Abstract
Background
Access and adherence to artemisinin-based combination therapy (ACT) are key challenges to effective malaria treatment. A secondary analysis of the Sierra Leone malaria Knowledge, Attitudes, and Practices (mKAP) survey was conducted to investigate access and adherence to ACT for the treatment of fever in children under-five.
Methods
The mKAP was a nationally representative, two-stage cluster-sample survey, conducted in 2012. Thirty primary sampling units per district were randomly selected using probability proportionate to size, based on national census estimates; 14 households were subsequently randomly selected and enrolled per sampling unit. The analysis was restricted to children under-five with fever in the past two weeks. Factors associated with access and adherence were assessed using multivariate logistic regression.
Results
Of 5169 enrolled households, 1456 reported at least one child under-five with fever in the past two weeks. Of the 1641 children from these households, 982 (59.8%) received any treatment for fever and were analysed for access to ACT; 469 (47.6%) received ACT and 466 were analysed for treatment adherence. Only 222 (47.4%) febrile children received ACT and completed 3-day treatment. In an adjusted analysis, factors associated with ACT access included knowledge of ACT (odds ratio [OR] 2.78, 95% CI 2.02–3.80; p < 0.001), knowledge of insecticide-treated nets (ITNs) (OR 1.84, 95% CI 1.29–2.63; p = 0.001), source of care (public health facility vs. other; OR 1.86, 95% CI 1.27–2.72, p = 0.001), geographic region (East vs. West; OR 2.30, 95% CI 1.20–4.44; p = 0.025), and age (24–59 vs. 0–23 months; OR 1.45, 95% CI 1.07–1.96; p = 0.016). The only factor associated with ACT adherence was time to treatment; children treated within 24 h were less likely to adhere (OR 0.55, 95% CI 0.34–0.89; p = 0.015).
Conclusions
In 2012, access and adherence to ACT remained low in Sierra Leone. Knowledge of ACT and ITNs, and seeking care in the public sector, were most strongly associated with ACT access. National surveys provide important information on anti-malarial access and could be expanded to measure treatment adherence.
Keywords: Malaria, Antimalarial, Artemisinin-based combination therapy (ACT), Sierra Leone, Prompt treatment, Access, Adherence, Treatment completion
Background
Malaria remains a serious health problem in sub-Saharan Africa and is particularly dangerous for children under-five [1–3]. Prompt access to effective treatment is critical to malaria control. The World Health Organization (WHO) malaria treatment guidelines recommend that all laboratory-confirmed malaria cases be treated promptly (within 24 h of onset) using artemisinin-based combination therapy (ACT) [4]. Despite this recommendation, access to prompt and effective treatment remains sub-optimal (on average, 65%) across sub-Saharan Africa [3]; with access to ACT influenced by availability, affordability, and acceptability [5]. Weak health systems are adversely impacted during global public health emergencies, such as Ebola and COVID-19, leading to disruptions in service provision, supply chain, and health-seeking behaviour [6, 7]. During the Ebola outbreak 2014–2015 in Sierra Leone, pre-existing gaps in reporting and service delivery worsened [8], and changes to service delivery had a lasting impact on access in neighbouring Liberia [9]. Although access to ACT is essential, multiple factors influence the effectiveness of treatment, including the efficacy of ACT regimens, targeted testing and treatment, and patient (or caregiver) adherence to treatment [10, 11].
In Sierra Leone, malaria is the leading cause of morbidity and mortality in children under-five, accounting for 47% of outpatient visits [12]. Although amodiaquine plus artesunate (AQ + AS) was recommended as the first-line treatment for uncomplicated malaria in 2004 [13], access to ACT remained low, with only 19.2% of children with fever receiving ACT in 2010 [14]. Furthermore, probable adherence to co-packaged AQ + AS in Sierra Leone was reported to be only 48.7% in 2008 [15]. In the 2016 Malaria Indicator Survey (MIS), 40% of children aged 6–59 months tested positive for malaria; parasite prevalence was twice as high in rural compared to urban areas (49% vs. 25%) and was highest in the northern region (52%) [16].
In 2010, the government of Sierra Leone recognized the importance of improving access to essential medications, including anti-malarials, to reduce childhood morbidity and mortality. Two initiatives were rolled out to improve access to health care: (1) The Free Health Care Initiative (FHCI), which provides services and medications free of charge to pregnant women, lactating mothers and children under five at government health facilities along with supportive supply-side interventions, such as improved drugs and medical supply chains; health workforce strengthening; governance; infrastructure for service delivery; communication; monitoring and evaluation; and health financing [17]; and (2) a malaria treatment policy advocating for free malaria testing and treatment with ACT for all malaria cases [18].
These initiatives only addressed health system factors that impact access and targeting of ACT, steps at the beginning of the effectiveness pathway, and did not focus on the quality of care provided [10, 11, 19]. To be truly effective, health workers must follow malaria treatment guidelines, and patients and caregivers must adhere to the prescribed ACT regimens. If these last steps of the pathway, focusing on adherence, are not realized, effective treatment and control of malaria cannot be achieved. Therefore, it is critical to measure and understand factors associated not only with access, but also adherence to ACT.
Although a number of national surveys between 2013 and 2016 documented access to ACT in Sierra Leone, ranging from 77–97% [14, 16, 20, 21], none of these surveys measured treatment adherence nor factors associated with access and adherence to ACT. In 2012, a nationwide malaria knowledge, attitudes, and practices (mKAP) survey was carried out in Sierra Leone, which included not only questions about treatment access but also treatment adherence [22]. To further explore access and adherence to ACT, a secondary analysis of the mKAP dataset was conducted to quantify, and determine factors associated with, access and adherence to ACT in Sierra Leone.
Methods
Study design
The mKAP was conducted in 2012 by Catholic Relief Services in partnership with the National Malaria Control Programme (NMCP), supported by Statistics Sierra Leone. The primary objective of the survey was to gather information to inform and update the national malaria communication strategy. Additionally, data from the survey was used to establish a baseline for malaria control activities that were to be subsequently implemented with support from the Global Fund to Fight AIDS, Tuberculosis, and Malaria [22]. This study is a secondary analysis of a subset of data collected from the mKAP survey.
The mKAP survey was a nationally representative two-stage cluster sample survey conducted in all 14 districts of Sierra Leone. Thirty primary sampling units (PSU) per district were randomly selected using probability proportional to size (PPS) based on estimates from the National Census [23]. This resulted in 5880 randomly chosen households (14 households per PSU; 420 households per district). The questionnaire was based on the Roll Back Malaria standardized guidelines for core population-level indicators [24]. All respondents answered questions about household demographics and assets, malaria knowledge and prevention practices, recent pregnancy experiences, and whether the household contained one or more children aged under-five who had a fever in the previous two weeks. Information on fever treatment was collected on up to three such children per household. The mKAP survey was conducted in 2012 before a question about receiving a blood test was universally introduced into national surveys, and therefore did not include a question on whether the child had “blood taken from his/her finger or heel for testing.”
Data collection
Data were collected by trained field staff using Apple iPhones provided by Catholic Relief Services. The devices were programmed using the iFormBuilder mobile platform (Zerion Software, Inc., Herndon, VA, USA) [25]. All electronic data were transferred from the Apple devices into a cloud database regularly while in the field using the local 3G mobile network. Upon completion of the fieldwork, any remaining forms that needed to be transferred were uploaded via wireless internet connections at Statistics Sierra Leone and Catholic Relief Services offices in Freetown.
Paper questionnaires were provided to teams to use only as a backup in case of electronic equipment failure. When necessary, data entered onto paper forms were then entered into an iPhone as soon as it was possible. Backup files of the database were stored on two external servers (iFormBuilder and a specially created Google email account). Additionally, data were stored on the iPhones until completion of the study. For quality control, validation and built-in skip logic were written into the iFormBuilder program.
Outcome variables and predictors
The objectives of this study were: (1) to quantify the level of access and adherence to ACT in children less than five in Sierra Leone, (2) to assess factors associated with access to ACT for children under-five with fever in the two weeks preceding the survey, and (3) to identify factors associated with adherence to ACT in those children that received ACT for their fever. Access was defined as receiving ACT for the treatment of the most recent fever. Adherence was defined as taking the treatment for the recommended 3 days. The methodology used to assess adherence was similar to that used in two previous cross-sectional household studies in Kenya, which utilized a self-report question to assess whether the duration of treatment with ACT was correct (i.e. 3 days) [26, 27]. Those taking ACT for 3 days were considered to have completed treatment and were classified as adherent, while those taking ACT for less than 3 days or more than 3 days were classified as non-adherent.
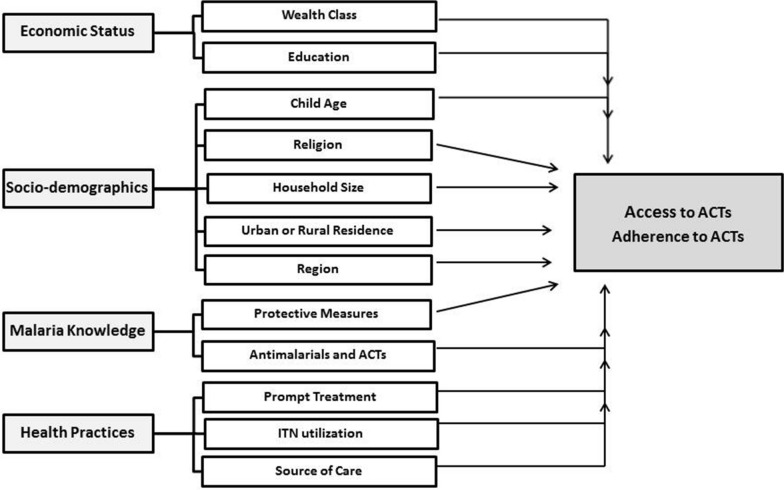
Using the available variables from the survey dataset, along with the factors identified in the ACT adherence literature, a list of a priori predictors were identified and evolved into a conceptual framework (Fig. 1). Four categories of potential predictors of access and adherence were identified: (1) socioeconomic status (i.e. wealth class and education); socio-demographic characteristics (i.e. child age, religion, household size, place of residence); (2) knowledge of malaria (i.e. knowledge of protective measures and treatments); and (3) health practices (i.e. accesses prompt treatment for fever, ITN utilization, and source of health care).
Fig. 1.
Conceptual framework. A summary of hypothesized socio-demographic factors associated with access and adherence to ACT
Data analysis
Stata Version 12 (StataCorp, College Station, TX USA) and Excel (Microsoft Corp. Redmond, WA, USA) were used for data processing and analysis. In all analyses, the “svy” commands were used to account for the survey design, including clustering by PSU and stratification by the location of the PSU (urban/rural). Descriptive statistics were used to summarize household and respondent characteristics as well as treatment-seeking behaviour for children with fever. Household socioeconomic status was based on a principal component analysis (PCA) of household assets, split into tertiles [28].
Logistic regression models were used to estimate the crude and adjusted odds ratios and their 95% confidence intervals to assess the strength of the association between the a priori predictors and the two outcomes (access and adherence to ACT). All predictor variables were included in multivariable analyses regardless of p-values, with the exception that for any pair of covariates identified to be strongly correlated (Pearson’s correlation r ≥ 0.8), one was removed from the final model. Associations between the predictors and outcomes were considered significant if the p-value was < 0.05.
Ethical considerations
The original study protocol was approved by the Sierra Leone Ethics and Scientific Review Committee prior to the commencement of activities. Ethical approval to conduct this secondary analysis of the mKAP data set was obtained from the London School of Hygiene & Tropical Medicine. Permission to use this data for secondary analysis was obtained from CRS and the NMCP in Sierra Leone.
Results
Survey profile
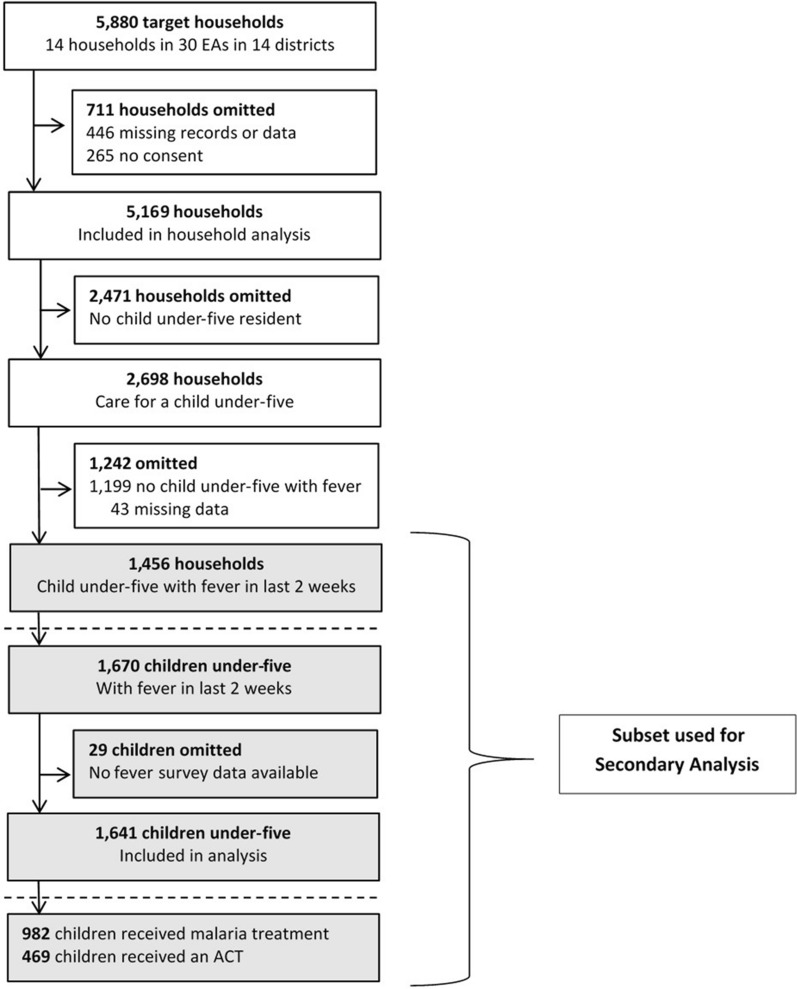
Of 5169 enrolled households, 1456 reported at least one child under-five with fever in the past two weeks and were included in the analysis (Fig. 2). Access to ACT was assessed in 1641 children residing in these households who had a recent history of fever and data on treatment available. Of these children, factors associated with access to ACT were estimated for children whose caregiver sought and received any treatment for their child’s fever (n = 982). Factors associated with adherence to ACT were assessed for children who received ACT and had data on treatment duration (n = 466).
Fig. 2.
mKAP survey profile
Characteristics of households and their children
Overall, households that reported fever in a child under-five within the past two weeks were similar to other households, as were the adult respondents from these households (Table 1). Households with febrile children were primarily located in rural areas (87.6%), practiced the Islamic faith (81.0%), and owned at least one bed net of any type (87.2%). The average age of the adult caregiver respondents was 39.2 years, and 66.8% reported not having any formal education. Three out of four adult respondents (76.6%) reported sleeping under an insecticide-treated net (ITN) the previous night. Although most respondents were knowledgeable about malaria, some information gaps were identified, specifically regarding the prevention and treatment of malaria. Only 606 (41.8%) respondents were aware that ACT was the recommended treatment for malaria.
Table 1.
Household and individual-level characteristics of survey participants, stratified by the presence or absence of a child under five with fever in the preceding 2 weeks
| Variable | Categories | Under-five Households with fever (N = 1456) | Under-five Households without fever (N = 1199) | ||
|---|---|---|---|---|---|
| n(%) | 95% CI | n(%) | 95% CI | ||
| Household | |||||
| Location | Urban | 191 (12.4%) | 10.6–14.4% | 184 (14.8%) | 12.8–17.0% |
| Rural | 1265 (87.6%) | 85.6–89.4% | 1015 (85.2%) | 83.0–87.2% | |
| Region of Sierra Leone | North | 672 (46.9%) | 41.4–52.5% | 368 (30.6%) | 25.8–35.9% |
| South | 371 (25.4%) | 21.1–30.3% | 427 (35.7%) | 30.4–41.4% | |
| East | 291 (19.5%) | 15.7–24.1% | 236 (19.8%) | 15.9–24.5% | |
| West | 122 (8.2%) | 5.8–11.3% | 168 (13.9%) | 10.6–18.0% | |
| Number of household residents | 1–5 | 430 (29.3%%) | 26.5–32.2% | 440 (36.6%) | 33.6–39.8% |
| 6–10 | 795 (54.7%) | 51.8–57.5% | 602 (50.4%) | 47.3–53.5% | |
| 11+ | 231 (16.1%) | 13.9–18.5% | 157 (12.9%) | 11.0–15.2% | |
| Religion | Christian | 284 (19.0%) | 16.2–22.2% | 261 (21.9%) | 18.9–25.4% |
| Muslim | 1172 (81.0%) | 77.8–83.8% | 938 (78.1%) | 74.8–81.2% | |
| Socio-economic status | 1 (poorest) | 508 (35.4%) | 31.9–39.0% | 400 (33.4%) | 30.0–37.1% |
| 2 | 532 (36.2%) | 33.3–39.1% | 410 (34.2%) | 31.2–37.4% | |
| 3 (least poor) | 416 (28.5%) | 25.2–31.9% | 389 (32.3%) | 28.6–36.3% | |
| Ownership of bed nets | Own any net | 1272 (87.2%) | 10.1–15.1% | 1045 (86.9%) | 84.4–89.1% |
| Net is an ITN | 1214 (83.1%) | 80.5–85.5% | 989 (82.2%) | 79.2–84.9% | |
| Mean ITNs | 2.6 | 2.5–2.8 | 2.5 | 2.4–2.6 | |
| Household adult respondents | |||||
| Mean age (years) | 39.2 | 38.4–40.1 | 38.7 | 37.8–39.6 | |
| Gender | Male | 684 (47.1%) | 44.0–50.2% | 577 (48.2%) | 45.0–51.5% |
| Female | 772 (52.9%) | 49.8–56.0% | 622 (51.8%) | 48.5–55.0% | |
| Education | None | 967 (66.8%) | 63.9–69.6% | 779 (64.7%) | 61.4–67.9% |
| Primary | 173 (11.8%) | 10.1–13.8% | 124 (10.7%) | 8.9–12.7% | |
| Secondary/highera | 279 (18.7%) | 16.4–21.2% | 277 (23.0%) | 20.3–25.9% | |
| Arabic/Other | 37 (2.7%) | 1.9–3.8% | 19 (1.7%) | 1.0–2.8% | |
| ITN use previous night | Respondent | 1129 (76.6%) | 73.7–79.3% | 913 (76.2%) | 72.9–79.2% |
| Knowledge of malaria-related topics | |||||
| Ever hear of the illness called “Malaria” | Yes | 1492 (98.1%) | 97.0–98.8% | 1167 (97.4%) | 96.1–98.3% |
| At least one sign or symptom of malaria | Yes | 1374 (94.2%) | 92.5–95.6% | 1116 (93.0%) | 91.1–94.6% |
| All are susceptible to malaria | Yes | 1065 (73.2%) | 70.3–75.9% | 884 (73.4%) | 70.3–76.3% |
| At least one malaria protective measure | Yes | 1244 (85.2%) | 82.6–87.5% | 1026 (85.4%) | 82.7–87.8% |
| ITNs can prevent malaria | Yes | 865 (59.6%) | 56.3–62.9% | 741 (60.9%) | 57.1–64.5% |
| At least one antimalarial drug | Yes | 1268 (86.9%) | 84.7––88.8% | 1021 (85.1%) | 82.5–87.3% |
| Recommended treatment with ACTs | Yes | 606 (41.8%) | 38.5–45.3% | 527 (43.7%) | 40.2–47.2% |
aSecondary or higher includes technical and vocational school
Treatment for children under‐five with fever
Of the 1641 children under-five with a fever episode in the last 2 weeks, care was sought for 1038 (63.4%) (Table 2). The majority of children (82.2%) were taken to a public health facility. Of those children for whom treatment was sought, most (n = 982) received any treatment, the majority of which were anti-malarials (n = 780). Of these children, 469 (47.6%) received ACT, most of whom (n = 314; 67.8%) were treated within 24 h of onset. Only 222 (47.4%) children with fever received ACT and completed 3-day treatment, and only 137 (31.6%) received prompt treatment (within 24 h) with ACT and completed the 3-day treatment.
Table 2.
Treatment and treatment-seeking behaviors for children under-five with fever (n = 1641)
| Observations | ||||
|---|---|---|---|---|
| n/N | % | Linearized SE | 95% CI | |
| Treatment seeking for fever | ||||
| Caregiver sought treatment | 1038/1641 | 63.4 % | 1.67% | 60.1–66.6% |
| Caregiver sought prompt treatment (≤ 24 h) | 571/1038 | 55.9% | 1.87% | 52.2–59.6% |
| First treatment sourcea | ||||
| Public health facility | 854/1038 | 82.2% | 1.48% | 79.1–84.9% |
| Otherb | 184/1038 | 17.8% | 1.48% | 15.1–20.9% |
| Treatment of fever | ||||
| Received any treatment | 982/1038 | 94.6% | 0.83% | 92.7–96.1% |
| Type of treatment | ||||
| Artemisinin combination therapy (ACT) | 469/982 | 47.6% | 2.11% | 43.4–51.7% |
| Chloroquine | 472/982 | 48.7% | 2.19% | 44.5–53.1% |
| Sulfadoxine-pyrimethamine (SP) | 153/982 | 15.0% | 1.37% | 12.5–17.9% |
| Paracetamol | 770/982 | 78.4% | 1.67% | 74.3–80.9% |
| Herbs | 141/982 | 14.4% | 1.36% | 11.6–17.0% |
| Otherc | 136/982 | 13.5% | 1.32% | 11.1–16.3% |
| ACT treatment timed | ||||
| Received ACT same/next day ( within 24 h) | 314/469 | 67.8% | 2.33% | 63.0–72.2% |
| Received ACT on day 2 | 93/469 | 19.4% | 2.00% | 15.8–23.7% |
| Received ACT 3 + days | 29/469 | 6.0% | 1.07% | 4.2–8.5% |
| ACT treatment time unknown | 33/469 | 6.8% | 1.21 | 4.8–9.6% |
| ACT durationd | ||||
| Took ACT for 3 days (correct duration) | 222/469 | 47.4% | 2.56% | 42.4–52.4% |
| Did not take ACT for 3 days | 244/469 | 52.0% | 2.53% | 47.1–57.0% |
| ACT duration unknown | 3/469 | 0.57% | 0.03% | 0.19–1.74% |
| Mean duration of ACT treatment (days) | 3.62 | 0.80 | 3.47–3.78 | |
| Prompt and effective treatment with ACTe | ||||
| Children who received ACT < 24 h & Completed 3-day treatment course | 137/433 | 31.6% | 2.57% | 27.3–37.4% |
aThe denominator is the number seeking treatment for the fever in the last 2 weeks (n = 1038)
bOther sources of treatment include: Community Health workers [Community Health Worker (CHW), Traditional Birth Attendant (TBA), Blue Flag Volunteer (BFV)] = 39; Informal Health workers (drug peddler, traditional healer) = 31; Drug shops/Pharmacy = 96; private clinics/doctors = 9 and self-treatment = 9
cOther drugs received include other antimalarial–mono-therapy: quinine and amodiaquine (4), antibiotics (34), antidiarrheal/ORS (17), other antipyretic (7), cough medicine (2), deworm (1), vitamins (12), iron (16), unnamed syrup (6), injection (11), routine medication (10), unspecified/unknown (16)
dThe denominator is the number who received ACT (n = 469)
eThe denominator is the number who received ACT and who have information on the timing and duration of treatment (n = 433)
Factors associated with access
In a multivariate analysis restricted to children who received any treatment for fever (n = 982), five factors were found to be significantly associated with receiving ACT (access), including geographic region, the knowledge that ITNs protect against malaria, knowledge of ACT, older child age, and seeking care at a public health facility (Table 3). ACT access was highest in the eastern region. Knowledge of ACT was the strongest predictor; children with caregivers who were knowledgeable about ACT had almost three times the odds of receiving an artemisinin-based combination for treatment of fever than children whose caregivers lacked this knowledge (OR: 2.78; 95% CI 2.02–3.80; p < 0.001). Similarly, children whose caregiver knew that ITNs provide protection from malaria were more likely to receive ACT (OR: 1.84; 95% CI 1.29–2.63; p = 0.001). Children treated at a public health facility had nearly twice the odds of receiving an artemisinin-based combination compared to those treated elsewhere (OR: 1.86; 95% CI 1.27–2.72; p = 0.001), and older children (24–59 months) were more likely to receive ACT than children aged 0–23 months (OR: 1.45; 95% CI 1.07–1.96; p = 0.016).
Table 3.
Factors associated with receiving an ACT among febrile children under-five who received any treatment (n = 982)
| Variable | n/N | % | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Location | ||||||
| Rural | 386/835 | 46.3% | Ref | 0.181 | Ref | 0.899 |
| Urban | 83/147 | 54.9% | 1.41 (0.85–2.33) | 1.03 (0.63–1.69) | ||
| Region | ||||||
| West | 36/75 | 47.8% | Ref | 0.253 | Ref | 0.025 |
| North | 203/452 | 45.0% | 0.89 (0.51–1.56) | 1.20 (0.64–2.26) | ||
| South | 125/268 | 45.9% | 0.93 (0.51–1.70) | 1.48 (0.79–2.83) | ||
| East | 105/187 | 56.4% | 1.41 (0.75–2.66) | 2.30 (1.20–4.44) | ||
| Household size | ||||||
| 1–5 | 146/269 | 52.2% | Ref | 0.058 | Ref | 0. 178 |
| 6–10 | 257/548 | 47.6% | 0.83 (0.59–1.17) | 0.95 (0.68–1.33) | ||
| 11+ | 66/165 | 39.9% | 0.61 (0.41–0.91) | 0.69 (0.46–1.04) | ||
| Household religion | ||||||
| Muslim | 375/796 | 46.9% | Ref | 0.402 | Ref | 0.579 |
| Christian | 94/186 | 50.7% | 1.17 (0.81–1.67) | 0.90 (0.62–1.30) | ||
| Socio-economic status | ||||||
| 1 (poorest) | 131/317 | 41.0% | Ref | 0.001 | Ref | 0.237 |
| 2 | 175/382 | 46.1% | 1.23 (0.87–1.73) | 1.00 (0.68–1.47) | ||
| 3 (least poor) | 163/283 | 57.0% | 1.91 (1.31–2.77) | 1.41 (0.88–2.24) | ||
| Respondent education | ||||||
| None | 290/644 | 45.1% | Ref | 0.059 | Ref | 0.930 |
| Primary | 63/123 | 51.2% | 1.28 (0.87–1.89) | 1.03 (0.68–1.58) | ||
| Secondary or higher | 107/196 | 53.8% | 1.42 (0.99–2.03) | 1.00 ( 0.66–1.50) | ||
| Arabic school or Other | 9/19 | 47.9% | 1.12 (0.44–2.87) | 1.41 (0.49–4.08) | ||
| Everyone is at risk | ||||||
| No | 120/250 | 47.4% | Ref | 0.948 | Ref | 0.380 |
| Yes | 349/732 | 47.6 | 1.01 (0.73–1.39) | 0.85 (0.60–1.22) | ||
| ITNs protect from malaria | ||||||
| No | 129/370 | 35.0% | Ref | < 0.001 | Ref | 0.001 |
| Yes | 340/612 | 55.2% | 2.29 (1.66–3.15) | 1.84 (1.29–2.63) | ||
| Under 5 slept under ITN | ||||||
| No | 119/287 | 40.2% | Ref | 0.009 | Ref | 0.061 |
| Yes | 350/695 | 50.6% | 1.53 (1.11–2.10) | 1.41 (0.98–2.02) | ||
| Knowledge of ACTs | ||||||
| No | 187/537 | 34.7% | Ref | < 0.001 | Ref | < 0.001 |
| Yes | 282/445 | 63.0% | 3.20 (2.36–4.35) | 2.78 (2.02–3.80) | ||
| Child age (months) | ||||||
| 0–23 months | 136/321 | 42.6% | Ref | 0.047 | Ref | 0.016 |
| 24–59 months | 333/661 | 49.9% | 1.34 (1.00–1.79) | 1.45 (1.07–1.96) | ||
| Prompt treatment (< 24 h) | ||||||
| No | 209/440 | 47.2% | Ref | 0.842 | Ref | 0.866 |
| Yes | 260/542 | 47.9% | 1.03 (0.77–1.37) | 1.03 (0.76–1.38) | ||
| Public health facility | ||||||
| No | 57/159 | 36.4% | Ref | 0.004 | Ref | 0.001 |
| Yes | 412/823 | 49.7% | 1.73 (1.19–2.52) | 1.86 (1.27–2.72) | ||
Factors associated with adherence
In a multivariate analysis restricted to children who received ACT and had data on treatment duration (n = 466), only one factor was found to be significantly associated with adherence (Table 4). Children receiving ACT within 24 h of symptom onset were less likely to complete treatment than those who received ACT treatment beyond this window (OR: 0.55; 95%CI 0.34–0.89; p = 0.015). Due to collinearity with ‘knowledge of ACT,’ ‘knowledge of at least one anti-malarial’ and ‘knowledge of at least one sign or symptom of malaria’ were removed from the multivariable models. Similarly, ITN use was retained, while ITN ownership and knowledge of any malaria protective measures were removed. As knowledge of the term ‘malaria’ was collinear with both knowledge of ACT and ITN utilization, it was removed from both final multivariable models.
Table 4.
Factors associated with adherence to ACTs among febrile children under-five who received treatment with an artemisinin-based combination (n = 466)
| Variable | n/N | % | Unadjusted analysis | Adjusted analysis | ||
|---|---|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |||
| Location | ||||||
| Rural | 178/385 | 46.2% | Ref | 0.233 | Ref | 0.245 |
| Urban | 44/81 | 55.3% | 1.44 (0.79–2.64) | 1.49 (0.76–2.95) | ||
| Region | ||||||
| West | 11/35 | 30.1% | Ref | 0.355 | Ref | 0.211 |
| North | 98/202 | 47.9% | 2.13 (0.83–5.50) | 2.58 (0.99–6.77) | ||
| South | 60/124 | 49.3% | 2.26 (0.87–5.89) | 2.83 (1.04–7.68) | ||
| East | 53/105 | 50.8% | 2.40 (0.91–6.32) | 2.76 (1.04–7.32) | ||
| Household size | ||||||
| 1–5 | 63/144 | 45.3% | Ref | 0.487 | Ref | 0.620 |
| 6–10 | 122/256 | 47.3% | 1.08 (0.72–1.62) | 1.13 (0.73–1.73) | ||
| 11 + | 37/66 | 54.2% | 1.43 (0.79–2.59) | 1.35 (0.73–2.50) | ||
| Household religion | ||||||
| Muslim | 172/373 | 46.0% | Ref | 0.158 | Ref | 0.058 |
| Christian | 50/93 | 54.7% | 1.42 (0.87–2.30) | 1.61 (0.98–2.65) | ||
| Socio-economic status | ||||||
| 1 (poorest) | 62/131 | 46.5% | Ref | 0.883 | Ref | 0.930 |
| 2 | 83/175 | 47.0% | 1.02 (0.64–1.63) | 1.07 (0.66–1.75) | ||
| 3 (least poor) | 77/160 | 49.4% | 1.13 (0.68–1.86) | 1.12 (0.60–2.08) | ||
| Respondent education | ||||||
| None | 138/288 | 48.1% | Ref | 0.984 | Ref | 0.854 |
| Primary | 30/63 | 48.5% | 1.02 (0.58–1.80) | 0.87 (0.46–1.64) | ||
| Secondary or higher | 50/106 | 46.5% | 0.94 (0.57–1.54) | 0.80 (0.46–1.38) | ||
| Arabic or other | 4/9 | 42.3% | 0.79 (0.18–3.49) | 0.74 (0.17–3.26) | ||
| Know everyone is at risk | ||||||
| No | 60/120 | 51.3% | Ref | 0.390 | Ref | 0.329 |
| Yes | 162/346 | 46.4% | 0.82 (0.53–1.29) | 0.79 (0.49–1.27) | ||
| Know ITNs protect | ||||||
| No | 62/128 | 48.4% | Ref | 0.844 | Ref | 0.943 |
| Yes | 160/338 | 47.4% | 0.96 (0.63–1.47) | 0.98 (0.63–1.54) | ||
| U5 slept under ITN | ||||||
| No | 58/118 | 48.8% | Ref | 0.779 | Ref | 0.829 |
| Yes | 164/348 | 47.3% | 0.94 (0.61–1.44) | 0.95 (0.60–1.50) | ||
| Knowledge ACTs | ||||||
| No | 88/187 | 46.9% | Ref | 0.805 | Ref | 0.866 |
| Yes | 134/279 | 48.2% | 1.05 (0.70–1.57) | 1.04 (0.67–1.60) | ||
| Child age (months) | ||||||
| 0–23 months | 60/135 | 43.9% | Ref | 0.276 | Ref | 0.270 |
| 24–59 months | 162/331 | 49.2% | 1.24 (0.84–1.81) | 1.26 (0.83–1.90) | ||
| Prompt treatment (< 24 h) | ||||||
| No | 102/207 | 49.5% | Ref | 0.744 | Ref | 0.150 |
| Yes | 119/258 | 45.9% | 1.05 (0.79–1.39) | 1.25 (0.92–1.70) | ||
| Public health facility | ||||||
| No | 28/57 | 48.3% | Ref | 0.926 | Ref | 0.951 |
| Yes | 194/409 | 47.6% | 0.97 (0.55–1.74) | 0.98 (0.53–1.81) | ||
| ACT within 24 h | ||||||
| No | 85/154 | 55.0% | Ref | 0.047 | Ref | 0.015 |
| Yes | 137/312 | 44.1% | 0.65 (0.42–0.99) | 0.55 (0.34–0.89) | ||
Data for ACT duration was available for 466 children out of the 469 that received ACT
Discussion
The pathway to effective malaria case management depends on timely access to ACT and patient (or caregiver) adherence to treatment regimens. ACT has been recommended as the first-line treatment in Sierra Leone since 2004; however, in 2012, uptake remained suboptimal. To further investigate access to ACT and adherence to treatment guidelines in Sierra Leone, a secondary analysis of the national 2012 mKAP survey was undertaken. In a previous nationwide survey conducted in 2010, only 19.2% of febrile children received ACT, and only 50.3% received any anti-malarial within 24 h [14]. Assuming that a large proportion of febrile illness in children under five in Sierra Leone is due to malaria, these results suggest that challenges to delivering prompt and effective malaria treatment still remained in 2012.
In 2012, most children under-five with fever in the 2 weeks before the survey did not receive an artemisinin-based combination. ACT access varied geographically and was highest in the eastern region of the country. Similar to findings reported from Thailand, Kenya, and Uganda [29–31], this study found that children were more likely to receive ACT if their caregiver had prior knowledge of ACT and ITNs, suggesting that health education interventions could improve patient access to ACT. Additionally, this analysis found that seeking care from a public health facility doubled the odds of receiving ACT, suggesting that price or limited availability may impact access to ACT in the private sector. This is similar to other studies in sub-Saharan Africa, which have reported improved access to ACT for children treated in the public sector [32, 33].
This study also demonstrates a difference in access based on age, with older children (2–4 years) more likely to receive ACT than younger children (< 2 years). While removing user-fees removes the cost barrier of accessing care for individuals, it can strain weak health systems when demand increases. Higher patient loads require more resources to provide adequate service delivery. Although Sierra Leone had a relatively high availability of amodiaquine + artesunate (the ACT of choice at the time) compared to other post-conflict countries [34], the number of infant doses has often been insufficient due to improper forecasting and provision of pediatric formulations [35, 36]. Such challenges could be even more significant with the recent Ebola outbreak and ongoing pandemic [7, 8].
Of those febrile children who received artemisinin-based combination, less than half completed the recommended 3-day course of treatment (47.6%). However, no significant association between knowledge of ACT, malaria, or prevention practices and adherence was found, despite suggestions that patient knowledge, attitudes, and beliefs may be strong predictors of adherence [15, 31, 37–40]. Although Bruxvoort et al. reported that age, higher household income, higher education level, malaria knowledge, and treatment-seeking behavior are factors facilitating anti-malarial adherence [41], none of the a priori socioeconomic or demographic factors were associated with adherence in this study.
Unlike the findings reported for access, this analysis found no association between the source of care and treatment adherence. Unexpectedly, poor adherence to ACT was associated with accessing ACT promptly (within 24 h from the onset of symptoms). Lemma et al.. reported similar results; participants that delayed 1 day before seeking treatment were more adherent than those seeking prompt treatment (OR: 5.39; 95% CI 1.83–15.88) [37]. In contrast, a study in Uganda reported that prompt access to ACT was associated with higher treatment adherence [30]. Similarly, patients in Kenya seeking treatment greater than 1 day after the start of fever were 27% less likely to be adherent [31]. Given these mixed results, the association between prompt treatment for fever and lower ACT adherence should be interpreted with caution. Those accessing treatment early may have had lower parasite loads, which was cleared more quickly, resulting in fewer symptoms and possibly lower treatment adherence. Moreover, as the mKAP survey did not capture information on confirmatory malaria diagnosis, the child may have had a non-malaria febrile illness, and their symptoms may have resolved at the same time as receiving ACT, thus leading the caregiver to discontinue the treatment.
National cross-sectional household surveys such as the Demographic Health Survey (DHS), Multiple Indicator Cluster Survey (MICS), and the Malaria Indicator Survey (MIS) routinely collect information on the treatment of fever. These surveys including questions on treatment-seeking behavior for fever, medications received for that fever, and how soon after the onset of symptoms, the treatment was initiated. Additionally, since 2013, most surveys have gathered information on whether a blood test was received. However, the test question is not malaria-specific, nor is information on the test result collected due to concerns about the reliability of the data [42, 43]. Without this vital specific information, caution should be taken when interpreting results on fever case management from these surveys, as these data would represent the treatment of fever and not necessarily malaria. Ashton et al.. recently found that caregiver recall surrounding testing and diagnosis to be valid; therefore, the recommendation regarding confirmed malaria cases may need to be reviewed [44].
The fever case management sections of these national surveys could be expanded to include questions related to ACT treatment adherence, such as duration and/or completion of treatment, as was done in this study. Including these additional questions would allow a population-level estimate of adherence to be measured, as well as information on access to ACT, providing a more complete picture of the malaria treatment pathway. Collecting ACT adherence data through national surveys would be simple to implement, sustainable, and cost-effective. However, the use of national surveys to assess ACT adherence has several limitations: (1) the method assumes that respondents know and recognize which anti-malarial or artemisinin-based combination was prescribed for their child; (2) the data is relying on self-reported outcomes; and (3) unless surveys collect information about diagnostic testing specifically for malaria along with those test results, then the utility of adherence data would be limited as it would apply only to children with fever who may or may not have malaria.
Although this was a national survey, which allows generalizability of the findings to the entire country, there were some limitations. First, the data presented here were collected in 2012. Despite the delay in reporting these results, ACT adherence at the national level remains unknown in 2020, and factors impacting access and adherence to ACT have yet to be evaluated in Sierra Leone. Second, the study cannot provide a causal relationship between improved access and government programs rolled out to improve access to health services. However, the successful implementation of the FHCI in certain districts may have contributed to better access to medicines and services [19]. Third, the analysis was limited to the variables collected and may not have captured all the factors plausibly associated with receiving or completing treatment with ACT. In particular, this secondary analysis did not contain information on ACT and malaria rapid diagnostic tests stock-outs, confirmatory diagnosis of malaria, the quality of care at the health facility (including if the health worker provided information on how to administer the medication), or treatment completion. Additionally, questions on the number of tablets taken or whether treatment was completed were not included in this survey and would have contributed to a more precise quantification of adherence. Finally, although the sample size was large, it may not have provided the optimal power needed to detect associations between adherence and the a priori socioeconomic or demographic factors identified.
Conclusions
This study demonstrates that poor access and adherence to ACT remained key challenges to ensuring effective malaria case management in Sierra Leone in 2012 and continues to be a challenge in the face of public health emergencies such as Ebola and COVID-19. While efforts have been made to improve access to key health services in Sierra Leone, such as malaria treatment, further emphasis on ACT adherence is still needed. Optimizing the supply chain, implementation of the Free Health Care Initiative, and scaling up malaria communication campaigns to include messages on adherence could improve malaria treatment effectiveness in Sierra Leone. Finally, national household surveys could be expanded to capture key indicators on ACT access and adherence to help guide malaria case management in the future.
Acknowledgements
The Authors thank the initial study team for their hard work in completing the initial survey. In particular, we thank the CRS program manager Nancy Mansary and our college at Statistics Sierra Leone Sahr Yambasu for leading the field teams and assisting with the sampling frame. We are also very grateful for the support of the National Malaria Control Programme and the district health teams. Finally, we are grateful for the participants for agreeing to take part in the initial study.
Abbreviations
- ACT
Artemisinin-based combination therapy
- AL
Artemether–lumefantrine
- AOR
Adjusted odds ratio
- AQAS
Fixed-dose combination amodiaquine–artesunate
- AQ+AS
Co-packaged amodiaquine plus artesunate
- CRS
Catholic Relief Services
- DHS
Demographic Health Survey
- FHCI
Free Health Care Initiative
- GFATM
The Global Fund to Fight AIDS, Tuberculosis, and Malaria
- ITN
Insecticide-treated net
- KAP
Knowledge, attitudes, and practices
- MIS
Malaria Indicator Survey
- MICS
Multiple Indicator Cluster Survey
- mKAP
Malaria Knowledge, Attitudes, and Practices Survey
- NMCP
National Malaria Control Programme
- OR
Odds ratio
- PCA
Principal component analysis
- PPS
Probability proportional to size
- PSU
Primary sampling units
- WHO
World Health Organization
- 95% CI
95% confidence interval
Authors’ contributions
KB was the principal investigator of the initial study and subsequent secondary analysis. KB, EBD, SJS were involved in the initial survey design, implementation, and data analysis. KB conceived, designed, conducted the secondary analysis, interpretation, and first draft of the paper. ELW provided essential statistical expertise during the design, analysis, and interpretation. SS provided overall guidance and a critical review of the first draft. KB, ELW, EBD, SJS, DC, and SS revised and edited the manuscript. All authors read and approved the final manuscript.
Funding
The initial survey was funded by The Global Fund to Fight AIDS, Tuberculosis, and Malaria. KB was supported for the analysis and writing of this paper by the American Association of University Women (AAUW) and the NIH Research Training Grant# D43009340 funded by the NIH Fogarty International Center, NHBLI, NINDS, NCI, NINR, NIAID, and NIEHS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
The data used for this study were made available by Catholic Relief Services, specifically for this analysis. Data are available from the authors upon reasonable request and with permission of Catholic Relief Services.
Ethics approval and consent to participate
The original survey protocol was reviewed and approved by the Sierra Leone Ethics and Scientific Review Committee. All participants provided informed consent at the time of the mKAP interview. Catholic Relief Services and the National Malaria Control Program granted permission to use the survey data for this secondary analysis. The LSHTM Research Ethics Committee approved the secondary analysis protocol.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . World malaria report 2017. Geneva: World Health Organization; 2017. [Google Scholar]
- 2.WHO . World malaria report 2018. Geneva: World Health Organization; 2018. [Google Scholar]
- 3.WHO . World malaria report 2019. Geneva: World Health Organization; 2019. [Google Scholar]
- 4.WHO . Guidelines for the treatment of malaria. Third. Geneva: World Health Organization; 2015. [PubMed] [Google Scholar]
- 5.Chuma J, Okungu V, Molyneux C. Barriers to prompt and effective malaria treatment among the poorest population in Kenya. Malar J. 2010;9:144. doi: 10.1186/1475-2875-9-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quaglio G, Tognon F, Finos L, Bome D, Sesay S, Kebbie A, et al. Impact of Ebola outbreak on reproductive health services in a rural district of Sierra Leone: a prospective observational study. BMJ Open. 2019;9:e029093. doi: 10.1136/bmjopen-2019-029093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.WHO . The potential impact of health service disruptions on the burden of malaria: a modelling analysis for countries in sub-Saharan Africa. Geneva: World Health Organization; 2020. [Google Scholar]
- 8.Moses F, Tamang D, Denisiuk O, Dumbuya U, Hann K, Zachariah R. Management of malaria in children with fever in rural Sierra Leone in relation to the 2014–2015 Ebola outbreak. Public Health Action. 2017;7:22-6. doi: 10.5588/pha.16.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunbar NK, Richards EE, Woldeyohannes D, Van den Bergh R, Wilkinson E, Tamang D, et al. Knockdown and recovery of malaria diagnosis and treatment in Liberia during and after the 2014 Ebola outbreak. Public Health Action. 2017;7:76–81. doi: 10.5588/pha.16.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Banek K, Lalani M, Staedke SG, Chandramohan D. Adherence to artemisinin-based combination therapy for the treatment of malaria: a systematic review of the evidence. Malar J. 2014;13:7. doi: 10.1186/1475-2875-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.malERA Consultative Group on Health Systems and Operational Research A research agenda for malaria eradication: health systems and operational research. PLoS Med. 2011;8:e1000397. doi: 10.1371/journal.pmed.1000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Government of Sierra Leone Ministry of Health and Sanitation . Sierra Leone Malaria Control Strategic Plan 2016–2020: “access to malaria control interventions for all”. Freetown: Government of Sierra Leone Ministry of Health and Sanitation; 2015. [Google Scholar]
- 13.Checchi F, Roddy P, Kamara S, Williams A, Morineau G, Wurie AR, et al. Evidence basis for antimalarial policy change in Sierra Leone: five in vivo efficacy studies of chloroquine, sulphadoxine–pyrimethamine and amodiaquine. Trop Med Int Health. 2005;10:146–53. doi: 10.1111/j.1365-3156.2004.01367.x. [DOI] [PubMed] [Google Scholar]
- 14.UNICEF . Sierra Leone Multiple Indicator Cluster Survey 4 (MICS4) New York: UNICEF; 2011. [Google Scholar]
- 15.Gerstl S, Dunkley S, Mukhtar A, Baker S, Maikere J. Successful introduction of artesunate combination therapy is not enough to fight malaria: results from an adherence study in Sierra Leone. Trans R Soc Trop Med Hyg. 2010;104:328–35. doi: 10.1016/j.trstmh.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 16.National Malaria Control Programme (NMCP) [Sierra Leone], Statistics Sierra Leone,University of Sierra Leone, Catholic Relief Services, and ICF International . Sierra Leone malaria indicator survey. Freetown: National Malaria Control Programme (NMCP) [Sierra Leone]; 2016. [Google Scholar]
- 17.Witter S, Brikci N, Harris T, Williams R, Keen S, Mujica A, et al. The Sierra Leone Free Health Care Initiative (FHCI): process and effectiveness review (Report). Health and Education Advice & Resource Team, 2016.
- 18.National Malaria Control Programme (NMCP) [Sierra Leone] Guidelines for case management of malaria. Freetown: Government of Sierra Leone Ministry of Health and Sanitation; 2015. [Google Scholar]
- 19.Witter S, Brikci N, Harris T, Williams R, Keen S, Mujica A, et al. The free healthcare initiative in Sierra Leone: evaluating a health system reform, 2010–2015. Int J Health Plann Manag. 2018;33:434–48. doi: 10.1002/hpm.2484. [DOI] [PubMed] [Google Scholar]
- 20.Statistics Sierra Leone - SSL, ICF International . Sierra Leone Demographic and Health Survey 2013. Freetown: Statistics Sierra Leone - SSL; 2014. [Google Scholar]
- 21.Statistics Sierra Leone - SSL, ICF Macro . Sierra Leone Demographic and Health Survey 2008. Calverton: Statistics Sierra Leone - SSL; 2009. [Google Scholar]
- 22.Catholic Relief Services [Sierra Leone]. National Malaria Control Programme (NMCP) [Sierra Leone], Statistics Sierra Leone. Sierra Leone Malaria Knowledge, Attitudes and Practices (KAP) Study Final Report. Freetown, Sierra Leone; 2012.
- 23.Statistics Sierra Leone (SSL) Sierra Leone population and housing census. Freetown: Statistics Sierra Leone; 2004. [Google Scholar]
- 24.Roll Back Malaria, Measure Evaluation, USAID, UNICEF, WHO, MACEPA, CDC . MACEPA, CDC. Guidelines for core population-based indicators. Calverton: MEASURE Evaluation; 2009. [Google Scholar]
- 25.Mendoza G, Okoko L, Morgan G, Konopka S. USAID mHealth Compendium, Volume Two. African Strategies for Health project. USA: Management Sciences for Health, Arlington; 2013. [Google Scholar]
- 26.Onyango EO, Ayodo G, Watsierah CA, Were T, Okumu W, Anyona SB, et al. Factors associated with non-adherence to Artemisinin-based Combination Therapy (ACT) to malaria in a rural population from holoendemic region of western Kenya. BMC Infect Dis. 2012;12:143. doi: 10.1186/1471-2334-12-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watsierah CA, Jura WGZO, Raballah E, Kaseje D, Abong’o B, Ouma C. Knowledge and behaviour as determinants of anti-malarial drug use in a peri-urban population from malaria holoendemic region of western Kenya. Malar J. 2011;10:99. doi: 10.1186/1475-2875-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vyas S, Kumaranayake L. Constructing socioeconomic status indices. how to use principal components analysis. Health Policy Plan. 2006;21:459–68. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 29.Khantikul N, Butraporn P, Kim HS, Leemingsawat S, Tempongko MASB, Suwonkerd W. Adherence to antimalarial drug therapy among vivax malaria patients in northern Thailand. J Health Popul Nutr. 2009;27:4–13. doi: 10.3329/jhpn.v27i1.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalyango JN, Rutebemberwa E, Karamagi C, Mworozi E, Ssali S, Alfven T, et al. High adherence to antimalarials and antibiotics under integrated community case management of illness in children less than five years in Eastern Uganda. PLoS One. 2013;8:e60481. doi: 10.1371/journal.pone.0060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawford H, Zurovac D, O’Reilly L, Hoibak S, Cowley A, Munga S, et al. Adherence to prescribed artemisinin-based combination therapy in Garissa and Bunyala districts, Kenya. Malar J. 2011;10:281. doi: 10.1186/1475-2875-10-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simba DO, Warsame M, Kakoko D, Mrango Z, Tomson G, Premji Z, et al. Who gets prompt access to artemisinin-based combination therapy? A prospective community-based study in children from rural Kilosa, Tanzania. PLoS One. 2010;5:e12104. doi: 10.1371/journal.pone.0012104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Littrell M, Gatakaa H, Evance I, Poyer S, Njogu J, Solomon T, et al. Monitoring fever treatment behaviour and equitable access to effective medicines in the context of initiatives to improve ACT access: baseline results and implications for programming in six African countries. Malar J. 2011;10:327. doi: 10.1186/1475-2875-10-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amuasi JH, Diap G, Nguah SB, Karikari P, Boakye I, Jambai A, et al. Access to artemisinin-combination therapy (ACT) and other anti-malarials: national policy and markets in Sierra Leone. PLoS One. 2012;7:e47733. doi: 10.1371/journal.pone.0047733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Médecins Sans Frontières Brussels. Full Prescription: better malaria treatment for more people, MSF’s experience; 2008. https://www.msf.org/full-prescription-better-malaria-treatment-more-people-msfs-experience. Accessed 2 Dec 2020.
- 36.Yeka A, Harris JC. Treating uncomplicated malaria in children: comparing artemisinin-based combination therapies. Curr Opin Pediatr. 2010;22:798–803. doi: 10.1097/MOP.0b013e32833fac44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lemma H, Lofgren C, San Sebastian M. Adherence to a six-dose regimen of artemether-lumefantrine among uncomplicated Plasmodium falciparum patients in the Tigray Region, Ethiopia. Malar J. 2011;10:349. doi: 10.1186/1475-2875-10-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anyanwu P, Fulton J, Paget T, Evans E. Socioeconomic determinants of antimalarial drug use behaviours: a systematic review. J Community Public Health Nurs. 2016;2:2. [Google Scholar]
- 39.Gore-Langton GR, Alenwi N, Mungai J, Erupe NI, Eves K, Kimwana FN, et al. Patient adherence to prescribed artemisinin-based combination therapy in Garissa County, Kenya, after three years of health care in a conflict setting. Malar J. 2015;14:125. doi: 10.1186/s12936-015-0645-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steiner JF. Rethinking adherence. Ann Intern Med. 2012;157:580–5. doi: 10.7326/0003-4819-157-8-201210160-00013. [DOI] [PubMed] [Google Scholar]
- 41.Bruxvoort K, Goodman C, Kachur SP, Schellenberg D. How patients take malaria treatment: a systematic review of the literature on adherence to antimalarial drugs. PLoS One. 2014;9:e84555. doi: 10.1371/journal.pone.0084555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Survey and Indicator Task Force of the Roll Back Malaria Monitoring & Evaluation Reference Group (RBM MERG). MEASURE Evaluation, MEASURE DHS, President’s Malaria Initiative, Roll Back Malaria Partnership, UNICEF, World Health Organization. Household Survey Indicators for Malaria Control. 2018.
- 43.Eisele TP, Silumbe K, Yukich J, Hamainza B, Keating J, Bennett A, et al. Measuring coverage in MNCH: accuracy of measuring diagnosis and treatment of childhood malaria from household surveys in Zambia. PLoS Med. 2013;10:e1001417. doi: 10.1371/journal.pmed.1001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashton RA, Doumbia B, Diallo D, Druetz T, Florey L, Taylor C, et al. Measuring malaria diagnosis and treatment coverage in population-based surveys: a recall validation study in Mali among caregivers of febrile children under 5 years. Malar J. 2019;18:3. doi: 10.1186/s12936-018-2636-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used for this study were made available by Catholic Relief Services, specifically for this analysis. Data are available from the authors upon reasonable request and with permission of Catholic Relief Services.