Abstract
Purpose:
The purpose of the study is to describe visual and anatomic outcomes of 5774nm micropulse laser photocoagulation in eyes with either treatment-naïve or refractory diabetic macular edema (DME) at 3 months.
Methods:
This was a prospective case series that recruited 23 consecutive patients (33 eyes) with center-involved DME that was either treatment-naïve or had not responded to prior treatment. Micropulse therapy was performed with the Easy Ret 577 (Quantel Medical, Cournon d’Auvergne, France) diode laser in a high-density manner in eyes with treatment-naïve or refractory DME. The primary outcome was the change of best-corrected visual acuity (BCVA; logMAR) at 1 and 3 months. Secondary outcomes were changes in the central macular thickness (CMT), thickness area, macular volume, and macular capillary leakage at 1 and 3 months.
Results:
There were no significant changes in BCVA at 3 months, with mean ± standard deviation (SD) of −0.08 ± 0.01 (p = 0.228) and + 0.01 ± 0.01 (p = 0.969) for treatment-naïve and refractory groups, respectively. The change in CMT at 3 months was statistically but not clinically significant in the treatment-naïve group only (mean ± SD; –30 ± 130 µm; p = 0.011). The macular volume and area thickness change were not statistically significant (p = 0.173 and p = 0.148 for macular volume and area thickness, respectively) in the treatment-naïve group. There was no difference concerning the leakage area in both groups. No adverse events were reported.
Conclusion:
We concluded that micropulse 577nm laser therapy maintained the visual acuity and macular thickness at 3 months in both treatment-naïve and refractory DME.
Keywords: best-corrected visual acuity, central macular thickness, diabetic macular edema, 577 nm, macular capillary leakage, macular volume, photocoagulation, Subthreshold Micropulse Laser
Introduction
The main cause of visual loss in patients with diabetes mellitus (DM) is diabetic macular edema (DME), with a 10-year cumulative incidence of 20.1% and 25.4% for patients with type 1 and 2 diabetes, respectively.1–3 Argon laser treatment was the treatment of choice for many years, as it resulted in a 50% reduction in moderate visual loss.4,5 Intravitreal anti-vascular endothelial growth factor (VEGF) and steroids are the current treatment of choice, and their efficacy is demonstrated by several clinical trials; 6–11 however, DME is sometimes resistant to these therapies and may require other treatment modalities. Recent trials showed higher visual improvement rates (gain of ⩾10 letters in up to 44% of patients at 3 years) with conventional macular laser, suggesting that this procedure is still useful and can improve vision.12,13
The conventional laser uses continuous-wave energy producing a visible burn on the retina, which has several complications such as visual field loss, expanding scars, choroidal neovascularization, and subretinal fibrosis.5 In an attempt to improve the efficacy and reduce these adverse events, the subthreshold diode micropulse laser has been introduced.14 This strategy has two properties, which are shorter exposure time and a subvisible clinical endpoint, delivering the energy by dividing the beam into a series of short pulses (100–300 µs). Every single pulse has an “on and off” duration (duty cycle (DC)), enabling tissues to cool down before the next pulse.15,16 This is a tissue-sparing technique as it avoids protein coagulation and induces a controlled thermal elevation of the retina that theoretically stimulates the retinal pigment epithelium (RPE) only,17,18 reducing biomarkers (expressed by Müller cells) in treated DME eyes. In addition, a significantly smaller amount of pro-inflammatory molecules produced by the microglia-like macrophage inflammatory proteins (MIP)-1α, Fas ligand (FasL), regulated on activation normal T cell expressed and secreted (RANTES), and VEGF have also been found in eyes with DME treated with this technology, suggesting that this treatment strategy acts by reducing Müller cells activation.19,20 Friberg and Karatza15 first showed the clinical application of micropulse (810 nm) laser for DME. Since then, many studies have revealed the apparent efficacy of this method in stabilizing visual acuity and reducing macular edema.16,21–26 The 577 nm yellow wavelength is not absorbed by the xanthophyll pigment in the macula and is different from the 810 nm wavelength (used very widely) because it is better absorbed by RPE melanin.23 Its safety was also demonstrated, allowing retreatment sessions and application directly to the center of the fovea.22,27
The study aimed to evaluate short-term visual and anatomic effects of subthreshold 577 nm micropulse laser photocoagulation in eyes with treatment-naïve and refractory DME.
Patients and methods
Study design
This was a prospective case series conducted on 33 eyes of 23 consecutive patients diagnosed with either treatment-naïve or refractory DME at the retina service of the Mexican Institute of Ophthalmology, Queretaro, Mexico, from October 1, 2018, to July 2019. The study complied with the Declaration of Helsinki, and the ethics committee of the Mexican Institute of Ophthalmology approved the study (CEl/028-1/2019). We explained the purpose of our study to the patients, and all participants gave a written informed consent.
Eligibility and exclusion criteria
The eligibility criteria included patients with at least 18 years or more with type 1 or 2 DM, best-corrected visual acuity (BCVA) of 20/400 or better, and center-involved DME defined as a central macular thickness (CMT) of >250 but <700 µm measured by spectral-domain optical coherence tomography (SD-OCT; Revo NX; Optopol Technology SA, Zawiercie, Poland). Both treatment-naïve and refractory DME (two groups) were included. The latter was defined as less than five letters gain in visual acuity or reduction of less than 50 µm or 10% of retinal thickness on SD-OCT with persistent intraretinal and subretinal fluid measured 1 month after at least three ranibizumab (0.3 mg of Lucentis; Novartis, Pharma AG, Basel, Switzerland) injections that were given at monthly intervals.7,28 Patients with any level of non-proliferative diabetic retinopathy or proliferative diabetic retinopathy with adequate panretinal photocoagulation (PRP) and no signs of disease activity determined by fluorescein angiography (FA) were also included. Exclusion criteria included monocular eyes, chronic renal failure or renal transplant because of diabetic nephropathy, glycated hemoglobin (HbA1c) of more than 10%, vitreomacular traction syndrome, epiretinal membrane, PRP within 4 months before the treatment, and intraocular surgery within 6 months, including cataract or vitreoretinal operation. Patients with other retinal vascular diseases, rubeosis iridis, severe glaucoma, high-risk proliferative diabetic retinopathy, poor dilation, increased foveal avascular zone, or any condition that could interfere with OCT measurement or visual acuity were also excluded.
Subjects, follow-up, and measure outcome
Thirty-three eyes of 23 patients with a diagnosis of DME were included. Twenty-two (66.7%) were female. Mean ± standard deviation (SD) age was 61 ± 8.9 (range = 39–77) years and mean ± SD of HbA1c was 8.7 ± 0.8 (range = 7–9.9%). Baseline demographic and clinical characteristics among groups (15 and 18 eyes with treatment-naïve and refractory DME respectively) are described in Table 1.
Table 1.
Baseline characteristics among groups (N = 33).
| Variable | Mean ± SD | p a | |
|---|---|---|---|
| Treatment-naïve (n = 18) | Refractory (n = 15) | ||
| Age (years) | 59 ± 7.6 | 63 ± 10.3 | 0.308 |
| Sex, n (%) | |||
| Female | 13 (72.2) | 9 (60) | 0.458 |
| Male | 5 (27.8) | 6 (40) | 0.425 |
| HbA1c (%) | 7.8 ± 0.5 | 7.6 ± 0.9 | 0.457 |
| Lens status, n (%) | 0.678 | ||
| Phakic | 12 (66.7) | 11 (73.3) | |
| Pseudophakic | 6 (33.3) | 4 (26.6) | |
| DME type, n (%) | 0.665 | ||
| Cystoid macular edema | 14 (77.8) | 13 (86.7) | |
| Neuroepithelial detachment | 4 (22.2) | 2 (13.3) | |
| DR, n (%) | 0.85 | ||
| No DR | 0 (0) | 1 (6.7) | |
| Mild NPDR | 1 (5.6) | 1 (6.7) | |
| Moderate NPDR | 1 (5.6) | 0 (0) | |
| PDR with previous laser | 16 (88.9) | 13 (86.7) | |
| Laser parameters | |||
| Number of spots | 525.9 ± 197.5 | 489.4 ± 277.3 | 0.662 |
| Fluence, J/cm2 | 28.6 ± 8 | 26.5 ± 10 | 0.508 |
| Power, mW | 626.5 ± 135 | 670 ± 187.4 | 0.445 |
DME, diabetic macular edema; DR, diabetic retinopathy; HbA1c, glycosylated hemoglobin; J/cm2, Joule/square centimeters; LogMAR, logarithm of the minimum angle of resolution; µm, micrometer; mm3, cubic millimeter; mW, milli watt; n, number; NPDR, non-proliferative diabetic retinopathy; %, percentage; PDR, proliferative diabetic retinopathy; SD, standard deviation.
Student’s T test for quantitative variables and χ2 and Fisher’s exact test for qualitative variables, as appropriate.
Baseline ophthalmic examination included measurement of the BCVA by a Snellen chart (converted to the logarithm of the minimum angle of resolution (logMAR) for statistical analysis), complete ophthalmologic examination, FA, and SD-OCT. Angiograms were provided by the fundus camera (Zeiss Fundus Camera FF 450 plus, Jena, Germany). OCT evaluation was performed with the SD-OCT (Revo NX; Optopol Technology SA). CMT was described as the mean thickness of the neurosensory retina in the central 1 mm diameter area determined by the three-dimensional (3D) macular protocol, and high-definition linear scans were analyzed for the evaluation of structural macular changes. Area thickness and macular volume were defined as the overall average macular thickness and macular volume over the entire grid area which were obtained from the software output. Color fundus photography (45°) and FA were used to evaluate reduction of exudates and macular capillary leakage areas, respectively. One author (A.A.-G.) performed the OCT and FA analyses, whereas the two others (J.Q.-M. and D.V.-C.) performed the laser treatments, reducing the observer bias. All examinations were performed at baseline, 1 and 3 months.
Objectives
The primary endpoint was the change of BCVA (logMAR) at 1 and 3 months in both groups, whereas the secondary endpoints were changes of CMT, area thickness, and macular volume (determined by the OCT 3D protocol) at 1 and 3 months. Other secondary outcomes were changes in macular capillary leakage observed with FA. All adverse events were reported.
Laser treatment technique
All laser procedures were performed in a darkened room. At first, 0.8% tropicamide and 5% phenylephrine (T-P Ofteno©, Sophia Laboratories, Guadalajara, Mexico) were used to dilate the pupil 20 minutes before the procedure. All eyes were anesthetized with topical 0.5% tetracaine hydrochloride (Ponti-Ofteno©; Sophia Laboratories) drops. Volk Area Centralis contact lens (Volks Optical Inc., Mentor, Ohio, USA) was used in all the patients. The eyes were treated with the Easy Ret 577 (Quantel Medical, Cournon d’Auvergne, France) diode laser. The program was set in subliminal mode (micropulse), and a first test laser burn (continuous-wave mode of 200 ms) with 100–150 µm spot size was applied outside the vascular arcade and then the power was increased until a mild visible laser burn was seen. The power was reduced to 50% (micropulse mode), and a 5% DC was used. The laser was delivered together in an 8 × 8 pattern mode with high density (0 µm of spacing) over the entire area of macular edema, including the foveal center and unthickened (200 µm) retina with no attempt to target or avoid microaneurysms. The laser treatment was performed once in both treatment-naïve and refractory DME group, without any retreatment sessions.
Rescue therapy
Rescue treatment (with intravitreal dexamethasone implant or anti-VEGFs injection as appropriate) was allowed in both groups if the CMT increased more than 200 μm from baseline at any point during the study or if a loss of more than one Snellen line occurred related to DME.
Statistics
Numerical data were expressed as measures of central tendency and dispersion while categorical variables as absolute and relative frequencies. Data were tested for normality using a Shapiro–Wilk test. Fisher’s exact and Student’s T test were used for comparison between the groups. The p value below 0.05 was considered statistically significant. Statistical analysis was performed using Stata® version 15.1 (StataCorp. 2015, Stata Statistical Software: Release 15, College Station, Texas, USA: StataCorp LP) and GraphPad Prism software Version 8.4.2 (GraphPad Software Inc., La Jolla, CA, USA).
Results
There were no statistically significant differences in baseline values of BCVA (p = 0.093), CMT (p = 0.208), macular volume (p = 0.901), and area thickness (p = 0.819). Similarly, HbA1c values (p = 0.457) and DME type (p = 0.423) were not statistically different between the both groups.
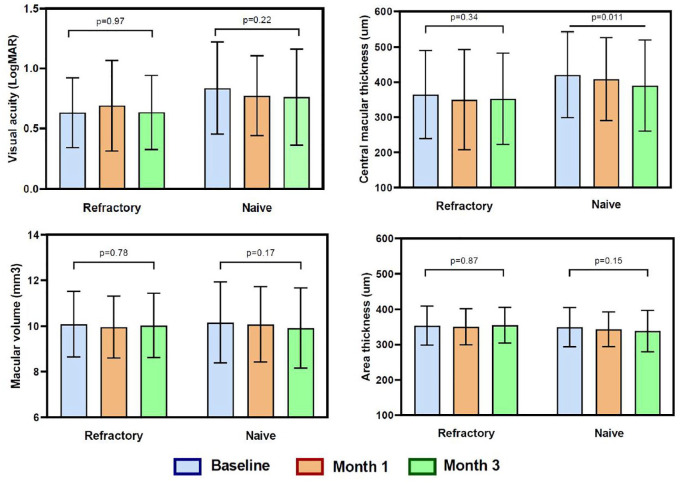
For eyes with treatment-naïve DME, baseline visual acuity improved from (mean ± SD) 0.84 ± 0.4 to 0.76 ± 0.4 at 3 months, and for eyes with refractory DME, visual acuity changed from 0.63 ± 0.3 to 0.64 ± 0.3. In both groups, there were no significant changes in BCVA at 3 months (mean ± SD: –0.08 ± 0.01, p = 0.228 and mean ± SD: + 0.01 ± 0.01, p = 0.969, for treatment-naïve and refractory groups, respectively) (Figure 1).
Figure 1.
Main outcome measures among groups (visual acuity in LogMAR, central macular thickness, area thickness, and macular volume) at baseline, 1, and 3 months. P values show the difference between baseline measure and the third month.
Baseline CMT improved from (mean ± SD) 420 ± 121 to 390 ± 130 µm at 3 months in the treatment-naïve group, and in the refractory group, CMT reduction was less (from 364 ± 125 to 352 ± 130 µm) at 3 months. The mean CMT change (mean ± SD) was −30 ± 125 and −12 ± 128 µm after 3 months in naïve and refractory groups, respectively (p = 0.011 and p = 0.34, respectively; Figure 1). The change in CMT at 3 months was statistically significant only in the treatment-naïve group (p = 0.011) (Figure 1).
The macular volume and area thickness showed no variation at 3 months in the refractory group, but a tendency to reduction was observed in the treatment-naïve group; however, the variations were not significant (p = 0.173 and p = 0.148, respectively in the treatment-naïve group). There was no significant difference concerning the leakage area and exudation after the treatment in both groups; however, a few eyes (two eyes) showed a minimal decrease in exudation (Figure 2).
Figure 2.
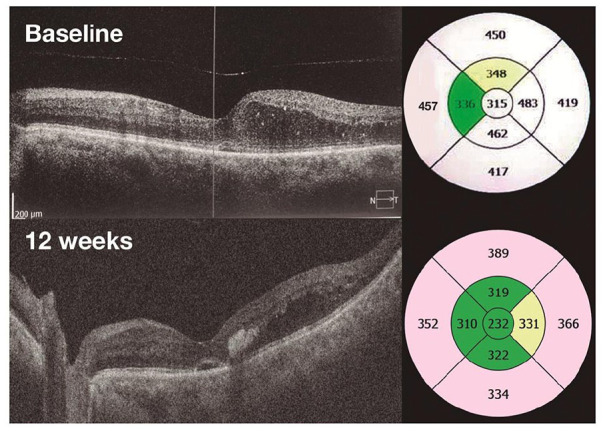
Optical coherence tomography (3D macular scan): left eye of a patient with center-involved treatment-naïve diabetic macular edema at baseline and good anatomical response at 3 months (central macular thickness reduction of −83 µm).
The difference among both groups was not significant for BCVA (p = 0.31) and CMT values (p = 0.41) at 3 months, as well as other parameters such as macular volume (p = 0.82) and area thickness (p = 0.38) at 3 months. Six eyes had moderate visual loss (two and four in the treatment-naïve and refractory group, respectively) at the third month and needed rescue therapy; no other severe adverse events related to treatment were reported. Fundus color photographs and FA images were compared, and no laser damage was detected (Figure 3). Other complications like choroidal neovascularization, intraretinal hemorrhage, foveal burn, and subretinal fibrosis were not observed. A slightly improved fluorescein leakage was observed in few eyes with treatment-naïve DME.
Figure 3.
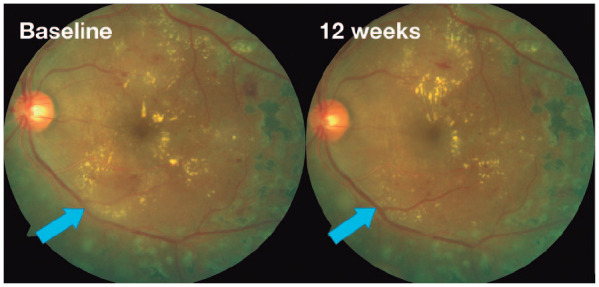
Fundus color photographs images: left eye of a patient with center-involved treatment-naïve diabetic macular edema at baseline and mild changes on fundus exam at 3 months (decrease on focal hard exudates, blue arrow).
Discussion
Subthreshold micropulse laser is a tissue-sparing modality to treat DME. Unlike conventional focal laser therapy, no standard protocol for laser settings in micropulse treatments has been elucidated.26,29 Theoretically, yellow (577 nm) wavelengths are ideal for macular tissue because they are better absorbed by melanin and hemoglobin and much less by macular xanthophylls. Its wavelength is outside the absorption spectrum of macular xanthophylls, which allows the treatment to be applied near or even directly to the fovea.21,24
Regarding our primary outcome (BCVA changes at 3 months; Figure 1), we found no significant and nonclinical change after 3 months of follow-up in both groups without any intergroup difference (mean ± SD: −0.08 ± 0.01, p = 0.228 and mean ± SD: + 0.01 ± 0.01, p = 0.969, for treatment-naïve and refractory patients, respectively), which could be interpreted as a stabilization of the visual function, with no changes during this short period of time. Many authors reported visual function data after the procedure, showing that it remained stable at 6 and 12 months of follow-up;15,22,29–36 these results are very similar to ours and to some reported clinical trials.24,37 From the morphological point of view, no visible lesions were detected on fundus examination and other imaging modalities (color fundus photos, FA, and SD-OCT). Our study did not demonstrate any clinically significant difference (despite the statistical difference in the treatment-naïve group at 12 weeks) in CMT (mean ± SD: −30 ± 125 and −12 ± 128 µm in the treatment-naïve and refractory groups, respectively; p = 0.011 and p = 0.34), thickness area, and macular volume at 3 months and between the groups, which is very similar to many studies.22,24,29–35,37–39 Although, by now, we know that an anatomical reduction of macular edema is not always followed by an improvement in visual acuity, the relationship between these two variables is weak.40 On the contrary, few reports showed a more important visual and anatomic response,25,29 but some of them did not use micropulse laser as a monotherapy.36,41
As this modality of treatment does not apparently produce any tissue damage, it can be repeated, which is very important. The time of retreatment period in most studies was about 2–4 months (Tables 2 and 3), and some studies reported that almost 77% of patients even needed two retreatment sessions.25,32,35 This observation could be related to the maximum effect of the procedure, during the first months. Regarding the safety of the procedure, we did not find any visible lesion in any patient, which could be related to the DC (5%) and wavelength (577 nm) used. Lavinsky and colleagues26 performed an analysis of structural retinal changes under several fluence reductions and reported that 30% of threshold energy does not create any defects on the tissue. Other authors showed an increased burn risk using an 810-nm sub-threshold laser with >5% of DC.21,29 We did not perform a quantitative analysis of the amount of leakage measured by FA because only very few eyes (two eyes) showed a small reduction of the leakage in the treatment-naïve group (Figure 4).
Table 2.
Previous non-comparative clinical studies..
| Author and year | Study design | Eyes | Laser wave length/spot/duty cycle | Power setting/power change | BCVA change; mean ± SD (LogMAR or letters) | CMT change; mean ± SD (microns/µm) | Last follow-up | Time to retreatment/% of eyes requiring retreatments |
|---|---|---|---|---|---|---|---|---|
| Latalska and colleagues42 | PCS | 75 | 577 nm/100 µm/5% | Titrated \(power × 2) |
+ 0.1 ± 0.3 | −154 ± 150 µm | 6 months | 2 months/—% |
| Abouhussein30 | PCS | 20 | 577 nm/200 µm/5% | Fixed 400 mW |
−0.1 ± 0.15 | −44 ± 40 µm | 6 months | — |
| Kwon and colleagues31 | RCS | 14 | 810 nm/100 µm /15% | Titrated | −0.08 ± 0.1 | −58.4 ± 103 µm | 7 months | 1–2 months/—% |
| Luttrull and Sinclair22 | RCS | 39 | 810 nm /125–200 µm/5% | Titrated | −0.03 ± 0.09 | −18.0 ± 58 µm | 13–16 months | — |
| Othman and colleagues32 | PCS | 187 | 810 nm/75–125 µm/15% | Fixed (800 ± 200 mW) |
+ 0.6 ± 0.3 | −42 ± 35 µm | 12 months | 3–4 months/80% |
| Ohkoshi and Yamaguchi33 | PCS | 43 | 810 nm/75 µm/15% | Titrated | Within 0.2 change | −51 ± 120 µm | 12 months | 3 months/18% |
| Friberg and Karatza15 | PCS | 40 | 810 nm/75–200 µm/30% | Titrated | Within 0.1 change | No | 6 months | No |
BCVA, best-corrected visual acuity; CMT, central macular thickness; —, no data; LogMAR, logarithm of the minimum angle of resolution; µm, micrometer; nm, nanometer; n, number; %, percentage; PCS, prospective case series; RCS, retrospective case series; SD, standard deviation.
Table 3.
Previous comparative clinical studies.
| Author and year | Study design | Eyes | Laser wave length/spot/duty cycle | Power setting/power change | BCVA change; mean ± SD (LogMAR or ETDRS letters) | CMT change; mean ± SD (microns/µm) | Last follow-up | Time to retreatment/% of eyes requiring retreatments |
|---|---|---|---|---|---|---|---|---|
| Akhlaghi and colleagues36, a | PCSa | 21 Anti-VEGF 21 SDM + anti-VEGF |
810 nm/200 µm/5% | Titrated | −0.19 ± 0.1 | −105 ± 100 µm | 3 months | — |
| Chhablani and colleagues29 | PCI | 10 CLP 10 SDM/5% 10 SDM/15% |
577 nm/100 µm/5% 577 nm/100 µm/15% |
Titrated /(–30%) |
−0.7 ± 6 letters +2.11 ± 2.5 letters |
−12.4 ± 36 µm + 0.6 ± 213 µm |
3 months | — |
| Fazel and colleagues37 | RCT | 34 CLP 34 SDM |
810 nm/75–125 µm/15% | Titrated/ (power × 2) |
−0.06 ± 0.3 | −18.0 ± 56 µm | 4 months | — |
| Vujosevic and colleagues34 | PCI | 27 Infrared 26 Yellow |
810 nm/125 µm/5% 577 nm/125 µm/5% |
Fixed 750 mW 250 mW |
−1.3 ± 4 letters –1.0 ± 4 letters |
−5 ± 40 µm −17 ± 40 µm |
6 months | 3 months/85–88% |
| Inagaki and colleagues41, b | PCIb | 24 Infrared 29 Yellow |
810 nm/200 µm/15% 577 nm/200 µm/15% |
Titrated /(–60%) |
−0.05 ± — –0.02 ± — |
−124 ± — µm −76 ± — µm |
12 months | 3 months/16% 3 months/3% |
| Venkatesh and colleagues38 | PCI | 22 CLP 23 SDM |
810 nm/125 µm/5% | Titrated/ (–50%) |
+0.02 ± 0.3 | −23 ± 55 µm | 6 months | — |
| Lavinsky and colleagues25 | RCT | 39 ND SDM 42 HD SDM |
810 nm/125 µm/15% | Titrated/ (+20%) |
−0.03 ± 0.22 –0.25 ± 0.31 |
−32 ± 107 µm −154 ± 157 µm |
12 months | 3–6 months/77–21% |
| Vujosevic and colleagues35 | PCI | 30 CLP 32 SDM |
810 nm/125 µm/5% | Fixed 750 mW |
+0.02 ± 0.1 | −46.6 ± 73 µm | 12 months | 3 months/— % |
| Figueira and colleagues24 | RCT | 40 CLP 44 SDM |
810 nm/125 µm/15% | Titrated | −6.6 letters | +41.9 ± 103.8 µm | 12 months | 4 months/— % |
| Laursen and colleagues39 | PCI | 11 CLP 12 SDM |
810 nm/125 µm/5% | Titrated/ (–50%) |
+0.9 (–8 to + 19) letters | +10 (–155 to + 66 µm) | 6 months | — |
Anti-VEGF, anti-vascular endothelial growth factor; BCVA, best-corrected visual acuity; CLP, conventional laser photocoagulation; CMT, central macular thickness; —, no data; ETDRS, Early Treatment Diabetic Retinopathy Study; HD, high density; LogMAR, logarithm of the minimum angle of resolution; µm, micrometer; ND, normal density; PCI, prospective comparative interventional; RCT, randomized clinical trial; SD, standard deviation; SDM, subthreshold diode laser micropulse.
Intravitreal Bevacizumab + subthreshold diode laser micropulse. bDirect focal conventional photocoagulation + subthreshold diode laser micropulse.
Figure 4.
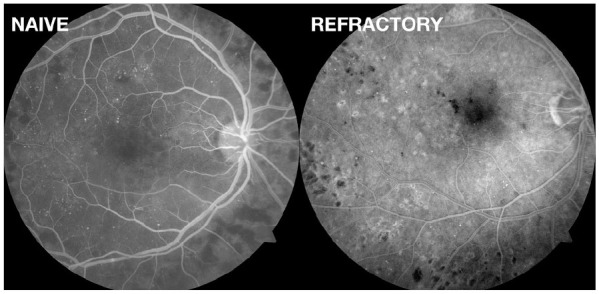
Fluorescein angiography images: right eyes of patients with treatment-naïve and refractory center-involved diabetic macular edema 3 months after the procedure showing the absence of retinal lesions (choroidal neovascularization, foveal burn, or subretinal fibrosis) in either of the eyes.
Regarding the wavelength used, some authors believe that the 810 nm laser is ideal for micropulse therapy because its absorption is maximum by the RPE, has less scattering and better penetration, and is not absorbed by hemoglobin (intraretinal hemorrhage, retinal vessels) or foveal pigment, nor absorbed or attenuated by the thickened retina. On the contrary, shorter wavelengths (yellow: 577 nm and green: 532 nm) could present an increased risk of inadvertent damage to the retinal tissue because of an increased scatter and media absorption, which will require a patient-specific adjustment for laser parameters that are hard to titrate because of the absence of a visible treatment endpoint. Besides, shorter wavelengths are more energetic, proportionally narrowing the therapeutic window, thus increasing the risk of retinal damage.16,18,21,22,35
Our study had several limitations, including its design, the small size, short duration, and the absence of controls. Another limitation is related to the inclusion of thicker macular thickness (>400 µm), which is not the ideal parameter to receive micropulse therapy. However, our results are consistent with the findings of other previous reports (Tables 2 and 3). To date, there is no consensus regarding dose-response on clinical studies that address better pulse fluence, energy, duration, and the ideal endpoint of injury. Therefore, proper laser application targeting invisible or sub-visible lesions is not well determined. Like our report, many studies22, 24, 29, 30, 32-34, 37, 39 concluded that micropulse therapy can stabilize the edema and visual acuity in patients with center-involved DME. Ultra-short pulse nanosecond lasers are also reported and deliver a fraction of the energy from the traditional continuous-wave lasers, selectively targeting the RPE with less damage to surrounding tissues. Clinical results are promising with this ultra-short modality.43–47
In cases in which therapy was switched for unresponsive patients, many observational studies used a minimum washout period of 1 month.28 Although we would expect to have lower but still persistent intravitreal levels from prior anti-VEGF doses in the refractory group, the absence of a real washout period affects our outcomes and this is another limitation. The impact of the significant additional effect from prior anti-VEGF therapy may be confounded by the direct effects of the micropulse laser treatment because these effects could not be estimated separately and therefore may bias our results. The aforementioned effect would be a combination of previous antiangiogenic therapy and laser treatment, not only the laser effect so there would be a potential bias for a greater anti-VEGF effect. However, we do not suspend treatment in patients with DME, whether they have naïve or refractory edema because persistent fluid can cause irreversible vision loss as a result of chronic tissue damage and permanent disruption of retinal architecture.48 A controlled trial is mandatory for the evaluation of the real effects of micropulse laser in this group of patients.
In an attempt to improve retinal structure and function, micropulse and subthreshold laser therapy also called “laser retinal restoration therapy” has become more popular.49 Molecular and clinical observations regarding this tissue-sparing modality have improved our understanding of the mechanisms related to laser therapy, replacing old concepts (requiring the need of a laser-induced retinal lesion) with the current ones of laser-induced stimulation and restoration.50
Recent studies related to the application of micropulse therapy beyond conventional indications (DME and central serous chorioretinopathy) have shown favorable clinical outcomes. Diseases such as open-angle glaucoma, hereditary retinal diseases, and age-related macular degeneration in which electrophysiological and perimetric improvement were observed could significantly expand the applications of retinal laser therapy,51–53 but validation by larger randomized trials are needed.
Our results revealed no statistical or clinical changes regarding BCVA and CMT at 3 months in patients with naïve and refractory center–involved DME. However, micropulse therapy treatment leads to no retinal scars or visible lesions. There is still a need for a randomized clinical trial comparing intravitreal anti-VEGF drugs, micropulse laser, and the combination of both to demonstrate its true long-term efficacy in eyes with naïve and refractory DME.
Footnotes
Conflict of interest statement: The authors declared no conflicts of interest. All authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Diego Alejandro Valera-Cornejo  https://orcid.org/0000-0001-5125-1342
https://orcid.org/0000-0001-5125-1342
Marlon García-Roa  https://orcid.org/0000-0003-1970-799X
https://orcid.org/0000-0003-1970-799X
Contributor Information
Diego Alejandro Valera-Cornejo, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Marlon García-Roa, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Jaime Quiroz-Mendoza, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Alejandro Arias-Gómez, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Paulina Ramírez-Neria, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Yolanda Villalpando-Gómez, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Veronica Romero-Morales, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
Renata García-Franco, Retina department, Instituto Mexicano de Oftalmología I.A.P., Santiago De Querétaro, Querétaro, México.
References
- 1. Bhagat N, Grigorian RA, Tutela A, et al. Diabetic macular edema: pathogenesis and treatment. Surv Ophthalmol 2009; 54: 1–32. [DOI] [PubMed] [Google Scholar]
- 2. Klein R, Klein BE, Moss SE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology 1995; 102: 7–16. [DOI] [PubMed] [Google Scholar]
- 3. Klein R, Knudtson MD, Lee KE, et al. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XXIII. The Twenty-Five-Year Incidence of Macular Edema in Persons with Type 1 Diabetes. Ophthalmology 2009; 116: 497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol Chic Ill 1960. 1985; 103: 1796–1806. [PubMed] [Google Scholar]
- 5. Early photocoagulation for diabetic retinopathy. ETDRS report number 9. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1991; 98: 766–785. [PubMed] [Google Scholar]
- 6. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- 7. Gonzalez VH, Campbell J, Holekamp NM, et al. Early and long-term responses to anti-vascular endothelial growth factor therapy in diabetic macular edema: analysis of protocol I data. Am J Ophthalmol 2016; 172: 72–79. [DOI] [PubMed] [Google Scholar]
- 8. Bressler NM, Beaulieu WT, Maguire MG, et al. Early response to anti-vascular endothelial growth factor and two-year outcomes among eyes with diabetic macular edema in protocol T. Am J Ophthalmol 2018; 195: 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Elman MJ, Aiello LP, Beck RW, et al. ; Diabetic Retinopathy Clinical Research Network. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010; 117: 1064–1077e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wells JA, Glassman AR, Ayala AR, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016; 123: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cai S, Bressler NM. Aflibercept, bevacizumab or ranibizumab for diabetic macular oedema: recent clinically relevant findings from DRCR.net protocol T. Curr Opin Ophthalmol 2017; 28: 636–643. [DOI] [PubMed] [Google Scholar]
- 12. Aiello LP, Edwards AR, Beck RW, et al. Factors associated with improvement and worsening of visual acuity 2 years after focal/grid photocoagulation for diabetic macular edema. Ophthalmology 2010; 117: 946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Three-year follow up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol 2009; 127: 245–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dorin G. Evolution of retinal laser therapy: minimum intensity photocoagulation (MIP). Semin Ophthalmol 2004; 19: 62–68. [DOI] [PubMed] [Google Scholar]
- 15. Friberg TR, Karatza EC. The treatment of macular disease using a micropulsed and continuous wave 810-nm diode laser. Ophthalmology 1997; 104: 2030–2038. [DOI] [PubMed] [Google Scholar]
- 16. Luttrull JK, Musch DC, Mainster MA. Subthreshold diode micropulse photocoagulation for the treatment of clinically significant diabetic macular oedema. Br J Ophthalmol 2005; 89: 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu AK, Merrill KD, Truong SN, et al. The comparative histologic effects of subthreshold 532- and 810-nm diode micropulse laser on the retina. Invest Ophthalmol Vis Sci 2013; 54: 2216–2224. [DOI] [PubMed] [Google Scholar]
- 18. Luttrull JK, Dorin G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: a review. Curr Diabetes Rev 2012; 8: 274–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Midena E, Micera A, Frizziero L, et al. Sub-threshold micropulse laser treatment reduces inflammatory biomarkers in aqueous humour of diabetic patients with macular edema. Sci Rep 2019; 9: 10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Midena E, Bini S, Martini F, et al. Changes of aqueous humor Müller cells’ biomarkers in human patients affected by diabetic macular edema after subthreshold micropulse laser treatment. Retina Phila Pa 2018; 40: 126–134. [DOI] [PubMed] [Google Scholar]
- 21. Luttrull JK, Sramek C, Palanker D, et al. Long-term safety, high-resolution imaging, and tissue temperature modeling of subvisible diode micropulse photocoagulation for retinovascular macular edema. Retina 2012; 32: 375–386. [DOI] [PubMed] [Google Scholar]
- 22. Luttrull JK, Sinclair SH. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 2014; 34: 2010–2020. [DOI] [PubMed] [Google Scholar]
- 23. Sivaprasad S, Prevost AT, Vasconcelos JC, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet Lond Engl 2017; 389: 2193–2203. [DOI] [PubMed] [Google Scholar]
- 24. Figueira J, Khan J, Nunes S, et al. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol 2009; 93: 1341–1344. [DOI] [PubMed] [Google Scholar]
- 25. Lavinsky D, Cardillo JA, Melo LAS, et al. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Invest Ophthalmol Vis Sci 2011; 52: 4314–4323. [DOI] [PubMed] [Google Scholar]
- 26. Lavinsky D, Sramek C, Wang J, et al. Subvisible retinal laser therapy: titration algorithm and tissue response. Retina 2014; 34: 87–97. [DOI] [PubMed] [Google Scholar]
- 27. Inagaki K, Ohkoshi K, Ohde S, et al. Comparative efficacy of pure yellow (577-nm) and 810-nm subthreshold micropulse laser photocoagulation combined with yellow (561-577-nm) direct photocoagulation for diabetic macular edema. Jpn J Ophthalmol 2015; 59: 21–28. [DOI] [PubMed] [Google Scholar]
- 28. Kodjikian L, Bellocq D, Mathis T. Pharmacological management of diabetic macular edema in real-life observational studies. BioMed Res Int 2018; 2018: 8289253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chhablani J, Alshareef R, Kim DT, et al. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol 2018; 18: 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Abouhussein M. Micropulse laser for diabetic macular edema. Delta J Ophthalmol 2016; 17: 167. [Google Scholar]
- 31. Kwon YH, Lee DK, Kwon OW. The short-term efficacy of subthreshold micropulse yellow (577-nm) laser photocoagulation for diabetic macular edema. Korean J Ophthalmol 2014; 28: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Othman IS, Eissa SA, Kotb MS, et al. Subthreshold diode-laser micropulse photocoagulation as a primary and secondary line of treatment in management of diabetic macular edema. Clin Ophthalmol 2014; 8: 653–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohkoshi K, Yamaguchi T. Subthreshold micropulse diode laser photocoagulation for diabetic macular edema in Japanese patients. Am J Ophthalmol 2010; 149: 133–139. [DOI] [PubMed] [Google Scholar]
- 34. Vujosevic S, Martini F, Longhin E, et al. Subthreshold micropulse yellow laser versus subthreshold micropulse infrared laser in center-involving diabetic macular edema: morphologic and functional safety. Retina 2015; 35: 1594–1603. [DOI] [PubMed] [Google Scholar]
- 35. Vujosevic S, Bottega E, Casciano M, et al. Microperimetry and fundus autofluorescence in diabetic macular edema: subthreshold micropulse diode laser versus modified early treatment diabetic retinopathy study laser photocoagulation. Retina 2010; 30: 908–916. [DOI] [PubMed] [Google Scholar]
- 36. Akhlaghi M, Dehghani A, Pourmohammadi R, et al. Effects of subthreshold diode micropulse laser photocoagulation on treating patients with refractory diabetic macular edema. J Curr Ophthalmol 2019; 31: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fazel F, Bagheri M, Golabchi K, et al. Comparison of subthreshold diode laser micropulse therapy versus conventional photocoagulation laser therapy as primary treatment of diabetic macular edema. J Curr Ophthalmol 2016; 28: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Venkatesh P, Ramanjulu R, Azad R, et al. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: a prospective, randomized study using multifocal electroretinography. Photomed Laser Surg 2011; 29: 727–733. [DOI] [PubMed] [Google Scholar]
- 39. Laursen ML, Moeller F, Sander B, et al. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol 2004; 88: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bressler NM, Odia I, Maguire M, et al. Association between change in visual acuity and change in central subfield thickness during treatment of diabetic macular edema in participants randomized to aflibercept, bevacizumab, or ranibizumab: a post hoc analysis of the protocol t randomized clinical trial. JAMA Ophthalmol 2019; 137: 977–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Inagaki K, Shuo T, Katakura K, et al. Sublethal photothermal stimulation with a micropulse laser induces heat shock protein expression in ARPE-19 cells. J Ophthalmol 2015; 2015: 729792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Latalska M, Prokopiuk A, Wróbel-Dudzińska D, et al. Subthreshold micropulse yellow 577 nm laser therapy of diabetic macular oedema in rural and urban patients of south-eastern Poland. Ann Agric Environ Med 2017; 24: 96–99. [DOI] [PubMed] [Google Scholar]
- 43. Casson RJ, Raymond G, Newland HS, et al. Pilot randomized trial of a nanopulse retinal laser versus conventional photocoagulation for the treatment of diabetic macular oedema. Clin Exp Ophthalmol 2012; 40: 604–610. [DOI] [PubMed] [Google Scholar]
- 44. Chidlow G, Shibeeb O, Plunkett M, et al. Glial cell and inflammatory responses to retinal laser treatment: comparison of a conventional photocoagulator and a novel, 3-nanosecond pulse laser. Invest Ophthalmol Vis Sci 2013; 54: 2319–2332. [DOI] [PubMed] [Google Scholar]
- 45. Chidlow G, Plunkett M, Casson RJ, et al. Investigations into localized re-treatment of the retina with a 3-nanosecond laser. Lasers Surg Med 2016; 48: 602–615. [DOI] [PubMed] [Google Scholar]
- 46. Jobling AI, Guymer RH, Vessey KA, et al. Nanosecond laser therapy reverses pathologic and molecular changes in age-related macular degeneration without retinal damage. FASEB J 2015; 29: 696–710. [DOI] [PubMed] [Google Scholar]
- 47. Pelosini L, Hamilton R, Mohamed M, et al. Retina rejuvenation therapy for diabetic macular edema: a pilot study. Retina 2013; 33: 548–558. [DOI] [PubMed] [Google Scholar]
- 48. Lang GE, Berta A, Eldem BM, et al. Two-year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology 2013; 120: 2004–2012. [DOI] [PubMed] [Google Scholar]
- 49. Chhablani J, Roh YJ, Jobling AI, et al. Restorative retinal laser therapy: present state and future directions. Surv Ophthalmol 2018; 63: 307–328. [DOI] [PubMed] [Google Scholar]
- 50. Wells-Gray EM, Doble N, Ohr MP, et al. Structural integrity of individual cone photoreceptors after short-wavelength subthreshold micropulse laser therapy for diabetic macular edema. Ophthalmic Surg Lasers Imaging Retina 2018; 49: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Luttrull JK, Margolis BWL. Functionally guided retinal protective therapy for dry age-related macular and inherited retinal degenerations: a pilot study. Invest Ophthalmol Vis Sci 2016; 57: 265–275. [DOI] [PubMed] [Google Scholar]
- 52. Luttrull JK, Chang DB, Margolis BWL, et al. Laser resensitization of medically unresponsive neovascular age-related macular degeneration: efficacy and implications. Retina 2015; 35: 1184–1194. [DOI] [PubMed] [Google Scholar]
- 53. Luttrull JKK, Lum BJ, Kent D, et al. Improved VEP and visual fields following panmacular subthreshold diode micropulse laser (SDM) in open angle glaucoma. Invest Ophthalmol Vis Sci 2017; 58: 5849–5849.29141080 [Google Scholar]