Abstract
Background: Open carpal tunnel release typically requires several weeks of recovery. A less invasive, ultrasound-guided percutaneous technique of releasing the transverse carpal ligament using a thread (thread carpal tunnel release [TCTR]) has been described. To date, its clinical effectiveness and safety have been evaluated exclusively by the group that developed the technique, using a single outcome measure without a control comparison. The objective of this study was to independently evaluate the safety and effectiveness of TCTR using multiple outcome measures and a control comparison. Methods: A convenience sample of 20 participants with refractory moderate or severe carpal tunnel syndrome underwent TCTR of their most symptomatic hand. Outcome measures included pre-TCTR and 1-, 3-, and 6-month post-TCTR Boston questionnaire; pre-, 3-, and 6-month post-TCTR monofilament sensibility, strength, ultrasound, and electrodiagnostic testing; weekly post-TCTR phone interviews for 1 month; and satisfaction surveys at 3 and 6 months post-TCTR. Results: No complications were reported. During the month post-TCTR, significant prompt improvements in hand pain and dysfunction occurred. The following significant improvements were demonstrated in the treated versus control hand: Boston Questionnaire scores, median nerve distal motor latency, transcarpal tunnel motor and sensory conduction velocities and sensory nerve action potential amplitudes. No significant differences in sensibility, pinch or grip strength, median nerve cross-sectional area (CSA) at the carpal tunnel inlet, or wrist: forearm median nerve CSA ratio were documented between TCTR and control sides. Satisfaction with the TCTR procedure was high (85%-90%). Conclusions: This study supports previous reports that the TCTR procedure is safe and effective.
Keywords: carpal, invasive, minimally, syndrome, tunnel, ultrasound
Introduction
Open carpal tunnel release to treat carpal tunnel syndrome (CTS) is commonly performed and has a high success rate. However, postoperative recovery ranges between 3 and 8 weeks, and postoperative pillar pain can occur.1,2 In hopes to shorten recovery time and minimize pillar pain, a variety of strategies to reduce the invasiveness of carpal tunnel release have been described. These strategies include endoscopic release and ultrasonographic (US)-guided percutaneous release using a variety of cutting instruments including a needle, knife, and saw blade.3-7 A recently described ultraminimally invasive technique used a cutting thread that is percutaneously looped around the transverse carpal ligament (TCL) through a needle under US guidance.8 The ends of the cutting thread are reciprocally pulled back and forth like a Gigli saw, transecting the TCL beneath the skin. The original thread carpal tunnel release (TCTR) technique involved inserting the guide needle just proximal to the carpal tunnel inlet and exiting distal to the carpal tunnel outlet at the mid palm. The TCL was looped and cut from distal to proximal. This original TCTR technique was initially performed on a single cadaveric hand resulting in complete transection of the TCL and no injury to other structures. The same procedure was then performed on 34 hands of 20 patients. The Boston Carpal Tunnel Questionnaire (BCTQ) was completed 3 months after the procedure, and scores were found to be comparable with those in the literature following open and endoscopic release. No post-TCTR complications were reported. Our research group subsequently completed a cadaveric evaluation of the original TCTR technique on 14 specimens.9 Ultrasonography accurately defined the critical boundaries and contents of the carpal tunnel. Complete transection of the TCL was achieved in 9 of the 14 specimens, and an average of 69% complete transections in the remaining 5 specimens. No trauma was sustained by any structure within the carpal tunnel other than the TCL. If the release was incomplete, it was typically the distal portion of the TCL that remained intact.
The originators of the TCTR procedure addressed the problem of incomplete release of the distal portion of the TCL by revising the surgical approach. The originators placed the guide needles from distal to proximal allowing the TCL to be cut from proximal to distal. A cadaveric study using this revised TCTR technique confirmed effectiveness and safety.10 The outcomes of a cohort of 159 hands in 116 patients who underwent the revised TCTR technique were published by the originators. Using the BCTQ as the sole outcome measure, results were excellent and prompt. Significant improvement in symptoms occurred within 1 day and in function within 1 week. The revised TCTR resulted in earlier and greater improvements in BCTQ symptoms and function scores when compared with open and endoscopic carpal tunnel release results in existing literature.11
To date, the clinical effectiveness and the safety of the TCTR procedure have been evaluated solely by the originators of the technique using only a single outcome measure and without a control comparison. The objective of this study was to independently evaluate the safety and effectiveness of the TCTR using multiple outcome measures and a control comparison.
Materials and Methods
This study was approved by the Health Research Ethics Board of the University of Alberta (Pro00060817).
Participants
A convenience sample of 20 participants (mean age = 52.8 years; male: female = 9:11; 18 right handed; 13 bilateral and 7 unilateral CTS) was recruited from the electrodiagnostic practice of author R.S.B. An ultrasound examination of both carpal tunnels was also performed at the time of the prerecruitment electrodiagnostic assessment. Selection criteria included the following: >18 years of age; symptoms (hand numbness, tingling, weakness or pain aggravated by repetitive or sustained gripping and at night) and signs (median nerve distribution hand sensory disturbance or weakness, positive median nerve Tinel sign, or Phalen test) compatible with CTS present for at least 3 months; refractory to conservative treatment (ie, wrist splints, activity modification, nonsteroidal anti-inflammatory drug, intracarpal tunnel corticosteroid injection); and nerve conduction study abnormalities compatible with moderate to severe median neuropathy at the carpal tunnel (motor and sensory conduction slowing across the carpal tunnel with or without evidence of axonal loss) present in at least one hand. Exclusion criteria included the following: bifid median nerve or persistent median artery seen on ultrasound at the carpal tunnel inlet; clinical or electrodiagnostic evidence of a neurological disorder affecting the upper extremity besides CTS (ie, proximal median neuropathy, ulnar or radial neuropathy, brachial plexopathy, cervical radiculopathy, or generalized polyneuropathy); inability to understand the informed consent or the BCTQ; coagulopathy or anticoagulation treatment that could not be stopped for the TCTR procedure; allergy to local anesthetic; and systemic infection or local infection at the procedure site. All participants signed an informed consent prior to participation in the study.
A sample size of 20 participants was selected based on data available in the literature describing nerve conduction study changes from before the procedure to 6 months after the procedure following both open carpal tunnel release surgery and intracarpal tunnel corticosteroid injection. Assuming an α of .05, power of .8, a 2-tailed model, and considering the reported effect size for distal median nerve motor latency, the required sample size for the open carpal tunnel release surgery was 6 and for intracarpal tunnel corticosteroid injection was 26. Assuming the looped thread technique would result in improvement somewhere between open carpal tunnel release surgery and corticosteroid injection, a required sample size of 20 was conservatively estimated.
The TCTR procedure was performed on only one hand. The other hand functioned as the control. For participants with unilateral CTS, the TCTR procedure was performed on the affected side only. Participants with bilateral CTS self-selected the side of the TCTR.
Interventional Procedure
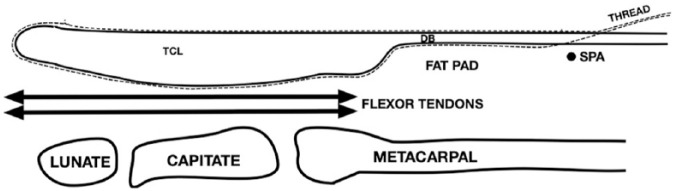
While lying in a supine position, the patient’s arm was extended onto a padded arm board with the forearm supinated and the wrist extended. A preliminary US examination of the carpal tunnel was performed using a high-frequency (15-6 MHz) linear array transducer. The skin overlying the following structures was marked with indelible ink markers: the carpal tunnel inlet (a line connecting the pisiform and scaphoid tubercle), carpal tunnel outlet (the “duck’s beak” shaped distal end of the TCL10,11 or, if not clearly visualized, a line connecting the distal edge of the hamate and trapezium), median nerve, ulnar artery, and superficial palmar arterial arch. The forearm, wrist, and hand were cleansed with a chlorhexidine/alcohol solution. A sterile drape was placed to cover the forearm and distal half of the hand. The first 5 procedures were performed using the original TCTR technique, as the revised technique had not yet been described. The subsequent 15 participants underwent the revised TCTR technique described hereafter. Using a 30-gauge (G), 1-inch needle and 1% lidocaine without epinephrine, the skin was infiltrated 1 to 2 cm proximal to the carpal tunnel inlet medial to the path of the median nerve and lateral to the path of the ulnar artery (the “safe zone”). Using the same needle and local anesthetic solution, the skin was anesthetized at a point in line with the safe zone and 1 cm distal to the superficial palmar arch. A 27-G needle was inserted through the anesthetized point of the palm and advanced proximally under continuous US visualization. The needle was advanced volar to the superficial palmar arch, through the superficial palmar aponeurosis, the deep layer of the palmar aponeurosis, and into the distal margin of the carpal tunnel between the tip of the “duck’s beak” and the superficial flexor tendon. After injecting local anesthetic, the 27-G needle was removed and replaced with a slightly curved 18-G, 3.5-inch long Tuohy spinal needle. The Tuohy needle was advanced proximally between the dorsal surface of the TCL and the superficial flexor tendon in the plane of the previously marked safe zone. With the wrist dorsiflexed, the needle was delivered through the anesthetized skin proximal to the carpal tunnel inlet. A sterile cutting thread was advanced through the 18-G Tuohy needle, and the needle was withdrawn leaving the thread in the carpal tunnel dorsal to the TCL. A straight 3.5-inch long 18-G Tuohy spinal needle was placed into the same entry point at the palm and was advanced along the volar surface of the TCL. This needle was also delivered through the same point proximal to the carpal tunnel inlet, and the cutting thread was passed through the needle. The needle was withdrawn, looping the cutting thread around the proximal border of the TCL (Figure 1). Needle placement and advancement were done under US guidance and hydrodissection using a solution of local anesthetic. Safety checks were done prior to cutting the TCL by tugging on the 2 ends of the thread simultaneously while visualizing the TCL and superficial palmar arch. If movement of the superficial palmar arch was seen with each tug, the thread was removed and repositioned until only movement of the TCL with tugging was seen. The fingers were also inspected to ensure they did not flex when the thread was tugged. This confirmed a flexor tendon was not ensnared. Once these safety checks were satisfactorily completed, the hand was secured and the 2 ends of the thread were pulled distally and oscillated using a sawing motion until the TCL was cut through and the thread was delivered through the distal portal. The hand was cleansed, dried, and a waterproof bandage was placed over each of the 2 needle puncture sites. The cutting thread consisted of a sterile uncoated multifilament stainless steel wire (35 N-LT Alloy; 7 × 7 × 0.043; 0.389 inches; Fort Wayne Metals, Indiana). Similar suture thread has demonstrated excellent strength characteristics.12 All TCTR procedures were performed by the same 2 practitioners who performed the previous cadaveric procedures.9
Figure 1.
Thread looped around TCL.
Note. TCL = transverse carpal ligament; dotted line = thread; DB = ducks bill; SPA = superficial palmar arterial arch.
Outcome Measures
On the day of the procedure and at weekly intervals for the first month post-TCTR, each participant was contacted by a research assistant to report on their recovery using standardized questions quantifying pain, disability, employability, and adverse events. Within a week prior to and at 3 and 6 months post-TCTR, the following outcome measures were collected: subjective measure of symptom and functional limitation severity (BCTQ), hand monofilament sensibility, grip and pinch strength, US-derived median nerve cross-sectional area (CSA) at the carpal tunnel inlet and at the pronator quadratus, and nerve conduction studies of the median nerve across the carpal tunnel. The BCTQ was also completed 1-month post-TCTR. At 3 and 6 months post-TCTR, participants also rated their overall satisfaction with their TCTR procedure. All 1-month post-TCTR recovery phone interviews and BCTQs were conducted by the same research coordinator. He was not blinded to which hand was treated. A second research assistant administered all of the hand sensibility, pinch, and grip strength testing. He was blinded to which hand was treated. A third research assistant performed all median nerve CSA measurements and nerve conduction studies. He was also blinded to which hand was treated and was a different physician than who had done the prerecruitment screening electrodiagnostic and ultrasound assessments. Participants were instructed not to inform the assessors which side had been treated with TCTR. At 3 and 6 months post-TCTR, when the hand sensibility, strength, CSA, and electrodiagnostic reassessments were done, there was no residual visible evidence of the procedure to indicate which was the treated hand.
Details of the outcome measures are as follows:
Recovery questions (for each hand treated with the TCTR procedure): How much pain are you having in your hand (0-10 numerical rating scale of pain intensity)? Are you experiencing any fever, chills, hand redness, or discharge? How close to normal is your hand function now? Have you resumed normal life activities including work (if applicable)? How many days did you work in the past week? Have you noticed any complications from the TCTR procedure? If so, do you want our doctor to evaluate you?
BCTQ. This is a self-assessment questionnaire evaluating the severity of symptoms and functional limitation of the hand. Each hand was assessed separately. The scores range between 1 and 5 with 5 being the most severe symptoms or functional limitation.13 Psychometric evaluation has confirmed that the BCTQ is a valid, reliable, responsive, and acceptable outcome tool for clinical and research purposes.14
Overall satisfaction with the TCTR procedure was assessed using a 5-item Likert scale. Possible responses included the following: very dissatisfied, dissatisfied, neither satisfied nor dissatisfied, satisfied or very satisfied.
Hand sensibility testing was performed using Semmes-Weinstein monofilaments. The participants’ hand was placed palm up and the sensation at the pulp of the volar aspect of the third digit distal phalanx was tested. The participant was instructed to say yes when they felt the pressure of the monofilament on the skin. The participant closed their eyes and the monofilament was held perpendicular to the skin and pressure was applied to the monofilament until it bent into a C-shape. This position was held for 1.5 seconds and was repeated 3 times for monofilaments 2.83 g and 3.61 g. Perception of pressure with any of the 3 repetitions was considered a positive response. For monofilaments larger than 4.31 g through 6.65 g, the stimulus was applied only once. The testing started with the smallest monofilament size and progressed incrementally to larger filaments until sensation was perceived. The monofilament gauges (from smallest to largest) were 2.83 g, 3.61 g, 4.31 g, 4.56 g, and 6.65 g. Monofilament testing has been shown to have excellent intra- and intertest reliability, validity, and responsiveness.15
Grip and key pinch strength were measured using electronic grip and mechanical pinch gauge dynamometers, respectively. For each, the participant was seated with their shoulder adducted, elbow flexed at 90°, forearm in neutral position, and wrist between 0° and 30° dorsiflexion and between 0° and 15° ulnar deviation. The key pinch measurement was made by pressing the volar part of the thumb together with the radial side of the index finger. For each strength test, the scores of 3 successive trials were recorded for each hand and the average of the scores was recorded.16
US-derived median nerve CSA measurements were made at the wrist in the volar transverse plane between the pisiform and the scaphoid tubercle approximating the carpal tunnel inlet and at the level of the pronator quadratus muscle. A direct tracing method was used outlining the inner border of the hyperechoic epineurium. Median nerve CSA at the carpal tunnel inlet and wrist: forearm CSA ratio have been shown to be reliable and valid diagnostic tools for CTS and responsive to carpal tunnel release.17,18
Nerve conduction studies: All testing was done by the same practitioner using the same electrodiagnostic machine (Sierra Wave Cadwell, Kennewick, Washington). Skin temperature was maintained at ≥31°C. Median nerve motor and sensory conduction studies were performed according to the protocol previously published by the principal author.19 The thresholds for median nerve conduction abnormality (>2 SD beyond the mean) for the practitioner’s electrodiagnostic lab are motor to distal motor latency >4.2 ms, wrist to palm conduction velocity <42 m/s, and sensory to wrist to palm conduction velocity <43 m/s.
Analyses: 2-way (side × time), repeated measures (time) analysis of variance was performed for each quantitative outcome variable (BCTQ score, monofilament sensory testing, grip and pinch dynamometry strength measurement, median nerve CSA, transcarpal tunnel motor and sensory conduction velocities and proximal: distal amplitude ratios). Descriptive statistics were used to summarize recovery, adverse events, and patient satisfaction.
Results
Data collection was complete except for the 6-month post-TCTR measurements on one participant who was lost to follow-up. For that missing data, an intention to treat analysis was applied with the 3-month post-TCTR observations being carried forward. Tables 1 through 5 summarize the outcome measure results for both the treated and the control hands. The F statistics and P values are for the interaction effects of side × time.
Table 1.
Early Recovery Following TCTR: Mean (SD).
| Outcome measure | Hand | Pre-TCTR | 1 week post | 2 weeks post | 3 weeks post | 4 weeks post | F a | P a |
|---|---|---|---|---|---|---|---|---|
| Painb | TCTR | 6.0 (2.8) | 4.1 (2.6) | 2.7 (2.4) | 2.5 (2.2) | 2.5 (2.4) | 5.3 | <.05 |
| Control | 3.8 (2.5) | 3.3 (2.6) | 3.3 (2.8) | 3.3 (2.8) | 2.5 (2.2) | |||
| Functionc | TCTR | 5.6 (2.1) | 4.6 (2.4) | 3.0 (2.2) | 3.2 (2.4) | 2.6 (2.3) | 5.7 | <.05 |
| Control | 3.8 (2.4) | 3.3 (2.3) | 3.0 (2.6) | 3.4 (2.5) | 3.0 (2.5) | |||
| Work,d d/w | TCTRc | 4.6 (1.1) | 2.3 (2.0) | 3.7 (1.8) | 3.7 (1.9) | 4.2 (1.7) | 9.1 | <.05 |
| Control | 4.8 (0.7) | 4.3 (1.6) | 4.4 (1.4) | 4.3 (1.6) | 4.3 (1.6) |
Note. TCTR = thread carpal tunnel release.
Interaction effects of time × side.
Pain Numerical Rating Scale (0-10): 0 = no pain; 10 = worst possible pain.
Function (0-10): 0 = completely normal; 10 = not normal at all.
Work (0-7): Days worked in the past week.
Table 5.
Median Nerve Electrodiagnostic Changes Following TCTR: Mean (SD).
| Test | Hand | Pre-TCTR | 3 months post | 6 months post | F a | P a |
|---|---|---|---|---|---|---|
| Motor | ||||||
| Distal motor latency, ms | TCTR | 6.1 (1.8) | 4.6 (0.7) | 4.7 (0.7) | 7.2 | <.05 |
| Control | 5.0 (1.3) | 4.8 (1.6) | 4.9 (1.7) | |||
| Conduction velocity, m/s | TCTR | 22.2 (9.2) | 27.7 (10.8) | 29.6 (7.7) | 3.6 | <.05 |
| Control | 28.6 (9.0) | 29.1 (6.0) | 29.9 (10.7) | |||
| Wrist: palm CMAP amplitude ratio | TCTR | 0.95 (0.5) | 0.88 (0.5) | 1.2 (0.8) | 0.4 | >.05 |
| Control | 0.92 (0.4) | 0.76 (0.3) | 1.3 (0.7) | |||
| CMAP amplitude at wrist, mV | TCTR | 5.5 (2.1) | 6.2 (2.8) | 5.7 (3.2) | 0.1 | >.05 |
| Control | 6.1 (2.6) | 6.6 (3.7) | 6.0 (3.3) | |||
| Sensory | ||||||
| Conduction velocity, m/s | TCTR | 25.1 (9.9) | 31.2 (9.5) | 33.6 (11.7) | 3.4 | <.05 |
| Control | 30.7 (10.4) | 34.4 (16.2) | 31.7 (15.2) | |||
| Wrist: palm SNAP amplitude ratio | TCTR | 0.73 (0.4) | 0.74 (0.4) | 0.94 (0.4) | 0.1 | >.05 |
| Control | 0.86 (0.4) | 0.74 (0.4) | 1.0 (1.0) | |||
| SNAP amplitude at wrist, µV | TCTR | 16.5 (13.0) | 17.6 (13.1) | 19.4 (13.5) | 5.5 | <.05 |
| Control | 22.7 (14.0) | 19.8 (12.8) | 19.0 (13.5) | |||
Note. TCTR = thread carpal tunnel release; CMAP = compound motor action potential; SNAP = sensory nerve action potential.
Interaction effects of time × side.
Table 1 summarizes the 1-month post-TCTR subjective experience. No adverse events were reported. Pain and dysfunction significantly decreased for the treated hand within 1 week and plateaued by 2 to 3 weeks post-TCTR. Ability to work with the treated hand decreased within the first week, returned to close to normal by 2 weeks, and to normal by 4 weeks post-TCTR. The BCTQ results are summarized in Table 2. Significant improvements of both symptoms and function were seen particularly within the first month post-TCTR with slight continued improvement for 6 months post-TCTR. Interestingly, the improvement was significant for both the treated and the control hands, although the magnitude of improvement was significantly greater in the treated hand (F = 41; P < .05). As outlined in Table 3, there was no significant change in hand sensibility or grip strength of either hand. There was a statistically significant improvement in pinch strength, but it was not disproportionate on either side (F = 6.4; P < .05). The CSA of the median nerves at the carpal tunnel inlet tended to decrease within the first 3 months post-TCTR. This decrease was significant for both median nerves as measured by wrist: forearm ratio (F = 14.4; P < .05) but was not disproportionate on either side (Table 4). Several improvements in electrodiagnostic function of the median nerves were documented as summarized in Table 5. Significant disproportionate treated hand improvements in distal motor latency, transcarpal tunnel motor conduction velocity, transcarpal tunnel sensory conduction velocity and sensory nerve action potential (SNAP) amplitude occurred. In general, improvement continued over the 6 months post-TCTR. Statistically significant improvement in wrist: palm compound motor action (CMAP) amplitude ratio was documented (F = 9.2; P < .05) but it was not disproportionate for the treatment hand.
Table 2.
Boston Carpal Tunnel Questionnaire Following TCTR: Mean (SD).
| Outcome measure | Hand | Pre-TCTR | 1 month post | 3 months post | 6 months post | F a | P a |
|---|---|---|---|---|---|---|---|
| Symptomsb | TCTR | 3.4 (0.9) | 1.8 (1.0) | 1.6 (0.9) | 1.5 (0.8) | 15.1 | <.05 |
| Control | 2.5 (0.7) | 2.4 (0.8) | 2.1 (0.9) | 1.9 (1.0) | |||
| Functionc | TCTR | 3.2 (1.0) | 2.1 (1.0) | 1.6 (1.1) | 1.4 (0.9) | 5.2 | <.05 |
| Control | 2.6 (0.7) | 2.1 (1.1) | 2.1 (1.1) | 1.9 (1.1) | |||
| Totald | TCTR | 6.6 (1.1) | 3.9 (1.8) | 3.2 (1.9) | 2.9 (1.7) | 11.3 | <.05 |
| Control | 5.1 (1.8) | 4.5 (2.1) | 4.2 (1.9) | 3.7 (2.0) |
Note. TCTR = thread carpal tunnel release.
Interaction effects of time × side.
Symptoms (1-5): 1 = no symptoms; 5 = maximum symptoms.
Function (1-5): 1 = no functional difficulty; 5 = nonfunctional.
Total: Sum of symptom and function scores.
Table 3.
Hand Sensation and Strength Following TCTR: Mean (SD).
| Outcome measure | Hand | Pre-TCTR | 3 months post | 6 months post | F c | P a |
|---|---|---|---|---|---|---|
| Sensibility | TCTR | 3.2 (0.9) | 3.1 (0.8) | 3.2 (0.9) | 0.2 | >.05 |
| Control | 3.0 (0.6) | 2.9 (0.4) | 3.0 (0.6) | |||
| Pinch, kg | TCTR | 7.5 (3.3) | 7.6 (3.2) | 8.2 (3.7) | 0.03 | >.05 |
| Control | 7.8 (3.4) | 7.9 (3.2) | 8.6 (3.7) | |||
| Grip, kg | TCTR | 34.4 (18.3) | 33.2 (19.4) | 35.1 (19.8) | 1.0 | >.05 |
| Control | 35.1 (18.3) | 35.8 (19.3) | 36.2 (19.2) |
Note. TCTR = thread carpal tunnel release.
Interaction effects of time × side.
Table 4.
Median Nerve CSA Following TCTR: Mean (SD).
| CSA | Hand | Pre-TCTR | 3 months post | 6 months post | F a | P a |
|---|---|---|---|---|---|---|
| At carpal tunnel inlet, mm2 | TCTR | 13.2 (3.9) | 11.6 (4.5) | 11.9 (4.6) | 1.1 | >.05 |
| Control | 13.8 (5.6) | 12.5 (2.6) | 12.6 (4.3) | |||
| Wrist: forearm ratio | TCTR | 1.4 (0.5) | 1.0 (0.3) | 1.0 (0.3) | 1.6 | >.05 |
| Control | 1.2 (0.4) | 1.0 (0.3) | 1.0 (0.2) |
Note. CSA = cross-sectional area; TCTR = thread carpal tunnel release.
Interaction effects of time × side.
Of overall satisfaction with the TCTR procedure, at 3 months, one participant was very dissatisfied, one was dissatisfied, 6 were satisfied, and 12 were very satisfied. At 6 months, 2 were very dissatisfied, one was neither satisfied nor dissatisfied, and 15 were very satisfied. Overall, 85% to 90% of participants were satisfied or very satisfied with their TCTR procedure. Both very dissatisfied participants went on to have open carpal tunnel release. The TCTR procedure of one of these participants had been done with the original technique involving a distal to proximal cut of the TCL. Follow-up ultrasound suggested that the distal portion of the TCL had been incompletely transected. The open release completed the decompression and the participant experienced prompt complete relief of her CTS symptoms. The other very dissatisfied participant was a 95-year-old man with severe CTS symptoms and electrophysiologic abnormalities (nonfunctioning sensory and barely functioning motor conductions). At open surgery, he was found to have a very thick TCL with severe compression and atrophy of the median nerve at the carpal tunnel. At 6 months post-open release, he had not experienced any symptom improvement.
Discussion
The objective of this study was to independently evaluate the safety and the effectiveness of the TCTR procedure using multiple outcome measures and a control comparison. Like the experience of Guo et al, we found the procedure to be safe and well tolerated.8,10,11 The TCTR procedure also effectively reduced the severity of CTS symptoms and dysfunction as measured by the BCTQ. The magnitude of the BCTQ score improvements at 1, 3, and 6 months post-TCTR were comparable with those reported by Guo et al. When compared with the BCTQ scores recorded longitudinally over 12 months following endoscopic and open carpal tunnel releases documented in the literature, Guo et al found that the TCTR symptom and function improvements were superior and occurred earlier after the procedure.11,20 Similarly, a randomized controlled trial by Rojo-Manaute et al documented greater disability improvement and faster return to normal daily activities with an ultrasound-guided ultraminimally invasive carpal tunnel release technique using a hook knife than a blind mini-open carpal tunnel release.4
It was interesting to observe that our unilateral TCTR had beneficial effects on the untreated hand both subjectively (BCTQ) and objectively (pinch strength, median nerve CSA at the carpal tunnel inlet and wrist: palm CMAP amplitude ratio). Thirteen of the 20 untreated “control hands” had symptomatic CTS. It is possible the subjective and objective control hand improvements reflected decreased compensatory demand of the control hand as the function of the treated hand improved post-TCTR.
Some, but not all of the objective measures of median nerve function and morphology validated the effectiveness of TCTR. We found the procedure had no significant impact on hand sensibility or strength, which is contrary to the report of Jerosch-Herold et al who found monofilament sensibility sensory testing to be moderately responsive to open carpal tunnel release.21 We documented a trend toward reduced median nerve CSA at the carpal tunnel inlet post-TCTR, which has also been reported by others.18 We also documented objective electrophysiologic improvements in median nerve function. Motor and sensory conduction velocities improved steadily over 6 months as has been reported by other investigators following open carpal release.18,22 SNAP amplitude also steadily improved post-TCTR but the CMAP amplitude did not. This is compatible with the observation that after carpal tunnel decompression, CMAP amplitude transiently decreases before starting to increase.22
The strengths of this study include the use of a control, multiple outcome measures, and blinding of some of the assessors. Weaknesses of the study include that the control was not independent, and one assessor was not blinded. The functional status of the treatment side likely had an effect on the subjective and objective status of the control side, especially considering the control side was symptomatic in the majority of participants. However, from a statistical perspective, this would make it less likely to commit a type I error. Accordingly, conclusions regarding between hand differences are conservative.
In conclusion, this study supports previous reports claiming ultrasound-guided, ultraminimally invasive percutaneous thread carpal tunnel release procedure is safe and effective. Future studies directly comparing this technique with open and/or endoscopic carpal tunnel release will be of interest.
Acknowledgments
The authors acknowledge Loren Jacobs, MSc, and Joe Burnham, MBA, for their assistance with data collection and project management, and Shaun Gray, MD, PhD, for his assistance with article review.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008.
Statement of Informed Consent: Informed consent was obtained from all participants in the study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Eldon Y. Loh  https://orcid.org/0000-0001-5063-4827
https://orcid.org/0000-0001-5063-4827
Larry D. Playfair  https://orcid.org/0000-0003-0794-826X
https://orcid.org/0000-0003-0794-826X
References
- 1. Huisstede BM, Randsdorp MS, Coert H, et al. Carpal tunnel syndrome. Part II: effectiveness of surgical treatments—a systematic review. Arch Phys Med Rehabil. 2010;91:1005-1024. [DOI] [PubMed] [Google Scholar]
- 2. Rodner CM, Katarincic J. Open carpal tunnel release. Tech Orthop. 2006;21(1):3-11. [Google Scholar]
- 3. McShane JM, Slaff S, Gold JE, Nazarian LN. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: preliminary report. J Ultrasound Med. 2012;31(9):1341-1349. [DOI] [PubMed] [Google Scholar]
- 4. Rojo-Manaute J, Capa-Grasa A, Chana-Rodriguez F, et al. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016;35:1149-1157. [DOI] [PubMed] [Google Scholar]
- 5. Petrover D, Silvera J, De Baere T, et al. Percutaneous ultrasound-guided carpal tunnel release: study upon clinical efficacy and safety. Cardiovasc Intervent Radiol. 2017:40568-40575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Buncke G, McCormack B, Bodor M. Ultrasound-guided carpal tunnel release using the Manos CTR system. Microsurgery. 2013;33(5):362-366. [DOI] [PubMed] [Google Scholar]
- 7. Markison RE. Percutaneous ultrasound-guided MANOS carpal tunnel release technique. Hand (N Y). 2013;8(4):445-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo D, Tang Y, Ji Y, et al. A non-scalpel technique for minimally invasive surgery: percutaneously looped thread transection of the transverse carpal ligament. Hand (N Y). 2015;10(1):40-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burnham R, Playfair L, Loh E, et al. Evaluation of the effectiveness and safety of ultrasound-guided percutaneous carpal tunnel release—a cadaveric study. Am J Phys Med Rehabil. 2017;96(7):457-463. [DOI] [PubMed] [Google Scholar]
- 10. Guo D, Guo D, Guo J, et al. A cadaveric study for the improvement of thread carpal tunnel release. J Hand Surg Am. 2016;41(10):e351-e357. [DOI] [PubMed] [Google Scholar]
- 11. Guo D, Guo D, Guo J, et al. A clinical study of the modified thread carpal tunnel release (TCTR). Hand. 2017;12(5):453-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McDonald E, Gordon JA, Buckley JM, Gordon L. Comparison of a new multifilament stainless steel suture with frequently used sutures for flexor tendon repair. J Hand Surg Am. 2011;36(6):1028-1034. [DOI] [PubMed] [Google Scholar]
- 13. Levine D, Simmons BP, Koris MJ, et al. A self-administered questionnaire for the assessment of severity of symptoms and functional status in carpal tunnel syndrome. J Bone Joint Surg Am. 1993;75(11):1585-1592. [DOI] [PubMed] [Google Scholar]
- 14. Leite JC, Jerosch-Herold C, Song F. A systematic review of the psychometric properties of the Boston Carpal Tunnel Questionnaire. BMC Musculoskelet Disord. 2006;7(null):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Novak C. Evaluation of hand sensibility—a review. J Hand Ther. 2001;14(4):266-272. [DOI] [PubMed] [Google Scholar]
- 16. Mathiowetz V, Kashman N, Volland G, et al. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69-74. [PubMed] [Google Scholar]
- 17. Cartwright M, Hobson-Webb L, Boon A, et al. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46(2):287-293. [DOI] [PubMed] [Google Scholar]
- 18. Mondelli M, Filippou G, Aretini A, et al. Ultrasonography before and after surgery in carpal tunnel syndrome and relationship with clinical and electrophysiological findings. A new outcome predictor? Scand J Rheumatol. 2008;37:219-224. [DOI] [PubMed] [Google Scholar]
- 19. Burnham R, Steadward R. Upper extremity peripheral nerve entrapments among wheelchair athletes: prevalence, location, and risk factors. Arch Phys Med Rehabil. 1994;75(5):519-524. [PubMed] [Google Scholar]
- 20. Trumble TE, Diao E, Abrams RA, Gilbert-Anderson MM. Single-portal endoscopic carpal tunnel release compared with open release: a prospective, randomized trial. J Bone Joint Surg Am. 2002;84-A(7):1107-1115. [DOI] [PubMed] [Google Scholar]
- 21. Jerosch-Herold C, Shepstone L, Miller L, Chapman P. The responsiveness of sensibility and strength tests in patients undergoing carpal tunnel decompression. BMC Musculoskelet Disord. 2011;12:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ginanneschi F, Milani P, Reale F, Rossi A. Short-term electrophysiological conduction change in median nerve fibres after carpal tunnel release. Clin Neurol Neurosurg. 2008;110(10):1025-1030. [DOI] [PubMed] [Google Scholar]