Abstract
Purpose
To investigate the effect of short-wavelength light (SL) on guinea pigs with lens-induced myopia (LIM) and the possible retinoic acid (RA)–related mechanisms.
Methods
Two-week-old guinea pigs (n = 60) with monocular −5D lenses were reared under white light (WL, 580 lux) or SL (440 nm, 500 lux). The left eyes were uncovered as control. Refractive error (RE) and axial length (AL) were measured at baseline, one week, two weeks, and four weeks after intervention. Retinal RA was measured from four guinea pigs after two and four weeks of treatment with HPLC. Two-week-old guinea pigs (n = 52) with monocular −5D lens were fed with either RA or its synthesis inhibitor citral every third day in the morning, and half from each group were reared under WL or SL conditions. RE and AL were recorded at baseline and two and four weeks after intervention. Retinal RA was measured after four weeks of intervention.
Results
At the end of treatment, guinea pigs exposed to SL were less myopic than to WL (2.06 ± 1.69D vs. −1.00 ± 1.88D), accompanied with shorter AL (P = 0.01) and less retinal RA (P = 0.02). SL reduced retinal RA even after exogenous RA supplementation (P = 0.02) and decelerated LIM compared to WL (1.66 ± 1.03D vs. −3.53 ± 0.90D). Citral slowed ocular growth, leading to similar RE in W+CI and S+CI groups (3.39 ± 1.65D vs. 5.25 ± 0.80D).
Conclusions
Overall, SL reduced LIM in guinea pigs, even in those supplemented with oral RA, accompanied by reduced retinal RA levels. Oral RA accelerated eye elongation, but citral equally decelerated eye elongation under SL and WL with no significant retinal RA reduction.
Keywords: short-wavelength light, lens-induced myopia, retinoic acid, guinea pig
Eye growth can be altered by lenses placed in front of the eyes, which has been demonstrated in a range of vertebrate species including rhesus monkey,1 marmoset,2,3 guinea pig4,5 and chicks.6 Similarly, different wavelengths of light can also affect eye growth, as demonstrated in studies on guinea pigs, where short-wavelength light (SL) slowed ocular growth in guinea pigs, even after exposure to negative defocus or middle, and long-wavelength light accelerated eye growth and myopia progression.7,8 Longitudinal chromatic aberration (LCA) may be one of the possible mechanisms behind SL slowing down lens-induced myopia progression, because light with shorter wavelengths is focused more anteriorly in comparison to light with longer wavelengths.9 LCA has been calculated to be about 1.23D in chicks between 470 nm and 680 nm and 3.10D in rats.10 Further evidence that supports this theory can be found in studies on chicks, where those reared under red light were 1.41D more myopic than those reared under SL.11 However, in other studies, red light slowed eye growth and induced hyperopia in young tree shrews12 and rhesus monkeys.13 Furthermore, it is documented that guinea pig14,15 and chick16 eyes can emmetropize but did not match the prediction of LCA in monochromatic light, which suggests that LCA is not the only signal to direct eye growth.
Visual signals reaching the retina require transduction to induce chemical and physical changes in the sclera that drive eye elongation. However, the mechanism behind this process is unclear. One possible mediator is retinoic acid (RA), because retinal RA levels have been shown to be elevated in form-deprived chick eyes.17 Retinal RA levels also increased in chicks during accelerated eye growth while wearing a −15D lens and decreased when wearing a +15D lens that slowed axial elongation.18 This would allow us to infer that supplementing chicks with RA would increase the rate of eye elongation, and vice versa if they were supplemented with citral, an inhibitor of RA synthesis, as observed by McFadden et al.19 Similar results have been observed in guinea pigs, in which retinal RA increased in myopic eyes and RA supplementations resulted in rapid eye elongation.4
RA is the acid derivative of vitamin A (retinol) and in vertebrate animal models is synthesized locally within the developing eye, with high concentrations ventrally and lesser concentrations dorsally in chicks and mice.20,21 RA can also modulate cone differentiation by reducing the differentiation of short wavelength-sensitive cones and promoting the differentiation of long-wavelength–sensitive cones in vertebrates.22 Guinea pig retina is dominated by M-cones dorsally and S-cones ventrally.23 Exposure to SL over 10 weeks has been shown to increase S-cone density and decrease M-cone density, with accompanying relative hyperopia, which might be driven by a decrease in RA.24 Because the mechanism behind eye growth deceleration with SL remains unclear, the current study attempted to investigate the effect of SL on guinea pigs with lens-induced myopia and the possible associated role of RA.
Materials and Methods
Animals and Housing
All research procedures were approved by the Institutional Animal Care and Ethics Committee at the Eye and ENT Hospital of Fudan University and were in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. The indoor temperature of the animal facility was maintained at 22°C to 26°C, and relative humidity was at 55% to 65%. The peripheral walls and ceiling were all installed with LED light bars, either SL (peak value 440 nm, half bandwidth 10 nm; Ruiliu Light & Electronic Co., Shanghai, China) or WL (color temperature 5000K). The light illumination was set to be comparable in each group, which was 500 lux and 580 lux (LX-101 lux meter; Lutron Electronics Enterprise Co., Taiwan) in SL and WL, respectively. The intensity of illumination in each group was controlled and adjusted by a voltage regulator. All groups underwent a 12-hour/12-hour light/dark cycle (light: 6 AM to 6 PM).
Lens Wearing
A −5D lens (PMMA, diameter 20 mm, optical zone 12 mm), mounted onto a piece of Velcro, was glued around the right eye of the guinea pig, aligned with the center of the pupil, as described by Howlett and McFadden.5 The lenses were worn continuously during the experiments except when they were removed for cleaning once a day or during ocular measurements. In addition, the lens would be replaced whenever visible scratches were found at its optical zone.
Experiment 1. Effect of SL on Lens-Induced Myopia and Retinal Retinoic Acid
Sixty pigmented guinea pigs (Cavia porcellus, two weeks old) were randomly divided into a WL (n = 30) group and a SL (n = 30) group. Guinea pigs in both groups wore a −5D lens on the right eye. The left eyes were uncovered and set as control. Refractive error (RE), axial length (AL) and corneal curvature were measured before and at one, two, and four weeks after intervention; four guinea pigs in each group were sacrificed at two weeks and four weeks after treatment, respectively, for retinal RA measurement.
Experiment 2. Effect of Supplementing Retinoic Acid or Its Inhibitor
Preparation and Administration of Retinoic Acid and Citral
Fifty-two pigmented guinea pigs (two weeks old) were divided into the WL group and the SL group, half of them in each group were supplemented with RA (W+RA group, n = 11, S+RA group, n = 15) or its synthesis inhibitor, citral (W+CI group, n = 16, S+CI group, n = 10). The animals were raised under WL or SL with a −5D lens on the right eye. RE, AL, and corneal curvature were measured before and two and four weeks after intervention; retinal RA level was measured four weeks after lens treatment.
Guinea pigs from the RA groups were fed 24 mg/kg (guinea pigs were weighed before each RA administration) all-trans retinoic acid (atRA; Sigma, St. Louis, MO, USA) mixed with 0.4 mL peanut oil; the method we used to prepare the RA–peanut oil suspension has been reported in previous studies.4,19 Guinea pigs in the citral group were fed 445 mg/kg citral (mixture of cis and trans; Sigma) dissolved in 0.4 mL peanut oil (using the chick model as a reference19). Guinea pigs were administered the appropriate substance by gavage orally every three days at approximately 11 AM while they were awake.
Biometric Measurements
RE was measured while the guinea pigs were awake using retinoscopy (66 Vision-Tech Co., Ltd., Suzhou, Jiangsu Province, China) 15 minutes after cycloplegia. Guinea pigs were given a total of three drops of cycloplegic agent (1% cyclopentolate hydrochloride; Alcon Ophthalmika GmbH, Austria) in five-minute intervals to dilate pupils and inhibit ocular accommodation. RE was recorded after averaging the results from two independent experienced technicians, who measured RE in the two meridians and calculated the spherical equivalent power (spherical power +1/2 cylindrical power). No correction was made for the artefact of retinoscopy, which is relatively small in guinea pigs.25
AL, including anterior chamber depth (ACD), lens thickness and vitreous chamber depth (VCD), was measured using A-scan ultrasonography with a 10-MHz probe (KN-1800; Kangning Medical Device Co., Ltd., Wuxi, Jiangsu Province, China) with one drop of 0.5% proparacaine hydrochloride (Alcaine, Alcon). Results from 10 readings were averaged for each eye.
Corneal radius of curvature (CRC) was measured using a keratometer (OM-4; Topcon, Tokyo, Japan) with a +8D lens attached to the anterior surface. A set of stainless-steel ball bearings with diameters ranging from 5.5 to 11.0 mm was used for calibration, and CRC was deduced by averaging three readings on the balls with known radii.26 The mean of the horizontal and vertical measurements was recorded as the final data.
Retinoic Acid Level Measurement
The retinal retinoic acid level at weeks 2 and 4 was detected after corresponding biometric measurements were completed. The level of RA in the retina was detected using high performance liquid chromatography (HPLC, LC-8A, SHIMADZU.co, Japan) with an electrochemical detector (Couloehem111, ESA, USA). After the guinea pigs were sacrificed by cervical dislocation, eyes were removed and hemisected on the line of the ora serrata. The retinal layer was carefully dissected from the RPE, and all pigmented cells were removed to ensure RPE was not collected. Procedures were carried out on ice and under dim red light. The atRA standard sample was purchased from Sigma (C20H28O2; molecular mass, 300.44). Concentration of retinal RA (µg) in each tissue was determined using methods reported in previous studies.27
Statistical Analysis
All statistical analyses were performed using SPSS 20.0 software (SPSS, Inc., Armonk, NY, USA), and the results are presented as mean ± standard deviation (SD) unless otherwise stated. Two-way repeated measures analysis of variance (ANOVA) was used to compare the mean RE, VCD, and AL and intraocular difference (IOD) among the guinea pigs reared under SL or WL conditions with different diets subgroups across time. Least significant difference (LSD) correction was applied for post hoc analysis to find differences of refraction and ocular biometric parameters among different diet groups (routine diet, added RA, or citral) at each timepoint. Pearson's correlation analyses was performed to characterize the relationship between the change in RE and AL. Mann-Whitney U test was used to analyze retinal RA results among different groups. A P value <0.05 was considered to indicate a statistically significant difference.
Results
Experiment 1. SL Slowed Myopia Progression and Reduced Endogenous Retinal Retinoic Acid
The Effect of SL on Lens-Induced Myopia
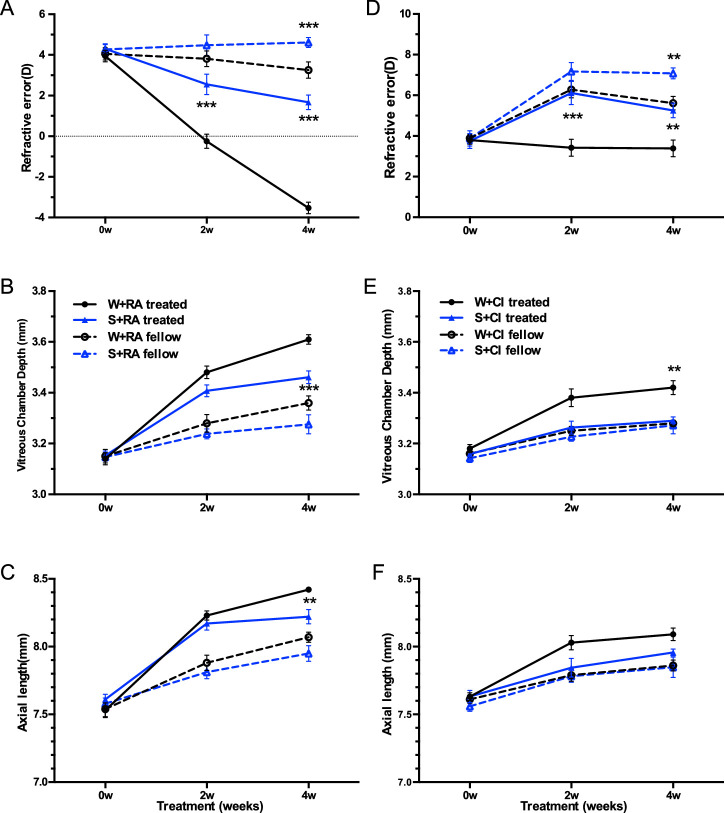
At the end of the experiment, both defocus and lighting characteristics together influenced the refractive error outcome (F[1, 50] = 6.13, P = 0.004, two-way repeated measures ANOVA), as well as AL (F[1, 50] = 6.37, P = 0.015) and VCD (F[1, 50] = 7.76, P = 0.008), but the effect did not change across ages (RE: F[2.28, 113.97] = 2.46, P = 0.08, AL: F[2.58, 128.95] = 0.25, P = 0.86, VCD: F[2.50, 124.75] = 1.77, P = 0.17). Eyes wearing a −5D lens for four weeks under WL were on average −1.00 ± 1.88D more myopic than eyes exposed to SL, which was 2.06 ± 1.69D (post hoc LSD, P < 0.001, see Supplementary Table S1 for detailed data, Fig. 1A), accompanied by a longer VCD (post hoc, P = 0.046, Supplementary Table S1, Fig. 1B) and longer AL (post hoc, P = 0.01, Supplementary Table S1, Fig. 1C). Moreover, treated eyes, as well as fellow eyes in SL, were relatively more hyperopic than WL from the second week of lens treatment (post hoc, treated: P < 0.001; fellow: P < 0.01), as shown in Figure 1A.
Figure 1.
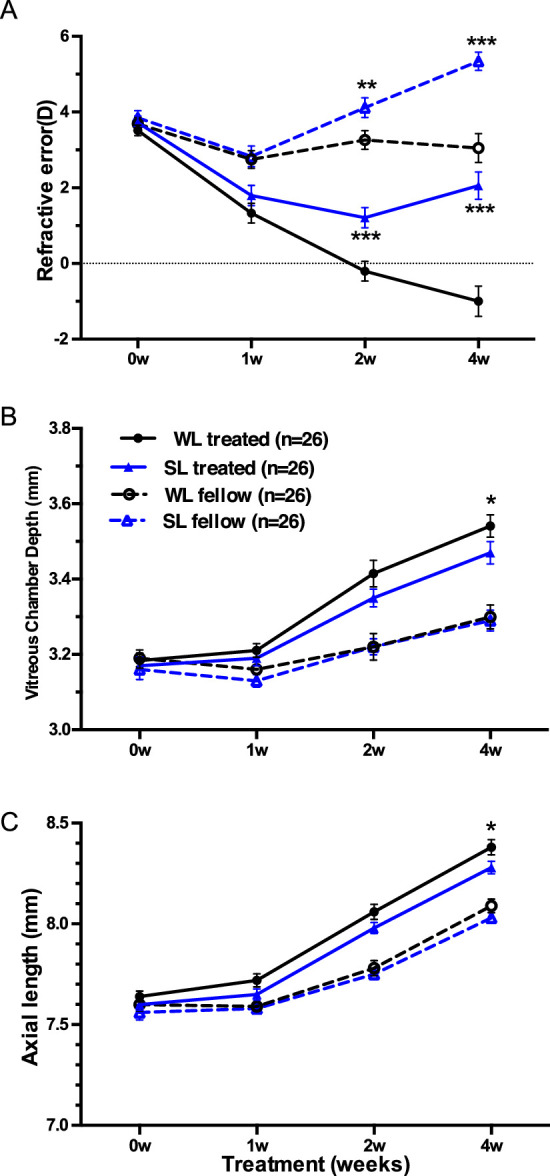
Biometric parameters (Mean± SEM), including (A) refractive error (Diopter, D), (B) vitreous chamber depth (mm), and (C) axial length (mm) of treated (solid lines) and fellow (dashed lines) eyes plotted against treatment time (weeks) in guinea pigs wearing monocular −5D lenses (treated eyes) reared under white light (WL, black lines) or short-wavelength light (SL, blue lines) conditions. Asterisks refer to the significant difference between guinea pigs under WL or SL conditions. *P < 0.05, **P < 0.01, ***P < 0.001. n = 26 across all treatment time.
The interaction between different lighting conditions and lens treatment did not change significantly between the two- and four-week time points for IOD in RE (F[2.28, 113.97] = 2.46, P = 0.08, two-way ANOVA); however, SL reduced lens-induced myopia significantly at the end of the experiment (post hoc, P = 0.004, Supplementary Fig. S1A). However, IOD of AL and IOD of VCD growth was not influenced by interaction of time and color signals (AL: F[2.58, 128.97] = 0.244, P = 0.84, VCD: F[2.50, 124.75] = 1.77, P = 0.17; Supplementary Figs. S1B, S1C).
ACD and lens thickness had no significant difference between the two groups at all-time points (P > 0.05, Supplementary Table S1). CRC in all groups increased gradually with treatment time compared to pretreatment (P < 0.001), but no intergroup differences were found at each time-point (P > 0.05).
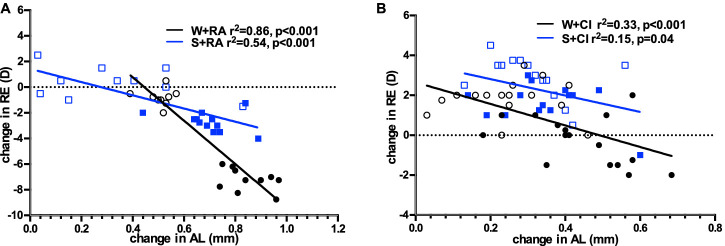
The correlation analysis showed that the change in RE after 4 weeks of treatment significantly correlated with the change in AL in both WL (R2 = 0.73, P < 0.0001, Fig. 2) and SL (R2 = 0.41, P < 0.0001) conditions, and the slopes were not significantly different between WL and SL groups (−10.92 vs. −7.22 in WL and SL, respectively; P = 0.36).
Figure 2.
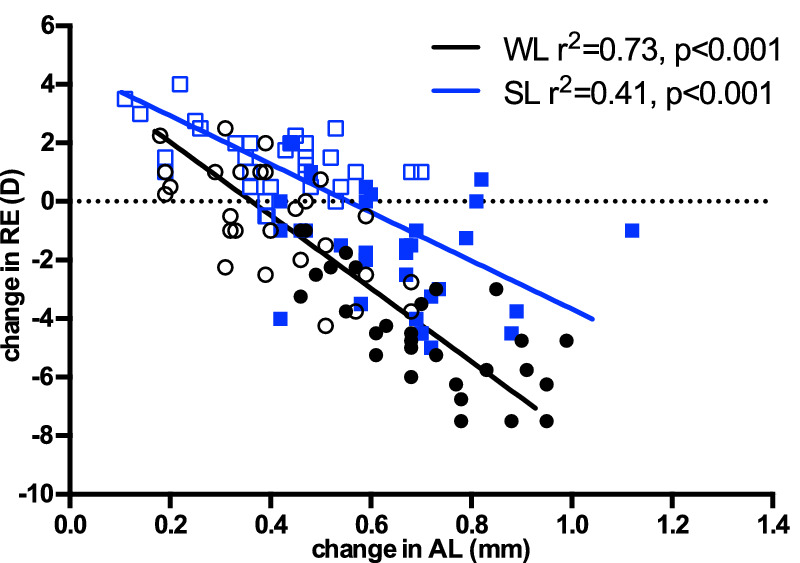
Linear regression of change in refractive error (change in RE) and change in axial length (change in AL) after four-week treatment period, in guinea pigs reared under white light (WL, black circle/line) or short wavelength light (SL, blue box/line). Open circles/boxes represent fellow eyes, closed circles/boxes represent treated (wearing −5D lens) eyes. The line is fitted to both lens defocus and fellow eyes. AL changed in parallel with a change in RE in both groups (P < 0.001).
Endogenous Retinal Retinoic Acid Level Change
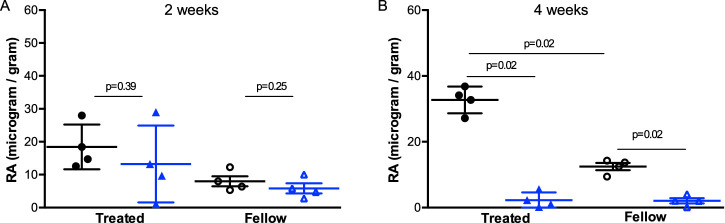
Eyes which had experienced hyperopic defocus developed a myopic RE, a longer AL and had an increased level of retinal RA relative to their fellow eye in WL group from the second week to the end of the treatment (Figs. 3A, 3B). Guinea pigs exposed to SL had lower retinal RA level in both eyes compared to those under WL illumination at the end of the intervention (Fig. 3B).
Figure 3.
Retinal retinoic acid level (RA concentration/weight of retina, µg/g) in guinea pigs reared under short wavelength or white light conditions at two weeks (A) and four weeks (B) after monocular lens treatment. Each black circle represent RA level from each guinea pig under white light; each blue triangle represents RA level from each guinea pig under short wavelength light. Closed symbols represent RA level from lens treated eyes, open symbols represent RA level from fellow eyes. Error bars denote SD. Treated: eyes with −5D lens wearing; fellow: left eyes set as control. P values calculated by Mann-Whitney U test are presented above the corresponding bars.
Retinal RA levels decreased from two to four weeks in both eyes under SL (OD: 13.24 ± 11.68 µg/g to 2.26 ± 2.37 µg/g; OS: 5.85 ± 3.07 µg/g to 2.07 ± 1.63 µg/g, respectively), whose retinal RA levels were three to six times higher at two weeks than at four weeks. This phenomenon suggested that SL could reduce RA levels by inhibiting the synthesis or increasing the degradation of RA in the retina.
To analyze the data further, eyes with two weeks of lens-wearing under SL were relatively hyperopic, accompanied with a slightly lower retinal RA level compared to under WL. By the time the difference in retinal RA levels between WL and SL groups reached significance (i.e., after four weeks of treatment), the difference in RE between these two groups were greater. In addition, IOD of retinal RA level (OD-OS) under SL was smaller than WL after four weeks of intervention (M-W test, P = 0.02), which is concordant with lower myopia progression in SL compared to WL.
Experiment 2. Effect of Feeding Retinoic Acid or Its Inhibitor
At the end of the experiment, the interaction of defocus, lighting characteristics, drug administration, and treatment time all together influenced the refractive error outcome (two-way ANOVA, F[8.81, 172.73] = 10.88, P < 0.001). Correspondingly, the interaction effect influenced axial elongation (F[10, 196] = 3.25, P = 0.001) and VCD (F[10, 196] = 2.84, P = 0.003).
The Effect of SL on Guinea Pigs Fed With Retinoic Acid
For guinea pigs fed with RA, post hoc analysis showed that SL slowed down the myopic shift of the lens-treated eye compared with WL (P < 0.001, Supplementary Table S2, Fig. 4A), as well as reduced VCD or AL significantly (P < 0.001 for VCD, P < 0.01 for AL, Figs. 4B, 4C).
Figure 4.
Biometric parameters between guinea pigs fed with retinoic acid (Left column, A, B, C) or fed with citral (Right column, D, E, F) under white and short wavelength light conditions: Mean ± SEM values in A and D, refractive error in D, vitreous chamber depth (mm) in B and E, and axial length (mm) in C and F of treated (solid lines) and fellow (dashed lines) eyes plotted against treatment time (weeks) in guinea pigs wearing monocular −5D lenses (treated) reared under WL (black lines) or SL (blue lines) conditions. Asterisks refer to the significant difference between guinea pigs under WL or SL conditions. * P < 0.05, ** P < 0.01, *** P < 0.001.
Similarly, IOD of RE was significantly affected by the interaction of color signals, drug administration and treatment time (F[8.81, 172.73] = 10.88, P < 0.001), as well as IOD of AL (F[10, 196] = 3.25, P = 0.001) and IOD of VCD (F[10, 196] = 2.84, P = 0.003). Lens-induced myopia was significantly decreased by SL (−2.94D ± 1.01D vs. −6.78 ± 1.11D, post hoc, P < 0.001, Supplementary Fig. S1D), but AL or VCD had no significant difference between W+RA and S+RA groups (Supplementary Figs. S1E, S1F).
The correlation analysis showed that the change in RE after four weeks of treatment significantly correlated with the change in AL in guinea pigs fed with RA in both WL (R2 = 0.86, P < 0.001) and SL (R2 = 0.54, P < 0.001, Fig. 5A) conditions, and the slopes were significantly different between W+RA and S+RA groups (−16.92 vs. −5.15 in W+RA and S+RA, respectively; P = 0.008).
Figure 5.
Linear regression of change in refractive error (change in RE) and change in axial length (change in AL) during the four-week treatment period, in guinea pigs fed with RA (A) or citral (B) and reared under white light (black circle/line) or short-wavelength light (blue box/line). The line is fitted to both the lens defocus and fellow eye. Closed circles/boxes represent treated eyes, open circles/boxes represent fellow eyes. AL changed in parallel with a change in RE in all groups (P < 0.05).
Biometric Changes After Feeding Citral
For guinea pigs fed with citral, hyperopic shift was significantly enhanced by SL compared with WL (post hoc, P = 0.002, Fig. 4D), and significant VCD reduction was observed under SL (post hoc, VCD: P = 0.01, Fig. 4E). However, lens-induced myopia (IOD of RE) was not significantly different between S+CI and W+CI groups at the end of treatment (post hoc, P = 0.27, Supplementary Fig. S1G), and no significant difference in IOD of AL was observed (post hoc, P = 0.06, Supplementary Fig. S1I).
Similar to guinea pigs fed with RA, guinea pigs fed with citral also showed a significant association between change in RE and change in AL under both WL (R2 = 0.33, P < 0.001) and SL (R2 = 0.15, P = 0.04; Fig. 5B), but the slopes were not significantly different between W+CI and S+CI groups (−5.39 vs. −4.11 in W+CI and S+CI, respectively; P = 0.32).
The Effect of Different Diets on Guinea Pigs Reared Under White Light
Under WL, citral decelerated myopia progression at the end of the experiment compared to routine diet or oral RA (post hoc, WL vs. W+CI and W+RA vs. W+CI; both P < 0.001; Supplementary Fig. S2A). Correspondingly, citral slowed VCD extension (post hoc, WL vs. W+CI, P = 0.004; W+RA vs. W+CI, P = 0.007; Supplementary Fig. S2B) and axial elongation (post hoc, WL vs. W+CI, P = 0.002; W+RA vs. W+CI, P = 0.02; Supplementary Fig. S2C) as well.
Lens-induced myopia (IOD of RE) was significantly increased by oral RA and decreased by oral citral (post hoc, W+RA vs. WL, WL vs. W+CI; both P < 0.001; Supplementary Fig. S2G), and IOD of VCD was correspondingly lessened with citral supplement (post hoc, WL vs. W+CI and W+RA vs. W+CI; both P < 0.001; Supplementary Fig. S2H).
The Effect of Different Diets on Guinea Pigs Reared Under SL
Under SL, for guinea pigs wearing a monocular −5D lens and fed with a different diet, oral RA did not accelerate myopia development compared to a normal diet (SL vs. S+RA; P = 0.62, post hoc). However, citral induced a hyperopic shift at the end of the treatment compared to RA or routine diet (post hoc, S+CI vs. S+RA and S+CI vs. SL; both P < 0.001; Supplementary Fig. S2D). Correspondingly, AL elongation in S+CI group was less than that in S+RA or SL groups (post hoc, S+CI vs. S+RA, P = 0.021; S+CI vs. SL, P = 0.021; Supplementary Fig. S2F).
Exogenous citral could reduce IOD of RE (post hoc, S+RA vs. S+CI, P = 0.04; SL vs. S+CI, P < 0.001; Supplementary Fig. S2G), while RA could induce and citral could reduce IOD of VCD (post hoc, S+RA vs. SL, P = 0.04; SL vs. S+CI, P = 0.02; Supplementary Fig. S2H) and IOD of AL (post hoc, S+RA vs. SL, P = 0.001; SL vs. S+CI, P = 0.005; Supplementary Fig. S2I).
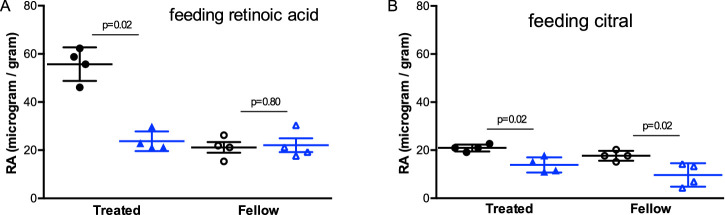
Endogenous Retinal Retinoic Acid Level After Feeding Retinoic Acid or Citral
Similar to Experiment 1, SL reduced retinal RA level in the lens-wearing eye even after guinea pigs were fed with RA (S+RA vs. W+RA: M-W test, Z = 2.31, P = 0.02; Fig. 6A), and for guinea pigs fed with citral (lower RA levels), SL also affected RA concentration significantly (S+CI vs. W+CI: Z = 2.32, P = 0.02; Fig. 6B). As expected, retinal RA level in the lens-wearing eye increased 72% with oral RA but decreased almost 40% with feeding citral in WL groups (W+RA: 55.72 ± 6.96 vs. W+CI: 20.94 ± 1.43). Also, in SL groups, retinal RA increased ten times after RA supplement in the lens-wearing eyes (S+RA: 23.75 ± 4.09 µg/g vs. SL: 2.26 ± 2.37 µg/g). An unexpected finding was that citral did not decrease retinal RA levels under SL (13.87 ± 3.18 µg/g).
Figure 6.
Retinal retinoic acid level (RA concentration/weight of retina, ug/g) between guinea pigs fed with retinoic acid (A) or fed with citral (B) reared under white (A, W+RA; B, W+CI) or short-wavelength light (A, S+RA; B, S+CI) conditions four weeks after monocular lens treatment. Error bars denote SD. Each white circle represent RA levels from each guinea pig under white light; each blue triangle represent RA levels from each guinea pig under SL. Closed symbols: treated eyes with −5D lens wearing; open symbols: fellow eyes set as control. P values calculated by Mann-Whitney U test were distributed above the corresponding bars.
IOD of retinal RA level (OD-OS) in guinea pigs fed with RA under SL was much smaller than WL after four weeks of intervention (M-W test, P = 0.002), which is concordant with lower myopia progression in S+RA compared to W+RA. However, for guinea pigs fed with citral, IOD of retinal RA did not show a difference between WL and SL groups (P = 0.68), and IOD of RE did not show a significant difference between S+CI and W+CI groups simultaneously.
Discussion
The principal finding in the current study is that guinea pigs exposed to negative lens defocus and reared under SL experienced a lower myopia progression and a reduced retinal RA level compared to their counterparts under WL, even after exogenous RA supplementation. Furthermore, oral RA administration induced accelerated myopia progression under WL, but not under SL; on the contrary, feeding citral stopped myopia progression and slowed down eye elongation under WL, and the inhibition was almost equally strong when combined with SL.
The effect of SL in emmetropization has remained controversial for a long time. Chickens28,29 and guinea pigs7,30 became more hyperopic in SL; however, the effects of SL on tree shrew refraction is more complicated because flickering SL drove the eye to significant myopia whereas steady SL had little effect.31 In the current study, we observed a hyperopic shift after exposure to SL in guinea pigs and confirmed the inhibitory effect of SL on lens-induced myopia development compared to WL, as previous studies have demonstrated.7,30 In addition, the reduction of myopia progression under SL was also accompanied with a shorter AL.
Inhibited eye growth under SL may be mediated by reduced retinal RA levels observed during SL exposure. RA may be one of the possible mediators that translates the visual signals from the retina to sclera to modulate eye growth, as multiple studies have shown elevated retinal RA levels during myopia development in defocus induced guinea pigs and these RA changes preceded ocular elongation.32–34 Only one previous study examining monochromatic light in animal models of myopia included RA testing.35 The results demonstrated that guinea pig eyes elongated faster after four weeks’ exposure to green light in comparison to SL, along with a higher retinal RA level. In the current study, lens-wearing eyes under WL expressed more RA in the retina at four weeks than at two weeks (Fig. 3), and the difference between lens-wearing eyes and the fellow eyes was greater at four weeks than two weeks. These results are consistent with the above-mentioned lens-induced myopia studies. Additionally, retinal RA level in eyes exposed to SL with or without lens-wearing were lower than those exposed to WL at two weeks, and further reduced to reach statistical significance at four weeks. This observation suggests that SL might reduce the synthesis or increase the degradation of retinal RA to slow down myopia development, but it needs further research to confirm this hypothesis.
RA is known to affect extracellular matrix metabolism in many systems and is involved in the expression of hundreds of genes and transcription factors influencing eye development.36,37 RA has been shown to inhibit the differentiation of blue sensitive cones and enhance red sensitive cones.22,38 Also, ventral/dorsal gradient retinal RA level determines the dorsal-ventral gradient of middle-wavelength sensitive and short-wavelength sensitive opsin expression via specific receptors.39,40 In guinea pigs, s-opsin in the retina was upregulated, and m-opsin was downregulated when exposed to SL, with a shorter AL compared to WL exposure, suggesting the involvement of RA.24,41 In addition, RA has been demonstrated to directly inhibit scleral proteoglycan synthesis in chicks and primates.27,42,43
In the second experiment, we observed that oral RA administration in guinea pigs under WL significantly increased retinal RA level and accelerated myopia progression in the lens-wearing eye, but not the fellow eye (Fig. 4A). Similar to a previous study,4 feeding RA had no significant effect on RE in eyes not exposed to negative lens defocus. However, in lens-wearing eyes, supplementing with RA showed longer AL, as well as increased magnitude of myopia, suggesting that RA could enhance the myopiagenic effect of negative lenses. Contrary to these results, McFadden et al.19 observed that chicks wearing −6 D lenses compensated normally for the lenses despite the enhanced ocular elongation caused by feeding RA, which may indicate species differences. In guinea pigs reared under SL, although retinal RA increased after oral RA administration, their levels were lower than those seen in guinea pigs exposed to WL (Fig. 6A). Correspondingly, guinea pigs from the S+RA group developed less lens-induced myopia compared to guinea pigs from the W+RA group, but was similar with their counterparts under a routine diet (S+RA vs. SL: −2.94D vs. −3.28D).
Feeding guinea pigs with RA synthesis inhibitor (citral) resulted in significant reduction of retinal RA and inhibition of myopia development in the lens-wearing eye of guinea pigs reared under WL. In addition, citral inhibited myopia progression starting at the second week of treatment, stabilizing the RE in both eyes, especially the lens-wearing eye, resulting in more hyperopia and shorter VCD and AL than WL and W+RA groups. However, retinal RA level in the fellow eye did not decrease in W+CI group as expected, despite eye growth inhibition, suggesting that citral may work through inhibiting RA synthesis, as well as affecting RA receptors and subsequently decelerate eye growth. Citral not only inhibits the mitochondrial isozyme of rat aldehyde dehydrogenase to reduce RA synthesis, it could also work as an antagonist to alter a variety of genes expression through retinoid receptors because it bears a structural resemblance to RA.44,45 Therefore we speculate that both the above two mechanisms may come into play in eye growth inhibition by citral. Further research is needed to determine the mechanisms of citral.
An unexpected and interesting finding in this study was that retinal RA did not decrease after citral intake in all eyes under SL, although shorter axial elongation was observed. This could be due to a feedback mechanism that attempted to compensate for the inhibitory effect of citral, because citral not only reduces RA levels but also combines retinoid receptors as an antagonist.44,45
The limitations of this study include the following: first, no choroidal thickness or retinal thickness data was collected during the experiment, which could provide us with more information to understand their relationship with changes in RE and AL. Second, detecting the downstream molecules of RA may give us more clues to understand how SL and RA works to influence eye growth. Third, the lack of a vehicle control group fed only with peanut oil in Experiment 2, however, based on the effect of RA and citral on eye growth, it is reasonable to deduce that the vehicle would not have affected eye growth.
Conclusion
In summary, although the specific mechanisms of our observations regarding the influence of SL in modulating ocular elongation remain obscure, this preliminary study led us to the conclusion that in the guinea pig, retinal RA levels are affected by visual conditions and can enhance ocular elongation in a negative defocus model. Such changes are inhibited in the presence of SL, even with exogenous RA supplement. Furthermore, feeding RA to young guinea pigs rapidly enhanced ocular elongation, with significant changes in refraction observed in the lens-wearing eye, but not in the fellow eye. On the contrary, citral stopped myopia progression and slowed eye elongation, but it may act via pathways other than those altering RA levels.
Supplementary Material
Acknowledgments
Supported by Grant 81470657 and Grant 81970831 from the National Natural Science Foundation of China, Grant KW-201875334 from Shanghai Science and Technology Commission and Grant 2018PT32019 from the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences. The sponsor or funding organization had no role in the design or conduct of this research.
Disclosure: M. Yu, None; W. Liu, None; B. Wang, None; J. Dai, None
References
- 1. Hung LF, Crawford MLJ, Smith EL.. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nat Med. 1995; 1(8): 761–765. [DOI] [PubMed] [Google Scholar]
- 2. Graham B, Judge SJ.. The effects of spectacle wear in infancy on eye growth and refractive error in the marmoset (Callithrix jacchus). Vision Res. 1999; 39(2): 189–206. [DOI] [PubMed] [Google Scholar]
- 3. Whatham AR, Judge SJ.. Compensatory changes in eye growth and refraction induced by daily wear of soft contact lenses in young marmosets. Vision Res. 2001; 41(3): 267–273. [DOI] [PubMed] [Google Scholar]
- 4. Mcfadden SA, Howlett MHC, Mertz JR.. Retinoic acid signals the direction of ocular elongation in the guinea pig eye. Vision Res. 2004; 44: 643–653. [DOI] [PubMed] [Google Scholar]
- 5. Howlett MHC, McFadden SA.. Spectacle lens compensation in the pigmented guinea pig. Vision Res. 2009; 49(2): 219–227. [DOI] [PubMed] [Google Scholar]
- 6. Wildsoet C, Wallman J.. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995; 35(9): 1175–1194. [DOI] [PubMed] [Google Scholar]
- 7. Jiang L, Zhang S, Schaeffel F, et al.. Interactions of chromatic and lens-induced defocus during visual control of eye growth in guinea pigs (Cavia porcellus). Vision Res. 2014; 94: 24–32. [DOI] [PubMed] [Google Scholar]
- 8. Qian YF, Dai JH, Liu R, Chen MJ, Zhou XT, Chu RY.. Effects of the chromatic defocus caused by interchange of two monochromatic lights on refraction and ocular dimension in guinea pigs. PLoS One. 2013; 8(5): e63229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rucker FJ. The role of luminance and chromatic cues in emmetropisation. Ophthalmic Physiol Opt. 2013; 33(3): 196–214. [DOI] [PubMed] [Google Scholar]
- 10. Mandelman T, Sivak JG.. Longitudinal chromatic aberration of the vertebrate eye. Vision Res. 1983; 23(12): 1555–1559. [DOI] [PubMed] [Google Scholar]
- 11. Seidemann A, Schaeffel F.. Effects of longitudinal chromatic aberration on accommodation and emmetropization. Vision Res. 2002; 42(21): 2409–2417. [DOI] [PubMed] [Google Scholar]
- 12. Gawne TJ, Ward AH, Norton TT.. Long-wavelength (red) light produces hyperopia in juvenile and adolescent tree shrews. Vision Res. 2017; 140: 55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith ELI, Hung L, Arumugam B, Holden BA, Neitz M, Neitz J.. Effects of long-wavelength lighting on refractive development in infant rhesus monkeys. Invest Ophthalmol Vis Sci. 2015; 56(11): 6490–6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li W, Lan W, Yang S, et al.. The effect of spectral property and intensity of light on natural refractive development and compensation to negative lenses in guinea pigs. Investig Ophthalmol Vis Sci. 2014; 55(10): 6324–6332. [DOI] [PubMed] [Google Scholar]
- 15. Liu R, Qian YF, He JC, et al.. Effects of different monochromatic lights on refractive development and eye growth in guinea pigs. Exp Eye Res. 2011; 92(6): 447–453. [DOI] [PubMed] [Google Scholar]
- 16. Rucker FJ, Wallman J.. Cone signals for spectacle-lens compensation: Differential responses to short and long wavelengths. Vision Res. 2008; 48(19): 1980–1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seko Y, Shimizu M, Tokoro T.. Retinoic acid increases in the retina of the chick with form deprivation myopia. Ophthalmic Res. 1998; 30(6): 361–367. [DOI] [PubMed] [Google Scholar]
- 18. Mertz JR, Howlett MHC, McFadden S, Wallman J.. Retinoic acid from both the retina and choroid influences eye growth. In: Invest Ophthalmol Vis Sci. 1999:40(4);S849. [Google Scholar]
- 19. McFadden SA, Howlett MHC, Mertz JR, Wallman J.. Acute effects of dietary retinoic acid on ocular components in the growing chick. Exp Eye Res. 2006; 83(4): 949–961. [DOI] [PubMed] [Google Scholar]
- 20. Mey J, McCaffery P, Klemeit M.. Sources and sink of retinoic acid in the embryonic chick retina: Distribution of aldehyde dehydrogenase activities, CRABP-I, and sites of retinoic acid inactivation. Dev Brain Res. 2001; 127(2): 135–148. [DOI] [PubMed] [Google Scholar]
- 21. McCaffery P, Wagner E, O'Neil J, Petkovich M, Dräger UC. Dorsal and ventral retinal territories defined by retinoic acid synthesis, break-down and nuclear receptor expression. Mech Dev. 1999; 85(1-2): 203–214. [DOI] [PubMed] [Google Scholar]
- 22. Prabhudesai SN, Cameron DA, Stenkamp DL.. Targeted effects of retinoic acid signaling upon photoreceptor development in zebrafish. Dev Biol. 2005; 287(1): 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Röhlich P, van Veen T, Szél Á.. Two different visual pigments in one retinal cone cell. Neuron. 1994; 13(5): 1159–1166. [DOI] [PubMed] [Google Scholar]
- 24. Zou L, Zhu X, Liu R, et al.. Effect of altered retinal cones/opsins on refractive development under monochromatic lights in guinea pigs. J Ophthalmol. 2018; 2018: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Howlett MHC, Mcfadden SA.. Emmetropization and schematic eye models in developing pigmented guinea pigs. Vision Res. 2007; 47(9): 1178–1190. [DOI] [PubMed] [Google Scholar]
- 26. Norton TT, McBrien NA.. Normal development of refractive state and ocular component dimensions in tree shrew (Tupaia belangeri). Vis Res. 1992; 32(5): 833–842. [DOI] [PubMed] [Google Scholar]
- 27. Mertz JR, Wallman J.. Choroidal retinoic acid synthesis: a possible mediator between refractive error and compensatory eye growth. Exp Eye Res. 2000; 70: 519–527. [DOI] [PubMed] [Google Scholar]
- 28. Foulds WS, Barathi VA, Luu CD.. Progressive myopia or hyperopia can be induced in chicks and reversed by manipulation of the chromaticity of ambient light. Investig Ophthalmol Vis Sci. 2013; 54(13): 8004–8012. [DOI] [PubMed] [Google Scholar]
- 29. Wang M, Schaeffel F, Jiang B, Feldkaemper M.. Effects of light of different spectral composition on refractive development and retinal dopamine in chicks. Invest Ophthalmol Vis Sci. 2018; 59(11): 4413–4424. [DOI] [PubMed] [Google Scholar]
- 30. Qian Y-F, Liu R, Dai J-H, Chen M-J, Zhou X-T, Chu R-Y.. Transfer from blue light or green light to white light partially reverses changes in ocular refraction and anatomy of developing guinea pigs. J Vis. 2013; 13(11): 16–16. [DOI] [PubMed] [Google Scholar]
- 31. Gawne TJ, Siegwart JT, Ward AH, Norton TT.. The wavelength composition and temporal modulation of ambient lighting strongly affect refractive development in young tree shrews. Exp Eye Res. 2017; 155: 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mao J-F, Liu S-Z, Dou X-Q.. Retinoic acid metabolic change in retina and choroid of the guinea pig with lens-induced myopia. Int J Ophthalmol. 2012; 5(6): 670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang S, Liu S, Mao J, Wen D.. Effect of retinoic acid on the tight junctions of the retinal pigment epithelium-choroid complex of guinea pigs with lens-induced myopia in vivo. Int J Mol Med. 2014; 33(4): 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang J, Qu X-M, Chu R-Y.. Expressions of cellular retinoic acid binding proteins I and retinoic acid receptor-β in the guinea pig eyes with experimental myopia. Int J Ophthalmol. 2011; 4(2): 131–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang J, Qu X, Chu R.. Research on retinoic acid signals in retina of guinea pig eyes with different monochromatic illumination. zhong hua yan ke za zhi. 2011; 47(10): 938–943. [PubMed] [Google Scholar]
- 36. Cvekl A, Wang WL.. Retinoic acid signaling in mammalian eye development. Exp Eye Res. 2009; 89(3): 280–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Das BC, Thapa P, Karki R, et al.. Retinoic acid signaling pathways in development and diseases. Bioorganic Med Chem. 2014; 22(2): 673–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitchell DM, Stevens CB, Frey RA, et al.. Retinoic acid signaling regulates differential expression of the tandemly-duplicated long wavelength-sensitive cone opsin genes in zebrafish. PLoS Genet. 2015; 11(8): 1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mccaffery P, Lee M, Wagner MA, Sladek NE, Drager UC.. Asymmetrical retinoic acid synthesis in the dorsoventral axis of the retina. Development. 1992; 115: 371–382. [DOI] [PubMed] [Google Scholar]
- 40. Roberts MR, Hendrickson A, McGuire CR, Reh TA.. Retinoid X receptor γ is necessary to establish the S-opsin gradient in cone photoreceptors of the developing mouse retina. Investig Ophthalmol Vis Sci. 2005; 46(8): 2897–2904. [DOI] [PubMed] [Google Scholar]
- 41. Hu M, Hu Z, Xue L, et al.. Guinea pigs reared in a monochromatic environment exhibit changes in cone density and opsin expression. Exp Eye Res. 2011; 93(6): 804–809. [DOI] [PubMed] [Google Scholar]
- 42. Troilo D, Nickla DL, Mertz JR, Rada JAS.. Change in the synthesis rates of ocular retinoic acid and scleral glycosaminoglycan during experimentally altered eye growth in marmosets. Investig Ophthalmol Vis Sci. 2006; 47(5): 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rada JAS, Hollaway LR, Lam W, Li N, Napoli JL.. Identification of RALDH2 as a visually regulated retinoic acid synthesizing enzyme in the chick choroid. Investig Ophthalmol Vis Sci. 2012; 53(3): 1649–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kikonyogo A, Abriola DP, Dryjanski M, Pietruszko R.. Mechanism of inhibition of aldehyde dehydrogenase by citral, a retinoid antagonist. Eur J Biochem. 1999; 262: 704–712. [DOI] [PubMed] [Google Scholar]
- 45. Gudas LJ. Retinoids and vertebrate development. J Biol Chem. 1994; 269(22): 15399–15402. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.