Abstract
Diabetes mellitus (DM) has been treated with herbs for centuries and many herbs reported to exert antidiabetic activity. Laurus nobilis is an aromatic herb belonging to the Lauraceae family, commonly known as bay. This study aimed to investigate the activity of Laurus nobilis leave extracts on histopathological and biochemical changes in β-cells of streptozotocin (STZ)-induced diabetic rats. Thirty healthy adult male albino rats were included in the study and divided equally into 5 groups for 4 weeks as follow; control group (C), diabetic group (D), diabetic Laurus nobilis extract group (DLN), Laurus nobilis extract group (LN) and diabetic acarbose (DA) group. Histopathologically, D group rats exhibited various degenerative and necrotic changes in their liver, pancreas and kidney, whereas the DLN rats had nearly normal histology. Insulin immunostaining in the pancreatic beta cells was decreased in the D group compared to the C group, whereas the DLN group was similar to the C group. The glucose concentration decreased significantly in both diabetic rats treated with L. nobilis and acarbose (p < 0.05). Additionally, the levels of aspartate aminotransferase (AST), gamma-glutamyltransferase (GGT) and alanine aminotransferase (ALT) enzyme were significantly decreased in both diabetic rats treated with L. nobilis and acarbose, compared to the D group (p ˃ 0.05). Outcomes of this study said that leave extracts of L. nobilis has valuable effect on blood glucose level and ameliorative effect on regeneration of pancreatic islets, it also restored the altered liver enzymes, urea, creatine kinase, total protein levels, calcium and ferritin to near normal.
Keywords: Diabetes mellitus, Laurus nobilis, Streptozotocin, Histopathology, Blood glucose
Highlights
-
•
Diabetes mellitus has been treated with herbs for centuries and many herbs reported to exert antidiabetic activity.
-
•
Historically, traditional herbal treatments have been shown to possess successful pharmacological activity, such as in the case with metformin, isolated from Galega officinalis.
-
•
Laurus nobilis has a valuable effect on blood glucose level and ameliorative effect on regeneration of pancreatic islets.
-
•
Laurus nobilis have also been shown to display insulin-enhancing activity in vitro.
-
•
Laurus nobilis also restored the altered liver enzymes, urea, creatine kinase, total protein levels, calcium and ferritin to near normal.
1. Introduction
Diabetes mellitus (DM) is a chronic endocrine disorder of multiple etiologies distinguished by hyperglycemia resulting from defects in insulin secretion, insulin action, or both [1]. The clinical diagnosis of diabetes is often indicated by the presence of symptoms such as polyuria, polydipsia, unexplained weight loss, and is confirmed by documented hyperglycemia [2,3]. Diabetes complications can be classified as microvascular complications such as nervous system damage (neuropathy), renal system damage (nephropathy) and eye damage (retinopathy), and macrovascular complications for example cardiovascular disease, stroke, and peripheral vascular disease [[4], [5], [6]].
Natural supplements are widely used around the world to treat diabetes, but medical research does not support their effectiveness. DM has been treated with herbs for centuries and there are many herbs that have been reported to exert antidiabetic activity [[7], [8], [9]]. Traditional plants have been used as a cure for diabetes for a long time before the introduction of modern medicine. Historically, traditional herbal treatments have been shown to possess successful pharmacological activity, such as in the case with metformin, isolated from Galega officinalis [[10], [11], [12]].
The dried leaves of Laurus nobilis are used as a spice or flavoring agent in the culinary and food industries [13]. The essential oil (EO) prepared from the leaves has been reported to have antibacterial, antioxidant and anti-inflammatory activities [14,15]. Further leaves of Laurus nobilis have been also used to treat rheumatism, neuralgia, and scabies [16,17].
The main volatile compounds in laurel herb extract are usually 1,8-cineole, methyl eugenol, α-terpinyl acetate. α-pinene, β-pinene, sabinene, and linalool. Generally, leaves and berries are widely utilized, oxygenated monoterpene 1,8-cineole is one of the major constituents of leaves and berry fruits essential oil obtained from Laurus nobilis [18]. The leaves contains about 1.3% essential oils. The EOs obtained of berry fruit depending on provenance and storage conditions. The oil extracted from berries contain fatty acids, which include lauric (54%) linoleic (17%) oleic (15%) and palmitic (5%) and volatile compounds such as β-ocimene (22%), 1,8-cineole (9.5%), bicyclogermacrene (4.5%) and β-elemene (2%) [19,20]. The bioactive components in bay leaves have been shown to have effects on insulin sensitivity, glucose uptake, antioxidant status, inflammatory response, and glucose emptying.
Laurus nobilis is an aromatic herb belonging to the Lauraceae family known as bay, daphne, bay laurel, true bay, or sweet bay. It is an evergreen tree or shrub, is a native plant from the Southern Mediterranean region, found in warm climate regions with high rainfall, especially distribution in Turkey, Greece, Spain, Portugal, Italy, France, Morocco and Mexico [21,22]. In Turkey, Laurus nobilis grows in the Marmara, Aegean and Mediterranean regions [23,24]. It's one of the oldest known spices, widely used as a condiment and spice. With relevant medicinal properties due to its important chemical composition and its potential therapeutic effects [14]. Afifi et al. [25] reported that aqueous extracts of Laurus nobilis seeds were effective in reducing ethanol-induced gastric ulcer in rats.
Traditionally bay leave extract have been used orally to treat the symptoms of gastrointestinal problems, such as epigastric bloating, impaired digestion, eructation, and flatulence [25]. As a traditional medicine, the decoction or tea of bay leaves is often used as therapy, intestinal and gastric antispasmodic, against diarrhea, for rheumatic pains, also diseases of the respiratory tract, as a cough, asthma, and cardiac diseases [26].
Bay leaves have also shown that display insulin-enhancing activity in vitro [27], however these enhance glucose metabolism and the overall condition of individuals with diabetes not only by hypoglycemic effects but also by improving lipid metabolism, antioxidant status, and capillary function [28]. Bay leaves reduced serum glucose, total cholesterol, LDL cholesterol and triglycerides, and increased HDL-cholesterol levels in people with type 2 diabetes [27]. This study aimed to evaluate the effects of Laurus nobilis leave extracts on biochemical and histopathological changes of pancreas, liver and kidney on diabetic rats.
2. Materials and methods
2.1. Materials
2.1.1. Animals
All experimental protocols were approved by the Experimental Animal Center of Yuzuncu Yil University, Van, Turkey. In the present study, thirty male Wistar rats, weighting about 160–200 g with averagely 7 weeks old were randomly divided into five groups based on treatment each group containing 6 animals. All animals were housed under safe laboratory conditions in a temperature-controlled room (22–24 °C) and kept on a 12 h light/dark cycle. Blood glucose and body weight were monitored before treatment once a week throughout 4 weeks of experimental period.
2.1.2. Equipments
All measurements were performed by using Automatic tissue processor (LEICA TP 1020 Semi-enclosed Benchtop), Centrifuge (Hettich TD4, Shanghai, China), Nikon digital camera (DXM-1200F), Glucometer (Accu-Chek, Taiwan), Tissue imbedded paraffin (LEICA Eg115° H Shanghai, China).
2.2. Methods
2.2.1. Preparation of plant material and diet
The bay leave purchased from traditional herbal markets, a specimen was deposited at the herbarium of the Hatay. The laurus nobilis leaves were ground to powder by electric grinder and extracted with ethanol, and the extraction oils were stored at room temperature in dark place. 200 mg kg −1 of bay oils was administered daily orally using intragastric tube at the time of work [29].
2.2.2. Diabetes model with streptozotocin (STZ)
Diabetes mellitus was induced by single intraperitoneal (IP) injection of freshly prepared STZ (Sigma-aldrich, Saint Louis, MO) at dose of 70 mg kg −1 b.w. dissolved in 0.01 M citrate buffer, pH 4.5 [30]. After 72 h of STZ injection, and overnight fast, blood was taken from tail artery of the rats. Accu-Chek monitoring used to rapidly changing blood glucose level, when rats with blood glucose higher than 250 mg dl −1 were selected for the diabetic groups and involved to the examination. Strict monitoring of all diabetic group rats was done for blood glucose after 24–48 h of STZ administration.
Injection of STZ and attack on pancreas cause hypersecretion of insulin and this lead to intensive hypoglycemia and this may cause death to many animals, to avoid this, drinking water containing 10% dextrose were given to rats directly after I.P of STZ. In addition for taken care about rats, blood glucose was measured at 3rd, 15th and 28th days of throughout experimental model in blood taken from tail artery.
2.2.3. Experimental protocols
Experimental animals were randomly divided into 5 groups; each group was included 6 animals. The examination period was continuous for four weeks as below:
-
1.
Control group (C): did not receive any other kind of co-supplementation. Rats were given a standard diet.
-
2.
Diabetes group (D): in this group diabetes was induced by administered 70 ml/kg single dose of STZ IP injection [30], and given standard diet.
-
3.
Diabetes treated with Laurus nobilis leave extract group (DLN) given 200 mg kg −1 of bay extract that administered every day orally using intragastric tube for 28 days during the examination [29].
-
4.
Laurus nobilis extract group (LN): 200 mg kg −1 of bay leave was administered every day orally using intragastric tube [29].
-
5.
Diabetes with drug (Acarbose) group (DA): The rats of this group were treated with 150 mg kg −1 dose of Acarbose tablet (Glucobay), (Bayer Türk Kimya San) each day orally using intragastric tube [31].
2.2.4. Blood sample collection and biochemical analysis
At the end of the treatment period, all rats were fasted for 18 h, weighed and then anaesthetized via IP injection of ketamine hydrochloride (50 mg kg −1 b.w.) and xylazine (8 mg kg −1 b.w.). Blood samples were collected from the heart puncture of rats and transferred to suitable tubes for biochemical analysis using Merck commercial diagnostic kits (Darmstadt, Germany) on a blood chemistry analyzer (BTS-350, BioSystems S.A. Barcelona, Spain).
2.2.5. Histopathological studies
Histopathological investigation were performed at last day of the experiment. Pancreas, liver and kidneys were removed and kept in 10% formaldehyde and embedded in paraffin. Blocks of the preserved tissues were sectioned (3–5 μm) on a microtome (Leica RM 2135: Leica Biosystems Nussloch GmbH, Nussloch, Germany) and mounted on glass slides. Hematoxylin and eosin (H&E) staining was done and histopathological examination was done in accordance with the method adopted by Nagy and Ewais [32].
2.2.6. Immunohistochemical investigation
Insulin expressions were stained using the streptavidin-peroxidase method (ABC), with the streptavidin / biotin immunoperoxidase kit (Histostain-Plus Bulk Kit; Zymed, South San Francisco, CA, USA) in accordance with the staining procedures of the manufacturer companies. After the sections taken in 4–5 micron thick by microtome had been placed on adhesive slides, they were passed through xylene and alcohol series. In order to remove the endogenous peroxidase activity, the sections were kept in 3% Hydrogen peroxide (H2O2) for 20 minutes after being washed with PBS (phosphate buffer solution). After placing the antigen in the retrieval solution (citrate buffer), it was heated twice in the microwave oven for 20 minutes. Then it was taken out of the oven and it was left to cool until it reached room temperature. After being washed again with PBS, the sections were blocked by protein blocking (non-immune serum) for 20 minutes. Insulin antibody (Catalog no: ab-181547; Abcam, Toronto, Canada, diluted 1:1.000) were dropped into each tissue and left overnight at +4°C. Sections were washed again with PBS and incubated for 20 minutes at room temperature with biotinylated secondary antibody. The sections washed again with PBS were left in streptavidin-peroxidase for 20 minutes and then washed in the same way as PBS. After washing, 3,3'-Diaminobenzidine (DAB) was dropped and left for 1–2 minutes. Then all sections were kept in Mayer’s hematoxylin (Bio-Optika, 05-06002E) for 1–2 minutes and washed in tap water. Sections were passed through of 70%, 80%, 90%, 96% alcohol for 3 minutes, respectively, and 100% alcohol for 10 minutes and of the xylol series for 5 minutes were closed using entellan. Negative controls reacted with PBS were used instead of primary antibodies to confirm staining. Sections were examined and photographed under a light microscope. The immunoreactivity staining intensities of the testicular samples obtained from the groups with primary antibodies were scored as mild (+), moderate (++) and strong (+++).
2.2.7. Statistical analysis
Statistical analyses were performed by using ‘IBM SPSS Alan C. Elliott software. ANOVA test was applied to analysis the significant differences in all groups. (Kruskal-Wallis) huc Dunn's test was done to compare among groups, differences were considered significant when the p < 0.05.
3. Results
3.1. Effect on body weight
The diabetic rats exhibited profound body weight loss as compared to normal rats. The initial, half-way and final body weights of the rats are shown in (Table 1).
Table 1.
Results of body weight (g) in different groups (mean ± Standard deviation).
| G | 1st day | 15th day | 28th day |
|---|---|---|---|
| C | 195.33 ± 15.31a | 233.66 ± 17.90 b | 268.00 ± 13.91 b |
| D | 204.33 ± 19.28b | 195.83 ± 33.18a,b | 189.66 ± 24.76a |
| DLN | 217.33 ± 19.00a | 211.66 ± 10.38a | 214.33 ± 10.46a |
| LN | 212.33 ± 13.10a | 229.83 ± 17.26b | 262.16 ± 21.16b |
| DA | 215.66 ± 18.85a | 213.83 ± 24.11a,b | 211.16 ± 34.56a |
*G; groups were C (control group), D (diabetic group), DLN (diabetic with Laurus nobilis trated group), DA (diabetic with drug treated group), LN (Laurus nobilis fed group).
Data are means ± S.D. b,c,dSignificantly different from Control group in initial.
The final body weights in diabetic group (D), diabetes Laurus nobilis group (DLN), and diabetic Acarbose group (DA) were significantly decreased when they compared with measuring at 14 days and also higher decreased compared with baseline weight at the beginning of the study. In contrast, body weights in control group (C) were significantly higher in comparison to other groups during experimental period while laurus nobilis group (LN) was not significantly changed in body weight. Diabetes seemed to be more effective in decreasing body weight (Table 1).
3.2. Effect on serum glucose
The serum glucose concentration (mg dl −1) in the Group C was significantly (p < 0.05) lower, whereas Group D showed significantly (p < 0.05) higher concentration as compared to other groups throughout the experiment (Table 2). On the other hand, L. nobilis-fed group rats showed relative to control (C) rats during the first, second and fourth week, respectively.
Table 2.
Results of blood glucose (mg dl −1) in different groups (mean ± Standard deviation).
| G | 1st day | 15th day | 28th day |
|---|---|---|---|
| C | 128.16 ± 10.87a | 157.50 ± 30.73a | 138.33 ± 24.23a |
| D | 600.00 ± 0.00 d | 592.50 ± 18.37c | 457.66 ± 170.57c |
| DLN | 563.16 ± 53.21c | 479.16 ± 164.36 b | 287.33 ± 109.83 b |
| LN | 120.00 ± 4.97a | 127.33 ± 20.61a | 140.00 ± 7.29a |
| DA | 521.66 ± 14.26 b | 587.33 ± 18.61c | 316.16 ± 70.00 b |
*G; groups were C (control group), D (diabetic group), DLN (diabetic with Laurus nobilis trated group), DA (diabetic with drug treated group), LN (Laurus nobilis fed group).
Data are means ± S.D. b,c,dSignificantly different from Control group.
The two diabetic treated Groups (DLN and DA) showed significant (p < 0.05) decrease in glucose concentration on last week and as compared to Group C; the decrease was the highest in Groups C and LN. All Higher blood glucose concentrations were observed in group D, DLN and DA group after 72 h of STZ injection, and gradually decreased on day 14th and 28th also significant difference between group D and treated diabetes groups (DLN, DA). Laurus nobilis inhibited the development of diabetes induced by STZ treatment (Table 2).
3.3. Histopathological findings
3.3.1. Liver
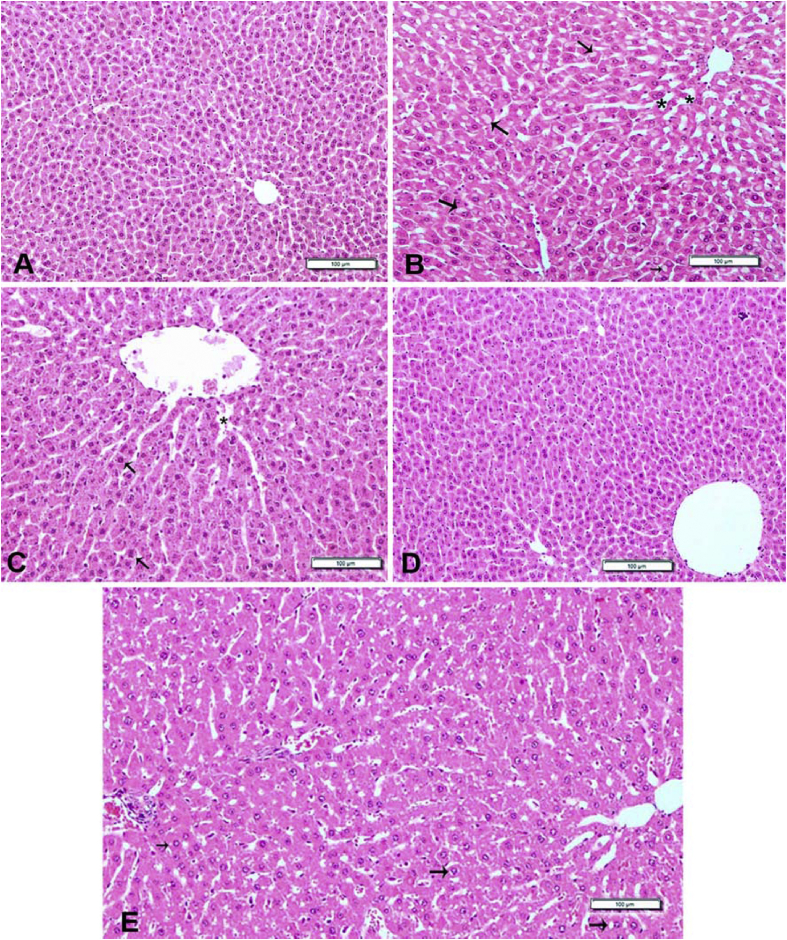
The normal histological view of the liver was observed in the Control and LN groups (Fig. 1-A and D). Degeneration and necrosis in the hepatocytes were detected in Diabetes group. Additionally, disrupted hepatic cords and sinusoidal architecture were detected. Varying in size vacuoles were determined in the cytoplasm of degenerated hepatocytes (Fig. 1-B). These findings were found to be significantly reduced in the liver of rats in DLN group such as degeneration and necrosis (Fig. 1-C). Similar histological appearance to the Control group were found in DA group except for slight hydropic degeneration and dilation of sinusoids (Fig. 1-E).
Fig. 1.
Hematoxylin and eosin-stained sections of liver. A) Control group: Normal histological appearance. B) Diabetic group: Disseminated vacuolization (arrows) in the hepatocytes and dilation of sinusoids (stars). C) Diabetic + L. nobilis treated group: Slight hydropic degeneration in the some hepatocytes (arrows) and dilation of sinusoids (star). D)L. nobilis-fed group: Almost normal histological appearance of the liver. E) Diabetic + drug- treated group: rat administered with acarbose showing slight hydropic degeneration (arrows) and dilation of sinusoids. Bar = 100 μm.
3.3.2. Pancreas
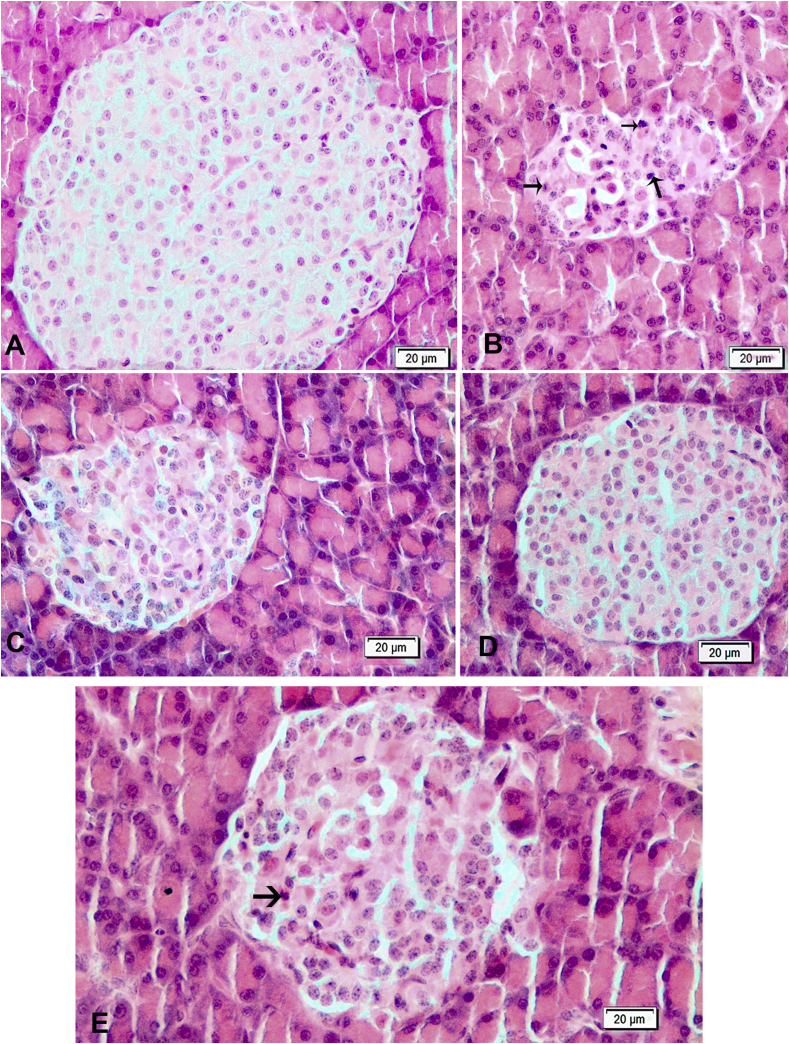
Histological views in cells of Langerhans islet in a Control and LN groups were normal (Fig. 2-A and D). The diabetic rats had degenerative and necrotic changes in the cells of Langerhans islet. As a result, atrophied islets which is cells with degenerative and picnotic nucleus had deteriorated and shrunken architecture (Fig. 2-B). Partially, Langerhans islet were preserved in rats in DLN group (Fig. 2-C). A significant recovery in DA group was observed in the islets (Fig. 2-E).
Fig. 2.
Hematoxylin and eosin-stained sections of pancreas. A) Control group: Normal histological appearance of islets of Langerhans. B) Diabetic group: Note that islets of Langerhans is atrophic, and there are hydropic degeneration and necrosis (arrows) of some cells of islets of Langerhans. C) Diabetic + L. nobilis treated group: Almost normal histological appearance of islets of Langerhans. D)L. nobilis group: Normal histological appearance of pancreas. E) Diabetic + Acarbose treated group: Showing slight hydropic degeneration and a few necrotic cells of islets of Langerhans. Bar = 20 μm.
3.3.3. Kidney
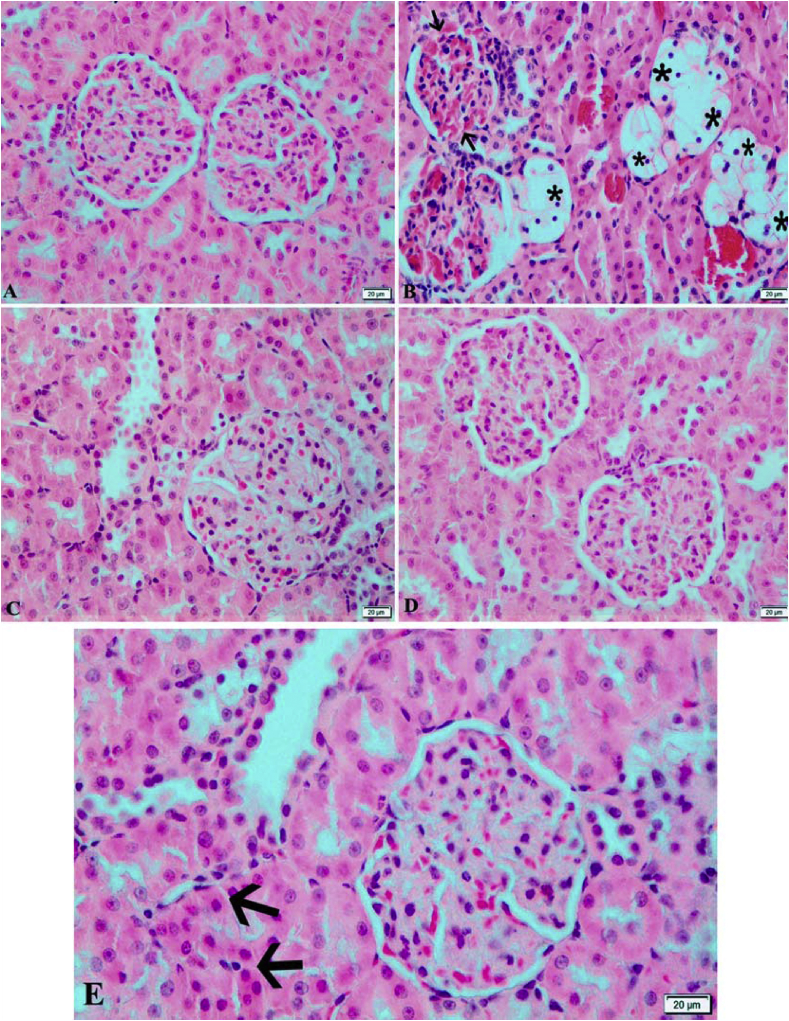
Kidney had normal histological view in both Control and LN groups (Fig. 3-A and D). Severe balloon degeneration and necrosis of tubular epithelium, vascular congestion, degeneration and necrosis of podocytes of glomeruli of kidney of diabetic rats. Inflammatory cells were focally detected in the periglomerular areas. It were adhesion Bowman capsule in some Glomeruli (Fig. 3-B). Slight degeneration and necrosis were determined in some parts of the tubular epithelial cells and dilation of some lymphatic in the kidney of rats in DLN group. Additionally, seldom adhesions in bowman capsule of some glomeruli were observed (Fig. 3-C). DA group were similar to DLN group (Fig. 3-E).
Fig. 3.
Hematoxylin and eosin-stained sections of kidney. A) Control group: Normal histological appearance. B) Diabetic group: Showing adhesions (arrows) between the glomerulus and Bowman capsule, and severe degeneration (stars) of tubular epithelium. C) Diabetic + L. nobilis treated group: Almost normal histological appearance of tubular epithelium and glomerulus. D)L. nobilis group: Normal histological appearance of tubular epithelium and glomerulus. E) Diabetic + Acarbose treated group: showing degenerative and necrotic changes (arrows) in some tubular epithelium. Bar = 20 μm.
3.4. Immunohistochemical evaluation
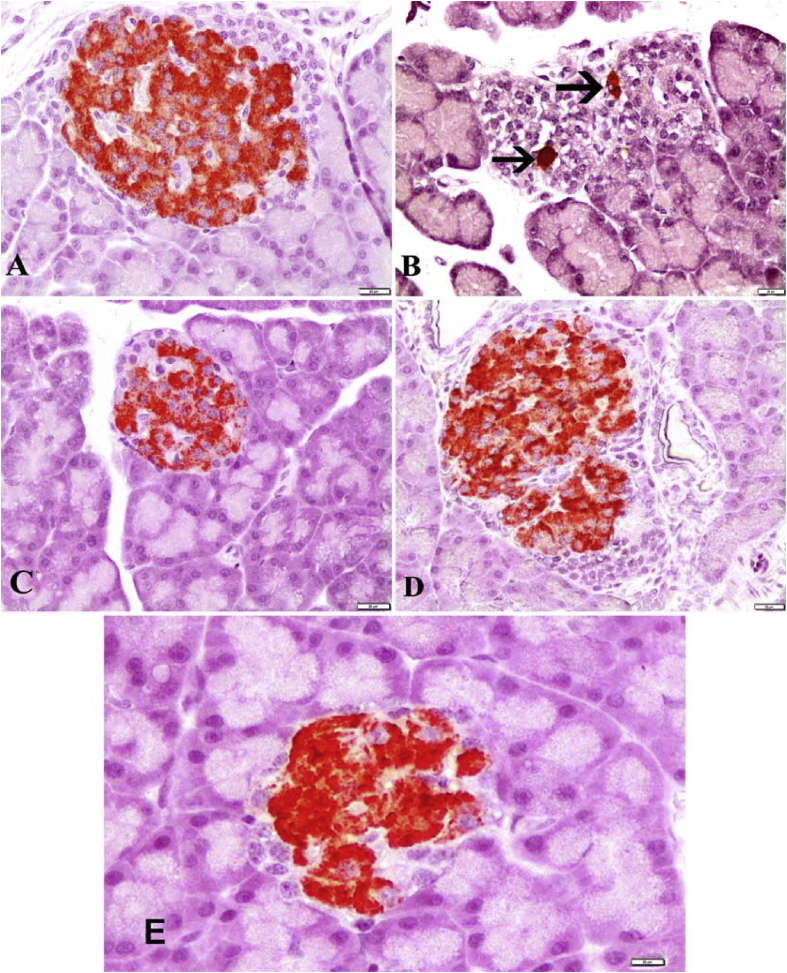
The insulin secreting β-cells represented the major cell population of the islets, occupying mainly the central zone in the Langerhans islets. Positive insulin expression was seen in the form of dark brown granules present in the cytoplasm of β-cells. Strong insulin immunoreaction were found in the Langerhans islets of Control and LN groups (Fig. 4-A and D). Immunoreactivity in the Diabetes group had dramatically reduced according to the control group and only a few β-cells displayed minimal insulin immunoreaction (Fig. 4-B). In diabetic rats treated L. nobilis extracts was increased in the number and percentage area of reactive β-cells an apparent observed, as compared with the diabetic group. Moderate insulin immunoreaction in the DLN group were detected compared to diabetic rats. (Fig. 4-C). DA group showed significantly recovery compared to Diabetes group (Fig. 4-E).
Fig. 4.
Immunohistochemical staining of islets of Langerhans of pancreas by streptavidin-peroxidase (ABC) method. A) Control group: Strong insulin immunoreactivity in β cells, which occupy most of the islet. B) Diabetic group: Weak insulin immunoreactivity in a few β cells (arrows). C) Diabetic + L. nobilis treated group: Moderate insulin immunoreactivity in β cells. D)L. nobilis group: Strong insülin immunoreactivity in β cells. E) Diabetic + Acarbose treated group: Moderate insulin immunoreactivity in β cells. Bar = 20 μm.
3.5. Biochemical results
The glucose concentration decreased significantly in both diabetic rats treated with L. nobilis and acarbose (p < 0.05) (Table 3). The levels of alanine aminotransferase (ALT) enzyme were significantly (p < 0.05) decreased in L. nobilis diabetic treated rats compared to diabetic group rats. Also ALT level was significantly (p < 0.05) lower in acarbose diabetic treated group as compared to that of diabetes group (Table 3).
Table 3.
Changes in the serum of Glucose(mg dL −1), ALT(U l −1), AST(U l −1), GGT(U l −1), ALP(U l −1), Urea(mg dL −1), Calcium(mg dL −1), Magnesium(mg dL −1), Phosphor(mg dL −1), Total protein(g dL −1), Albumin(mg dL −1), Creatinine(mg dL −1), and Iron(μmol L −1) levels in different groups under study (mean ± standard error).
| BC |
G |
||||
|---|---|---|---|---|---|
| C | D | DLN | DA | LN | |
| ALT | 49.16 ± 1.07a | 251.83 ± 46.91b | 51.83 ± 10.99a | 34.16 ± 1.53a | 77.66 ± 23.41a |
| AST | 136.50 ± 7.53a | 547.33 ± 139.46 b | 98.50 ± 6.92a | 110.33 ± 1.28a | 216.33 ± 41.20a |
| GGT | 0.51 ± .0.14a | 9.16 ± 2.08 b | 2.18 ± 1.16a | 0.16 ± 0.16a | 1.08 ± .0.40a |
| ALP | 203.16 ± 9.14a | 678.50 ± 120.73 b | 654.33 ± 139.94 b | 281.16 ± 25.29a | 366.50 ± 79.07a,b |
| Urea | 31.61 ± 0.91c | 53.76 ± 1.22 d | 19.90 ± 0.90 b | 15.05 ± 0.27a | 30.56 ± 2.69c |
| Ca | 11.10 ± 0.12a | 10.21 ± 0.22a | 12.58 ± 0.93 b | 11.35 ± 0.25a,b | 10.98 ± 0.30a |
| Mg | 3.52 ± 0.08 b | 3.50 ± 0.18 b | 3.13 ± 0.39a,b | 2.64 ± 0.04a | 3.51 ± 0.22 b |
| P | 9.03 ± 0.11 b | 9.10 ± 0.45 b | 7.39 ± 1.59 b | 4.49 ± 0.13a | 9.75 ± 1.02 b |
| TP | 6.64 ± 0.07c | 6.23 ± 0.10a,b | 5.90 ± 0.13a | 5.87 ± 0.05a | 6.57 ± 0.21 b,c |
| ALB | 3.65 ± 0.02 b | 3.17 ± 0.07b | 3.56 ± 0.10 b | 3.35 ± 0.01a | 3.58 ± 0.09 b |
| CK | 725.6 ± 85.10 b | 664.1 ± 60.77 b | 282.1 ± 51.01a | 331.6 ± 16.01a | 809.6 ± 52.38 b |
| Fe | 32.08 ± 1.57 b | 17.20 ± 3.55a | 32.20 ± 6.95 b | 35.15 ± 3.60 b | 40.30 ± 5.60 b |
*BC; biochemical tests were ALT (alanine aminotransferase), AST (aspartate aminotransferase), GGT (gamma-glutamyltransferase), ALP (alkaline phosphatase), Ca (calcium), Mg (magnesium), P (phosphor), TP (total protein), ALB (albumin), CK (creatinine kinase), Fe (iron).
*G; groups were C (control group), D (diabetic group), DLN (diabetic with Laurus nobilis trated group), DA (diabetic with drug treated group), LN (Laurus nobilis fed group).
*a - c Means with the same column are not significantly different P ˃ 0.05. *a-c Means with different column are statistically significant P ˂ 0.05.
The mean values of aspartate aminotransferase (AST) activities were significantly decreased in both diabetic rats treated with L. nobilis and acarbose (p < 0.05), the level of AST was lower in L. nobilis treated rats as comparison other rat groups (Table 3). As shown in (Table 3), the mean values of Gamma-glutamyltransferase (GGT) activities were significantly decreased in all groups when compared with diabetes group (p < 0.05). However, the differences within other groups were not significant (p ˃ 0.05).
A statistically non-significant decrease (p > 0.05) found in alkaline phosphatase (ALP) level L. nobilis diabetes treatment group in comparison with diabetes groups. In addition, statistically significant decrease (p < 0.05) can be seen in both diabetes drug treatment acarbose and undiabetes treatment of L. nobilis extracts as compared with diabetes group (Table 3).
The mean values of Urea level were significantly decreased in DLN extracts group as compared with diabetic groups (p < 0.05). Diabetic treated group of rats showed a significant high level of calcium as compared to other group of rats (p < 0.05). The L. nobilis-fed group was no significant when compared with control group (Table 3). No significant difference in magnesium level was observed in treated rats group with the diabetic group (p > 0.05), (Table 3). In the phosphor (p) level did not show any significant difference in L. nobilis extract compared to both diabetic and control group of rats (p > 0.05) (Table 3).
Statistically non-significant and slight reduction found in the total protein (TP) levels as compared with diabetic group. L. nobilis leave extract and control group are relative and significant when compared with diabetic group (p > 0.05), (Table 3).
A insignificantly (p > 0.05) increased can be seen in the Albumin (ALB) level in the L. nobilis leave extract group in comparison with diabetic group (Table 3). The level of creatinine kinase (CK) was decreased significantly (p < 0.05) in the diabetic L. nobilis group in comparison with the diabetic group (Table 3). Iron (Fe) levels in the diabetic rats were treated with L. nobilis significantly increased when compared to diabetic group rats, there was no significant difference in the treated diabetes groups with control group in the levels of Fe (p > 0.05), (Table 3).
4. Discussion and conclusion
Conventional therapies for DM have many side effects and high rate of secondary failure. On the other hand herbal extracts are expected to have similar efficacy with fewer side effects than conventional drugs [33]. Nowadays, more than 1200 plant species are used to treat symptoms of DM, the hypoglycemic property of almost 50% of these traditionally consumed medicines has been experimentally tested [34].
In our study detected weight loss in all STZ-induced rats group when comparisons with control group, and exhibited hyperglycaemia in STZ-induced rats with decrease in serum glucose levels in the treated diabetic groups. L. nobilis leaves extracts (LNLE) inhibit the development of diabetes induced by STZ and decrease serum glucose levels. LNLE treatment did not induce a significant change in the body weight of the diabetic rats, however, LNLE had a significant decrease in the blood glucose levels for 28 days of the diabetic rats’ treatment group.
In this study, we observed histopathological and biochemical changes in STZ-induced rats and these changes were reduced with LNLE treatment. The promising mechanism by which LNLE mediated its antidiabetic effect could be by potentiation of pancreatic secretion of insulin from existing β-cells of islets, as was evident by the significant decrease in the level of glucose in the extract treated animals.
The hypoglycemic activity of LNLE was compared with acarbose, a standard hypoglycemic drug, since the results of the present study, it may be suggested that the mechanism of action of L. nobilis may be similar to acarbose action. In this essay indicated that the pathological effects in rats liver and pancreas tissues induced by STZ were reversible and normalized by received extracts bay leaves for 4 weeks of experiment, this could be attributed to its antidiabetic effects. Several studies have reported that the hepatocytes of STZ-induced diabetic rats showed cytoplasmic alterations, sinusoidal dilation and congestion, periportal inflammation, showed kupffer cells activation, cytoplasmic vacuolization of hepatocytes and necrosis [35].
The hepatocyte nuclei were generally enlarged and sometimes showed irregular contours and intranuclear inclusions [36]. In pancreatic sections of untreated diabetic rats disclosed that the islets were comparatively small and shrunken and severe degenerative changes in the pancreatic islets, mainly at the center of the islets and karyolysis of the nuclei was visible [37]. The L. nobilis treated diabetic rats were reversed and the normalization of pancreatic architecture revealed vacuolations of β-cells was observed.
In addition regarding the immunohistochemistry, different sizes of islets of pancreas were observed with increasing immunoreaction to insulin antibody in β-cells treated with L. nobilis leaves extracts comparison to diabetic group, langerhans with increasing insulin immunoreactivity in cytoplasm of its β-cells, in the number and percentage area of reactive β-cells, areas of dark brown staining (strong positive) for insulin antibody were seen in islet β-cells cytoplasm from diabetic treated by L. nobilis.
In STZ-induced diabetic rats the liver was necrotized. An increase in the activities of ALT, AST and GGT in plasma might be mainly an indication of the hepatotoxic effect of STZ [38]. ALT, AST and GGT were significantly decreased in diabetic bay leaves treatment, and related to normal control group. In contrast diabetic group significantly higher level of enzymatic liver function test observed [39]. Al Chalabi et al. [40] reported that the bay leave extract resulted in a decrease in fasting blood glucose and a higher level of fasting insulin, also LDL, ALT, and AST were also decreased, whereas HDL and body weight increased in groups of diabetic rats relative to control not treated groups. In another study recommended that the L. nobilis tea consumption in healthy volunteers can improve blood lipid profile (HDL level increased and a small decrease in levels of LDL and triglycerides) and this implies a possible positive impact on the risk reduction of coronary heart disease [41]. Similar results were reported by Casamassima et al. [42] and investigated a substantial reduction in blood lipid profile, glycemic profile and liver enzymes, with decreased levels of LDL, ALT and AST, and increased HDL, has resulted from dietary incorporation of dried bay leaves meal.
Treatment of the diabetic rats with LNLE reduced the activity of these enzymes in plasma compared to the diabetic untreated group and consequently alleviated liver damage caused by STZ-induced diabetes and indicated the hepato protective role in preventing diabetic complications. The diabetic rats treated with bay leave extract displayed a statistically significant reduction in the urea and creatinine levels with respect to diabetic rats. In this sense, kidney failure is manifested by increasing urea and creatinine but a decrease indicates clinical improvement [43].
There's still clearly a significant dispute about the use of natural or cultivated plants, which has both positive and negative aspects in biophysical terms, as well as in terms of economics. L. nobilis is a significant socioeconomic evergreen tree belonging to the Lauraceae family. L. nobilis are used as antihyperglycaemic herbs, used to treat bacterial and fungal contaminations, to treat eructation, flatulence and gastrointestinal problems. It also exhibits anti-inflammatory, anticonvulsive, antiepileptic and antioxidant properties [44,45].
In conclusion, from this study, based on the experimental findings, it was suggested that administration of L. nobilis leave extracts, at a safe dose level, significantly suppressed STZ-induced diabetic rats. We believe that further preclinical research into the utility of L. nobilis treatment may indicate its suitability as a potential treatment in diabetic patients, our results expressed that leave extracts of L. nobilis has valuable effect on blood glucose level and ameliorative effect on regeneration of pancreatic islets. It also restored the altered liver enzymes (ALT, AST, and GGT), urea, creatine kinase, total protein levels calcium and Fe to near normal. It may be used as a therapeutic agent in the management of diabetes mellitus.
Author contributions
All authors contribute to this research study.
Rebin R. Mohammed, Abdullah K. Omer, Avin K, Ahmed: Data collection and analysis, original draft preparation, Zabit Yener, Ahmet Uyar: Study design, conceptualization and writing, review and editing.
5. Provenance and peer review
Not commissioned, externally peer reviewed.
Funding
The authors wish to acknowledge Van Yuzȕncu Yıl University for the support (Grant number 2015-SBE-YL309).
Ethical approval
All experimental protocols were approved by the Experimental Animal Center of Van Yȕzȕncȕ Yıl University, Turkey.
Consent
All experimental protocols were approved by the Experimental Animal Center of Van Yȕzȕncȕ Yıl University, Turkey.
Guarantor
Rebin R.Mohammed (on behalf of all authors).
Declaration of competing interest
There is no conflict of interest.
Acknowledgements
The authors wish to acknowledge Van Yȕzȕncȕ Yıl University for the support (Grant number 2015-SBE-YL309).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2020.11.051.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.American Diabetes Association Standards of medical care in diabetes--2014. Diabetes Care. 2014;37(3):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention . Centers for Disease Control and Prevention, US Dept of Health and Human Services; Atlanta, GA: 2015. Diabetes Report Card 2014. [Google Scholar]
- 3.Donath M.Y. Targeting inflammation in the treatment of type 2 diabetes: time to start. Nat. Rev. Drug Discov. 2014;13(6):465–476. doi: 10.1038/nrd4275. [DOI] [PubMed] [Google Scholar]
- 4.Afroz A., Zhang W., Wei Loh A.J., Jie Lee D.X., Billah B. Macro- and micro-vascular complications and their determinants among people with type 2 diabetes in Bangladesh. Diabet. Metab. Syndr. 2019;13(5):2939–2946. doi: 10.1016/j.dsx.2019.07.046. [DOI] [PubMed] [Google Scholar]
- 5.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 6.Massaro J.D., Polli C.D., Costa E.S.M., Alves C.C., Passos G.A., Sakamoto-Hojo E.T. Post-transcriptional markers associated with clinical complications in Type 1 and Type 2 diabetes mellitus. Mol. Cell. Endocrinol. 2019;490:1–14. doi: 10.1016/j.mce.2019.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Dehghan H., Sarrafi Y., Salehi P. Antioxidant and antidiabetic activities of 11 herbal plants from Hyrcania region, Iran. J. Food Drug Anal. 2016;24(1):179–188. doi: 10.1016/j.jfda.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han X., Yang Y., Metwaly A.M., Xue Y., Shi Y., Dou D. The Chinese herbal formulae (Yitangkang) exerts an antidiabetic effect through the regulation of substance metabolism and energy metabolism in type 2 diabetic rats. J. Ethnopharmacol. 2019;239 doi: 10.1016/j.jep.2019.111942. [DOI] [PubMed] [Google Scholar]
- 9.Lian F., Li G., Chen X., Wang X., Piao C., Wang J. Chinese herbal medicine Tianqi reduces progression from impaired glucose tolerance to diabetes: a double-blind, randomized, placebo-controlled, multicenter trial. J. Clin. Endocrinol. Metab. 2014;99(2):648–655. doi: 10.1210/jc.2013-3276. [DOI] [PubMed] [Google Scholar]
- 10.Bailey C.J., Turner R.C. Metformin. N. Engl. J. Med. 1996;334(9):574–579. doi: 10.1056/NEJM199602293340906. [DOI] [PubMed] [Google Scholar]
- 11.Seetaloo A.D., Aumeeruddy M.Z., Rengasamy Kannan R.R., Mahomoodally M.F. Potential of traditionally consumed medicinal herbs, spices, and food plants to inhibit key digestive enzymes geared towards diabetes mellitus management — a systematic review. South Afr. J. Bot. 2019;120:3–24. [Google Scholar]
- 12.Tarabra E., Pelengaris S., Khan M. A simple matter of life and death—the trials of postnatal beta-cell mass regulation. Internet J. Endocrinol. 2012;2012 doi: 10.1155/2012/516718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akcan T., Estévez M., Serdaroğlu M. Antioxidant protection of cooked meatballs during frozen storage by whey protein edible films with phytochemicals from Laurus nobilis L. and Salvia officinalis. LWT (Lebensm.-Wiss. & Technol.) 2017;77:323–331. [Google Scholar]
- 14.Ozcan B., Esen M., Sangun M.K., Coleri A., Caliskan M. Effective antibacterial and antioxidant properties of methanolic extract of Laurus nobilis seed oil. J. Environ. Biol. 2010;31(5):637–641. [PubMed] [Google Scholar]
- 15.Taban A., Saharkhiz M.J., Niakousari M. Sweet bay (Laurus nobilis L.) essential oil and its chemical composition, antioxidant activity and leaf micromorphology under different extraction methods. Sustain. Chem. Pharm. 2018;9:12–18. [Google Scholar]
- 16.da Silveira S.M., Luciano F.B., Fronza N., Cunha A., Scheuermann G.N., Vieira C.R.W. Chemical composition and antibacterial activity of Laurus nobilis essential oil towards foodborne pathogens and its application in fresh Tuscan sausage stored at 7 °C. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2014;59(1):86–93. [Google Scholar]
- 17.Ramos C., Teixeira B., Batista I., Matos O., Serrano C., Neng N.R. Antioxidant and antibacterial activity of essential oil and extracts of bay laurel Laurus nobilis Linnaeus (Lauraceae) from Portugal. Nat. Prod. Res. 2012;26(6):518–529. doi: 10.1080/14786419.2010.531478. [DOI] [PubMed] [Google Scholar]
- 18.Abu-Dahab R., Kasabri V., Afifi F. Evaluation of the volatile oil composition and antiproliferative activity of laurus nobilis L . ( Lauraceae ) on breast cancer cell line models. Record Nat. Prod. 2014;8(2):136–147. [Google Scholar]
- 19.Bozan B., Karakaplan U. Antioxidants from laurel ( Laurus nobilis L.) berries: influence of extraction procedure on yield and antioxidant activity of extracts. Acta Aliment. 2007;36(3):321–328. [Google Scholar]
- 20.Kilic A., Hafizoglu H., Kollmannsberger H., Nitz S. Volatile constituents and key odorants in leaves, buds, flowers, and fruits of Laurus nobilis L. J. Agric. Food Chem. 2004;52(6):1601–1606. doi: 10.1021/jf0306237. [DOI] [PubMed] [Google Scholar]
- 21.Davis P.H., Cullen J., Coode M.J.E. University Press; Edinburgh: 1965. Flora of Turkey and the East Aegean Islands. [Google Scholar]
- 22.Hogg J.W., Terhune S.J., Lawrence B.M. Dehydro-1, 8-cineole: a new monoterpene oxide in Laurus nobilis oil. Phytochemistry. 1974;13(5):868–869. [Google Scholar]
- 23.Müller-Riebau F.J., Berger B.M., Yegen O., Cakir C. Seasonal variations in the chemical compositions of essential oils of selected aromatic plants growing wild in Turkey. J. Agric. Food Chem. 1997;45(12):4821–4825. [Google Scholar]
- 24.Tilki F. Influence of pretreatment and desiccation on the germination of Laurus nobilis L. seeds. J. Environ. Biol. 2004;25(2):157–161. [PubMed] [Google Scholar]
- 25.Afifi F.U., Khalil E., Tamimi S.O., Disi A. Evaluation of the gastroprotective effect of Laurus nobilis seeds on ethanol induced gastric ulcer in rats. J. Ethnopharmacol. 1997;58(1):9–14. doi: 10.1016/s0378-8741(97)00070-6. [DOI] [PubMed] [Google Scholar]
- 26.Hafizoglu H., Reunanen M. Studies on the components of Laurus nobilis from Turkey with special reference to laurel berry fat. Fett Wissenschaft Technol./Fat Sci. Technol. 1993;95(8):304–308. [Google Scholar]
- 27.Khan A., Zaman G., Anderson R.A. Bay leaves improve glucose and lipid profile of people with type 2 diabetes. J. Clin. Biochem. Nutr. 2009;44(1):52–56. doi: 10.3164/jcbn.08-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey C.J., Day C. Traditional plant medicines as treatments for diabetes. Diabetes Care. 1989;12(8):553–564. doi: 10.2337/diacare.12.8.553. [DOI] [PubMed] [Google Scholar]
- 29.Yanardağ R., Can S. Effects of Laurus nobilis L. leaves on blood glucose levels in normal and alloxan-diabetic rabbits. Chim. Acta Turc. (Istanb.) 1994;22:169–175. [Google Scholar]
- 30.Furman B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015;70 doi: 10.1002/0471141755.ph0547s70. 5.47.1-5..20. [DOI] [PubMed] [Google Scholar]
- 31.Salemi Z., Rafie E., Goodarzi M.T., Ghaffari M.A. Effect of metformin, acarbose and their combination on the serum visfatin level in nicotinamide/streptozocin-induced type 2 diabetic rats. Iran. Red Crescent Med. J. 2016;18(3) doi: 10.5812/ircmj.23814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nagy M.A., Ewais M.M. Antidiabetic and antioxidative potential of Cystoceria merica. Am. J. Biochem. 2014;4(4):59–67. [Google Scholar]
- 33.Gupta R., Bajpai K.G., Johri S., Saxena A.M. An overview of Indian novel traditional medicinal plants with anti-diabetic potentials. Afr. J. Tradit., Complementary Altern. Med. : AJTCAM. 2007;5(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- 34.Marles R.J., Farnsworth N.R. Antidiabetic plants and their active constituents. Phytomedicine. 1995;2(2):137–189. doi: 10.1016/S0944-7113(11)80059-0. [DOI] [PubMed] [Google Scholar]
- 35.Lenk S.E., Bhat D., Blakeney W., Dunn W.A., Jr. Effects of streptozotocin-induced diabetes on rough endoplasmic reticulum and lysosomes of rat liver. Am. J. Physiol. 1992;263(5 Pt 1):E856–E862. doi: 10.1152/ajpendo.1992.263.5.E856. [DOI] [PubMed] [Google Scholar]
- 36.Balázs M., Halmos T. Electron microscopic study of liver fibrosis associated with diabetes mellitus. Exp. Pathol. 1985;27(3):153–162. doi: 10.1016/s0232-1513(85)80037-2. [DOI] [PubMed] [Google Scholar]
- 37.Doi K., Yamanouchi J., Kume E., Yasoshima A. Morphologic changes in hepatocyte nuclei of streptozotocin (SZ)-induced diabetic mice. Exp. Toxicol. Pathol. 1997;49(3):295–299. doi: 10.1016/S0940-2993(97)80041-3. [DOI] [PubMed] [Google Scholar]
- 38.Sobeh M., Mahmoud M.F., Abdelfattah M.A.O., El-Beshbishy H.A., El-Shazly A.M., Wink M. Hepatoprotective and hypoglycemic effects of a tannin rich extract from Ximenia americana var. caffra root. Phytomedicine. 2017;33:36–42. doi: 10.1016/j.phymed.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 39.Esteghamati A., Eskandari D., Mirmiranpour H., Noshad S., Mousavizadeh M., Hedayati M. Effects of metformin on markers of oxidative stress and antioxidant reserve in patients with newly diagnosed type 2 diabetes: a randomized clinical trial. Clin. Nutr. 2013;32(2):179–185. doi: 10.1016/j.clnu.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Al Chalabi S., Majeed D., Jasim A., Al-Azzawi K. Benefit effect of ethanolic extract of Bay leaves (Laura nobilis) on blood sugar level in adult diabetic rats induced by alloxan monohydrate. Ann. Trop. Med. Publ. Health. 2020;23(16) [Google Scholar]
- 41.Chbili C., Maoua M., Selmi M., Mrad S., Khairi H., Limem K., Mrizek N., Saguem S., Ben Fredj M. Evaluation of daily Laurus nobilis tea consumption on lipid profile biomarkers in healthy volunteers. J. Am. Coll. Nutr. 2020 Nov-Dec;39(8):733–738. doi: 10.1080/07315724.2020.1727787. [DOI] [PubMed] [Google Scholar]
- 42.Casamassima D., Palazzo M., Vizzarri F., Coppola R., Costagliola C., Corino C., Di Costanzo A. Dietary effect of dried bay leaves (Laurus nobilis) meal on some biochemical parameters and on plasma oxidative status in New Zealand white growing rabbit. J. Anim. Physiol. Anim. Nutr. 2017 Oct;101(5):e175–e184. doi: 10.1111/jpn.12584. [DOI] [PubMed] [Google Scholar]
- 43.Kharbanda C., Sarwar Alam M., Hamid H., Bano S., Haider S., Nazreen S. Trapa natans L. root extract suppresses hyperglycemic and hepatotoxic effects in STZ-induced diabetic rat model. J. Ethnopharmacol. 2014;151(2):931–936. doi: 10.1016/j.jep.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 44.Dias M.I., Barros L., Dueñas M., Alves R.C., Oliveira M.B., Santos-Buelga C., Ferreira I.C. Nutritional and antioxidant contributions of Laurus nobilis L. leaves: would be more suitable a wild or a cultivated sample? Food Chem. 2014 Aug 1;156:339–346. doi: 10.1016/j.foodchem.2014.01.122. [DOI] [PubMed] [Google Scholar]
- 45.Hughes M.A., Black A., Smith J.A. First report of laurel wilt caused by raffaelea lauricola on bay laurel (Laurus nobilis) in the United States. Plant Dis. 2014 Aug;98(8):1159. doi: 10.1094/PDIS-02-14-0194-PDN. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.