Summary
Convalescent plasma (CP) is widely used to treat COVID-19, but without formal evidence of efficacy. Here, we report the beneficial effects of CP in a severely ill COVID-19 patient with prolonged pneumonia and advanced chronic lymphocytic leukemia (CLL), who was unable to generate an antiviral antibody response of her own. On day 33 after becoming symptomatic, the patient received CP containing high-titer (ID50 > 5,000) neutralizing antibodies (NAbs), defervesced, and improved clinically within 48 h and was discharged on day 37. Hence, when present in sufficient quantities, NAbs to SARS-CoV-2 have clinical benefit even if administered relatively late in the disease course. However, analysis of additional CP units revealed widely varying NAb titers, with many recipients exhibiting endogenous NAb responses far exceeding those of the administered units. To obtain the full therapeutic benefits of CP immunotherapy, it will thus be important to determine the neutralizing activity in both CP units and transfusion candidates.
Keywords: immunodeficiency, chronic lymphocytic leukemia, CLL, SARS-CoV-2, COVID-19, convalescent plasma, neutralizing antibodies, pneumonia, immunotherapy
Graphical Abstract

Highlights
Convalescent plasma clinically benefited a severely ill immunodeficient patient
The transfer of high-titer neutralizing antibodies led to rapid clinical recovery
Neutralizing activity was low in most convalescent plasmas but high in recipients
Neutralizing activity should be tested in both plasma donors and recipients
An immunodeficient patient with protracted COVID-19 unable to mount a humoral immune response rapidly recovered following transfusion with convalescent plasma. Honjo et al. found that immunotherapy with high-titer neutralizing antibodies was clinically beneficial. However, since neutralizing activity of convalescent plasmas varies widely, units should be tested prior to therapy.
Introduction
One strategy to treat coronavirus disease 2019 (COVID-19) is to use convalescent plasma (CP) from individuals who have successfully cleared severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections and established humoral immunity.1 Historical evidence indicates that CP mitigates human infectious diseases,2 including those caused by the related SARS-CoV-1 (SARS) and Middle East respiratory syndrome (MERS) coronaviruses.3,4 CP therapy is widely used and was initiated as an investigational new drug (IND) by the US Food and Drug Administration (FDA) through a nationwide expanded access treatment protocol.5 Considered safe,6,7 it was approved for emergency use authorization (EUA) on August 23, 2020.8 However, as of yet there is no evidence for efficacy,9, 10, 11, 12 thus rendering this decision controversial.13 This may be because correlates of immune protection are lacking, which render the identification of appropriate convalescing donors and recipients difficult. A key component of CP is neutralizing antibodies (NAbs) that impede SARS-CoV-2 entry into human cells, usually by inhibiting nanomolar affinity interactions between the receptor-binding domain (RBD) of the viral spike (S-protein) and the angiotensin-converting enzyme 2 (ACE2) receptor.14,15 Recent reports indicate, but do not prove, that CPs containing high NAb titers can be beneficial when administered within a few days of hospitalization.9, 10, 11 Given later or in lower amounts, CP NAbs may be unable to meaningfully supplement endogenous NAbs produced during seroconversion.
Here, we report the successful administration of CP to a COVID-19 patient who was unable to generate her own antiviral antibodies (Abs) due to underlying B cell chronic lymphocytic leukemia (CLL). The CP contained high-titer NAbs (ID50 >5,000) and was given on day 33 after symptom onset. Her prolonged clinical illness and fever resolved rapidly and she was discharged 4 days later, providing compelling evidence for a curative antiviral effect of the administered NAbs, even though they were given late in the disease course. To place this case in context, we quantified NAb titers in additional banked and remnant CP units used to treat COVID-19 patients at the University of Alabama at Birmingham (UAB), as well as in CP recipients before and after transfusion. Many CP units from convalescent donors had only low-titer NAbs and thus were unable to usefully supplement endogenously produced Abs post-seroconversion.
Results
Presentation of the case and clinical course
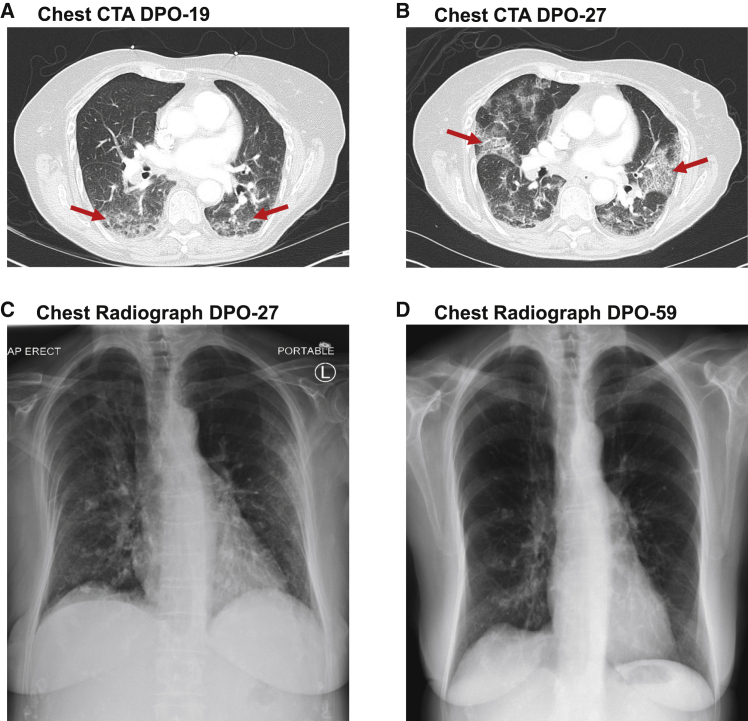
A 72-year-old female with a 20-year history of CLL developed a dry cough following exposure to her daughter (relative 1) who had contracted COVID-19 (Figure 1). The patient had a history of humoral immunodeficiency with chronic sinopulmonary infections requiring monthly intravenous immunoglobulin (IVIg) infusions for over 4 years. Given the progression of her CLL with Rai stage III disease in December 2019, obinutuzumab anti-CD20 B cell depletion immunotherapy was initiated. Her last treatment was 23 days before symptom onset and she had received IVIg 9 days earlier. Relative 1 developed a dry cough 17 days after a holiday in Key West, FL (Figure S1A), presented to a local hospital with headache, ageusia, and diarrhea, tested positive for SARS-CoV-2 by polymerase chain reaction (PCR) analysis of a respiratory swab 11 days post-symptom onset (DPO), and was admitted with fever, dyspnea, and psychataxia. Her spouse (relative 2), who had also traveled, developed myalgia and headache, and was SARS-CoV-2 virus positive on DPO 8 (Figure S1B). With worsening cough and fever, he was admitted from DPO 10–15. Following exposure to relative 1, the CLL patient tested positive for SARS-CoV-2 virus on DPO 8 (Figure 1). She was admitted on DPO 19 with dyspnea and an oxygen saturation of 86% on ambient air. Computed tomography angiography (CTA) showed ground glass opacities consistent with COVID-19-related pneumonia (Figure 2A). After 24 h, she was discharged on hydroxychloroquine. Despite receiving an IVIg infusion at DPO 24, she developed worsening dyspnea, fever, and loss of appetite with a 7.2-kg (15.8-lb) weight loss and was re-admitted on DPO 27. Laboratory studies showed albumin of 2.8 g per deciliter (reference range, 3.4–4.8), ferritin of 521.8 ng per milliliter (reference range, 4.6–204), high-sensitivity C-reactive protein of 67.2 mg per liter (reference range, ≤0.6), and fibrinogen of 729 mg per deciliter (reference range, 200–400). Repeat chest CTA and radiography (Figures 2B and 2C) revealed worsening bilateral infiltrates concerning for bacterial superinfection and piperacillin-tazobactam was started. A repeat SARS-CoV-2 PCR test was positive, indicating persistent infection. She remained febrile to 40°C, with poor nutrition status, elevated transaminases, and anemia (Figures 1B and 1C). Following their recovery, her relatives visited a blood donation center. Relative 2 had a complementary O Rh positive blood type. CP was designated for the patient, who received 218 mL (3.6 mL/kg) on DPO 33. She rapidly defervesced over the next 48 h, became SARS-CoV-2 virus negative on DPO 36, was discharged at DPO 37, recovered her weight, and her hepatic function and anemia resolved by DPO 119 (Figures 1A–1D). Repeat chest radiography showed resolution of her pneumonia at DPO 59 (Figure 2D).
Figure 1.
Timeline of exposure and clinical course of the patient
(A) SARS-CoV-2 infection was diagnosed by quantitative real-time polymerase chain reaction (PCR) of respiratory swab samples (A). Notable symptoms at days post onset (DPO), hospitalization course, relevant interventions, and body weight are indicated. IVIg denotes intravenous immunoglobulin; CT, computed tomography; PNA, pneumonia; CXR, chest radiograph; CP, convalescent plasma.
(B–D) Longitudinal analysis of serum albumin and aspartate aminotransferase (AST) (B), hemoglobin and platelet counts (C), and maximum body temperatures (D) are shown after symptom onset. The date of CP infusion (B–D) is indicated by a red arrow.
Figure 2.
Radiographic analysis of lung abnormalities
(A and B) Computed tomography angiography (CTA) of the chest on the day of initial admission at DPO 19 (A) and subsequent readmission at DPO 27 (B), demonstrating the evolution of diffuse patchy ground glass and interstitial opacities (red arrows in A) consistent with COVID-19 infection to moderately extensive bilateral patchy interstitial lung infiltrates (red arrows in B).
(C and D) An anteriorposterior chest radiograph at DPO 27 (C) demonstrates bilateral patchy interstitial lung infiltrates, which are resolved on a posterioranterior image at DPO 59 (D). Dense calcified granulomas are old.
Transfer of high-titer neutralizing antibodies has curative potential
Plasma samples from the CLL patient, convalescing relatives 1 and 2, a third unexposed relative (relative 3), and the remnant CP from relative 2 were tested for SARS-CoV-2 anti-S-protein IgG binding Abs by ELISA. High-titer Abs were present in plasma from relatives 1 and 2 as well as in the remnant CP from relative 2 but were absent in relative 3 (Table 1). As expected, anti-S-protein Abs were not initially present in the immunodeficient patient but became detectable 1 day after CP transfusion.
Table 1.
Anti-S-protein and neutralizing antibodies in plasma from the index case and her relatives
| Plasma sample | Collection date (DPO)a | SARS-CoV-2 S-Protein IgGb |
Neutralization (ID50)c | ACE2/RBD binding (1:25)d | |
|---|---|---|---|---|---|
| EC50 | Endpoint | ||||
| Relatives | |||||
| Relative 1 (infected daughter) | 52 | 44,770 | 762,070 | 6,710 | 7% |
| Relative 2 (infected son-in-law/donor) | 44 | 28,350 | 459,970 | 13,190 | 11% |
| Relative 3 (infection-naive daughter) | − | <100 | <100 | <20 | 109% |
| Remnant convalescent plasma (CP) | 38 | 24,360 | 266,470 | 5,720 | 16% |
| Index case recipient | |||||
| Before CP transfusion (day 0) | 33 | <100 | <100 | <20 | 106% |
| Day 1 after transfusion | 34 | 1,340 | 11,680 | 570 | 83% |
| Day 2 after transfusion | 35 | 1,150 | 12,230 | 500 | 89% |
| Day 3 after transfusion | 36 | 670 | 12,870 | 370 | 98% |
| Day 4 after transfusion | 37 | 820 | 12,880 | 360 | 102% |
DPO, days post-onset of symptoms.
SARS-CoV-2 IgG S-protein ELISA EC50 and endpoint titers are rounded to the nearest 10, and values of <100 are considered negative.
Neutralization ID50 titers are rounded to the nearest 10, and values <20 are considered negative.
A reduction in the binding of the angiotensin converting enzyme 2 (ACE2) to the receptor binding domain (RBD) to <90% of control indicates inhibition and values are rounded to the nearest whole number.
The same plasma samples were then tested for SARS-CoV-2 NAbs using an HIV-1-based pseudovirus system.16 An additional assay for Ab-mediated inhibition of the SARS-CoV-2 RBD/ACE2 interaction generated highly concordant data (Figure S2). Consistent with the ELISA findings, high-titer NAbs and ACE2/RBD inhibitory Abs were present in the plasma from relatives 1 and 2 and the remnant CP unit, but not in the pretreatment plasma from the CLL patient (Table 1; Figure S3). However, NAbs were clearly detectable on four consecutive days after CP transfusion with titers consistent with an ∼1:10 dilution. Analysis of the CP for receptor binding domain (RBD) reactivity revealed primarily IgG and IgM isotype responses, with IgG1 and IgG3 dominant among the IgG subclasses (Figure S4). The CP contained ∼112 μg/mL of RBD-specific IgG1, corresponding to ∼24 mg in the transfused unit. Thus, the transfer of a CP unit with a NAb ID50 titer of 5,700 from a convalescent donor to an immunodeficient recipient resulted in the loss of detectable virus in nasal swabs, a dramatic clinical improvement, discharge from the hospital within 4 days, and resolution of an advanced pneumonia (Figures 1 and 2).
Most CP units do not reach pre-transfusion neutralizing antibody levels of hospitalized recipients
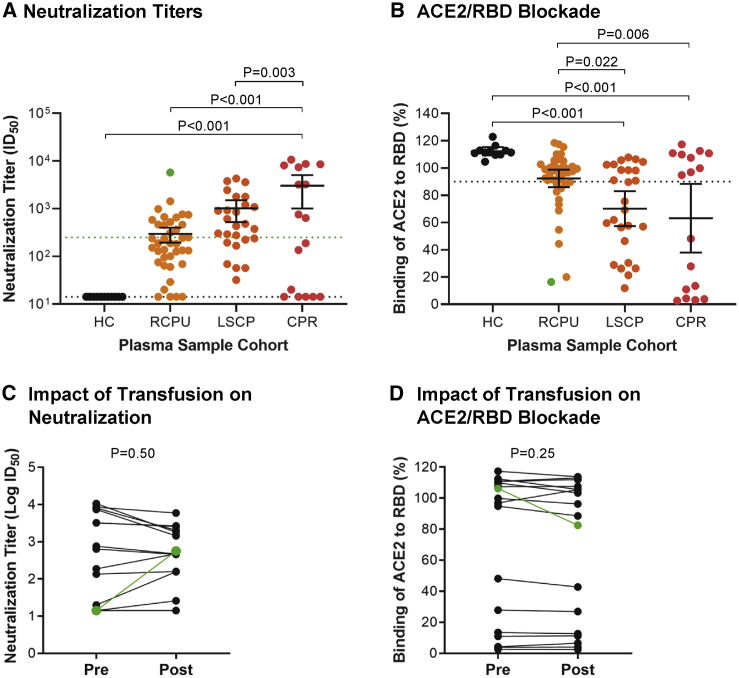
To assess how this case compared to other CP transfusions, we measured NAb titers in 38 remnant CP units (RCPUs) from the American Red Cross and other regional blood services that were transfused at UAB under the FDA IND indication for COVID-19 patients (Figure 3).5 All CP donors were SARS-CoV-2 PCR or Ab positive and donated plasma at least 14 days after symptom resolution.17 Only 37% of the RCPUs had NAb titers >250, and only 29% inhibited RBD/ACE2 binding. NAbs were undetectable in four RCPUs (ID50 <20). Based on the FDA criterion for ID50 titers >250,18 a high proportion of these plasmas were inadequate for transfusion. We also analyzed a different set of 26 CP units from LifeSouth Community Blood Centers (LSCPs) that were pre-screened with a SARS-CoV-2 spike immunoassay (Ortho-VITROS). Those CPs were generally of higher titer (median NAb ID50 456), with 47% exceeding the >250 FDA cutoff and 58% inhibiting ACE2/RBD binding. However, only 8 of the 26 LSCP units had ID50 values >1,000 and none exceeded the 5,700 titer of the CP plasma given to the CLL patient.
Figure 3.
Disparate neutralizing activity of convalescent plasma units and recipients
(A and B) Reciprocal half-maximal pseudovirus NAb titers (A) or ACE2/RBD inhibition at a 1:25 dilution (B) are shown for sera from healthy controls (HC) (n = 11), remnant CP units (RCPU) (n = 38), banked LifeSouth CP units (LSCP) (n = 26), and CP recipients (CPR) (n = 17). Bars indicate the mean with 95% confidence intervals (ID50, plasma dilution at which luminescence output was reduced to 50%); dotted black lines indicate assay sensitivity cutoffs, specifically an ID50 value of <20 in the neutralization assay (A) or 90% ACE2 binding in the RBD-inhibition assay (B). The green line in (A) indicates the FDA recommended NAb titer of 250. The p values were calculated by ANOVA with multiple comparisons.
(C and D) A pairwise comparison of log transformed pseudovirus ID50 titers (C) and ACE2/RBD binding (1:25 dilution) titers (D) is shown for 16 CP recipients from whom pre- and post-transfusion plasma samples were available. p values were calculated with a paired Student’s t test.
The samples labeled green (A–D) represent the CP unit and CPR samples from the immunodeficient index case patient; they were excluded from the statistical analysis.
To examine how CP transfusion affected plasma NAb activity, we analyzed 17 CP recipients (CPR) for whom pre- and/or post-transfusion blood samples were available. These patients were hospitalized, mostly in the intensive care unit (Table S1). At baseline, 53% had ID50 values >250, with 7 exceeding titers of 3,000. Overall, the mean ID50 for this cohort before transfusion was 10.2-fold higher than for the RCPU products (p < 0.001), 3-fold higher than the LSCP units (p = 0.003), and 5.2-fold higher than all units combined (p < 0.001) (Figure 3A). Similar results were obtained analyzing ACE2/RBD inhibition (Figure 3B). For 16 CPRs we could analyze, CP transfusion had no significant impact on the pre-existing NAb titers or ACE2/RBD inhibition (p = 0.50 and p = 0.25, respectively) (Figures 3C and 3D). In contrast, CP transfusion of our CLL patient resulted in an obvious rise in NAb titers (Figures 3A–3D, green samples) to levels that are protective in animal studies.19, 20, 21, 22
Discussion
Here, we report the effects of CP immunotherapy in an immunodeficient COVID-19 patient unable to mount a humoral response, who suffered a prolonged deteriorating illness with severe pneumonia and a high risk of death.23 A single CP transfusion (NAb ID50 5,700) led to viral clearance, her discharge from the hospital within 4 days, and ultimate resolution of her lung-related pathology. Such a prompt and profound improvement provides compelling evidence for a causative benefit of the transferred NAbs. Of note, we saw no indication of an adverse interaction between infused Abs and circulating virus that has been proposed to drive Ab-dependent enhancement and lung pathology.24
Our results have important implications for how CP therapy is being used now and how it may be improved. The low NAb titers in most CP donors raise concerns. The initial FDA guidelines for use of CP as an IND in May 2020 recommended a NAb titers of >160.5 According to this criterion, 53% of CPs transfused at UAB exceeded this cutoff. However, the use of CPs with low NAb titers lack demonstrable efficacy.25 Accordingly, the revised FDA guidance following EUA approval in August 2020 recommended the use of CP with titers >250,18 indicating that only 36.8% units met this criterion. Although the majority of LSCP samples (85%) had titers >160, only 47% had activity >250. Convalescing individuals with truly high-titer NAbs are rare,26 which underscores the need for a concerted effort to identify them but also poses the question of whether there are ample numbers of suitable CP donors.
Our results are consistent with data from other testing centers domestically and abroad. A recent analysis of 753 plasma donors from three different processing centers in the US27 found half maximal neutralization titers (NT50) of >1:160 in 76.4%, >1:320 in 61.6%, and >1:640 in 44.9% of CP units. In contrast, an analysis of CP units in the United Kingdom found NAb titers of >250 in only ∼26% of plasma samples (n = 436).28 Thus, while more than half of the units collected in the US study had titers >250, it is not yet clear how uniform the distribution of NAb-enriched CP is around the country and, most importantly, what NAb titer threshold provides sufficient efficacy.10
Our findings confirm the wide disparity in neutralizing activity found for donor plasma in other studies,25 including international randomized controlled clinical trials that failed to find efficacy for CP immunotherapy.11,12,29 Thus, beyond reliably identifying suitable high-titer plasma donors, clinical trials that investigate correlates of immune protection will be critical for defining titers more likely to achieve clinical efficacy. In this context, CP studies in non-human primates may also be informative.19 The evolving FDA guidelines and implementation of improved screening procedures that more accurately define neutralizing activity should improve the CP unit supply quality. However, since NAb levels gradually decline over time,30 retesting will be needed for repeat donations.
The strongest plasma NAb titers are found in the sickest patients, and they increase over time during the first ∼20–30 days after developing symptoms.31 Thus, the timing of CP transfusions is another critical consideration given that this treatment is likely to be most effective if given before endogenous NAbs develop. Indeed, other recent studies have shown that CP therapy may only be beneficial when it is used very early after hospitalization and is enriched with high-titer IgG.9, 10, 11 Ideally, CP use should consider the NAb titers in both donors and recipients, taking into account the substantial dilution factors involved. The failure to consider these issues may account for why early small-scale CP trials have generated non-definitive outcomes11,12,29 and why the recombinant NAb bamlanivimab lacked efficacy in hospitalized COVID-19 patients but may show promise by lowering viral loads in outpatients.32
While the clinical benefits of CP transfusion may extend beyond NAbs, our case demonstrates that CPs with high-titer NAbs may be particularly useful to immunocompromised patients. Vaccine efficacy is lower among the immunosuppressed, including those on therapies for transplant rejection, autoimmunity, or cancer.33 Inadequate humoral responses are a common problem in patients with lymphoproliferative disorders, who develop hypogammaglobulinemia secondary to immune dysregulation or B cell-targeted therapies. An estimated 25%–40% of these patients receive IVIg.34 Thus, passive immunotherapy, including cocktails of monoclonal NAbs,24,35,36 may be especially important in these populations if they acquire SARS-CoV-2 infection.
In summary, our case report demonstrates that transfusing a high NAb titer CP into a B cell-deficient patient rapidly resolved her severe COVID-19 illness. CP is a readily available therapeutic option that can potentially provide an immediate benefit. However, the generally low NAb titers in most donors, as well as high-titer baseline NAbs in many recipients, highlight the importance of first testing the CPs, and also the recipients. Doing so should optimize the clinical benefit and reduce the effort spent when CP therapy is not appropriate.
Limitations of study
One of the limitations of this report is the sample size of a single index patient. An analysis of neutralizing activity in a larger series of immunodeficient recipients transfused with CP units harboring well-defined NAb titers would be helpful for validating these findings. However, while NAbs may serve as a useful correlate of immune protection, the NAb titer threshold required to achieve clinical efficacy still remains unknown. Although the index case was unable to mount a humoral immune response, we did not examine whether the patient developed SARS-CoV-2-specific cell-mediated immunity and whether this contributed to her clinical improvement. Another limitation is that qPCR Ct values of the index case were not available, thus precluding quantitative analyses of SARS-CoV-2 viral loads prior to and after the CP infusion. Finally, we were unable to examine the clinical outcomes of CP recipients who received units with low NAb titers. Thus, it remains unclear whether CP transfusions have beneficial effects in addition to providing Abs capable of neutralizing SARS-CoV-2. These data will ultimately be required to determine the full clinical benefits of CP immunotherapy for COVID-19.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Goat anti-human IgG Fc-HRP | SouthernBiotech | Cat# 2048-05; RRID: AB_2795688 |
| Goat anti-human IgG-HRP | SouthernBiotech | Cat# 2045-05; RRID:AB_2795676 |
| Goat anti-human IgM-HRP | SouthernBiotech | Cat# 2023-05; RRID:AB_2795622 |
| Goat anti-human IgA-HRP | SouthernBiotech | Cat# 2053-05; RRID:AB_2795715 |
| Mouse anti-human IgG1-HRP (clone HP6069) | Invitrogen | Cat# A-10648; RRID:AB_2534051 |
| Mouse anti-human IgG1- HRP (clone 4E3) | SouthernBiotech | Cat# 9052-05; RRID:AB_2796619 |
| Mouse anti-human IgG2-HRP (clone 31-7-4) | SouthernBiotech | Cat# 9060-05; RRID:AB_2796633 |
| Mouse anti-human IgG3-HRP (clone HP6050) | SouthernBiotech | Cat# 9210-05; RRID:AB_2796699 |
| Mouse anti-human IgG4-HRP (clone HP6025) | SouthernBiotech | Cat# 9200-05; RRID:AB_2796691 |
| Mouse anti-human IgA1- HRP (clone B3506B4) | Southern Biotech | Cat# 9130-05; RRID:AB_2796654 |
| Goat anti-human ACE2-biotin | R&D systems | Cat# BAF933; RRID:AB_2305177 |
| Human IgG1 anti-Spike-RBD (clone CR3022) | Obtained from the lab of Dr. James Kobie | |
| Human IgM anti-Spike-RBD-hIgM (clone CR3022) | Invivogen | Cat# srbd-mab5 |
| Human IgA1 anti-Spike-RBD-hIgA1 (clone CR3022) | Invivogen | Cat# srbd-mab6 |
| Bacterial and virus strains | ||
| MAX Efficiency Stbl2 Competent Cells | ThermoFisher | Cat# 10268019 |
| Biological samples | ||
| Human serum | Millipore Sigma | Cat# H5667 |
| Chemicals, peptides, and recombinant proteins | ||
| 3,3′,5,5′-Tetramethylbenzidine (TMB) Liquid Substrate | Sigma-Aldrich | Cat# T0440-1L |
| 3,3′,5,5′-Tetramethylbenzidine (TMB) | BioLegend | Cat# 421501 |
| Sulfuric Acid (1N) | ICCA | Cat# 8300-32 |
| SARS CoV2 His-tag | Brouwer et al., Produced In House | PMID: 32540902 |
| SARS Cov2 Strep-tag | Brouwer et al., Produced In House | PMID: 32540902 |
| SARS CoV2 Avi-tag | Brouwer et al., Produced In House | PMID: 32540902 |
| Recombinant human ACE2 | Raybiotech | Cat# 230-30165 |
| Recombinant SARS-CoV-2 S1 subunit (RBD) | Raybiotech | Cat# 230-30162 |
| DMEM | ThermoFisher | Cat# 10566024 |
| Characterized Fetal Bovine Serum, US Origin | HyClone | Cat# SH30071.02 |
| Penicillin-Streptomycin-Glutamine (100X) | ThermoFisher | Cat# 10378016 |
| FuGENE 6 Transfection Reagent | Promega | Cat# E2692 |
| Critical commercial assays | ||
| Nano-Glo Luciferase Assay System | Promega | Cat# N1150 |
| SARS-CoV-2 IgG | Abbott Laboratories | Cat# 6R86 |
| Experimental models: cell lines | ||
| Human embryo kidney (HEK) 293T cells | ATCC | ATCC CRL-3216 |
| 293T-ACE2-Clone13 | Schmidt et al. | PMID: 32692348 |
| Recombinant DNA | ||
| pHIV-1NL4ΔEnv-NanoLuc | Schmidt et al. | PMID: 32692348 |
| pSARS-CoV-2-SΔ19 | Schmidt et al. | PMID: 32692348 |
| Software and algorithms | ||
| Graphpad Prism version 8 | Graphpad | https://www.graphpad.com:443/ |
| Other | ||
| Corning Clear Polystyrene 96-Well Microplates | FisherScientific | Cat# 07-200-642 |
| Corning 96-Well, High Binding, Flat-Bottom, Half-Area Microplate | FisherScientific | Cat# 07-200-37 |
| Streptavidin-HRP | SouthernBiotech | Cat# 7105-05 |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Randall S. Davis (rsdavis@uab.edu).
Materials availability
This study did not generate new unique reagents.
Data and code availability
This study did not generate any unique datasets or code.
Experimental model and subject details
Human samples
Blood samples were collected following institutional review board (IRB) approval by the University of Alabama at Birmingham (IRB #130821005 and 160125005) and written informed consent was obtained from all participants including the index patient (72 year old female) and relatives (Relative 1, 50 year old female; Relative 2, 58 year old male; and Relative 3, 53 year old female) (Table 1). Diagnosis of SARS-CoV-2 infection was determined by real-time polymerase-chain-reaction (PCR) analysis of respiratory swab specimens37 and/or detection of nucleocapsid IgG antibodies in the blood (Abbott)38 performed in a clinical diagnostic laboratory. Seronegative healthy controls (n = 11) were obtained from cryopreserved samples prior to Dec 31, 2020. Sera from CP recipients (n = 16) and units (n = 38) was obtained from remnant blood specimens or discarded plasma bags and tubing. Details of the gender and age for CP recipients is provided in Table S1. Deidentified samples from banked SARS-CoV-2 recovered donor CP units (n = 26) were obtained from LifeSouth Blood Bank (Gainesville, FL).
Cell lines
HEK293T (ATCC) and 293T-ACE2-Clone13 cells were cultured in DMEM supplemented with 10% fetal bovine serum, L-glutamine, and penicillin/streptomycin at 37°C and 5% CO2.
Method details
SARS-CoV-2 S-protein ELISA
The design of the soluble, His-tagged, stabilized SARS-CoV-2 spike (S)-protein (residues 1-1138 of the Wuhan strain) has been described previously.39 Test wells of Corning Clear Polystyrene 96-Well Microplates (FisherScientific #07-200-642) were coated with 300 ng of S-protein in PBS (100 μl) overnight at 4°C. All subsequent incubation steps were carried out at 37°C in volumes of 100 μl. The plates were blocked with blocking buffer (5% non-fat milk powder in PBS + 0.05% Tween 20) for 1 h incubation. Plasma samples were 5-fold serially diluted in blocking buffer and added to the plates for 1 h incubation. Plates were then washed five times with washing buffer (PBS + 0.1% Tween 20) and incubated for 1 h with a goat anti-human IgG horseradish peroxidase (HRP) conjugated antibody (SouthernBiotech #2048-05) diluted 1:5,000 in blocking buffer. Plates were again washed five times with washing buffer. For color development, the TMB substrate (Sigma-Aldrich #T0440-1L) was added for 10 min before the reaction was stopped with an equal volume of 1N H2SO4. Absorbance was read at 450 nm using a Synergy 4 (BioTek) spectrophotometer. For each serum dilution, the average OD450 value from three background control wells (no serum) was subtracted from the S-protein coated sample wells. In addition, the average OD450 value plus 2 standard deviation derived from 28 pre-pandemic sera from UAB was subtracted for each serum dilution. Midpoint (EC50) values were calculated by a nonlinear-regression fit of a 4-parameter sigmoid function to the corrected OD450 values and the logarithmic dilution factors (the lower plateau was set to 0; GraphPad Prism software). The end-point titers were read from the fitted curve at a corrected OD450 cut-off of 0.1.
SARS-CoV-2 RBD ELISA
High-binding 96-well plates (Corning #3690) were coated at 4°C overnight with 50 μl of recombinant RBD (RayBiotech) diluted at 1 μg/ml in PBS. The following day, plates were washed 3 times with 0.1% Tween 20 in PBS (PBST). A blocking solution of 100 μl per well of 3% non-fat dry milk in PBST was added and incubated at room temperature for 1 h, before washing plates 3 times with PBST. Plasma samples were inactivated at 56°C for 30 min and initially diluted at 1:25, then serially diluted 4-fold in 1% non-fat dry milk in PBST before adding 50 μl per well, and incubating at room temperature for 2 h. Plates were then washed 3 times with PBST, and a 50 μl per well mixture of horseradish peroxidase (HRP) conjugated goat anti-human IgG (1:8000), goat anti-human IgM (1:10000), goat anti-human IgA (1:5000), mouse anti-human IgG1 (clone 4E3, 1:1000), mouse anti-human IgG2 (31-7-4, 1:500), mouse anti-human IgG3 (HP6050, 1:4000), or mouse anti-human IgG4 (HP6025, 1:3000) (all from Southern Biotech) in PBST was added to the wells and incubated at room temperature for 1 h to measure S-RBD Ab responses. For quantitative measurements of RBD-specific antibodies, 50 μl of serially diluted human IgG1 anti-Spike-RBD (clone CR3022, a gift from Dr. James Kobie), human IgM anti-Spike-RBD (CR3022, Invitrogen), and human IgA1 anti-Spike-RBD (CR3022, Invitrogen) in 1% non-fat dry milk in PBST were used as standards, and plate bound Abs were detected by HRP conjugated goat anti-human IgM (1:20000, Southern Biotech), mouse anti-human IgG1 (HP6069, 1:750, Invitrogen), and mouse anti-human IgA1 (B3506B4, 1:1200, Southern Biotech) in PBST, respectively. Plates were washed 4 times with PBST, developed with 50 μl per well of HRP substrate 3, 3′, 5, 5′ tetramethyl benzidine (TMB, Biolegend) at room temperature for 10 min. The reaction was stopped by addition of 50 μl of 1N H2SO4. The optical density (OD) was measured at 450 nm with a SPECTROstar omega (BMG Labtech) microplate reader. EC50 values and endpoints were determined as detailed for the SARS-CoV-2 S-protein ELISA.
SARS-CoV-2 Pseudovirus Neutralization Assay
Plasma samples were tested for SARS-CoV-2 neutralizing antibodies as previously described.16 Briefly, pseudovirus was generated by co-transfection in HEK293T cells, using an HIV-1 nanoluciferase reporter construct and an expression plasmid for the SARS-CoV-2 Spike (Wuhan 1), containing a 19-amino acid cytoplasmic tail truncation. To test neutralization, 1.5x104 293T-ACE2 cells (clone 13) were seeded in 96-well plates. The following day, five-fold serial dilutions of plasma were incubated with pseudovirus for one hour at 37°C prior to the addition to target cells. Two days post infection, cells were washed with PBS, lysed, and nanoluciferase activity was determined according to manufacturer’s instructions (Nano-Glo Luciferase Assay System). Luciferase activity in wells with virus and no patient plasma were set to 100%, and the dilution of plasma at which luminescence was reduced to 50% (ID50) was calculated.
ACE2/RBD Binding Inhibition Assay
Plasma samples were analyzed with an ACE2/receptor binding domain (RBD) binding inhibition assay. High-binding 96-well plates (Corning #3690) were coated with 50 μl per well of recombinant RBD (RayBiotech) diluted at 1 μg/ml in PBS at 4°C overnight. The following day, plates were washed 3 times with PBS + 0.1% Tween 20 (PBST), and wells were blocked with 100 μl per well of 3% non-fat dry milk in PBST by incubation at room temperature for 1 h. After washing the blocked wells 3 times with PBST, either 50 μl of serum or plasma samples serially diluted in 1% non-fat dry milk in PBST, or 1% non-fat dry milk in PBST alone as a no inhibition control, was added to wells, and incubated at room temperature for 2 h. Heat-inactivated plasma samples (56°C for 30 min) were initially diluted at 1:25, then serially diluted 2-fold for the assay to 1:400. After incubation, plates were washed 3 times with PBST, then 50 μl per well of recombinant human ACE2 (RayBiotech) diluted at 0.1 μg/ml in PBST was added to the wells. Plates were incubated at room temperature for 1 h, washed 4 times with PBST, and 50 μl per well of biotinylated goat anti-human ACE2 (R&D) diluted at 0.1 μg/ml in PBST was added to wells. Plates were incubated at room temperature for 1 h, washed 4 times with PBST, and then 50 μl per well of HRP-conjugated streptavidin (Southern Biotech) (1:2000 in PBST) was added to the wells, and incubated at room temperature for 30 min. Plates were washed 5 times with PBST, developed with 50 μl per well of 3,3′,5,5′-tetramethylbenzidine TMB substrate (Biolegend) at room temperature for 8 min, and the reaction was stopped by addition of 50 μl of 1N H2SO4. The OD was measured at 450 nm with a SPECTROstar omega (BMG Labtech) microplate reader. ACE2 binding was expressed as a percentage of OD values relative to OD value of a no inhibition control. Binding values of < 90% at a 1:25 dilution of plasma were used to calculate inhibitory activity.
Quantification and statistical analysis
Statistical analysis was performed and visualized using GraphPad Prism v8 software. Correlations between ACE2/RBD binding (1:25 dilution) and neutralization activity (ID50) were analyzed using the Pearson correlation coefficient. Comparisons among plasma sample cohorts were analyzed by ANOVA with Tukey’s multiple comparisons and Brown-Forsythe test correction. Comparisons between CP recipients pre and post transfusion were analyzed with Student’s paired t test. Statistical parameters are provided in Figure S2 and Figure 3. P values below 0.05 were considered significant.
Acknowledgments
We thank the donors and patients who contributed samples to the study as well as Charles Henry, Lorinda Cruz, Christina Morelock, the staff at Sacred Heart Hospital Emerald Coast, and the staff of the UAB blood bank. We also thank Philip Brouwer, Rogier Sanders, Albert Cupo, Wilhem Leconet, and Victor Cruz-Portillo for producing S-proteins. We appreciate Ravi Bhatia, Robert Welner, and Mark Dransfield for helpful discussions. These studies were funded in part by the UAB School of Medicine Dean’s office COVID-19 research initiative, the UAB Cancer Immunobiology Program, and NIH/NIAID UM1AI069452, R01AI110553, R01AI036082, and R01AI150590.
Author contributions
Conceptualization, S.L.H., B.H.H., and R.S.D.; Methodology, K.H., T.J.K., T.H., P.D.B., J.P.M., and R.S.D.; Validation, K.H., R.M.R., W.L., and D.T.R.; Formal Analysis, K.H., R.M.R., D.T.R., and R.S.D.; Investigation, K.H., R.M.R., R.L., W.L., R.S., E.M.T., and Y.H.; Resources, L.P., A.N.K., S.S., M.B.M., J.L.L., C.M.L., T.P.M., T.J.K., T.H., P.D.B., J.P.M., P.A.G., S.L.H., and R.S.D.; Writing – Original Draft, R.S.D.; Writing – Review & Editing, M.B.M., J.P.M., P.A.G., S.L.H., B.H.H., and R.S.D.; Supervision, M.B.M., P.A.G., S.L.H., B.H.H., and R.S.D.; Funding Acquisition, J.P.M., P.A.G., S.L.H., B.H.H., and R.S.D.
Declaration of interests
R.S.D. and K.H. have submitted an intellectual property disclosure related to the ACE2/RBD assay.
Published: December 5, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.xcrm.2020.100164.
Contributor Information
Beatrice H. Hahn, Email: bhahn@pennmedicine.upenn.edu.
Randall S. Davis, Email: rsdavis@uab.edu.
Supplemental information
References
- 1.Casadevall A., Pirofski L.A. The convalescent sera option for containing COVID-19. J. Clin. Invest. 2020;130:1545–1548. doi: 10.1172/JCI138003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casadevall A., Scharff M.D. Return to the past: the case for antibody-based therapies in infectious diseases. Clin. Infect. Dis. 1995;21:150–161. doi: 10.1093/clinids/21.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng Y., Wong R., Soo Y.O., Wong W.S., Lee C.K., Ng M.H., Chan P., Wong K.C., Leung C.B., Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur. J. Clin. Microbiol. Infect. Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arabi Y.M., Hajeer A.H., Luke T., Raviprakash K., Balkhy H., Johani S., Al-Dawood A., Al-Qahtani S., Al-Omari A., Al-Hameed F. Feasibility of Using Convalescent Plasma Immunotherapy for MERS-CoV Infection, Saudi Arabia. Emerg. Infect. Dis. 2016;22:1554–1561. doi: 10.3201/eid2209.151164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FDA . 2020. Coronavirus (COVID-19) update: FDA coordinates national effort to develop blood-related therapies for COVID-19.https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-coordinates-national-effort-develop-blood-related-therapies-covid-19 [Google Scholar]
- 6.Joyner M.J., Bruno K.A., Klassen S.A., Kunze K.L., Johnson P.W., Lesser E.R., Wiggins C.C., Senefeld J.W., Klompas A.M., Hodge D.O. Safety Update: COVID-19 Convalescent Plasma in 20,000 Hospitalized Patients. Mayo Clin. Proc. 2020;95:1888–1897. doi: 10.1016/j.mayocp.2020.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salazar E., Perez K.K., Ashraf M., Chen J., Castillo B., Christensen P.A., Eubank T., Bernard D.W., Eagar T.N., Long S.W. Treatment of Coronavirus Disease 2019 (COVID-19) Patients with Convalescent Plasma. Am. J. Pathol. 2020;190:1680–1690. doi: 10.1016/j.ajpath.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.FDA . 2020. Emergency use authorization (EUA) of COVID-19 convalescent plasma for treatment of COVID-19 in hospitalized patients.https://www.fda.gov/media/141477/download [Google Scholar]
- 9.Salazar E., Christensen P.A., Graviss E.A., Nguyen D.T., Castillo B., Chen J., Lopez B.V., Eagar T.N., Yi X., Zhao P. Treatment of Coronavirus Disease 2019 Patients with Convalescent Plasma Reveals a Signal of Significantly Decreased Mortality. Am. J. Pathol. 2020;190:2290–2303. doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyner M.J., Senefeld J.W., Klassen S.A., Mills J.R., Johnson P.W., Theel E.S., Wiggins C.C., Bruno K.A., Klompas A.M., Lesser E.R. Effect of Convalescent Plasma on Mortality among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv. 2020 doi: 10.1101/2020.08.12.20169359. [DOI] [Google Scholar]
- 11.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., den Hollander J.G., Karim F., Mollema F.P.N., Stalenhoef J.E., Dofferhoff A., Ludwig I., Koster A. Convalescent Plasma for COVID-19. A randomized clinical trial. medRxiv. 2020 doi: 10.1101/2020.07.01.20139857. [DOI] [Google Scholar]
- 12.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P., PLACID Trial Collaborators Convalescent plasma in the management of moderate covid-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pau A.K., Aberg J., Baker J., Belperio P.S., Coopersmith C., Crew P., Glidden D.V., Grund B., Gulick R.M., Harrison C. Convalescent Plasma for the Treatment of COVID-19: Perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann. Intern. Med. 2020 doi: 10.7326/M20-6448. Published online September 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T., Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt F., Weisblum Y., Muecksch F., Hoffmann H.H., Michailidis E., Lorenzi J.C.C., Mendoza P., Rutkowska M., Bednarski E., Gaebler C. Measuring SARS-CoV-2 neutralizing antibody activity using pseudotyped and chimeric viruses. J. Exp. Med. 2020;217:e20201181. doi: 10.1084/jem.20201181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.FDA . 2020. Investigational COVID-19 Convalescent Plasma: Guidance for Industry.https://www.fda.gov/media/136798/download [Google Scholar]
- 18.FDA . 2020. Decisional Memo.https://www.fda.gov/media/141480/download [Google Scholar]
- 19.Cross R.W., Prasad A.N., Borisevich V., Woolsey C., Agans K.N., Deer D.J., Dobias N.S., Geisbert J.B., Fenton K.A., Geisbert T.W. Use of convalescent serum reduces severity of COVID-19 in nonhuman primates. bioRxiv. 2020 doi: 10.1101/2020.10.14.340091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun J., Zhuang Z., Zheng J., Li K., Wong R.L., Liu D., Huang J., He J., Zhu A., Zhao J. Generation of a Broadly Useful Model for COVID-19 Pathogenesis, Vaccination, and Treatment. Cell. 2020;182:734–743. doi: 10.1016/j.cell.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rogers T.F., Zhao F., Huang D., Beutler N., Burns A., He W.T., Limbo O., Smith C., Song G., Woehl J. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science. 2020;369:956–963. doi: 10.1126/science.abc7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng J., Wong L.R., Li K., Verma A.K., Ortiz M., Wohlford-Lenane C., Leidinger M.R., Knudson C.M., Meyerholz D.K., McCray P.B., Jr., Perlman S. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature. 2020 doi: 10.1038/s41586-020-2943-z. Published online November 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mato A.R., Roeker L.E., Lamanna N., Allan J.N., Leslie L., Pagel J.M., Patel K., Osterborg A., Wojenski D., Kamdar M. Outcomes of COVID-19 in patients with CLL: a multicenter international experience. Blood. 2020;136:1134–1143. doi: 10.1182/blood.2020006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klasse P.J., Moore J.P. Antibodies to SARS-CoV-2 and their potential for therapeutic passive immunization. eLife. 2020;9:e57877. doi: 10.7554/eLife.57877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bradfute S.B., Hurwitz I., Yingling A.V., Ye C., Cheng Q., Noonan T.P., Raval J.S., Sosa N.R., Mertz G.J., Perkins D.J., Harkins M.S. Severe Acute Respiratory Syndrome Coronavirus 2 Neutralizing Antibody Titers in Convalescent Plasma and Recipients in New Mexico: An Open Treatment Study in Patients With Coronavirus Disease 2019. J. Infect. Dis. 2020;222:1620–1628. doi: 10.1093/infdis/jiaa505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584:437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodhue Meyer E., Simmons G., Grebe E., Gannett M., Franz S., Darst O., Di Germanio C., Stone M., Contestable P., Prichard A. Selecting COVID-19 Convalescent Plasma for Neutralizing Antibody Potency Using a High-capacity SARS-CoV-2 Antibody Assay. medRxiv. 2020 doi: 10.1101/2020.08.31.20184895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harvala H., Mehew J., Robb M.L., Ijaz S., Dicks S., Patel M., Watkins N., Simmonds P., Brooks T., Johnson R. Convalescent plasma treatment for SARS-CoV-2 infection: analysis of the first 436 donors in England, 22 April to 12 May 2020. Euro Surveill. 2020;25:2001260. doi: 10.2807/1560-7917.ES.2020.25.28.2001260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang K., Long Q.X., Deng H.J., Hu J., Gao Q.Z., Zhang G.J., He C.L., Huang L.Y., Hu J.L., Chen J. Longitudinal dynamics of the neutralizing antibody response to SARS-CoV-2 infection. Clin. Infect. Dis. 2020:ciaa1143. doi: 10.1093/cid/ciaa1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang X., Guo X., Xin Q., Pan Y., Hu Y., Li J., Chu Y., Feng Y., Wang Q. Neutralizing Antibodies Responses to SARS-CoV-2 in COVID-19 Inpatients and Convalescent Patients. Clin. Infect. Dis. 2020:ciaa721. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen P., Nirula A., Heller B., Gottlieb R.L., Boscia J., Morris J., Huhn G., Cardona J., Mocherla B., Stosor V., BLAZE-1 Investigators SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2029849. Published online October 28, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kunisaki K.M., Janoff E.N. Influenza in immunosuppressed populations: a review of infection frequency, morbidity, mortality, and vaccine responses. Lancet Infect. Dis. 2009;9:493–504. doi: 10.1016/S1473-3099(09)70175-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Na I.K., Buckland M., Agostini C., Edgar J.D.M., Friman V., Michallet M., Sánchez-Ramón S., Scheibenbogen C., Quinti I. Current clinical practice and challenges in the management of secondary immunodeficiency in hematological malignancies. Eur. J. Haematol. 2019;102:447–456. doi: 10.1111/ejh.13223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinto D., Park Y.J., Beltramello M., Walls A.C., Tortorici M.A., Bianchi S., Jaconi S., Culap K., Zatta F., De Marco A. Cross-neutralization of SARS-CoV-2 by a human monoclonal SARS-CoV antibody. Nature. 2020;583:290–295. doi: 10.1038/s41586-020-2349-y. [DOI] [PubMed] [Google Scholar]
- 36.Baum A., Ajithdoss D., Copin R., Zhou A., Lanza K., Negron N., Ni M., Wei Y., Mohammadi K., Musser B. REGN-COV2 antibodies prevent and treat SARS-CoV-2 infection in rhesus macaques and hamsters. Science. 2020;370:1110–1115. doi: 10.1126/science.abe2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Accelerated emergency use authorization (EUA) summary FRL SARS-CoV-2 Test (UAB Fungal Reference Lab). https://www.fda.gov/media/139437/download.
- 38.Bryan A., Pepper G., Wener M.H., Fink S.L., Morishima C., Chaudhary A., Jerome K.R., Mathias P.C., Greninger A.L. Performance Characteristics of the Abbott Architect SARS-CoV-2 IgG Assay and Seroprevalence in Boise, Idaho. J. Clin. Microbiol. 2020;58:e00941-20. doi: 10.1128/JCM.00941-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brouwer P.J.M., Caniels T.G., van der Straten K., Snitselaar J.L., Aldon Y., Bangaru S., Torres J.L., Okba N.M.A., Claireaux M., Kerster G. Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science. 2020;369:643–650. doi: 10.1126/science.abc5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This study did not generate any unique datasets or code.