Abstract
Background
Studies have reported beneficial effects of exercise training on autoimmunity, and specifically on multiple sclerosis (MS) and experimental autoimmune encephalomyelitis (EAE). However, it is unknown whether different training paradigms affect disease course via shared or separate mechanisms.
Objective
To compare the effects and mechanism of immune modulation of high intensity continuous training (HICT) versus high intensity interval training (HIIT) on systemic autoimmunity in EAE.
Methods
We used the proteolipid protein (PLP)‐induced transfer EAE model to examine training effects on the systemic autoimmune response. Healthy mice performed HICT or HIIT by running on a treadmill. Lymph‐node (LN)‐T cells from PLP‐immunized trained‐ versus sedentary donor mice were transferred to naïve recipients and EAE clinical and pathological severity were assessed. LN cells derived from donor trained and sedentary PLP‐immunized mice were analyzed in vitro for T‐cell activation and proliferation, immune cell profiling, and cytokine mRNA levels and cytokine secretion measurements.
Results
Both HICT and HIIT attenuated the encephalitogenicity of PLP‐reactive T cells, as indicated by reduced EAE clinical severity and inflammation and tissue pathology in the central nervous system, following their transfer into recipient mice. HICT caused a marked inhibition of PLP‐induced T‐cell proliferation without affecting the T‐cell profile. In contrast, HIIT did not alter T‐cell proliferation, but rather inhibited polarization of T cells into T‐helper 1 and T‐helper 17 autoreactive populations.
Interpretation
HICT and HIIT attenuate systemic autoimmunity and T cell encephalitogenicity by distinct immunomodulatory mechanisms.
Introduction
Exercise training modulates various autoimmune diseases, such as systemic lupus erythematosus, rheumatoid arthritis, inflammatory bowel disease and others. 1 Accordingly, there is an increasing body of research on the beneficial outcomes of exercise among multiple sclerosis (MS) patients. 2 , 3 Although the favorable effects of exercise on general health and its immuno‐modulatory effects are well accepted, 4 a thorough understanding of the immunologic effects on autoimmune diseases is still lacking.
We recently demonstrated that moderate‐intensity continuous training (MICT) reduced the encephalitogenicity of autoreactive T cells and attenuated transfer experimental autoimmune encephalomyelitis (EAE), an animal model used for the study of autoimmune‐mediated disease of the central nervous system (CNS). 5 We further demonstrated that high‐intensity continuous training (HICT) induced superior benefits in preventing autoimmunity in EAE as compared to MICT, without increasing susceptibility to acute bacterial infection, suggesting a preserved innate immune system response. 6
In recent years, high intensity interval training (HIIT) has become popular. The main advantage of HIIT over HICT is the shorter time required to achieve similar or superior physiological and metabolic adaptations to training. 7 , 8 , 9 Beneficial effects of HIIT have also been documented in several autoimmune diseases, including rheumatoid arthritis and diabetes. 10 , 11 , 12 Several studies also indicate that HIIT is safe and effective in improving fitness in people with MS. 13 However, no studies have compared the clinical efficacy of HIIT and HICT on autoimmunity, nor their mechanisms by which they induce immune modulation.
Thus, the aim of this study was to compare the clinical and immunologic effects of HIIT and HICT on development of systemic autoimmune response that causes EAE. We employed the chronic‐relapsing transfer EAE model that enabled investigation of the effects of exercise selectively on the systemic immune system.
Materials and Methods
Experimental animals
Female SJL/JCrHsd mice (6‐7 weeks of age) were purchased from Envigo Inc, Israel. Animal experimentation was approved by the Institutional Animal Care and Use Committee. The studies were conducted in accordance with the United States Public Health Service's Policy on Humane Care and Use of Laboratory Animals.
Experimental design
The PLP 139‐151 transfer EAE model in mice was utilized as previously described. 5 , 6 , 14 This model enabled comparison of the effects of HICT and HIIT on systemic autoimmunity, as indicated by induction of lymph node (LN)‐derived T‐cell encephalitogenicity (Figure 1A). Healthy mice were subjected to one of two defined treadmill high‐intensity running paradigms, followed by immunization with a proteolipid protein (PLP) peptide (after 5 weeks of training). Three days after the last exercise bout, inguinal LN‐T cells were isolated, stimulated in culture with PLP peptide and injected into naïve recipient mice that subsequently developed EAE. Recipient mice that were injected with PLP‐reactive LN‐T cells from sedentary (SED) mice served as controls. Encephalitogenicity was evaluated: 1. in vivo by clinical and pathological severity of EAE following transfer of LN‐T cells from HICT or HIIT versus SED donor mice into recipient naïve mice; and 2. in vitro following secondary activation of lymph node cells (LNCs) by the PLP auto‐antigen.
Figure 1.
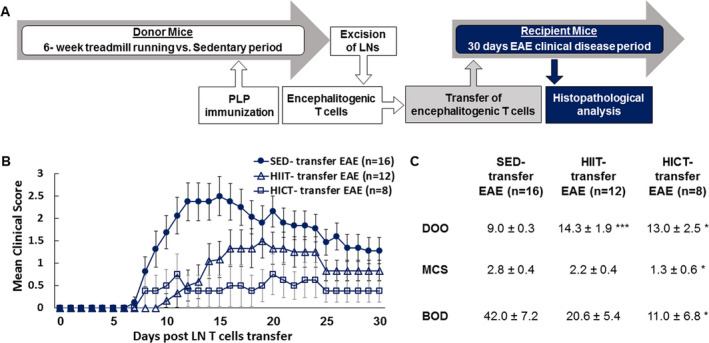
Superior inhibitory effect of high‐intensity continuous training on the encephalitogenicity of lymph node (LN) T cell derived from proteolipid (PLP) ‐ immunized mice in transfer model of experimental autoimmune encephalomyelitis (EAE). (A) Experimental protocol to investigate the effects of exercise training on the systemic immune system in transfer EAE model. Healthy mice were subjected to a 6‐week high‐intensity interval training (HIIT) or high‐intensity continuous training (HICT) treadmill‐running. At the end of the 5th week of training, HIIT, HICT, and sedentary (SED) mice were immunized with a PLP peptide and at the end of the 6th week their lymph nodes (LNs) were removed and stimulated in culture with PLP peptide. Donor HIIT‐, HICT‐, or SED‐ derived encephalitogenic T cells were injected to naïve recipient mice, which developed EAE and were scored daily for neurological symptoms up to 30 days post‐transfer. Mice were sacrificed for central nervous system histopathology analyses 12 days post EAE induction, at the acute phase of disease. Clinical course (B) and clinical parameters (C) of transfer EAE in mice that received PLP‐ reactive LN‐ T cells from high‐intensity interval trained (HIIT‐transfer EAE, n = 12), high‐intensity continuous trained (HICT‐transfer EAE, n = 8) or sedentary (SED‐ transfer EAE, n = 16) mice. The severity of EAE was scored on a 0–6 scale. Transfer of LN‐ T cells derived from both HIIT and HICT, PLP‐immunized mice to naïve recipients induced attenuated EAE, with better effects of HICT on the overall severity of the disease. DOO – day of onset, MCS – maximal clinical score, BOD – burden of disease. Data are mean ± SE. *P < 0.05, **P < 0.01, ***P < 0.001, as compared to SED‐ transfer EAE.
Treadmill exercise training
Healthy mice underwent 6 weeks of HICT or HIIT treadmill running, including pre‐ and post‐ training performance tests on a 5‐lane treadmill designed for mice (Panlab Harvard Apparatus, USA) as previously described. 5 , 6 The 6 weeks of training started with 3 weeks of an identical preparatory period in both HICT and HIIT protocols (see Table 1). The subsequent 3 weeks differed between the two protocols. The running speed in HICT and HIIT protocols were based on exhaustion speed performance tests (Table 2). HICT was defined as 70‐75% of exhaustion speed, while HIIT was performed at 85‐90% of exhaustion speed. Other aspects of the HIIT protocol (e.g., interval duration, rest speed, and duration etc.) were based on widely used training protocols for animals. 15 , 16 , 17
Table 1.
High‐intensity continuous and interval training programs.
| 1st week | 2nd week | 3rd week | 4th week | 5th week | 6th week | |||
|---|---|---|---|---|---|---|---|---|
| HICT | Duration per session | 10 min | 20 min | 30 min | 23 min | |||
| Speed per session | 23 cm/sec | 28 cm/sec | 30 cm/sec | |||||
| Sessions per week | 5 d/w | 3 d/w | ||||||
| HIIT | Duration per session | 10 min | 20 min | 30 min | Interval Speed | 34 cm/sec | 36 cm/sec | |
| Interval duration | 1 min | |||||||
| Rest Speed | 12 cm/sec | |||||||
| Rest duration | 2 min | |||||||
| Speed per session | 23 cm/sec | Repetitions per session | 5 | 7 | ||||
| Sessions per week | 5 d/w | Sessions per week | 3 d/w | |||||
HICT, High Intensity Continuous Training; HIIT, High Intensity Interval Training; Min, minutes; cm/sec, centimeters per second; d/w, days per week.
Table 2.
High‐intensity training programs improve performance.
| SED (n = 8) | HIIT (n = 5) | HICT (n = 8) | ||||
|---|---|---|---|---|---|---|
| Test type | Pre SED period | Post SED period | Pre ET | Post ET | Pre ET | Post ET |
| Exhaustion Speed (cm/sec) | 42.5 ± 1.1 | 43.0 ± 1.3 | 44.4 ± 0.7 | 53.6 ± 1.4*** | 43.0 ± 0.7 | 47.2 ± 0.5* |
| Exercise Tolerance (min:sec) | 15:11 ± 1:12 | 18:07 ± 1:28 | 16:17 ± 1:40 | 36:22 ± 0:59*** | 15:29 ± 0:44 | 30:08 ± 1:52*** |
SED, sedentary; HICT, High Intensity Continuous Training; HIIT, High Intensity Interval Training.
Data are represented as mean ± SE.
P < 0.05,
P < 0.001, as compared to pre‐training period.
Transfer experimental autoimmune encephalomyelitis (EAE)
The PLP 139‐151 transfer EAE model in mice was utilized as described previously. 5 , 6 , 14 EAE was developed in recipient mice, induced by a transfer of LN‐ T cells obtained from HICT, HIIT or SED donor mice (Figure 1A). Recipient EAE mice were assessed daily for neurological symptoms for up to 30 days after EAE induction.
Histopathology analyses
Twelve days after LN‐T cell transfer (at the acute phase of disease), a group of SED and HIIT EAE mice were sacrificed for histopathological analysis as described previously. 5 , 6 , 14 Serial paraffin‐embedded transverse sections were obtained from mid‐cervical, mid‐thoracic and mid‐lumbar levels of the spinal cords. Sections were stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB)/nuclear fast red to assess inflammation and demyelination, respectively. Immunohistochemistry was performed in adjacent serial sections for macrophages (rat anti‐mouse Mac3, 553322, 1:800, BD Pharmingen), T cells (monoclonal rabbit anti–CD3, RM‐9107‐SO; 1:800, Thermo‐Scientific), and non‐phosphorylated neurofilament H (anti‐mouse SMI32, NE1023, 1:2000, Calbiochem). For each staining, the whole white matter of three sections per mouse were quantified, one section per each spinal cord level. The number of immune cells in perivascular infiltrates were counted in H&E stained sections, and reported as the total average number per square millimeter. Mac3+ and CD3+ cells were counted in the perivascular infiltrates and parenchyma, and reported as total average number of each cell type per square millimeter. Demyelination and axonal damage were assessed by calculating the area of LFB loss and SMI32 protein expression, respectively. All pathology measurements were performed by using the Image J software analysis (ver. 1.51H, NIH, USA).
In vitro analyses of lymph node cells (LNCs) and encephalitogenic T cells
Lymph nodes were excised from HICT, HIIT or SED mice at 10 days after PLP immunization (Figure 3A). LNCs were cultured as single cell suspensions with either 10 μg/mL PLP peptide, 2.5 μg/mL concanavalin A (ConA) or no stimulation. 5 , 6 , 14 LNCs at day of excision and following 72 hours in culture were analyzed for proliferation and cell profiling (using flow cytometry), cytokine gene determination [real time‐PCR (RT‐PCR)] and cytokine secretion (MAGPIX System).
Figure 3.
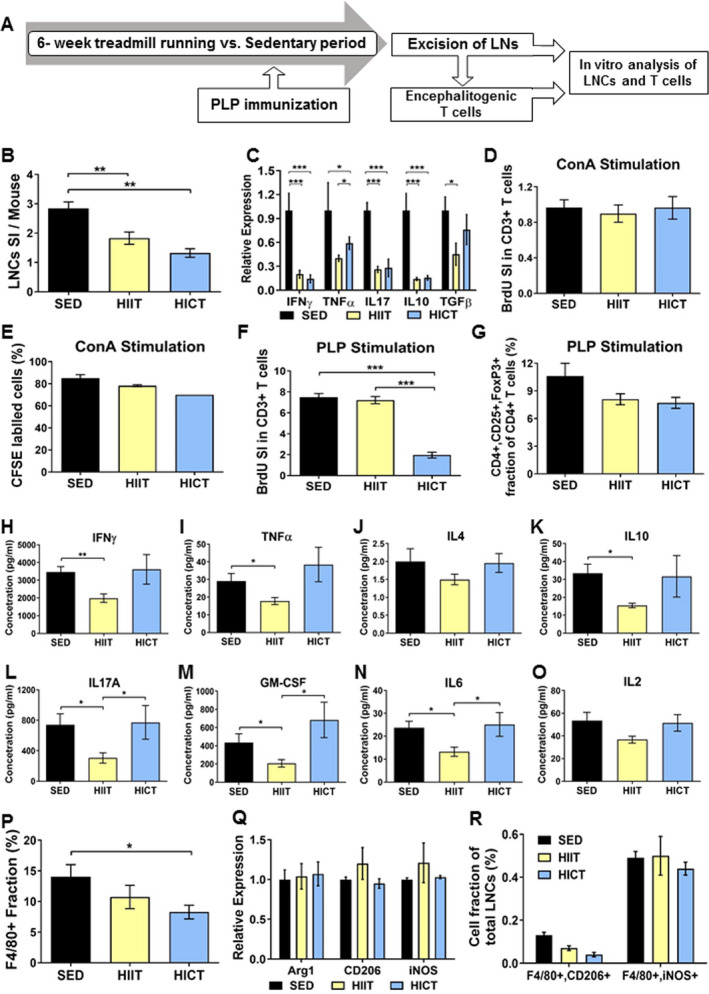
Distinct immuno‐modulatory effects of high‐intensity continuous and interval training on lymph node cells (LNCs), T cells, and macrophages derived from proteolipid (PLP) immunized mice. A: PLP‐ stimulated lymph node cells (LNCs) were excised from high‐intensity interval trained (HIIT), high‐intensity continuous trained (HICT), and sedentary (SED) mice at 10 days post PLP immunization and were stimulated in vitro for 72 h with PLP peptide or concanavalinA (ConA, n = 5‐8/group/experiment). B: Number of LNCs per mouse at day of LNs excision represented by stimulation index (SI): The number of LNCs in the experimental group divided by the number of LNCs in naïve, non‐immunized mice (n = 3). C: Cytokine mRNA levels in LNCs at day of LNs excision for interferon (IFN)‐ γ, tumor necrosis factor (TNF)‐ α, interleukin (IL)‐17, IL‐10 and transforming growth factor (TGF)‐β. Bromodeoxyuridine (BrdU) incorporation into CD3+ T cells at 72 h after ConA (D) or PLP (G) stimulation in vitro, represented by SI: The fraction of CD3+ BrdU+ T cells in the experimental group divided by the fraction of CD3+ BrdU+ T cells in naïve, non‐immunized mice. E: 5(6)‐carboxyfluorescein diacetate succinimidyl ester (CFSE) labeling at 72 h after ConA stimulation in vitro. F: CD4+, CD25+ FoxP3+ regulatory T cells at 72 h after PLP stimulation in vitro, represented as cell fraction of total T cells. H‐O: protein concentrations of IFNγ (H), TNFα (I), IL‐4 (J), IL‐10 (K), IL17A (L), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF, M), IL‐6 (N) and IL‐2 (O) in supernatants of PLP‐stimulated T cell cultures from PLP‐immunized HIIT, HICT and SED‐ mice. P: F4/80+ macrophage fractions of total LNCs at 72 h after PLP stimulation in vitro for. Q: mRNA levels of arginase (Arg)‐1 and CD206 M2 macrophages phenotype markers and inducible nitric oxidase synthase (iNOS) M1 type macrophages marker at 72 h after PLP stimulation in vitro. R: F4/80+, CD206+ M2 macrophages and F4/80+,iNOS + M1 macrophages fractions out of total LNCs population at 72 h after PLP stimulation in vitro. In response PLP immunization: (1) HICT induced reduction in the number of LNCs (B) and mRNA levels of cytokines (C) on day of LNs excision, and T cell proliferation (G) and number of macrophages (P) in response to PLP stimulation in vitro. (2) HIIT induced reduction in the number of LNCs (B) and mRNA levels of cytokines (C) on day of LNs excision, and Th1 (H, I), Th17 (L, M) and pro‐inflammatory IL6 (N) cytokines secretion in response to PLP stimulation in vitro. (3) Neither HICT nor HIIT affected the fraction of regulatory T cells (F), Th2‐ derived IL4 (J) and IL2 (O) cytokines secretion and macrophages phenotype (Q, R) after 72 h of PLP stimulation in vitro. (4) Training did not affect proliferation (D) and division cycles (E) of T cells in response ConA non‐specific stimulation. Data are mean ± SE. G, P: Relative expression to SED group = 1. * P < 0.05, **P < 0.01, ***P < 0.001.
In vitro activation and proliferation assays
Stimulation index (SI) was calculated as LNCs number in the experimental group divided by LNCs number in naïve, non‐immunized mice. The proliferation of T cells was evaluated by flow cytometry analysis for bromodeoxyuridine (BrdU) incorporation and for the incorporation of the cell division tracking dye 5(6)‐carboxyfluorescein diacetate succinimidyl ester (CFSE). 5 , 6 , 14 SI was calculated as the fraction of CD3+, BrdU+ cells (relative to total) in the experimental group divided by the fraction in naïve, non‐immunized mice with no secondary activation. All samples were analyzed in a Cytomics FC 500 apparatus (Beckman Coulter, Life Science) using the CXP analysis software (ver. 2.1; Informer Technologies, Inc).
Flow cytometry for immune cells profiling
LNCs from HIIT, HICT, and SED mice were analyzed for immune cell profiling, at day of excision or following their activation in vitro with PLP peptide, using flow cytometry. Triple staining with FITC‐labeled anti‐CD4 (eBiosciense), APC‐labeled anti‐CD25 (eBiosciense) and PE‐labeled anti‐FoxP3 (eBiosciense) were used to identify the fraction of regulatory CD4+, CD25+, FoxP3+ T cells. The fraction of macrophages was calculated following staining with FITC‐labeled anti‐F4/80 (eBioscience). FITC‐labeled anti‐F4/80 macrophages were analyzed for M1 and M2 phenotypes following double staining with APC‐labeled anti‐inducible nitric oxide synthase (iNOS, Invitrogen) or APC‐labeled anti‐CD206 (R&D), respectively. In all flow cytometry experiments, cells were pre‐coated with anti–mouse CD16/CD32 (BD Pharmingen), as an Fc blocker, to block non‐specific binding. In early experiments antibodies were tested for their specificity with an isotype control. All samples were analyzed in a Cytomics FC 500 apparatus (Beckman Coulter, Life Science) using the CXP analysis software (ver. 2.1; Informer Technologies, Inc).
Cytokine gene determination
Total RNA was prepared using the RNeasy Plus Mini Kit (QIAGEN) from LNCs that were excised from mice 10 days after PLP immunization or following an additional 72‐hour stimulation in culture with PLP. cDNA was prepared from 300 ng total RNA using qScript cDNA Synthesis Kit (Quanta Biosciences), according to the manufacturer’s instructions. Semiquantitative real‐time PCR was performed using the PerfeCTa SYBR Green FastMix, ROX (Quanta Biosciences).
Cytokine secretion assessment
Cytokine concentrations in PLP‐stimulated T‐cell supernatants were performed with MILLIPLEX® MAP mouse high sensitivity T‐cell magnetic bead panels, according to the manufacturer’s instructions (EMD Millipore Corp., MO, United States). The Luminex xMAP® technology was utilized, based on immunoassay performed on the surface of fluorescent‐coded magnetic beads MagPlex®‐C microspheres. Acquisition and data analysis were performed using the Luminex analyzer (MAGPIX®) software (Bio‐Rad Laboratories, Hercules, CA, USA).
Statistical analyses
For performance tests, the values before and after training for each experimental group were compared using Student’s paired t tests. When comparing >2 mean values, a one‐way analysis of variance (ANOVA) was used. If a significant F ratio was found, then the Newman–Keuls multiple comparison test was used to identify the location of significance. For pathology parameters the experimental groups were compared using two‐tailed Mann–Whitney test. Data were analyzed in GraphPad Prism software v.5. Differences were considered statistically significant at P < 0.05. All data are presented as mean ± standard error of mean (SE).
Results
High‐intensity training paradigms improve physical performance
HIIT and HICT significantly improved both maximal speed and exercise tolerance, whereas no significant changes were noted in SED mice (Table 2). As expected, the HIIT‐induced improvements in running performance were markedly greater than those induced by HICT for maximal speed (P < 0.001) and for exercise tolerance (P < 0.05). HIIT improved exhaustion speed and exercise tolerance significantly more than HICT (P < 0.001 and P < 0.01, respectively) and SED (P < 0.001 and P < 0.001, respectively).
High‐intensity training paradigms induce systemic immune‐modulation in donor mice to attenuate the clinical course of EAE in recipient mice
The transfer of encephalitogenic LN‐T cells derived from PLP‐immunized HICT and HITT mice induced a milder clinical course of EAE in recipient mice, as compared to mice that received LN‐T cells derived from PLP‐immunized SED mice (Figures 1B,C). Noteworthy is that the mildest overall clinical course of disease occurred in the HICT transfer group as assessed by maximal clinical score (MCS, P < 0.05) and burden of disease (BOD, P < 0.05, Figure 1C).
PLP‐reactive LN‐T cells from HIIT mice induce less tissue damage and milder inflammation in recipient EAE mice spinal cords
We previously showed that the transfer of encephlitogenic T cells from HICT mice caused ~80% less neuroinflammation, 65% reduction in the area of demyelination and ~10% decrease in non‐phosphorylated‐neurofilament SMI32‐ expressing injured axons than encephalitogenic T cells from SED mice. 6 We therefore examined whether the attenuated disease progression induced by LN‐T cells derived from HIIT mice was also associated with reduced tissue injury.
While SED‐transfer EAE mice exhibited extensive demyelination (Figure 2A‐C), axonal injury (Figure 2D‐F) and inflammation (Figure 2G‐O), tissue damage and inflammatory process were substantially attenuated in HIIT‐transfer EAE mice. Demyelination and axonal injury were decreased by ~60% in HIIT‐treated mice (P < 0.001 and P < 0.001, respectively; Figure 2A‐F). Total immune cell infiltration and CD3+ T‐cell infiltration were decreased by ~70‐75% (P < 0.01 and P < 0.001, respectively; Figure 2G‐L). An exception was in Mac3+ macrophages, where improvement amounted to ~25% (P < 0.05, Figure 2M‐O).
Figure 2.
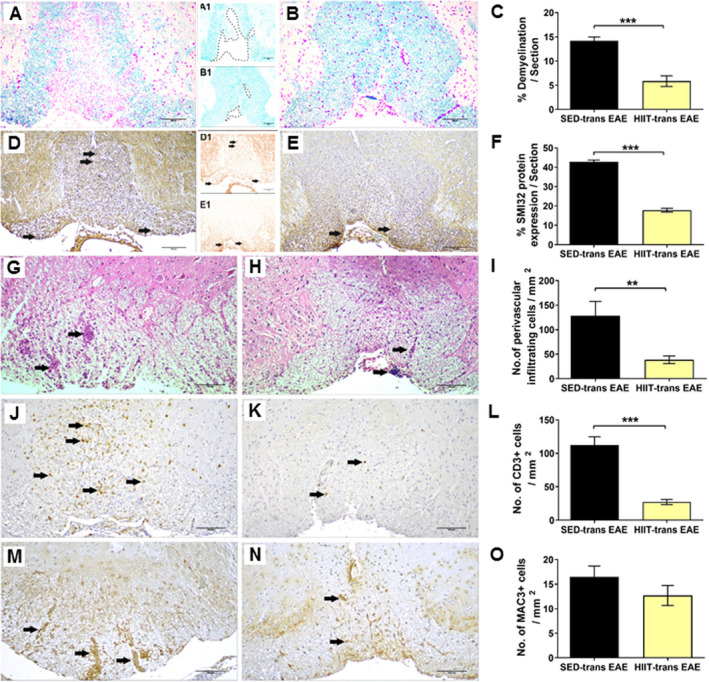
Attenuation of acute pathology in the spinal cords of experimental autoimmune encephalomyelitis (EAE) mice injected with proteolipid (PLP)‐reactive lymph node (LN) ‐T cells from high‐intensity interval trained mice. Evaluation of demyelination (A‐C), axonal damage (D‐F), and inflammation (G‐O) on cross sections of the spinal cords in EAE mice that were injected with LN‐ T cells from control sedentary (SED‐ transfer [trans] EAE; A, A1, D, D1, G, J, M; n = 6) or from high‐intensity interval trained mice (HIIT‐ trans EAE; B, B1, E, E1, H, K, N; n = 6) mice, at 12 days post EAE induction. C, F – quantification of tissue pathology in spinal cord white matter. I ‐ O – counts of inflammatory cell types in spinal cord white matter. A, B – luxol fast blue (LFB) histochemistry with periodic acid schiff (PAS) counterstaining, A1, B1 – LFB histochemistry without PAS counterstaining, dashed lines represent areas of demyelination shown in A and B, respectively; D, E – SMI32 immunohistochemistry with hematoxylin counterstaining, D1, E1 ‐ SMI32 immunohistochemistry without hematoxylin counterstaining, in brown SMI32+ injured axons. LFB staining showed reduction in the area of demyelination in HIIT‐transfer EAE (B, B1) versus SED‐transfer EAE mice (A, A1, C). SMI32 immunostainnings showed less axonal damage (F) in HIIT‐transfer EAE (E, E1) than in control SED‐transfer EAE mice (D, D1). In HIIT trans‐ EAE mice there was a reduction in total perivascular immune cell infiltrations (H), in CD3+ T cells (K) and non‐significant reduction in Mac3+ macrophages (N) versus SED tans‐EAE mice (G, J, M, respectively). G, H: arrows – indicate perivascular infiltrates; J, K: arrows – indicate perivascular CD3 + T cells; M, N: arrows – indicate Mac3 + macrophages. Scale bars = 100 μm. Data are mean ± SE. *P < 0.05, ***P < 0.001.
Distinct effects of high‐intensity continuous versus interval exercise training on PLP‐reactive LN‐T cell proliferation and cytokine secretion
As HIIT and HICT both attenuated the encephalitogenicity of PLP‐immunized LN‐T cells to induce neuroinflammation in vivo, we investigated the effects of training paradigms on LN‐T cell properties. First, the number and properties of freshly isolated LNCs were examined. The high basal stimulation index in LNCs derived from PLP‐immunized SED mice was significantly attenuated by HIIT (~ 35%, P < 0.01) and HICT (~55%, P < 0.01; Figure 3B). Furthermore, both HIIT and HICT induced large reductions in cytokine mRNA levels in LNCs from PLP‐immunized mice (ranging from P < 0.05 to P < 0.001, Figure 3C).
We next examined the proliferation and polarization of PLP‐immunized LN‐T cells in response to in vitro stimulation. To investigate the overall ability of T cells to respond to stimuli, we studied the proliferation of T cells derived from HIIT, HICT or SED, PLP‐immunized mice in response to the non‐specific mitogen ConA (Figure 3D). Neither training paradigm affected the proliferative response of T cells to ConA stimulation in vitro versus SED group (P > 0.05, Figure 3D). This finding was further confirmed when similar fractions (~80%) of T cells entered cell cycle in the three experimental groups (P > 0.05, Figure 3E). However, HICT markedly inhibited the proliferative response of LN‐T cells to the specific PLP autoantigen (~75%, P < 0.001, Figure 3F). Surprisingly, HIIT did not affect T‐cell proliferation in response to PLP autoantigen (P > 0.05). Similar fractions of CD4+, CD25+, FoxP3 + regulatory T cells were noted in all three experimental groups (8‐11%, P > 0.05; Figure 3G).
We further examined the effect of training on polarization into T helper (Th) cells following in vitro PLP stimulation, as reflected by measuring cytokine secretion (Figure 3H‐O). Whereas HICT had no effect on cytokine secretion (P > 0.05), compared to SED group, HIIT had a marked inhibitory effect on interferon γ (IFNγ, P < 0.01, Figure 3H), tumor necrosis factor (TNF) α (P < 0.05, Figure 3I), IL10 (P < 0.05, Figure 3K), interleukin (IL) 17A (P < 0.05, Figure 3L), granulocyte‐macrophage colony‐stimulating factor (GM‐CSF, P < 0.05, Figure 3M), and IL6 (P < 0.05, Figure 3N), but not on IL4 (P > 0.05, Figure 3J) and IL2 (P > 0.05, Figure 3O). Thus, HICT induced a marked reduction in PLP autoreactive T cells, without changing their polarization, whereas HIIT induced a marked reduction in the polarization of PLP autoreactive T cells into Th1 and Th17 phenotype, without affecting overall T‐cell proliferation.
Effects of high‐intensity training on macrophages
We then investigated the effects of high‐intensity training paradigms on LN‐ derived macrophages following PLP stimulation in vitro. HICT induced a ~40% reduction in F4/80 + macrophage fraction versus SED controls (P < 0.05, Figure 3P), whereas HIIT induced a smaller, nonsignificant reduction (~25%, P > 0.05) in this fraction.
Finally, we examined the effect of training on M1/M2 macrophage phenotypes. Similar Arg1, CD206 and iNOS mRNA levels (P > 0.05, Figure 3Q) and F4/80+, CD206+, and F4/80+, iNOS + cell fractions (P > 0.05, Figure 3R) were detected in the three experimental groups.
Discussion
The major findings of the present study are that both HICT and HIIT inhibited the encephalitogenicity of autoreactive T cells to induce EAE, albeit HICT is more effective than HIIT, but that the two training paradigms induce distinct immune‐modulatory effects against autoimmunity. Specifically, HICT induced a general reduction in autoreactive T‐cell proliferation and in macrophage fraction, but did not affect T‐helper polarization. In contrast, HIIT did not inhibit T‐cell proliferation nor macrophage fraction, but rather induced specific inhibition of polarization into autoreactive Th1 and Th17 cells.
Numerous studies have confirmed the beneficial effects of exercise training on fitness and reducing cardiovascular disease risk factors in healthy adults, with higher intensities producing a greater increase in fitness. 18 , 19 In the present study, both HIIT and HICT improved physical performance, but HIIT resulted in superior performance, as expected with training at higher intensity. Despite this, the beneficial effect of HIIT on clinical course of EAE was milder than that of HICT. The training volume (distance run) achieved with HIIT was 43% less than with HICT. Possibly the differences in training volume contributed to the difference in clinical outcomes between the training paradigms. Previously, we demonstrated that at matched training volumes, HICT provided a superior clinical outcome compared to MICT, 6 demonstrating that training intensity is also an important factor. There is likely an optimal intensity for affecting clinical outcome and once that intensity is achieved, duration of training may take on greater relevance. However, it is also possible that the excessive metabolic stress during HIIT (e.g., augmented cytokine response, catecholamines or ROS) may have negated some of the benefits of training. These results underscore the importance of identifying the mechanism(s) whereby exercise impacts positively (as well as negatively) on development of autoimmune diseases.
The results of this study demonstrated that both HICT and HIIT reduce pro‐ and anti‐ inflammatory cytokine gene expression by LNCs obtained from inguinal LNs 10 days after PLP immunization. However, BrdU incorporation assay indicated that HICT inhibited PLP‐reactive T‐cell proliferation following PLP stimulation in vitro, whereas HIIT did not, demonstrating differential immunomodulatory effects of the training paradigms. Macrophages are predominant inflammatory cells in EAE that present antigens to auto‐reactive T cells. 20 We therefore examined whether the differential effects of HICT and HIIT on PLP‐ reactive T‐cell proliferation was related to a decline in the macrophage population. Our results were supportive of this effect as HICT induced a significant reduction in macrophage fraction in LNCs from PLP‐immunized mice. The lack of a critical number of macrophages as antigen presenting cells is likely to be involved in the inhibitory mechanism of HICT on T‐ cell proliferation in response to the PLP autoantigen.
We also examined the effects of exercise on T‐cell polarization. Some data suggest a beneficial role of Foxp3+ regulatory T cells in EAE, 21 while other data suggest more complex dynamics. 22 It has been proposed that exercise may adjust the number and function of regulatory T cells. 23 In our experiments, similar fractions of CD4+, CD25+, FoxP3+ regulatory T cells were detected in PLP‐ reactive T cells obtained from HIIT, HICT, and SED mice.
A key finding was the distinct effects of HIIT and HICT on the polarization of T cells into Th populations. It is widely accepted that in MS and EAE, as well as in other autoimmune diseases, IFNγ‐producing Th1 cells and IL17 producing Th17 cells are pathogenic, while IL4 and IL10‐ producing Th2 cells are protective. 24 , 25 EAE studies showed the presence of both Th1 and Th17 cells infiltrating the CNS. 22 , 26 , 27 The encephalitogenicity of both Th1 and Th17 cells depends on GM‐CSF secretion, 28 while blockade of GM‐CSF resulted in resistance to EAE induction. 29 Human and animal studies suggest that exercise training induces a shift in the Th1/Th2 balance to a Th2 cell predominance. 30 , 31 In the present study, in PLP‐stimulated cultures of T cells obtained from the HICT group, cytokine secretion reached similar levels as in the SED groups. Strikingly, in the HIIT group there were marked decreases in Th1 and Th17 type cytokines and to lesser extent in Th2 cytokines (only in IL10, but not in IL4). Thus, there was a specific inhibitory effect of HIIT on polarization into Th1 and Th17 cell populations. IL6 plays a critical role in the induction phase of EAE by modulating Th1/Th2/ Th17 balance. 32 , 33 We showed that HIIT induced a marked reduction in IL6, whereas HICT did not affect IL6 levels. This finding suggests that the inhibitory effect of HIIT on IL6 secretion is responsible for the decrease in Th1 and Th17 populations. Interestingly, HIIT did not affect the secretion of IL4, and even inhibited IL10 secretion, which impacts on the development of EAE. These data suggest that the beneficial effect of HIIT on PLP autoimmunity is not mediated through a shift toward a Th2 response, nor enhancing protective, anti‐inflammatory processes.
The current study demonstrates that high‐intensity exercise training protocols modulate PLP‐autoimmunity rather than induce general immune suppression. The BrdU assay indicates that T cells from HIIT, HICT, and SED, PLP–immunized mice proliferated similarly in response to ConA‐stimulation in vitro. In addition, the CFSE assay indicated comparable fraction of cells that entered cell cycle when stimulated with ConA. These findings indicate that HICT and HIIT do not reduce the maximal capacity of T‐cell response to a non‐specific mitogen. Finally, no differences were measured in the secretion of IL2 cytokine by PLP‐stimulated HIIT, HICT and SED‐ derived T cells, providing another indication of preserved activation potential of T cells and no significant role of IL2 in the immuno‐modulatory effect of high‐intensity training against PLP.
In conclusion, our results show superior clinical effects of HICT versus HIIT in EAE attenuation. Moreover the two training paradigms modulate autoimmunity, and hence attenuate EAE by distinct mechanisms: HICT by decreasing T‐cell proliferation, and HIIT by decreasing T‐cell polarization into deleterious Th1 and Th17 cells. These finding may also be applicable to other autoimmune diseases. Future studies should focus on the molecular basis for these observations. In‐depth understanding of the cellular and molecular mechanisms underlying the beneficial effects of exercise training on EAE is essential for designing effective clinical treatments in MS patients and other patients with autoimmune diseases.
Conflict of Interests
The authors declare no competing financial interests.
Acknowledgments
This work was supported by the Chief Scientist Office of the Israeli Ministry of Health (no. 3‐15021) and by the Judy and Sidney Swartz Fund for research in Multiple Sclerosis.
Funding Information
Chief Scientist Office of the Israeli Ministry of Health Grant number: 3‐15021 and The Judy and Sidney Swartz Fund for research in Multiple Sclerosis
Funding Statement
This work was funded by Chief Scientist Office of the Israeli Ministry of Health grant 3‐15021; The Judy and Sidney Swartz Fund for research in Multiple Sclerosis grant .
References
- 1. Sharif K, Watad A, Bragazzi NL, et al. Physical activity and autoimmune diseases: get moving and manage the disease. Autoimmun Rev 2018;17(1):53–72. [DOI] [PubMed] [Google Scholar]
- 2. Heine M, Wens I, Langeskov‐Christensen M, et al. Cardiopulmonary fitness is related to disease severity in multiple sclerosis. Mult Scler 2016;22(2):231–238. [DOI] [PubMed] [Google Scholar]
- 3. Motl RW. Exercise and multiple sclerosis. Adv Exp Med Biol 2020;1228:333–343. [DOI] [PubMed] [Google Scholar]
- 4. Kruger K, Mooren FC, Pilat C. The immunomodulatory effects of physical activity. Curr Pharm Des 2016;22(24):3730–3748. [DOI] [PubMed] [Google Scholar]
- 5. Einstein O, Fainstein N, Touloumi O, et al. Exercise training attenuates experimental autoimmune encephalomyelitis by peripheral immunomodulation rather than direct neuroprotection. Exp Neurol 2018;299(Pt A):56–64. [DOI] [PubMed] [Google Scholar]
- 6. Fainstein N, Tyk R, Touloumi O, et al. Exercise intensity‐dependent immunomodulatory effects on encephalomyelitis. Ann Clin Transl Neurol 2019;6(9):1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Elliott AD, Rajopadhyaya K, Bentley DJ, et al. Interval training versus continuous exercise in patients with coronary artery disease: a meta‐analysis. Heart Lung Circ 2015;24(2):149–157. [DOI] [PubMed] [Google Scholar]
- 8. Milanovic Z, Sporis G, Weston M. Effectiveness of high‐intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta‐analysis of controlled trials. Sports Med 2015;45(10):1469–1481. [DOI] [PubMed] [Google Scholar]
- 9. Weston KS, Wisloff U, Coombes JS. High‐intensity interval training in patients with lifestyle‐induced cardiometabolic disease: a systematic review and meta‐analysis. Br J Sports Med 2014;48(16):1227–1234. [DOI] [PubMed] [Google Scholar]
- 10. Bartlett DB, Willis LH, Slentz CA, et al. Ten weeks of high‐intensity interval walk training is associated with reduced disease activity and improved innate immune function in older adults with rheumatoid arthritis: a pilot study. Arthritis Res Ther 2018;20(1):127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Nardi AT, Tolves T, Lenzi TL, et al. High‐intensity interval training versus continuous training on physiological and metabolic variables in prediabetes and type 2 diabetes: a meta‐analysis. Diabetes Res Clin Pract 2018;137:149–159. [DOI] [PubMed] [Google Scholar]
- 12. Stoa EM, Meling S, Nyhus LK, et al. High‐intensity aerobic interval training improves aerobic fitness and HbA1c among persons diagnosed with type 2 diabetes. Eur J Appl Physiol 2017;117(3):455–467. [DOI] [PubMed] [Google Scholar]
- 13. Campbell E, Coulter EH, Paul L. High intensity interval training for people with multiple sclerosis: a systematic review. Mult Scler Relat Disord 2018;24:55–63. [DOI] [PubMed] [Google Scholar]
- 14. Einstein O, Fainstein N, Vaknin I, et al. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann Neurol 2007;61(3):209–218. [DOI] [PubMed] [Google Scholar]
- 15. Li B, Liang F, Ding X, et al. Interval and continuous exercise overcome memory deficits related to beta‐Amyloid accumulation through modulating mitochondrial dynamics. Behav Brain Res 2019;376:112171. [DOI] [PubMed] [Google Scholar]
- 16. Marcinko K, Sikkema SR, Samaan MC, et al. High intensity interval training improves liver and adipose tissue insulin sensitivity. Mol Metab 2015;4(12):903–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naghibzadeh M, Ranjbar R, Tabandeh MR, Habibi A. Effects of two training programs on transcriptional levels of neurotrophins and glial cells population in hippocampus of experimental multiple sclerosis. Int J Sports Med 2018;39(8):604–612. [DOI] [PubMed] [Google Scholar]
- 18. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43(7):1334–1359. [DOI] [PubMed] [Google Scholar]
- 19. Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 2010;13(5):496–502. [DOI] [PubMed] [Google Scholar]
- 20. Rawji KS, Yong VW. The benefits and detriments of macrophages/microglia in models of multiple sclerosis. Clin Dev Immunol 2013;2013:948976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+CD25+ regulatory T cells suppress antigen‐specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J Immunol 2002;169(9):4712–4716. [DOI] [PubMed] [Google Scholar]
- 22. Korn T, Reddy J, Gao W, et al. Myelin‐specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med 2007;13(4):423–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dorneles GP, Dos Passos AAZ, Romao PRT, Peres A. New insights about regulatory T cells distribution and function with exercise: the role of immunometabolism. Curr Pharm Des 2020;26(9):979–990. [DOI] [PubMed] [Google Scholar]
- 24. Kuchroo VK, Anderson AC, Waldner H, et al. T cell response in experimental autoimmune encephalomyelitis (EAE): role of self and cross‐reactive antigens in shaping, tuning, and regulating the autopathogenic T cell repertoire. Annu Rev Immunol 2002;20:101–123. [DOI] [PubMed] [Google Scholar]
- 25. Hofstetter H, Gold R, Hartung HP. Th17 cells in MS and experimental autoimmune encephalomyelitis. Int MS J 2009;16(1):12–18. [PubMed] [Google Scholar]
- 26. Langrish CL, Chen Y, Blumenschein WM, et al. IL‐23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med 2005;201(2):233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stromnes IM, Cerretti LM, Liggitt D, et al. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med 2008;14(3):337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. El‐Behi M, Ciric B, Dai H, et al. The encephalitogenicity of T(H)17 cells is dependent on IL‐1‐ and IL‐23‐induced production of the cytokine GM‐CSF. Nat Immunol 2011;12(6):568–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McQualter JL, Darwiche R, Ewing C, et al. Granulocyte macrophage colony‐stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med 2001;194(7):873–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kakanis MW, Peake J, Brenu EW, et al. T helper cell cytokine profiles after endurance exercise. J Interferon Cytokine Res 2014;34(9):699–706. [DOI] [PubMed] [Google Scholar]
- 31. Xiang L, Rehm KE, Marshall GD Jr. Effects of strenuous exercise on Th1/Th2 gene expression from human peripheral blood mononuclear cells of marathon participants. Mol Immunol 2014;60(2):129–134. [DOI] [PubMed] [Google Scholar]
- 32. Ishihara K, Hirano T. IL‐6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev 2002;13(4–5):357–368. [DOI] [PubMed] [Google Scholar]
- 33. Okuda Y, Sakoda S, Fujimura H, et al. IL‐6 plays a crucial role in the induction phase of myelin oligodendrocyte glucoprotein 35–55 induced experimental autoimmune encephalomyelitis. J Neuroimmunol 1999;101(2):188–196. [DOI] [PubMed] [Google Scholar]


