Abstract
Heart transplantation improves quality of life and survival in patients with advanced heart failure. However, the shortage of available heart donors and technological advances for left ventricular assist devices (LVAD) have led to longer waiting times for transplantation, and long-term use of LVAD may increase the medical complexity of subsequent transplantation. We present the case of a 35-year-old man who underwent heart transplantation after being supported by an LVAD for 1490 days (∼4 years). He was sensitized with kidney dysfunction and recurrent infections, including candidemia, at the time of transplantation. He underwent a successful heart transplantation with pretransplant plasma exchange, intravenous immunoglobulin administration, early initiation of everolimus, and prompt management of infections.
<Learning objective: With a growing number of heart transplant candidates who are supported by left ventricular assist devices for long duration, managing such candidates is becoming increasingly complex and difficult to standardize. The present case had three problems that were linked to each other: (1) anti-HLA antibodies, (2) fungal infection, and (3) pre-transplantation renal dysfunction. Management of heart transplant candidates, including desensitization and immunosuppressive therapies, should be tailored to the individual and the clinical presentation to improve the survival and quality of life.>
Keywords: Heart transplantation, Left ventricular assist device, Sensitized patients
Introduction
Heart transplantation is an established therapy for advanced heart failure patients [1]. However, the shortage of available organ donors limits the number of transplantations performed. In Japan, the waiting times for transplantation are increasing and exceeded 1000 days in 2015 as the number of advanced heart failure patients on waiting lists increased, while the number of donor organs remains relatively constant [2]. In addition, left ventricular assist devices (LVAD) are increasingly used as a bridge to transplantation in over 90% of organ recipients [2].
As waiting times increase, transplant candidates on long-term LVAD support are more likely to experience complications such as aortic insufficiency, right ventricular failure, infection, bleeding, kidney dysfunction, and device malfunction [3], [4], [5]. Furthermore, as a result of LVAD implantation, transplant candidates may develop circulating antibodies, which are associated with increased waiting times and may lead to post-transplant rejection [6].
These complications have led to a growing number of candidates with medical complexity at the time of transplantation, which significantly affects patient morbidity and mortality post-transplantation. We managed a presensitized transplant candidate with candidemia and kidney dysfunction who was supported by an LVAD. With careful clinical decision-making and follow-up and the expertise of an infectious disease specialist and histocompatibility laboratory, the patient underwent successful heart transplantation.
Case report
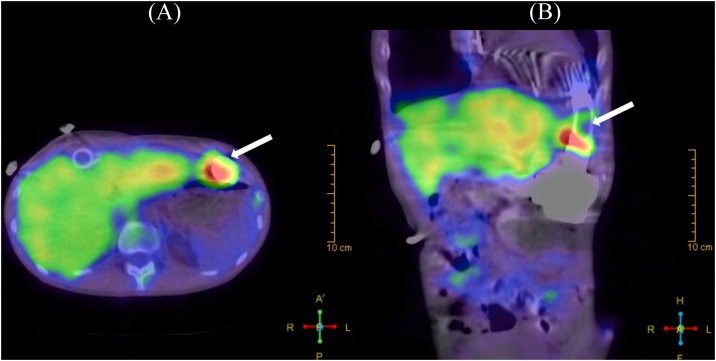
A 31-year-old man with advanced heart failure due to dilated cardiomyopathy was listed for heart transplantation. At the time of listing, his serum creatinine was normal (creatinine 0.54 mg/dL), and the panel reactive antibody (PRA) was negative (Class I: 0%, Class II: 3%). He received an LVAD (EVAHEART, Sun Medical Technology Research Corporation, Suwa, Nagano, Japan) as a bridge to transplantation. Although the early postoperative course was uneventful, he was hospitalized twice for driveline infection during the first 2 years after LVAD implantation. Cultures from the driveline site were positive for methicillin-resistant Staphylococcus aureus (MRSA) in both episodes. Three years after implantation, he was admitted to our hospital for fever and MRSA bacteremia. He was administered intravenous vancomycin, then was switched to linezolid because of repeated positive blood cultures for MRSA over 2 weeks. Three days after initiating linezolid, his blood culture was negative. However, 4 weeks after admission, he developed acute appendicitis requiring emergency appendectomy and transfusion of multiple packed red blood cells and platelets for intra-abdominal bleeding. Although annual PRA screening was negative, one month later after blood transfusions his PRA class I and II serum levels increased to 19% and 55%, respectively (Fig. 1). Despite continued intravenous antibiotics and prolonged hospitalization, repeat blood cultures demonstrated intermittent positive results for MRSA. Gallium single-photon emission computed tomography-computed tomography (Ga-SPECT-CT) demonstrated tracer concentration uptake around the LVAD (Fig. 2), suggesting that the LVAD was the source of the bacteremia, a phenomenon known as ventricular assist device (VAD) endocarditis. He then developed fever, and his blood culture was positive for Candida parapsilosis with no evidence of an infection source other than the VAD. He was treated with intravenous liposomal-amphotericin B (L-AMB) for the VAD-related bloodstream infection. He did well, and subsequent blood cultures were negative. However, he developed kidney dysfunction (transient maximum creatinine level: 1.96 mg/dL) and neutropenia (719/μL) due to adverse effects from the long-term use of L-AMB as well as a systemic fungal infection, requiring granulocyte-colony stimulating factor. After a 9-week course of L-AMB followed by intravenous fosfluconazole, his serum creatinine improved from 1.96 mg/dL (CCr: 32.7 mL/min) to 1.27 mg/dL (CCr: 52.5 mL/min). However, he developed a fever again and was restarted on intravenous L-AMB.
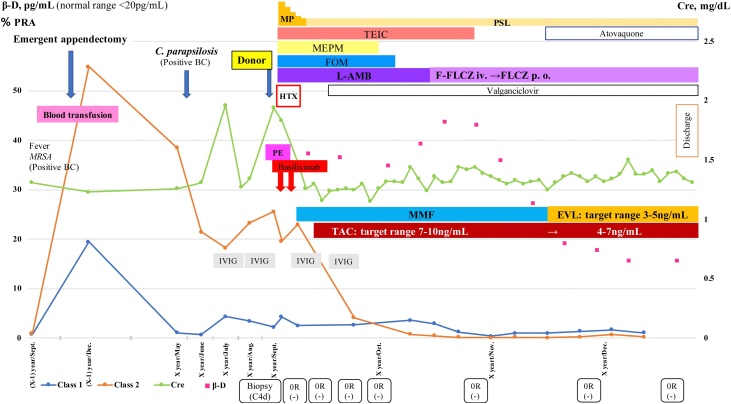
Fig. 1.
Clinical course and management pre- and post-heart transplantation.
BC, blood culture; β-D, beta-D-glucan; EVL, everolimus; FOM, fosfomycin; F-FLCZ, fosfluconazole; FLCZ, fluconazole; HTX, heart transplantation; IVIG, intravenous immunoglobulin; L-AMB, liposomal-amphotericin B; MEPM, meropenem; MMF, mycophenolate mofetil; MP, methylprednisolone; MRSA, methicillin-resistant Staphylococcus aureus; PE, plasma exchange; PRA, panel reactive antibody; PSL, prednisolone; TAC, tacrolimus; TEIC, teicoplanin. Biopsy results are shown in the order of ISHLT grade and deposition of C4d.
Fig. 2.
Gallium single-photon emission computed tomography-computed tomography transaxial (A) and coronal (B) images show intense uptake (arrows) around the left ventricular assist device.
For 10 months after the appendectomy, his PRA class II remained positive (Fig. 1), and he was administered 15 g intravenous immunoglobulin (IVIG) monthly. Finally, he underwent heart transplantation after more than 4 years (1490 days) on LVAD support.
Laboratory tests performed just before transplantation showed a white blood cell count of 2440/μL, hemoglobin of 9.9 g/dL, serum albumin of 3.3 g/dL, creatinine of 1.94 mg/dL, C-reactive protein of 0.26 mg/dL, PRA class I of 2.23%, and PRA class II of 25.58%. Subsequent testing for donor-specific antibody (DSA) was positive (Class II: DR15).
Because the patient showed high PRA levels, we initiated pre-transplant plasma exchanges to reduce antibody levels along with pre- and post-transplant IVIG. After desensitization therapy, the PRA class II decreased from 25.58% to 4.13%. He was also administered basiliximab, facilitating delayed initiation of calcineurin inhibitor (CNI) for kidney dysfunction (Fig. 1).
As the C. parapsilosis blood cultures were positive pre-transplantation, the patient was treated with L-AMB for the first month after the procedure, then with fluconazole for the next 4 months without endophthalmitis. Additionally, broad-spectrum antibiotics (teicoplanin, meropenem, and fosfomycin) were administered for 6 weeks post-transplantation because of the driveline site culture (MRSA and Pseudomonas aeruginosa) and possible VAD endocarditis due to MRSA.
Post-transplantation biopsies showed neither signs of cellular rejection nor antibody-mediated rejection (AMR). PRA levels decreased and remained low postoperatively (PRA class I: 2.66% and PRA class II: 4.13% at 3 weeks after transplantation). After wound healing, we initiated everolimus and reduced the trough levels of tacrolimus from the target level of 7–10 ng/mL to 4–7 ng/mL. Left heart catheterization at 3 months post-transplantation showed no evidence of cardiac allograft vasculopathy (CAV).
The patient was discharged 3 months post-transplantation; he has remained healthy, without recurrent infections or rejections during the 1-year follow-up.
Discussion
Our case illustrates the complexities, difficulties, and dilemmas encountered during pre- and post-transplant management of candidates on prolonged LVAD support.
The presence of circulating antibodies against HLA remains a challenge for transplantation and is associated with AMR, then CAV, and a less successful outcome [6]. Heart transplant candidates who develop circulating antibodies against HLA have a reduced chance of suitable donor matching and an increased risk of postoperative AMR. Consequently, presensitized candidates spend more time on the waiting list and are associated with poorer post-transplantation outcomes [6]. Sensitization occurs from blood-product transfusion, pregnancy, or infections [6], [7]. Patients with an LVAD have a greater risk of developing anti-HLA antibodies, and subsequently AMR, than do those without an LVAD [6], [7]. Therefore, PRA should be checked regularly, especially in patients with an LVAD, detectable circulating antibodies, or receiving blood transfusions. Although management of sensitization is not yet standardized, desensitization therapies may be considered in patients with PRA levels >25%. For these sensitized patients, desensitization therapy, including plasmapheresis, more effective immunosuppressive regimens, and IVIG prior to transplant, are believed to increase the frequency of suitable donor matching and improve post-transplantation outcomes [6].
In the present case, dynamic changes occurred in the PRA levels. After LVAD placement, transfusion, and infections, the PRA class I and class II increased from 0% and 3% to 19.41% and 54.86%, respectively. The patient was treated pre-transplant with plasma exchanges and IVIG, which led to decreased PRA. Pre-transplant desensitization therapy may contribute to negative cross-matching as in this case. We introduced the mammalian target of rapamycin (mTOR) inhibitor, everolimus, with early reduction of tacrolimus after complete wound healing to improve the patient’s kidney dysfunction. Arora et al. showed that conversion from a CNI to an mTOR inhibitor-based regimen with reduced CNI therapy significantly improved kidney function [8]. Furthermore, Asleh et al. reported that early conversion to an mTOR inhibitor was associated with fewer CAV-related events and lower mortality compared with continued CNI use [9]. Patients showing kidney dysfunction or a higher risk of CAV, such as those showing DSA, should be considered for an mTOR inhibitor regimen as soon as possible, unless they are at a high risk for wound complications.
Infection is the most common adverse occurrence 3 months after LVAD implantation and contributes to the mortality of patients on transplant waiting lists [4]. Koval et al. reported that more drug-resistant pathogens are emerging, and relapse occurs more often after treatment for driveline infections, especially progressive infections [10].
Fungal VAD infections are difficult to cure and are associated with high mortality rates, especially in terms of VAD-related blood stream infections. In the present case, because LVAD removal was the only viable treatment and the patient was still an acceptable candidate, we performed transplantation with desensitization and immunosuppressive strategies.
Finally, regarding renal dysfunction, the CCr worsened just before heart transplantation; however, we did not withdraw the patient from transplant eligibility. We considered that the renal function was reversible because the CCr was >40 mL/min at 1 month before heart transplantation. Further, we considered that we could discontinue the antimicrobial agents causing the renal impairment after removing the LVAD. The patient’s renal function improved after the heart transplantation, with a creatinine level of 1.36 mg/dL (CCr: 47.4 mL/min).
With donor shortages and excessive waiting times for heart transplants, the number of medically complicated cases due to prolonged LVAD support is expected to increase. The decision to receive a suitable donor for a “high-risk recipient” with a serious infection, anti-HLA antibodies, or kidney dysfunction, should be based on pre-transplant sensitization and the recipient’s clinical status. For long-term LVAD-supported candidates, complex decision-making processes and management will become increasingly necessary to improve the survival and quality of life.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Yancy C.W., Jessup M., Bozkurt B., Butler J., Casey D.E., Drazner M.H. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Fukushima N., Ono M., Saiki Y., Sawa Y., Nunoda S., Isobe M. Registry report on heart transplantation in Japan (June 2016) Circ J. 2017;81:298–303. doi: 10.1253/circj.CJ-16-0976. [DOI] [PubMed] [Google Scholar]
- 3.Kinugawa K., Nishimura T., Toda K., Saiki Y., Niinami H., Nunoda S. The second official report from Japanese registry for mechanical assisted circulatory support (J-MACS): first results of bridge to bridge strategy. Gen Thorac Cardiovasc Surg. 2020;68:102–111. doi: 10.1007/s11748-019-01227-y. [DOI] [PubMed] [Google Scholar]
- 4.Kirklin J.K., Pagani F.D., Kormos R.L., Stevenson L.W., Blume E.D., Myers S.L. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. doi: 10.1016/j.healun.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Tromp T.R., de Jonge N., Joles J.A. Left ventricular assist devices: a kidney’s perspective. Heart Fail Rev. 2015;20:519–532. doi: 10.1007/s10741-015-9481-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colvin M.M., Cook J.L., Chang P.P., Hsu D.T., Kiernan M.S., Kobashigawa J.A. Sensitization in heart transplantation: emerging knowledge: a scientific statement from the American Heart Association. Circulation. 2019;139:e553–78. doi: 10.1161/CIR.0000000000000598. [DOI] [PubMed] [Google Scholar]
- 7.Nunoda S. Impact of pretransplant antibodies on outcomes after heart transplantation. Curr Opin Organ Transplant. 2019;24:220–226. doi: 10.1097/MOT.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arora S., Gude E., Sigurdardottir V., Mortensen S.A., Eiskjær H., Riise G. Improvement in renal function after everolimus introduction and calcineurin inhibitor reduction in maintenance thoracic transplant recipients: the significance of baseline glomerular filtration rate. J Heart Lung Transplant. 2012;31:259–265. doi: 10.1016/j.healun.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Asleh R., Briasoulis A., Kremers W.K., Adigun R., Boilson B.A., Pereira N.L. Long-term sirolimus for primary immunosuppression in heart transplant recipients. J Am Coll Cardiol. 2018;71:636–650. doi: 10.1016/j.jacc.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 10.Koval C.E., Thuita L., Moazami N., Blackstone E. Evolution and impact of drive-line infection in a large cohort of continuous-flow ventricular assist device recipients. J Heart Lung Transplant. 2014;33:1164–1172. doi: 10.1016/j.healun.2014.05.011. [DOI] [PubMed] [Google Scholar]