Abstract
A 74-year-old man who had a history of paroxysmal atrial fibrillation, visited the emergency department because of syncope. An electrocardiogram showed atrial fibrillation with slow ventricular response and long pauses. A permanent pacemaker was implanted under oral anticoagulation. Two screw-in leads were positioned at the right atrial appendage and the right ventricular apex. Seven hours after the implantation, he collapsed with hypotension due to cardiac tamponade. Vital signs improved after urgent pericardial drainage, but blood was continuously drained from the pericardial catheter. Due to uncontrollable cardiac tamponade, surgical repair was indicated. We found neither of the leads perforated the myocardium, but there was intermittent bleeding from a pin hole injury in the atrial wall site of the right coronary artery. Redness was observed in the right atrial appendage, but there was no bleeding point. We supposed that the screw tip of the atrial lead might have perforated the atrial appendage, but was retracted spontaneously afterwards. The pin hole was closed with a patch and the postoperative course was uneventful. This is a rare case of cardiac tamponade due to the injury of the coronary artery by a screw-in lead positioned at the right atrial appendage.
<Learning objectives: Pacemaker implantation can cause cardiac tamponade due to coronary artery perforation. Right coronary artery perforation due to screwed-in atrial lead can be a cause of cardiac tamponade after pacemaker implantation, especially if proximal portion of right coronary artery meanders close to atrial appendage.>
Keywords: Pacemaker implantation, Cardiac tamponade, Coronary artery injury
Introduction
Pacemaker implantation (PMI) is a standard treatment for symptomatic bradyarrhythmia. Pneumothorax, aortic perforation, and pericardial effusion were reported as major complications of this procedure [1], [2]. A cohort study in Denmark found that the incidence was 9.5% of 5918 patients who were implanted with a cardiac implantable electronic device (CIED), among which cardiac perforation was 0.6%, the male to female ratio was 1.1% vs 0.4% [2]. In the study of the recipients of permanent pacemakers from 2008 to 2012 in the USA, cardiac tamponade occurred in 2595 cases, which was 0.28% for the implantation of 922,549 patients [3]. Many cases can be managed conservatively with drainage or lead revision, but sometimes surgical intervention is required. We report a rare case of cardiac tamponade due to injury of the coronary artery by an atrial screw-in lead.
Case report
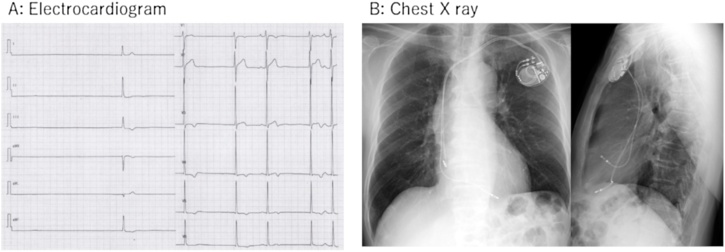
A 74-year-old man visited the emergency room because of syncope. He was diagnosed with bradycardia tachycardia syndrome two years previously with presyncope. At that time, he took no drug that could cause bradycardia. Ambulatory electrocardiographic monitoring revealed maximum of 5 sec sinus arrest after the termination of atrial fibrillation, but he refused PMI. At this time, heart rate was 35 beats/min and about 4 sec pause was found in the electrocardiogram (Fig. 1A). Consciousness was clear, and the other physical examination was not remarkable.
Fig. 1.
Electrocardiogram and chest X-ray. (A) Electrocardiogram showed atrial fibrillation with slow ventricular response and a long pause. (B) Chest X-ray after permanent pacemaker implantation.
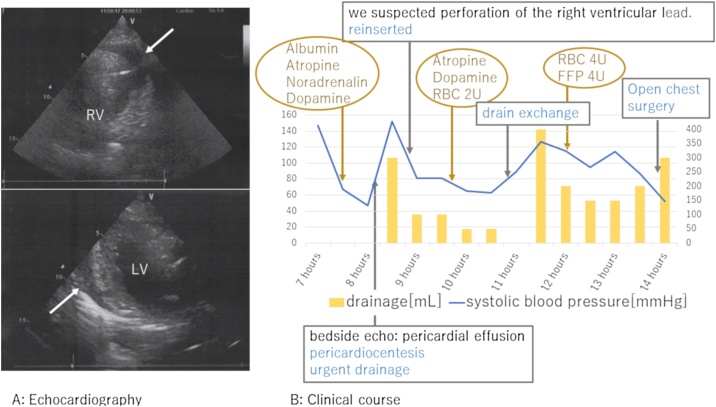
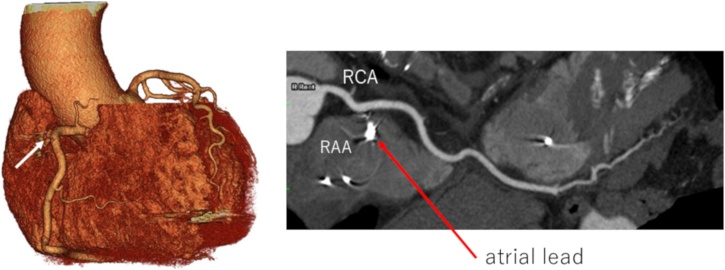
A temporary pacemaker lead was inserted from the right internal jugular vein to the right ventricle. Rivaroxaban 15 mg was started after hospitalization. A permanent pacemaker was implanted on the 6th day after the admission. The implantation procedure was conventional as follows: the left subclavian vein was punctured at the extrathoracic site under fluoroscopy and two screw-in leads were placed at the right atrial appendage and the right ventricular apex without any problems. Both leads were placed smoothly, and screwed in at the first attempt. Pacing threshold was 0.5 V in the atrium and 0.8 V in the ventricle. Electrocardiogram amplitude was 2.1 mV in the atrium and 12.0 mV in the ventricle. Lead impedance was 550 Ohm in the atrium and 630 Ohm in the ventricle. The post-operative chest X-ray showed no abnormality (Fig. 1B). Seven hours after the surgery, he was found collapsed. Systolic blood pressure (SBP) was decreased to 60 mmHg, and he was drowsy. Pericardial effusion was detected by a bedside echocardiogram and right ventricular perforation was suspected (Fig. 2A). Urgent pericardial puncture was performed. A 5Fr aspiration tube was inserted and about 300 ml of bloody pericardial effusion was drained. SBP recovered to 130 mmHg. The pacing threshold, sensitivity, and impedance of both leads showed no diagnostic changes in the clinical course. At first, we replaced the ventricular lead to the septal wall, because we suspected that cardiac tamponade was caused by a perforation of ventricular lead positioned at apex. However, SBP repeatedly decreased even after the replacement. An additional 300 ml of bloody effusion was drained. SBP was relatively stable at around 120 mmHg with appropriate transfusion, but bloody effusion was continuously drained even 14 h after the implantation (Fig. 2B). Although a contrast computed tomography (CT) image was obtained, the lesion could not be detected. Therefore, open chest surgery was performed to find hematoma and continuous bleeding in the pericardium. No leads had perforated the myocardium. The right atrial lead was not visible from the surface, but a spout of bleeding was observed from the right coronary artery near the tip of the electrode. The pinhole of the artery was closed using a polytetrafluoroethylene patch. We suspected a right coronary artery injury by the screw tip of the right atrial lead. After the operation, the patient fully recovered. Cardiac CT revealed that the proximal part of the right coronary artery formed a bend to come close to the atrial appendage (Fig. 3).
Fig. 2.
Transthoracic echocardiogram. (A) Transthoracic echocardiogram at the onset of cardiac tamponade (white arrow: pericardial effusion). In the left panel, it was suspected that the ventricular lead was protruding from the ventricle. (B) Clinical course until open heart surgery.
Fig. 3.
Post-operative coronary CT images. The proximal part of the right coronary artery of this patient forms a bend to come close to the atrial appendage. (arrow: a point of right coronary artery repair).
RAA, right atrial appendage; RCA, right coronary artery.
Discussion
We report a rare case of cardiac tamponade possibly due to a puncture of the coronary artery. Cardiac perforation is one of the major complications of PMI. A Danish national survey reported that lead revision or drainage was required in 0.3% of the patients who received CIED [2]. In a report from the USA, the frequency of cardiac tamponade increased from 0.26% in 2008 to 0.35% in 2012 [3]. The right ventricular apex has been reported to be the most frequent site of the perforation [4], [5], whereas the right atrial appendage is rarer. Notably there is only one case report on coronary artery injury by an atrial lead [6].
Complications with screw-in leads are observed more often than those with tined lead [4].
In many cases, perforation did not result in serious complications to require any form of intervention because of low pressure in the cardiac chamber. A study using cardiac CT [7] showed that the distance between atrial lead and coronary artery was large when the lead was placed at anterior or lateral wall. On the other hand, the distance was short when the lead tip was superior to the border of the tricuspid valve. Pericardial fluid retention could occur if the lead was placed at the right atrial free wall, and aortic perforation could be observed when it was placed to moderate to high atrial septum. The right coronary artery runs along the groove between the right atrium and the right ventricle. The right atrial lead is usually placed to the high center of the anterior wall of the appendage or the lateral wall apart from the coronary artery. In our case, the proximal right coronary artery ran close to the right atrial appendage (Fig. 3). Thus, the tip of the screw penetrated the right coronary artery when it was screwed in. Ideally, the right atrial lead should be screwed in at the upper edge of the right atrial appendage. But in this case, we screwed the lead at the lower position, where the right coronary artery curved to the direction of the right atrial appendage. In order to avoid this problem, it would be useful to select a tined lead or to screw in at the low atrial septum [6], [7].
In this case, cardiac tamponade could not be controlled, and blood transfusion was required several times. This condition suggested arterial injury, and surgical intervention was necessary. The definitive cause of tamponade could not be confirmed until surgical operation, because the tip of atrial lead was retracted spontaneously into atrial appendage. Bleeding was probably intermittent because the atrial appendage covered the artery as a lid, which opened and closed the bleeding point according to the atrial motion. It could take time until cardiac tamponade became evident.
The use of an anticoagulant also contributed to the exacerbation of bleeding. Although CIED can be implanted safely without interruption of anticoagulation [8], it would be safer to use a drug that can be neutralized when it is difficult to control bleeding and cardiac tamponade.
In conclusion, this is a rare case of cardiac tamponade due to injury of the right coronary artery by an atrial screw-in lead at the time of PMI.
Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
None.
References
- 1.Oginosawa Y., Haruhiko Abe., Nakashima Y. Right pneumothorax resulting from an endocardial screw-in atrial lead in an implantable cardioverter defibrillator system. Pacing Clin Electrophysiol. 2002;25:1278–1279. doi: 10.1046/j.1460-9592.2002.01278.x. [DOI] [PubMed] [Google Scholar]
- 2.Kirkfeldt R.E., Johansen J.B., Nohr E.A., Jørgensen O.D., Nielsen J.C. Complications after cardiac implantable electronic device implantations: an analysis of a complete, nationwide cohort in Denmark. Eur Heart J. 2014;35:1186–1194. doi: 10.1093/eurheartj/eht511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moazzami K., Dolmatova E., Kothari N., Mazza V., Klapholz M., Waller A.H. Trends in cardiac tamponade among recipients of permanent pacemakers in the United States: from 2008 to 2012. JACC Clin Electrophysiol. 2017;3:41–46. doi: 10.1016/j.jacep.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Cano Ó, Andrés A., Alonso P., Osca J., Sancho-Tello M.J., Olagüe J. Incidence and predictors of clinically relevant cardiac perforation associated with systematic implantation of active-fixation pacing and defibrillation leads: a single-centre experience with over 3800 implanted leads. Europace. 2017;19:96–102. doi: 10.1093/europace/euv410. [DOI] [PubMed] [Google Scholar]
- 5.Akbarzadeh M.A., Mollazadeh R., Sefidbakht S., Shahrzad S., Bahrololoumi Bafruee N. Identification and management of right ventricular perforation using pacemaker and cardioverter-defibrillator leads: a case series and mini review. J Arrhythm. 2017;33:1–5. doi: 10.1016/j.joa.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nakagawa E., Abe Y., Komatsu R., Naruko T., Itoh A. Right coronary artery perforation by an active-fixation atrial pacing lead resulting in life-threatening tamponade. J Arrhythm. 2015;31:313–315. doi: 10.1016/j.joa.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pang B.J., Joshi S.B., Lui E.H., Tacey M.A., Alison J., Seneviratne S.K. Proximity of pacemaker and implantable cardioverter-defibrillator leads to coronary arteries as assessed by cardiac computed tomography. Pacing Clin Electrophysiol. 2014;37:717–723. doi: 10.1111/pace.12330. [DOI] [PubMed] [Google Scholar]
- 8.Birnie D.H., Healey J.S., Wells G.A., Verma A., Tang A.S., Krahn A.D. Pacemaker or defibrillator surgery without interruption of anticoagulation. N Engl J Med. 2013;368:2084–2093. doi: 10.1056/NEJMoa1302946. [DOI] [PubMed] [Google Scholar]