Abstract
Background. Parkinson’s disease (PD) is neurodegenerative, causing motor, cognitive, psychological, somatic, and autonomic symptoms. Understanding PD patients’ preferences for novel neurostimulation devices may help ensure that devices are delivered in a timely manner with the appropriate level of evidence. Our objective was to elicit preferences and willingness-to-wait for novel neurostimulation devices among PD patients to inform a model of optimal trial design. Methods. We developed and administered a survey to PD patients to quantify the maximum levels of risks that patients would accept to achieve potential benefits of a neurostimulation device. Threshold technique was used to quantify patients’ risk thresholds for new or worsening depression or anxiety, brain bleed, or death in exchange for improvements in “on-time,” motor symptoms, pain, cognition, and pill burden. The survey elicited patients’ willingness to wait to receive treatment benefit. Patients were recruited through Fox Insight, an online PD observational study. Results. A total of 2740 patients were included and a majority were White (94.6%) and had a 4-year college degree (69.8%). Risk thresholds increased as benefits increased. Threshold for depression or anxiety was substantially higher than threshold for brain bleed or death. Patient age, ambulation, and prior neurostimulation experience influenced risk tolerance. Patients were willing to wait an average of 4 to 13 years for devices that provide different levels of benefit. Conclusions. PD patients are willing to accept substantial risks to improve symptoms. Preferences are heterogeneous and depend on treatment benefit and patient characteristics. The results of this study may be useful in informing review of device applications and other regulatory decisions and will be input into a model of optimal trial design for neurostimulation devices.
Keywords: medical devices, Parkinson’s disease, patient preferences, threshold technique
Parkinson’s disease (PD) is a progressive neurodegenerative disease, causing motor, cognitive, psychological, somatic, and autonomic symptoms. The average life expectancy for people with PD is 1 year less than those without PD, and incidence of PD is higher among men and individuals aged over 65 years.1,2 There is currently no cure for PD. Most PD treatments focus on alleviating motor symptoms, and PD patients indicate that there is a need to develop curative therapies and therapies that address additional symptoms.3 Novel neurostimulation devices target specific nerve structures to alleviate motor symptoms but can also contribute to depression, anxiety, brain bleed, and death. According to the Food and Drug Administration (FDA), understanding the personal experiences, preferences, and goals of patients is critical to clinical trial design, regulatory decision making, and treatment selection.4
Patient preference information (PPI) is defined as qualitative or quantitative statements of the relative desirability or acceptability to patients of specified alternatives or choices among outcomes or other attributes that differ among alternative health interventions.5,6 The FDA used PPI in support of recent approvals and label expansions for medical devices,7,8 and issued guidance about the submission of PPI in support of marketing applications.6 PPI may also provide valuable data for endpoint development and clinical trial design.9,10
Models of optimal clinical trial design have been proposed that use PPI to balance the urgency for new therapeutic options against potential risks posed by those therapies by adjusting clinical trial size and statistical significance thresholds to reflect the patient preferences.11–13 Such a model may result in smaller trials with less stringent significance threshold for patient populations with high unmet medical need who have high risk tolerance, and larger trials with more certainty about treatment outcomes for patient populations with fewer unmet needs and lower risk tolerance. Our objective was to elicit PD patients’ benefit-risk preferences for novel neurostimulation devices and their willingness to wait for development programs to be completed, including pivotal studies of efficacy and safety, to use as inputs into a model of optimal clinical trial design (S. E. Chaudhuri, PhD, unpublished data, 2019).
Methods
Survey Instrument
We used the threshold technique to elicit the tradeoffs patients would be willing to make between the benefits and risks of potential neurostimulation devices for PD along with the maximum time they would be willing to wait for a device offering different levels of benefit to become available. The five benefits included decreases in daily off-time, decreases in motor symptoms, decreases in PD pain, decreases in memory and thinking problems (whether due to PD or PD treatments), and decreases in the number of daily pills used to treat PD or the side effects of PD treatments. These benefits were determined to be important to patients (H. L. Benz, PhD, unpublished data, 2019). Each benefit was described as a 50% improvement from a baseline level reported by the patient. The three risks of harm were the risk of new or worsening depression and anxiety, the risk of brain bleed, and the risk of death. These harms reflected both the concerns of patients and the types of harms that FDA reviewers expected would be associated with neurostimulation devices. Starting values for these risks in the survey were determined primarily through consultation with FDA reviewers. Wait time was included as an additional burden to measure patient willingness to wait to receive the benefits of treatments in development. Therefore, the exercise included 20 possible pairwise tradeoffs (5 benefits × [3 risks of harm + wait time]; Table 1). Complete descriptions of the attributes are provided in Supplemental Appendix A.
Table 1.
Attributes in the Threshold Technique Scenarios
| Attribute | Benefit | Potential Threshold Values | |||
|---|---|---|---|---|---|
| Category | Label | Size of Benefit | Eligible Range of Baseline Values | Baseline Level | Alternate Levels |
| Benefits | Hours of “off-time” each day | 50% reduction in off-time from baseline | 0–13 hours | ||
| Severity of movement symptoms | 50% reduction in self-reported rating of average severity of movement symptoms during the past week | 3–10 rating | |||
| Severity of pain | 50% reduction in self-report rating of average pain severity during the past week | 3–10 rating | |||
| Difficulty thinking clearly, getting organized, or making plans | 50% reduction in self-reported difficulty thinking clearly, getting organized, or making plans during the past week | 3–10 rating | |||
| Number of pills you need to take | 50% reduction in self-reported number of pills or tablets taken each day to treat PD and the side effects of PD medicines | ≥3 pills per day | |||
| Risks of Harm | Risk of getting depression or anxiety after getting the device | 20% | 10% 15% 30% 40% |
||
| Risk of having bleeding in the brain after getting the device | 4% | 2% 3% 6% 8% |
|||
| Risk of dying within 1 year after getting the device | 2% | 1% 3% 4% |
|||
| Wait Time | Time until you get the device | 3 years | 1 year 2 years 5 years 6 years |
||
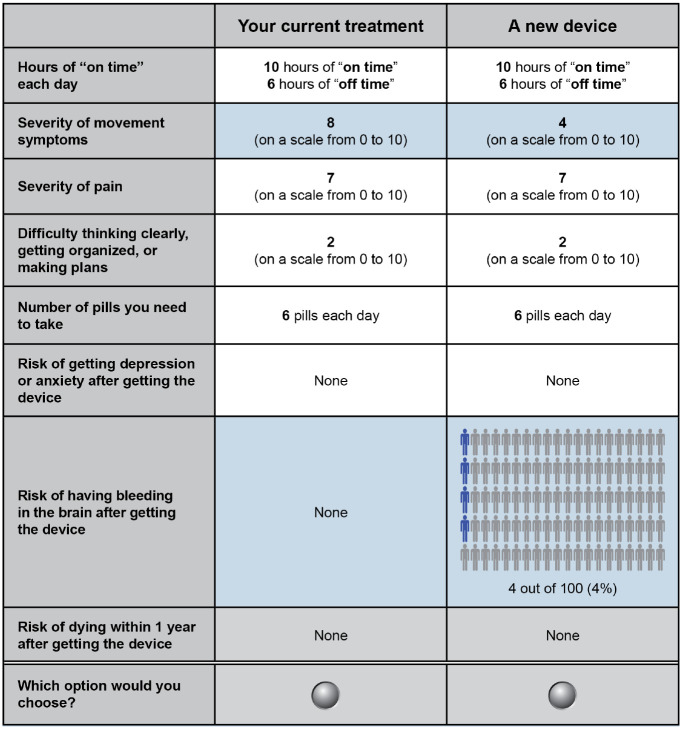
An example of a threshold technique question is presented in Figure 1. Using this technique, a patient is presented with two treatment options.14–16 Each option is defined by one or more treatment benefits and one or more treatment-related burdens (i.e., a risk of harm and/or wait time). The reference treatment typically represents no treatment, standard of care, or the patient’s current treatment. The target treatment offers some benefit relative to the reference treatment but is also associated with one or more additional burdens (i.e., risks of harm or wait time). If the patient chooses the reference treatment when presented with this initial choice, the target treatment is made more attractive in subsequent questions by decreasing the level of burden until the patient is willing to accept the target treatment. If the patient chooses the target treatment initially, the target treatment is made less attractive by increasing the level of burden until the patient is no longer willing to accept the target treatment. Thus, the level of burden at which a patient switches his or her choice represents the threshold level of burden that exactly offsets the treatment benefit.
Figure 1.
Example of a treatment choice question eliciting maximum risk of a brain bleed patients would accept in exchange for an improvement in motor symptoms.
The reference option was unique to each patient and defined by his or her self-reported levels of off-time each day, motor symptoms, pain, memory and thinking problems, and number of daily pills. The baseline number of hours of off-time was the difference between the number of waking hours per day (set at 16 hours) and the average number of hours of on-time (i.e., time without symptoms) each day at the time the survey was administered. Baseline levels of motor symptoms, PD pain, and memory and thinking problems were measured using an 11-point rating scale from 0 (no problems) to 10 (severe problems). Baseline daily pill burden was self-reported as the number of pills or tablets taken each day.
In Figure 1, the target treatment was defined as a neurostimulation device offering a 50% improvement from the patient’s baseline level for one benefit and a nonzero level of the risk of one harm. All other benefits were set to the patient’s baseline level in both the reference and target treatments. The risks of the two harms that were not included in that threshold series were set equal to zero for both the reference and target treatments.
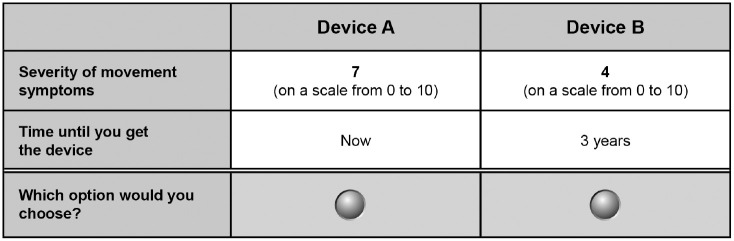
Tradeoffs between benefit and wait time were elicited separately. An example of a willingness-to-wait question is presented in Figure 2. The reference option in the willingness-to-wait questions was a device available today that would offer a one-unit improvement in symptom scale in a benefit from baseline. The target treatment was defined as a device that would be available at some time in the future that would offer a 50% improvement in the benefit from baseline. The wait time for the target treatment was adjusted until the patient switched from his or her initial choice, thus revealing the amount of time the patient would be willing to wait to achieve the corresponding increase in benefit.
Figure 2.
Example of a time tradeoff question eliciting maximum willingness to wait for an improvement in motor symptoms.
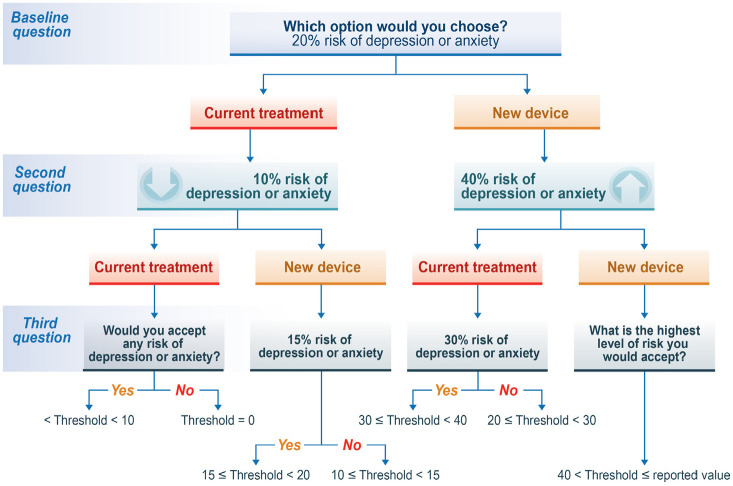
For each series of tradeoff questions, a patient was first asked whether they would be willing to accept the starting level of risk (40% for depression and anxiety, 4% for brain bleed, or 2% for death) or wait time (3 years) in exchange for a benefit. If the patient chose the reference option, thereby indicating that the benefit provided was not sufficient to justify the risk of harm or the wait time, the risk of harm or wait time in the target alternative was reduced systematically to determine the maximum level of acceptable risk of harm or wait time. If the patient chose the target alternative in the first question, the risk of harm or wait time was increased systematically. For each benefit-risk tradeoff, each patient was asked three questions. The pattern of questions for depression and anxiety is presented in Figure 3. The corresponding patterns for brain bleed, death, and wait time are presented in Supplemental Appendix B. The survey also included questions about the PD experience, questions to assess comprehension of the risk graphics, as well as demographic and clinical questions.
Figure 3.
Threshold levels and intervals for risk of new or worsening depression or anxiety.
Patients
Fox Insight (foxinsight.org) is an online longitudinal observational study sponsored by the Michael J Fox Foundation for Parkinson’s Research (MJFF). Fox Insight study participants have an online study dashboard from which they are asked regularly to complete a routine set of patient-reported outcome questionnaires. Participants are also invited to contribute additional data through additional surveys. PD patients enrolled in Fox Insight at the time of this study were invited by email and presented with the survey through their online study dashboard. Email notifications were sent to 10,682 individuals who had a self-declared PD diagnosis. In the initial screener, participants were only considered eligible if they qualified for at least two out of the five potential benefits offered in the survey. After 3 weeks of fielding, the survey screener was modified to allow respondents to complete the survey if they qualified for only one of the benefits offered in the survey. Reminder emails were sent after week 5 and week 6 to individuals who were previously invited but who had not completed the survey. Because study participants had previously provided informed consent to participate in research through Fox Insight, this study was determined to be exempt from institutional review board (IRB) review (RTI International IRB Number 14190, dated October 9, 2017). However, all pretest participants provided additional verbal informed consent prior to participating in pretest interviews.
Each patient was asked to evaluate two potential benefits. Therefore, each patient was asked to respond to 8 of the 20 possible tradeoff scenarios. A patient was excluded from answering a particular benefit question if a 50% improvement from his or her baseline was not possible. The range of acceptable baseline levels for each benefit attribute is presented in Table 1. For example, if a patient reported that their level of PD pain was 1 or 0 on a scale from 0 to 10, the patient was excluded from answering the pain tradeoff questions. Each patient was randomly assigned to two benefits from among those benefits from which he or she had not been excluded. Assignments were made sequentially across the sample to ensure that roughly equal numbers of patients answered threshold questions for each potential benefit.
Survey Pretesting
The survey was pretested by conducting 20 telephone interviews with a convenience sample of PD patients. Pretest participants were identified and recruited by MJFF. Prior to the interview, each participant provided baseline information needed to populate the reference treatment in the survey. Based on the baseline information provided, each patient was assigned to two sets of benefit-risk tradeoff questions, each describing one benefit for which a 50% improvement from baseline was possible. A Microsoft Word version of the survey was developed using this information and was sent electronically to each participant prior to the interview. An informed consent form was also sent to each participant electronically prior to the interview. The interviewer then asked each participant to read each page in the survey and to provide his or her response to each question. The interviewer also asked probing questions to better understand comments and observations provided by each participant and to ensure that the attribute descriptions and survey questions were understandable. The study team made modifications to the survey to reflect feedback provided by the interview participants.
Statistical Analysis
The series of threshold technique questions for each benefit-risk tradeoff resulted in a threshold interval that was analyzed using an interval regression model. When the questions from a survey eliciting pairwise tradeoffs result in an interval rather than a point estimate for one of the items in the tradeoff, the threshold for that item can be defined either as the midpoint of the interval17 or the interval itself. If the threshold is an interval, the data are interval censored because the actual threshold is unknown but is known to fall within an interval with fixed endpoints. An interval regression model is fit using a Tobit model to account for the fact that the interval has both a fixed upper bound, resulting in left-censored data, and a fixed lower bound, resulting in right-censored data.18
For each benefit, we regressed the final risk interval on the magnitude of the benefit and patient characteristics. Because each respondent saw a single level of benefit in each threshold series, the relationship between the level of benefit and the risk threshold was estimated cross-sectionally. The interval regression for risk r was specified as follows:
where is the absolute level of benefit in attribute offered to patient , is an independent and identically normally distributed random error term with mean 0 and variance σ2, and the remaining covariates are dummy variables, defined as follows:
= 1 if the age of patient i is 61 to 66 years
= 1 if the age of patient i is 67 to 71 years
= 1 if the age of patient i is >71 years
= 1 if patient i reported moderate or severe problems with balance and walking in the past week
= 1 if patient i reported difficulty thinking clearly, getting organized, or making plans in the past week because of PD
= 1 if patient i has had deep brain stimulation
= 1 if patient i experienced dyskinesia in the past week
= 1 if patient i reported motor symptoms of at least a 2 on a 10-point scale in the past week
Interaction terms between benefit and each age category are included in the model. The coefficient on the benefit is an estimate of the slope of the maximum acceptable risk (MAR) function, which can be interpreted as a percentage point increase in MAR associated with each one-unit increase in benefit for patients 60 years of age or younger. The coefficient of each age-benefit interaction captures the extent to which the incremental percentage point increase in MAR associated with each one-unit increase in benefit for each age group differs from the youngest age group. The remaining coefficients capture the effect of the presence or absence of each individual characteristics on risk tolerance, independent of the level of benefit. That is, for each age group, we estimate a unique slope of the risk tolerance curve. Each of the remaining patient-specific characteristics is assumed to shift the risk tolerance curve. Because we assume that patients would not tolerate any level of risk without a benefit, the model did not include a constant. Separate risk tolerance curves were estimated for all benefit-risk pairs.
For each benefit, the wait-time interval was regressed on the benefit and patient characteristics. The interval regression for wait time was specified as follows:
As in the benefit-risk interval regressions, the benefit is interacted with each age category and the remaining patient-specific characteristics enter as shift variables. In contrast to the benefit-risk model, the natural logarithm of the benefit was used as the explanatory variable in the benefit-wait time model. We used this specification because we hypothesized that willingness to wait was a diminishing function of the increase in benefit; that is, we assumed a diminishing marginal willingness to wait as the level of benefit increased. In addition, the benefit in the willingness-to-wait model was specified as a composite benefit measure by regressing the wait-time interval on all one-unit increases in benefit, regardless of the benefit. Finally, only the symptom benefits were included in the willingness-to-wait model. Decreases in pill burden were excluded from the model because decreases in pill burden are unlikely to be an endpoint that regulators would consider in clinical trials of PD treatments.
Results
Patients
Data were collected between November 26, 2017, and January 18, 2018. Of the 4203 individuals who responded to the invitation (a 39.3% response rate), 2752 (65.5%) were eligible, consented to participate, and completed the survey. Respondents who did not answer at least one set of benefit-risk tradeoff questions were excluded from the analysis. During data cleaning (see Supplemental Appendix C), 12 patients were removed from the sample. Two patients indicated that they would accept a 100% probability of death or brain bleed and answered the risk comprehension question incorrectly. One patient completed the study despite failing to provide a valid numeric entry for the number of daily pills but only provided tradeoff data related to reducing the number of daily pills. Finally, patients who reported taking 15 or more PD-related pills per day were excluded from the analysis of the medication threshold questions. Nine patients in this category were excluded from the analysis because they did not answer any threshold questions other than the medication threshold questions. The final analysis sample included 2740 respondents.
Respondents’ mean (standard deviation [SD]) age was 65.4 (9) years, and there was approximately an even split between men and women. Most patients were married and the majority of patients were retired. A large majority of patients had a 4-year college degree or graduate school experience. The overwhelming majority of patients (>90%) identified their race as being White or Caucasian. Information related to patient experience with PD, PD treatments, and the harms included in the benefit-risk survey are presented in Table 3. The large majority of patients reported having movement symptoms, which is consistent with known symptoms in this population, and the mean (SD) time since PD diagnosis was 5.3 (4.9) years; however, only 61.2% of patients reported experiencing off-time during which their PD medication was not working. Among those who reported having off-time, the mean (SD) number of hours of on-time in a 16-hour day was 10.8 (3.8) hours. Slightly fewer than half of all patients reported having PD pain or trouble with memory and thinking. The mean (SD) severity rating for movement symptoms, pain, and cognition was 4.4 (0.1), and the average ratings were about the same across these symptoms. The mean number of daily pills was greater than 7, and approximately 8% of patients in our sample had had prior deep brain stimulation (DBS). Approximately 41% of patients had depression or anxiety at the time of the survey. Finally, 2.4% of patients had experienced a brain bleed in the past, and more than a third of patients indicated that they knew someone who had died after having an operation (Table 2).
Table 3.
Patient Experience With PD and Parkinson’s Treatment
| Symptom | Number Reporting Symptom, Mean (SD) | Symptom Level, Mean (SD) |
|---|---|---|
| Average hours of on-timea | 1677 (61.2%) | 10.8 (3.78) |
| Severity of movement symptomsb | 2649 (96.7%) | 4.3 (2.06) |
| Severity of painb | 1348 (49.2%) | 4.5 (2.23) |
| Severity of cognitive symptomsb | 1217 (44.4%) | 4.4 (2.17) |
| Parkinson’s Related Characteristics | ||
| Number of daily pills | Mean (SD) | 7.6 (5.39) |
| Years since diagnosis | Mean (SD) | 5.3 (4.91) |
| Prior deep brain stimulation | n (%) | 219 (8%) |
| Biological relative with PD | n (%) | 569 (20.8%) |
| Experience With Risk Outcomes | N | Mean (SD) |
| Severity of current depression or anxietyb | 1,118 | 4.4 (2.09) |
| Prior brain bleed | 67 (2.4%) | |
| Know someone who died after an operation | 922 (33.6%) | |
Symptom was off-time; respondents reporting off-time were asked how many hours of on-time they had in 16 waking hours each day.
Symptoms rated on a scale from 0 to 10 in which 0 indicated no symptoms and 10 indicated very severe symptoms.
Table 2.
Patient Demographic Characteristics
| Demographics | All Respondents (N = 2740) |
|---|---|
| Age (years) | |
| Mean (SD) | 65.4 (9.01) |
| Median | 66.0 |
| Gender | |
| Male | 1461 (53.3%) |
| Female | 1279 (46.7%) |
| Marital status? | |
| Not married | 489 (17.8%) |
| Married/living as married/civil partnership | 2251 (82.2%) |
| Highest level of education | |
| Less than 4-year college degree | 828 (30.2%) |
| 4-year college degree or higher | 1912 (69.8%) |
| Which of the following best describes your employment status? | |
| Employed full-time | 411 (15.0%) |
| Employed part-time | 132 (4.8%) |
| Self-employed | 137 (5.0%) |
| Homemaker | 56 (2.0%) |
| Retired | 1576 (57.5%) |
| Disabled/unable to work | 391 (14.3%) |
| Unemployed | 37 (1.4%) |
| Race/ethnicity | |
| American Indian or Alaska Native | 24 (0.9%) |
| Asian | 34 (1.2%) |
| Black or African American | 21 (0.8%) |
| Hispanic or Latino | 56 (2.0%) |
| Native Hawaiian or other Pacific Islander | 7 (0.3%) |
| White or Caucasian | 2593 (94.6%) |
| Other | 19 (0.7%) |
| Prefer not to answer | 28 (1.0%) |
Threshold Models
Improvements in the level of benefit were consistently associated with statistically significant increases in risk threshold. Incremental increases in threshold for mortality risk were similar for improvements in movement symptoms, pain, and cognition. Incremental increases in risk threshold for depression and brain bleed were larger for improvements in movement symptoms and cognition than for improvements in pain. In addition, each one-unit improvement in benefit had a greater impact on risk threshold for depression than on risk threshold for brain bleed or death. For example, patients were generally willing to accept a 2.65 percentage-point increase in the annual risk of new or worsening depression for each 1-hour increase in daily on-time. The increases in risk threshold for brain bleed (0.47 percentage points) and treatment-related death (0.18 percentage points) associated with each 1-hour increase in daily on-time were substantially smaller (Tables 4–6).
Table 4.
Interval Regression Results for On-Time and Movement Tradeoffsa
| Benefit | On-Time (Hours) | On-Time (Hours) | On-Time (Hours) | Movement (Scale of 10) | Movement (Scale of 10) | Movement (Scale of 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk | Depression | Brain Bleed | Death | Depression | Brain Bleed | Death | ||||||
| N | n = 612 | n = 576 | n = 594 | n = 663 | n = 666 | n = 679 | ||||||
| Covariate | Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE |
| Benefit | 2.65 | 0.40 | 0.47 | 0.09 | 0.18 | 0.05 | 4.09 | 0.47 | 0.90 | 0.11 | 0.36 | 0.06 |
| Benefit * 61–66 years | −0.99 | 0.45 | −0.08 | 0.10 | 0.11 | 0.06 | −0.49 | 0.59 | −0.15 | 0.14 | 0.15 | 0.07 |
| Benefit * 67–71 years | −0.45 | 0.41 | 0.06 | 0.10 | 0.16 | 0.06 | 0.24 | 0.62 | −0.16 | 0.14 | 0.03 | 0.07 |
| Benefit * >71 years | −0.99 | 0.45 | −0.01 | 0.11 | 0.13 | 0.07 | −0.26 | 0.58 | 0.01 | 0.14 | 0.07 | 0.07 |
| Nonambulatory | 2.32 | 1.73 | 0.06 | 0.40 | −0.08 | 0.24 | 5.10 | 1.64 | 0.93 | 0.40 | 0.47 | 0.21 |
| Cognitive impairment | 0.00 | 1.14 | 0.23 | 0.28 | 0.27 | 0.16 | −0.28 | 1.11 | 0.29 | 0.26 | 0.27 | 0.14 |
| DBS | 3.98 | 2.01 | 0.78 | 0.51 | 0.45 | 0.26 | 11.35 | 2.17 | 0.46 | 0.54 | 0.61 | 0.27 |
| Dyskinesia | 0.88 | 1.25 | 0.37 | 0.30 | 0.43 | 0.17 | −2.10 | 1.20 | −0.02 | 0.30 | −0.04 | 0.15 |
| Motor ≥2 | 3.49 | 1.26 | 0.85 | 0.31 | 0.30 | 0.16 | — | — | — | — | — | — |
DBS, deep brain stimulation.
Boldface values denote estimated coefficients that are significant at the 5% level.
Table 5.
Interval Regression Results for Pain and Cognition Tradeoffsa
| Benefit | Pain (Scale of 10) | Pain (Scale of 10) | Pain (Scale of 10) | Cognition (Scale of 10) | Cognition (Scale of 10) | Cognition (Scale of 10) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk | Depression | Brain Bleed | Death | Depression | Brain Bleed | Death | ||||||
| N | n = 581 | n = 606 | n = 593 | n = 579 | n = 555 | n = 576 | ||||||
| Covariate | Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE | Coeff. | SE |
| Benefit | 3.26 | 0.64 | 0.56 | 0.14 | 0.35 | 0.07 | 3.87 | 0.64 | 1.18 | 0.17 | 0.39 | 0.09 |
| Benefit * 61–66 years | −0.54 | 0.53 | −0.03 | 0.12 | 0.09 | 0.06 | −0.55 | 0.61 | 0.01 | 0.16 | 0.14 | 0.08 |
| Benefit * 67–71 years | −0.08 | 0.55 | 0.01 | 0.12 | 0.06 | 0.07 | −1.23 | 0.59 | −0.06 | 0.16 | 0.20 | 0.09 |
| Benefit * >71 years | −1.32 | 0.62 | −0.20 | 0.13 | −0.02 | 0.07 | −1.45 | 0.61 | −0.14 | 0.16 | 0.16 | 0.08 |
| Nonambulatory | 1.52 | 1.60 | 0.90 | 0.35 | 0.11 | 0.19 | 1.71 | 1.64 | 0.29 | 0.40 | 0.23 | 0.21 |
| Cognitive impairment | 0.84 | 1.16 | 0.27 | 0.25 | 0.32 | 0.14 | — | — | — | — | — | — |
| DBS | 1.83 | 2.00 | 0.96 | 0.45 | 0.46 | 0.24 | −0.33 | 1.90 | 0.13 | 0.51 | 0.57 | 0.26 |
| Dyskinesia | 1.90 | 1.23 | 0.17 | 0.27 | −0.12 | 0.15 | 1.20 | 1.26 | 0.20 | 0.33 | −0.22 | 0.18 |
| Motor ≥2 | 1.06 | 1.63 | 0.21 | 0.35 | −0.02 | 0.18 | 2.43 | 1.55 | −0.29 | 0.41 | 0.19 | 0.23 |
DBS, deep brain stimulation.
Boldface values denote estimated coefficients that are significant at the 5% level.
Table 6.
Interval Regression Results for Medication Tradeoffsa
| Benefit | Medication (Pills) | Medication (Pills) | Medication (Pills) | |||
|---|---|---|---|---|---|---|
| Risk | Depression | Brain Bleed | Death | |||
| N | n = 676 | n = 674 | n = 684 | |||
| Covariate | Coeff. | SE | Coeff. | SE | Coeff. | SE |
| Benefit | 1.31 | 0.22 | 0.19 | 0.05 | 0.14 | 0.03 |
| Benefit * 61–66 years | −0.94 | 0.25 | −0.07 | 0.06 | −0.07 | 0.03 |
| Benefit * 67–71 years | −0.98 | 0.26 | −0.08 | 0.06 | −0.07 | 0.03 |
| Benefit * >71 years | −0.95 | 0.24 | −0.02 | 0.06 | −0.08 | 0.03 |
| Nonambulatory | 1.37 | 1.28 | −0.14 | 0.31 | 0.24 | 0.17 |
| Cognitive impairment | −1.00 | 0.75 | 0.04 | 0.18 | 0.19 | 0.09 |
| DBS | 3.48 | 1.54 | 1.15 | 0.36 | 0.42 | 0.21 |
| Dyskinesia | 1.40 | 0.91 | 0.23 | 0.21 | 0.18 | 0.11 |
| Motor ≥2 | 1.91 | 0.58 | 0.29 | 0.14 | 0.07 | 0.08 |
DBS, deep brain stimulation.
Boldface values denote estimated coefficients that are significant at the 5% level.
The effect of age on risk threshold was inconsistent and often not significant in the models for risk threshold associated with improvements in on-time, motor function, pain, and cognition. However, older patients were less willing to accept risks of depression or death than were younger patients in exchange for reductions in the number of daily pills. Patients who had moderate or severe problems with balance and walking (nonambulatory) were willing to accept higher levels of risk of depression, brain bleed, and death for improvements in motor symptoms than were patients with mild, slight, or no balance and walking problems (ambulatory). In addition, nonambulatory patients were willing to accept higher risks of brain bleed to achieve reductions in PD pain than were ambulatory patients. Prior DBS, however, was associated with a higher risk threshold for depression in most cases. Prior DBS was also a significant predictor of risk threshold for brain bleed and death in some cases. Current cognitive impairment, dyskinesias, and motor symptoms were rarely significant predictors of risk threshold.
Maximum Acceptable Risk Estimates
Changes in risk threshold associated with a one-unit improvement in symptoms or reduction in pill burden can be used to calculate the maximum level of acceptable treatment-related risk of harm for each possible level of benefit (Tables 4–6). For example, Figure 4 presents the maximum level of acceptable risk for each of the three harms included in the study across the range of possible improvements in motor symptoms. These results indicate that patients would, on average, be willing to accept more than a 5% annual risk of new or worsening depression or anxiety for a relatively modest improvement in motor symptoms (from 4 to 2 on the 11-point rating scale), and more than a 17% annual risk of new or worsening depression or anxiety to improve severe motor symptoms by 50%. In addition, Figure 4 shows that the threshold for new or worsening depression or anxiety is statistically significantly greater than the threshold for the risks of brain bleed and death for improvements in motor symptoms. Similar figures for the remaining benefits are available in Supplemental Appendix D.
Figure 4.
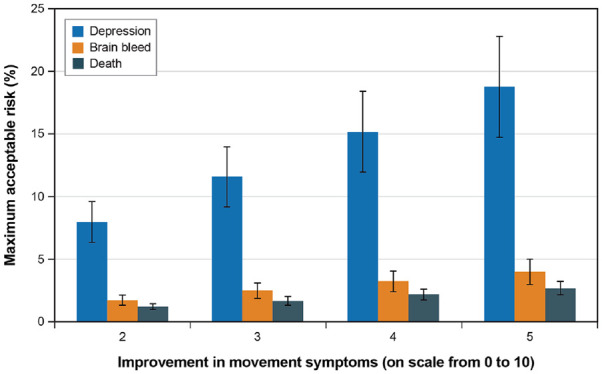
Maximum acceptable risks of treatment-related depression, brain bleed, and death for different levels of improvement in motor symptoms.
Figure 5 and Figure 6 present the MAR for depression for different improvements in motor symptoms for nonambulatory and DBS subgroups, respectively. In Figure 5, nonambulatory patients had a higher mean risk threshold for new or worsening depression or anxiety than ambulatory patients for all levels of improvement in motor symptoms. Differences in risk threshold between the nonambulatory and ambulatory subgroups were not statistically significantly different. Figure 6 shows that at all levels of motor symptom improvement, patients with prior DBS had statistically significantly greater threshold for depression risk than did patients without DBS.
Figure 5.
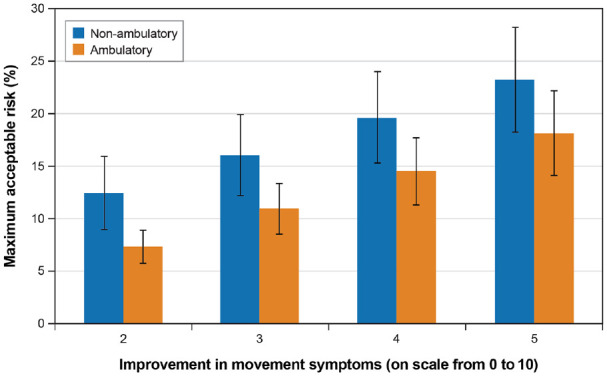
Maximum acceptable risk of new of worsening depression for different levels of improvement in motor symptoms for ambulatory and nonambulatory subgroups.
Figure 6.
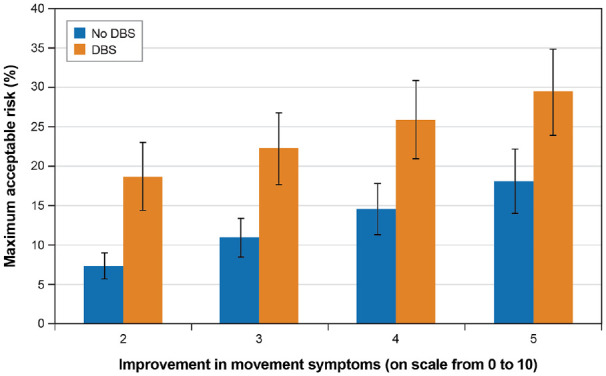
Maximum acceptable risk for new or worsening depression for improvements in motor symptoms for patients with and without prior deep brain stimulation.
Willingness-to-Wait Estimates
Maximum acceptable wait time for a neurostimulation device that offers different levels of improvement in any benefit is presented graphically in Figure 7. For a two-unit improvement in any benefit, the mean (SD) maximum acceptable wait time is 4.34 (0.14) years. For a five-unit improvement in any benefit (the maximum possible benefit for movement symptoms, pain, and cognition), the mean (SD) maximum acceptable wait time is 10.42 (0.39) years. For an eight-unit improvement in benefit (the maximum possible benefit for improvements in on-time), the average maximum acceptable wait time is more than 13 years. The maximum willingness-to-wait increases with the level of benefit, but at a decreasing rate, which is consistent with the specification of the wait-time threshold function (Table 7).
Figure 7.
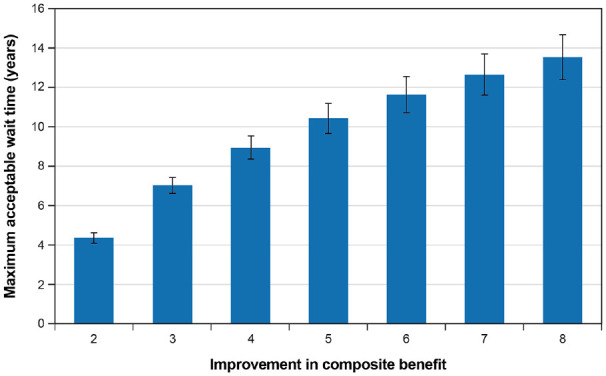
Maximum acceptable wait time for different levels of improvement in any benefit.
Table 7.
Interval Regression Results for Time Tradeoffsa
| Benefit | All Benefits | |
|---|---|---|
| N | n = 4657 | |
| Covariate | Coeff. | SE |
| ln(benefit) | 6.72 | 0.43 |
| Age | ||
| 61–66 years * ln(benefit) | −0.09 | 0.22 |
| 67–71 years * ln(benefit) | −0.84 | 0.21 |
| >71 years * ln(benefit) | −1.31 | 0.22 |
| Nonambulatory | −0.71 | 0.20 |
| Cognitive impairment | −0.36 | 0.16 |
| DBS | −0.62 | 0.25 |
| Dyskinesia | −0.40 | 0.17 |
| Motor ≥2 | 0.25 | 0.40 |
DBS, deep brain stimulation.
Boldface values denote estimated coefficients that are significant at the 5% level.
Discussion
We used a multi-stakeholder approach to eliciting patient preferences and risk tolerance for potential neurostimulation devices for PD. We found that risk tolerance increased with increases in benefits. Tolerance for new or worsening depression or anxiety was substantially higher than tolerance for brain bleed or death. Patient age, ambulation, and prior neurostimulation experience influenced risk tolerance. Patients were willing to wait an average of 4 to 13 years for devices that provide different levels of benefit. These results were developed not only to understand the tradeoffs patients with PD are willing to make between the benefits and burdens (i.e., risks of harm and wait time) of novel neurostimulation devices but also to be used as inputs to a model (S. E. Chaudhuri, PhD, unpublished data, 2019) designed to optimize clinical trial size and the acceptable level of statistical significance by balancing patient urgency for novel therapeutic options against tolerance for risk of harm.11–13
Patients were active participants in the identification of relevant benefits of PD treatment (H. L. Benz, PhD, unpublished data, 2019), the development of the survey instrument, and the analysis and interpretation of the results. It is often advantageous in a patient preference study to work with a well-established patient organization and individual patient scientists, and to recruit patients through an existing research registry, online study, or engaged cohort of research volunteers developed and hosted by the patient organization. However, patients recruited to participate in preference research by these organizations can often only self-report their diagnosis or symptoms.19,20 In addition, patients recruited through patient organizations may include fewer racial and ethnic minorities and be of higher socioeconomic status than the overall population of patients.
The threshold technique is a stated preference method.14 The structure of the threshold technique used here is similar to the structure of a discrete choice experiment (DCE) in which each treatment profile is composed of multiple attributes of varying levels, and the levels differ between profiles among which a respondent is asked to choose. However, in a DCE, all attribute levels vary simultaneously between options and across choice questions according to an experimental design. In contrast, the threshold technique varies the level of one attribute at a time until the threshold level of that attribute is determined. The DCE has the advantage of estimating the relationships among all attributes simultaneously; however, the DCE yields results for a sample rather than for each respondent, limiting the ability of DCEs to relate a choice to the individual characteristics of the respondents to the association between patient characteristics and latent segments in the data.
The threshold technique, however, has its own limitations. It may be subject to starting point bias—the starting level in the series of questions may systematically influence the estimated threshold. However, if the starting value of the threshold reflects reality, either current or expected, then the starting point bias is not necessarily unreasonable. In this case, the starting risk values reflected FDA reviewers’ expectations of the likely risks of harm of any new neurostimulation device. Another limitation of the threshold technique is that we cannot combine the results across pairwise comparisons. While we can directly compare risk tolerance across harms for any given benefit and compare the relative importance of different benefits for any risk of harm, we cannot directly estimate specific rates of tradeoff between different harms or between different benefits.
Finally, two additional study limitations should be noted. First, although the distribution of patients in our sample reflected the diversity in the Fox Insight study at the time the survey was administered, our sample was predominantly White and college educated. As a result, this sample is not representative of all PD patients, limiting the generalizability of our findings. In addition, during the first 3 weeks of screening, the study team noticed that a higher proportion of potential participants were excluded from completing the study because their symptoms were not severe enough for them to qualify for the minimum improvement in benefit included in the study. To maximize the number of study participants and not turn away patients who were interested in the study, we modified the screener to allow respondents to complete the survey if they qualified for only one the benefits offered in the study. We do not know the extent to which this modification to the screening criteria impacted our results.
In summary, we found that PD patients are willing to accept substantial risk to improve symptoms. However, patient preferences were heterogeneous and depended on treatment benefit and patient characteristics. These findings may help inform regulatory decisions and will provide valuable input into a model of optimal trial design for neurostimulation devices.
Supplemental Material
Supplemental material, sj-docx-1-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Supplemental material, sj-docx-2-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Supplemental material, sj-docx-3-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Supplemental material, sj-docx-4-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Acknowledgments
We would like to thank the Parkinson’s community for participating in this study to make this research possible. No compensation was provided to study respondents.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded in part by the Medical Device Innovation Consortium (MDIC) with funding provided to RTI Health Solutions and MIT. MDIC also provided funding to The Michael J. Fox Foundation for Parkinson’s Research (MJFF) to support survey programming and hosting. No funding was provided to members of the MJFF Patient Council or to FDA staff for participation in this research. The Fox Insight Study (FI) is funded by The Michael J. Fox Foundation for Parkinson’s Research.
ORCID iDs: Brett Hauber  https://orcid.org/0000-0003-3129-7268
https://orcid.org/0000-0003-3129-7268
Heather L. Benz  https://orcid.org/0000-0002-6707-9695
https://orcid.org/0000-0002-6707-9695
Anindita Saha  https://orcid.org/0000-0003-0306-4264
https://orcid.org/0000-0003-0306-4264
Supplemental Material: Supplementary material for this article is available on the Medical Decision Making Policy & Practice website at https://journals.sagepub.com/home/mpp.
Contributor Information
Brett Hauber, RTI Health Solutions, Research Triangle Park, North Carolina.
Brennan Mange, RTI Health Solutions, Research Triangle Park, North Carolina.
Mo Zhou, FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Shomesh Chaudhuri, MIT Sloan School of Management, Cambridge, Massachusetts.
Heather L. Benz, FDA Center for Devices and Radiological Health, Silver Spring, Maryland
Brittany Caldwell, FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
John P. Ruiz, FDA Center for Devices and Radiological Health, Silver Spring, Maryland
Anindita Saha, FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Martin Ho, FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Stephanie Christopher, Medical Device Innovation Consortium, Arlington, Virginia.
Dawn Bardot, Medical Device Innovation Consortium, Arlington, Virginia.
Margaret Sheehan, The Michael J. Fox Foundation for Parkinson’s Research, Patient Council, New York, New York.
Anne Donnelly, The Michael J. Fox Foundation for Parkinson’s Research, Patient Council, New York, New York.
Lauren McLaughlin, The Michael J. Fox Foundation for Parkinson’s Research, New York, New York.
Katrina Gwinn, FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
Andrew Lo, MIT Sloan School of Management, Cambridge, Massachusetts.
Murray Sheldon, FDA Center for Devices and Radiological Health, Silver Spring, Maryland.
References
- 1. Savica R, Grossardt BR, Bower JH, et al. Survival and causes of death among people with clinically diagnosed synucleinopathies with parkinsonism: a population-based study. JAMA Neurol. 2017;74(7):839–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15(12):1257–72. [DOI] [PubMed] [Google Scholar]
- 3. United States Food and Drug Administration. The voice of the patient. A series of reports from the US Food and Drug Administration’s (FDA’s) Patient-Focused Drug Development Initiative: Parkinson’s disease 2016. [cited December 24, 2018]. Available from: http://wayback.archive-it.org/7993/20171114201734/https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM498266.pdf [Google Scholar]
- 4. United States Food and Drug Administration. Plan for issuance of patient-focused drug development guidance under 21st Century Cures Act Title III Section 3002 [cited December 24, 2018]. Available from: https://www.fda.gov/downloads/ForIndustry/UserFees/PrescriptionDrugUserFee/UCM563618.pdf
- 5. Medical Device Innovation Consortium. Medical Device Innovation Consortium (MDIC) patient centered benefit-risk project report: a framework for incorporating information on patient preferences regarding benefit and risk in regulatory assessments of new medical technology [cited December 27, 2018]. Available from: https://mdic.org/wp-content/uploads/2018/05/MDIC_PCBR_Framework_Web.pdf
- 6. United Stated Food and Drug Administration. Patient preference information—voluntary submission, review in premarket approval applications, humanitarian device exemption applications, and de novo requests, and inclusion in decision summaries and device labeling: guidance for industry, Food and Drug Administration staff, and other stakeholders [cited December 27, 2018]. Available from: https://www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/UCM446680.pdf
- 7. Medical Device Innovation Consortium. A case study in how patient preference information contributed to regulatory decisions for medical devices [cited December 27, 2018]. Available from: https://mdic.org/event/a-case-study-in-how-patient-preference-information-contributes-to-regulatory-decisions-for-medical-devices/
- 8. Al-Faruque F. FDA weighs patients’ risk tolerance in approving obesity device. The Pink Sheet Daily [cited January 6, 2019]. Available from: https://www.pharmamedtechbi.com/publications/the-pink-sheet-daily/2015/1/20/fda-weighs-patients-risk-tolerance-in-approving-obesity-device
- 9. Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–93. [DOI] [PubMed] [Google Scholar]
- 10. Hauber AB, Fairchild AO, Johnson FR. Quantifying benefit-risk preferences for medical interventions: an overview of a growing empirical literature. Appl Health Econ Health Policy. 2013;11(4):319–29. [DOI] [PubMed] [Google Scholar]
- 11. Isakov L, Lo AW, Montazerhodjat V. Is the FDA too conservative or too aggressive? A Bayesian decision analysis of clinical trial design. J Econometrics. 2019;211(1):117–36 doi: 10.1016/j.jeconom.2018.12.009 [DOI] [Google Scholar]
- 12. Chaudhuri SE, Ho MP, Irony T, Sheldon M, Lo AW. Patient-centered clinical trials. Drug Discov Today. 2018;23(2):395–401. [DOI] [PubMed] [Google Scholar]
- 13. Montazerhodjat V, Chaudhuri S, Sargent D, Lo A. Use of Bayesian decision analysis to minimize harm in patient-centered randomized clinical trials in oncology. JAMA Oncol. 2017;3(9):e170123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hauber B, Coulter J. Using the threshold technique to elicit patient preferences: an introduction to the method and an overview of existing empirical applications. Appl Health Econ Health Policy. 2020;18(1):31–46. doi: 10.1007/s40258-019-00521-3 [DOI] [PubMed] [Google Scholar]
- 15. Llewellyn-Thomas HA. Threshold technique. In: Kattan MW, editor. Encyclopedia of Medical Decision Making. Sage; 2009. [Google Scholar]
- 16. Llewellyn-Thomas HA. Investigating patients’ preferences for different treatment options. Can J Nurs Res. 1997;29(3):45–64. [PubMed] [Google Scholar]
- 17. Watson SR. Decision Synthesis: The Principles and Practice of Decision Analysis. Cambridge University Press; 1987. [Google Scholar]
- 18. Cameron AC, Trivedi PK. Microeconometrics using Stata (Rev. ed.). Stata Press; 2010. [Google Scholar]
- 19. Mansfield C, Masaquel A, Sutphin J, et al. Patients’ priorities in selecting chronic lymphocytic leukemia treatments. Blood Adv. 2017;1(24):2176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bridges JF, Oakes AH, Reinhart CA, Voyard E, O’Donoghue B. Developing and piloting an instrument to prioritize the worries of patients with acute myeloid leukemia. Patient Prefer Adherence. 2018;12:647–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Supplemental material, sj-docx-2-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Supplemental material, sj-docx-3-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice
Supplemental material, sj-docx-4-mpp-10.1177_2381468320978407 for Parkinson’s Patients’ Tolerance for Risk and Willingness to Wait for Potential Benefits of Novel Neurostimulation Devices: A Patient-Centered Threshold Technique Study by Brett Hauber, Brennan Mange, Mo Zhou, Shomesh Chaudhuri, Heather L. Benz, Brittany Caldwell, John P. Ruiz, Anindita Saha, Martin Ho, Stephanie Christopher, Dawn Bardot, Margaret Sheehan, Anne Donnelly, Lauren McLaughlin, Katrina Gwinn, Andrew Lo and Murray Sheldon in MDM Policy & Practice