiccation and has some role in prevention of protein aggregation; and these proteins are also thought to participate in zygotic and somatic embryogenesis processes, particularly at the time of embryo maturation. Jing et al. (2016) reported that in Picea asperata conifer, the proteins differentially accumulated during desiccation-induced somatic embryo germination were associated with stress and these stress-related proteins were noted to be molecular chaperones, antioxidative-, and defence-related proteins, participated in several processes like osmosis, glyoxylate cycle and chitin metabolic activities. In sugar cane, Heringer et al. (2015) recently reported several proteins in somatic embryos; the LEA protein was reported to be synthesized at higher level at embryo maturation stage. Similarly, germin, another protein, is synthesized and plays an important role in both somatic and zygotic embryogenesis (Patnaik and Khurana 2001). In the present study, these proteins did not show any significant alteration in abundance at cotyledonary and maturation stages of embryos.
It is fact that several identified proteins are uncharacterized and the role of these proteins is still not ascertained. In this present study, these uncharacterized proteins were accumulated more in T2, and this enhanced accumulation suggests their immensely important role in embryo maturation process. Further studies are, however, needed to elucidate the precise role of these non-characterized proteins in embryogenesis especially at the time of embryo maturation. The schematic representation of some most accumulated and differentially accumulated proteins in T2 is summarized in Fig. 7.
Fig. 7.
The schematic representation of some of the most accumulated common proteins (proteins with yellow tags) and the proteins present exclusively (proteins with green tags) in maturation stage (T2). ETC electron transport chain, PI and PII photosystem I and photosystem II, direction of arrowheads points out the process or step where a particular protein has some association
The T1 and T2 are biochemically different, and the biochemical attributes like protein, sugar, proline, APX, SOD and CAT activities were high in T2 as compared to T1 (Fig. 4a–f). As pointed out earlier, the mature embryos have higher requirement of different kinds of existing proteins which were upregulated; there may be another sets of new proteins required exclusively for embryo maturation process to be accomplished successfully. In the present study, the antioxidant enzymes activities and the content of proline were high in T2 as compared to T1 (Fig. 4c–f) and these enhanced profiles may be due to higher level of stress in maturing embryos. Higher accumulation of proline and enhanced enzyme activities in response to stresses were noted in several medicinal plants and these may be due to over-expression of genes like P5C6 and Salt Overly Sensitive 1(SOS1) (Chen et al. 2009; Misic et al. 2012). In Citrus, Ma et al. (2014) reported transcriptional factor AP2/ ERF gene activation in 2,4-d-induced stress. This is the first ever proteomic map of two important somatic embryo forms of C. roseus which determine the success of plant regeneration. This proteomic outcome may be used in other plants for identifying tissues of important applications.
Conclusion
Somatic embryos of C. roseus matured with application of 2.0 mg/l GA3, and in this maturation process, the embryos synthesized and accumulated chlorophyll had increased level of glucose. Drastic changes occurred in protein profiles of cotyledonary embryos while attaining maturity. More than 60 proteins and with two or more folds increase in level were noted during the process of embryo maturation. The current study shows stress-expressed proteins drive transition from cotyledonary to maturation stage of embryo. The total protein, carbohydrate and proline content also increased during maturation, so were CAT, APX and SOD antioxidant enzyme activities. The carbohydrate metabolism was significantly improved during maturation. Further study is needed to completely elucidate the process and to unravel the commonality in different embryo-forming plants, which will broaden application to other important plants, needs fast propagation.
Acknowledgements
The first author is thankful to University Grant Commission (UGC) for receiving JRF. The authors are thankful to Department of Botany, Central Instrumentation facility, JamiaHamdard and University of Delhi for receiving necessary help.
Author contributions
BG, NZ, JM, MM, RS designed, performed and analysed the experimental data. BG also wrote the draft of manuscript. AM and MVR edited the whole article.
Compliance with ethical standards
Conflict of interest
There is no conflict of interest in this article.
Ethical approval
This article did not involve any experiment or study with human participants or animals.
References
- Aebi H. Catalase in vitro. Method Enzymol. 1984;105:121–126. doi: 10.1016/S0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Altamura MM, Rovere FD, Fattorini L, D'Angeli S, Falasca G. Recent advances on genetic and physiological bases of in vitro somatic embryo formation. In: Germana MA, editor. In vitro embryogenesis in higher plants. New York: Springer; 2016. pp. 47–86. [DOI] [PubMed] [Google Scholar]
- Amara I, Zaidi I, Masmoudi K, Ludevid MD, Pagès M, Goday A, Brini F. Insights into late embryogenesis abundant (LEA) proteins in plants: from structure to the functions. Am J Pl Sci. 2014;5:3440–3455. doi: 10.4236/ajps.2014.522360. [DOI] [Google Scholar]
- Bates LS, Waldren RP, Teare ID. Rapid determination of free proline for water stress studies. Plant Soil. 1973;39:205–207. doi: 10.1007/BF00018060. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal Biochem. 1976;72:248–253. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AA, Miki BL, Custers JBM, van Lookeren Campagne MM. Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell. 2002;14:1737–1749. doi: 10.1105/tpc.001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos NA, Panis B, Carpentier SC. Somatic embryogenesis in coffee: the evolution of biotechnology and the integration of omics technologies offer great opportunities. Front Plant Sci. 2017;8:1460. doi: 10.3389/fpls.2017.01460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cangahuala-Inocente GC, Steiner N, Santos M, Guerra MP. Morphological analysis and histochemistry of Feijoa sellowiana somatic embryogenesis. Protoplasma. 2004;224:33–40. doi: 10.1007/s00709-004-0055-5. [DOI] [PubMed] [Google Scholar]
- Chen J-B, Wang S-M, Jing R-L, Mao X-G. Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol. 2009;166:12–19. doi: 10.1016/j.jplph.2008.02.010. [DOI] [PubMed] [Google Scholar]
- Cunha ACG, Ferreira MF. Somatic embryogenesis, organogenesis and callus growth kinetics of flax. Plant Cell Tissue Org Cult. 1996;47(1):1–8. doi: 10.1007/BF02318959. [DOI] [Google Scholar]
- Curaba J, Moritz T, Blervaque R, Parcy F, Raz V, Herzog M, Vachon G. AtGA3ox2, a key gene responsible for bioactive gibberellin biosynthesis, is regulated during embryogenesis by Leafy cotyledon2 and FUSCA3 in Arabidopsis. Plant Physiol. 2014;136:3660–3669. doi: 10.1104/pp.104.047266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deMoura VE, Heringer AS, Barroso T, Ferreira AT, da Costa MN, Perales AJE, Santa-Catarina C, Silveira V. Comparative proteomic analysis of somatic embryo maturation in Carica papaya L. Proteome Sci. 2014;12:37. doi: 10.1186/1477-5956-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey PM. Methods in plant biochemistry. Carbohydrates. London: Academic Press; 1990. [Google Scholar]
- Dhindsa RH, Plumb-Dhindsa P, Thorpe TA. Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot. 1981;32:93–101. doi: 10.1093/jxb/32.1.93. [DOI] [Google Scholar]
- Fehér A. Somatic embryogenesis–stress-induced remodeling of plant cell fate. BiochimBiophysActa. 2015;1849(4):385–402. doi: 10.1016/j.bbagrm.2014.07.005. [DOI] [PubMed] [Google Scholar]
- Fehér A, Pasternak TP, Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult. 2003;74:201–228. doi: 10.1023/A:1024033216561. [DOI] [Google Scholar]
- Fraga HP, Vieria LN, Heringer AS, Puttkammer CC, Silveira V, Guerra MP. DNA methylation and proteome profiles of Araucaria angustfolia (Bertol) Kuntze embryogenic cultures as affected by plant growth regulators supplementation. Plant Cell Tissue Org Cult. 2016;125(2):353–374. doi: 10.1007/s11240-016-0956-y. [DOI] [Google Scholar]
- Gaj MD, Zhang S, Harada JJ, Lemaux PG. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta. 2005;222(6):977–988. doi: 10.1007/s00425-005-0041-y. [DOI] [PubMed] [Google Scholar]
- Gao J, Lan T. Functional characterization of the late embryogenesis abundant (LEA) protein gene family from Pinus tabuliformis (Pinaceae) in Escherichia coli. Sci Rep. 2015;6:19467. doi: 10.1038/srep19467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S, Tsuchiya Y, Lumba S, Okamoto M, McCourt P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the hormones gibberellin and abscisic acid. Dev Cell. 2004;7:373–385. doi: 10.1016/j.devcel.2004.06.017. [DOI] [PubMed] [Google Scholar]
- Ge X, Zhang C, Wang Q, Yang Z, Wang Y, Zhang X, Wu Z, Hou Y, Wu J, Li F. iTRAQ protein profile differential analysis between somatic globular and cotyledonary embryos reveals stress, hormone, and respiration involved in increasing plantlet regeneration of Gossypium hirsutum L. J Proteome Res. 2015;14(1):268–278. doi: 10.1021/pr500688g. [DOI] [PubMed] [Google Scholar]
- Ge F, Hu H, Huang X, Zhang Y, Wang Y, Li Z, Zou C, Peng H, Li L, Gao S, Pan G, Shen Y. Metabolomic and proteomic analysis of maize embryonic callus induced from immature embryo. Sci Rep. 2017;7(1):1004. doi: 10.1038/s41598-017-01280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan Y, Li SG, Fan XF, Su ZH. Application of somatic embryogenesis in woody plants. Front Plant Sci. 2016;7:938. doi: 10.3389/fpls.2016.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulzar B, Mujib A, Rajam MV, Frukh A, Zafar N. Identification of somatic embryogenesis (SE) related proteins through label-free shotgun proteomic method and cellular role in Catharanthusroseus (L.) G. Don. Plant Cell Tissue Org Cult. 2019;137(2):225–237. doi: 10.1007/s11240-019-01563-0. [DOI] [Google Scholar]
- Gulzar B, Mujib A, Malik MQ, Syeed R, Mamgain J, Ejaz B. Genes, proteins and other networks regulating somatic embryogenesis in plants. J Genet EngBiotechnol. 2020;18(1):1–15. doi: 10.1186/s43141-020-00047-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helleboid S, Hendriks T, Bauw G, InzeD VJ, Hilbert JL. Three major somatic embryogenesis related proteins in Cichorium identified as PR proteins. J Exp Bot. 2000;51:1189–1200. doi: 10.1093/jexbot/51.348.1189. [DOI] [PubMed] [Google Scholar]
- Heringer AS, Barroso T, Macedo AF, Santa-Catarina C, Souza GHMF, Floh EIS, Souza-Filho GA, Silveira V. Label-free quantitative proteomics of embryogenic and non-embryogenic callus during sugarcane somatic embryogenesis. PLoS ONE. 2015 doi: 10.1371/journal.pone.0127803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heringer AS, Santa-Catarina C, Silveira V. Insights from proteomic studies into plant somatic embryogenesis. Proteomics. 2018 doi: 10.1002/pmic.201700265. [DOI] [PubMed] [Google Scholar]
- http://www.uniprot.org
- Isaacson T, Damasceno CM, Saravanan RS, He Y, Catala C, Saladie M, Rose JK. Sample extraction techniques for enhanced proteomic analysis of plant tissues. Nat Protocol. 2006;2:769–774. doi: 10.1038/nprot.2006.102. [DOI] [PubMed] [Google Scholar]
- Jin F, Hu L, Yuan D, Xu J, Gao W, He L. Comparative transcriptome analysis between somatic embryos (SEs) and zygotic embryos in cotton: evidence for stress response functions in SE development. Plant Biotechnol J. 2014;12:161–173. doi: 10.1111/pbi.12123. [DOI] [PubMed] [Google Scholar]
- Jing D, Zhang J, Xia Y, Kong L, OuYang F, Zhang S, Zhang H, Wang J. Proteomic analysis of stress-related proteins and metabolic pathways in Piceaasperata somatic embryos during partial desiccation. Plant Biotechnol J. 2016 doi: 10.1111/pbi.12588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junaid A, Mujib A, Fatima S, Sharma MP. Cultural conditions affect somatic embryogenesis in Catharanthus roseus L. (G.) Don. Plant Biotechnol Rep. 2008;2:179–189. doi: 10.1007/s11816-008-0060-9. [DOI] [Google Scholar]
- Kumaravel M, Uma S, Backiyarani S, Saraswathi MS. Molecular analysis of somatic embryogenesis through proteomic approach and optimization of protocol in recalcitrant Musa spp. Physiol Plant. 2019;167:282–301. doi: 10.1111/ppl.12966. [DOI] [PubMed] [Google Scholar]
- Kumaravel M, Uma S, Backiyarani S, Saraswathi MS. Proteomic analysis of somatic embryo development in Musa spp. Cv. Grand Naine (AAA) Sci Rep. 2020 doi: 10.1038/s41598-020-61005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang S, Wang J. Transcriptomic and proteomic analyses of embryogenic tissues in Picea balfouriana treated with 6-benzylaminopurine. Physiol Plant. 2015;154(1):95–113. doi: 10.1111/ppl.12276. [DOI] [PubMed] [Google Scholar]
- Lu D, Wei W, Zhou W, Linda D, Yu XJ, L, Establishment of a somatic embryo regeneration system and expression analysis of somatic embryogenesis-related genes in Chinese chestnut (Castanea mollissima Blume) Plant Cell Tissue Org Cult. 2017;130(3):601–616. doi: 10.1007/s11240-017-1250-3. [DOI] [Google Scholar]
- Ma Q, Ding Y, Chang J, Sun X, Zhang L, Wei Q, Cheng Y, Chen L, Xu J, Deng X. Comprehensive insights on how 2,4-dichlorophenoxyacetic acid retards senescence in post-harvest citrus fruits using transcriptomic and proteomic approaches. J Exp Bot. 2014;65(1):61–74. doi: 10.1093/jxb/ert344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misic D, Siler B, Zivkovic NJ, Simonovic A, Maksimovic V, Budimir S, Janosevic D, Durickovic M, Nikolic M. Contribution of inorganic cations and organic compounds to osmotic adjustment in root cultures of two Centaurium species differing in tolerance to salt stress. Plant Cell Tiss Org Cult. 2012;108:389–400. doi: 10.1007/s11240-011-0050-4. [DOI] [Google Scholar]
- Misra RC, Sandeep KM, Kumar S, Ghosh S. A thaumatin-like protein of Ocimum basilicum confers tolerance to fungal pathogen and abiotic stress in transgenic Arabidopsis. Sci Rep. 2016;6:25340. doi: 10.1038/srep25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujib A, Ali M, Isah T, Dipti T. Somatic embryo mediated mass production of Catharanthus roseus in culture vessel (bioreactor)—a comparative study. Saudi J BiolSci. 2014;21(5):442–449. doi: 10.1016/j.sjbs.2014.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant. 1962;15(3):473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. [Google Scholar]
- Orłowska A, Igielski R, Łagowska K, Pczyska EK. Identification of LEC1, L1L and Polycomb Repressive Complex2 genes and their expression during the induction phase of Medicago truncatulaGaertn. somatic embryogenesis. Plant Cell Tissue Org Cult. 2017;129:119–132. doi: 10.1007/s11240-016-1161-8. [DOI] [Google Scholar]
- Park CJ, Seo YS. Heat shock proteins: a review of the molecular chaperones for plant immunity. Plant Path J. 2015;31(4):323–333. doi: 10.5423/PPJ.RW.08.2015.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patnaik D, Khurana P. Germins and germin like proteins: an overview. Ind J ExpBiol. 2001;39:191–200. [PubMed] [Google Scholar]
- Radoeva T, Lokerse AS, Llavata-Peris CI, Wendrich JR, Xiang D, Liao CY, Vlaar L, Boekschoten M, Hooiveld G, Datla R, et al. A robust auxin response network controls embryo and suspensor development through a basic helix loop helix transcriptional module. Plant Cell. 2019;31:52–67. doi: 10.1105/tpc.18.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupps A, Raschke J, Rümmler M, Linke B, Zoglauer K. Identification of putative homologs of Larix decidua to Babyboom (BBM), leafy cotyledon1 (LEC1), Wuschel-related Homeobox2 (WOX2) and somatic embryogenesis receptor-like kinase (SERK) during somatic embryogenesis. Planta. 2016;243:473–488. doi: 10.1007/s00425-015-2409-y. [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Guzzo F, Toonen MA, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Smulders MJM, Klerk GJ. Epigenetics in plant tissue culture. Plant Growth Reg. 2011;63(2):137–146. doi: 10.1007/s10725-010-9531-4. [DOI] [Google Scholar]
- Vale EM, Heringer AS, Barroso T, Ferreira ATDS, DaCosta MN, Perales JEA, Catarina CS, Silveira V. Comparative proteomic analysis of somatic embryo maturation in Carica papaya L. Proteome Sci. 2014;12:1–17. doi: 10.1186/1477-5956-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Li H, Fu S, Chen B, Sun W, Zhang J. AniTRAQ-based proteomics approach to clarify the molecular physiology of somatic embryo development in Prince Rupprecht's larch (Larix principis-rupprechtiiMayr) PLoS ONE. 2015 doi: 10.1371/journal.pone.0119987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen Y, Li C, Chen J, Chen Q, Shi J. Proteomics of embryogenic and non-embryogenic calli of a Liriodendron hybrid. ActaPhysiol Plant. 2015;37:211. doi: 10.1007/s11738-015-1963-z. [DOI] [Google Scholar]
- Zimmerman JL. Somatic embryogenesis: a model for early development in higher plants. Plant Cell. 1993;5:1411–1423. doi: 10.2307/3869792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Frugis G, Chua NH. The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J. 2002;30:349–359. doi: 10.1046/j.1365-313X.2002.01289.x. [DOI] [PubMed] [Google Scholar]