Abstract
Objective
Determine whether a treatment effect of ibudilast on brain atrophy rate differs between participants with primary (PPMS) and secondary (SPMS) progressive multiple sclerosis.
Background
Progressive forms of MS are both associated with continuous disability progression. Whether PPMS and SPMS differ in treatment response remains unknown.
Design/Methods
SPRINT‐MS was a randomized, placebo‐controlled 96‐week phase 2 trial in both PPMS (n = 134) and SPMS (n = 121) patients. The effect of PPMS and SPMS phenotype on the rate of change of brain atrophy measured by brain parenchymal fraction (BPF) was examined by fitting a three‐way interaction linear‐mixed model. Adjustment for differences in baseline demographics, disease measures, and brain size was explored.
Results
Analysis showed that there was a three‐way interaction between the time, treatment effect, and disease phenotype (P < 0.06). After further inspection, the overall treatment effect was primarily driven by patients with PPMS (P < 0.01), and not by patients with SPMS (P = 0.97). This difference may have been due to faster brain atrophy progression seen in the PPMS placebo group compared to SPMS placebo (P < 0.02). Although backward selection (P < 0.05) retained age, T2 lesion volume, RNFL, and longitudinal diffusivity as significant baseline covariates in the linear‐mixed model, the adjusted overall treatment effect was still driven by PPMS (P < 0.01).
Interpretation
The previously reported overall treatment effect of ibudilast on worsening of brain atrophy in progressive MS appears to be driven by patients with PPMS that may be, in part, because of the faster atrophy progression rates seen in the placebo‐treated group.
Introduction
We previously reported 1 a significant reduction in the rate of cerebral atrophy progression as determined by brain parenchymal fraction (BPF) assessments in people with progressive multiple sclerosis (MS) in a randomized phase 2 clinical trial, “SPRINT‐MS,” comparing ibudilast to placebo. Detailed results of the MRI outcomes from the SPRINT‐MS trial were recently reported. 2 Despite recognition as different clinical disease phenotypes, 3 primary progressive (PPMS) and secondary progressive (SPMS) are often viewed as being more similar than different pathologically. 4 Little is known about whether these disease phenotypes differ in treatment response. 5
The phase 2 trial of ibudilast included people with both PPMS and SPMS in roughly equal proportions using the same inclusion and exclusion criteria, thus facilitating the possibility to learn whether there were differences in treatment response outcomes between these two MS phenotypes. We hypothesized that ibudilast would have had a similar treatment effect in PPMS and SPMS.
Methods
SPRINT‐MS (clinicaltrials.gov NCT01982942) was a randomized, placebo‐controlled 96‐week phase 2 trial that evaluated the effect of ibudilast on brain measures of integrity in both PPMS (n = 134) and SPMS (n = 121) patients. Details of the trial design, imaging and statistical methodology, and main results have been published. 1 , 6 MRIs were acquired every 6 months; no gadolinium was administered. Human subject protection was provided by the NeuroNEXT central institutional review board at Massachusetts General Hospital.
Analyses for this manuscript focus on the differential effect of PPMS and SPMS clinical phenotypes on the treatment effect as measured by BPF decline. In the primary analyses of the SPRINT‐MS trial, the treatment effect was estimated as the difference in the rate of BPF decline between treatment groups. Differential treatment effect then refers to the difference in treatment effect across PPMS and SPMS disease phenotypes.
Similarly to the model in the original report, 1 the differential treatment effect of PPMS and SPMS on the rate of change of brain atrophy measured by BPF was examined by fitting a linear‐mixed model with a three‐way interaction of disease phenotype, treatment, and time with adjustment for immunomodulating therapy use. A random intercept and slope were included to account for the correlation between measurements and an unstructured covariance structure was assumed. The model also assumed a common intercept across treatment groups. The comparison of interest was a contrast of the treatment effect between PPMS and SPMS phenotypes. All analyses were based on a modified intention‐to‐treat approach which included all patients randomized to receive treatment and who also had at least one post‐randomization MRI assessment to measure BPF.
Models adjusting for differing baseline demographics and disease measures between the MS phenotypes were explored. A backward selection model (P < 0.05 as the cut‐off for inclusion) was also fit including all covariates that differed between the disease phenotypes at baseline. Additional analyses were performed to assess the impact of baseline brain size. Data were divided into “big” v. “small” brains using the overall median baseline BPF. Separate linear‐mixed models were fit for each disease type and the differential treatment effect between brain sizes was estimated within each MS phenotype. Alternatively, we also fit separate models for big and small brains and compared the effect of MS phenotype on the estimated treatment effect within each brain size.
In addition, we examined whether a differential treatment effect existed for short and long disease duration within each disease phenotype. We categorized patients into short and long disease duration based on the study‐wide median baseline disease duration (median = 10 years). Patients with disease duration less than or equal to 10 years were considered to have a “short” disease duration and patients with the disease over 10 years were termed to have a “long” disease duration. We then fit separate models for each disease phenotype including a three‐way interaction of time, treatment, and disease duration (short vs. long) along with adjustment for immunotherapy. Within each disease phenotype model, we estimated the treatment effect for short and long disease duration and the difference in effects.
In further exploratory analyses, differences in treatment effect between disease phenotypes for the major secondary outcome measures (cortical thickness (CTh); magnetization transfer ratio (MTR) in normal‐appearing brain tissue, transverse diffusivity and longitudinal diffusivity as measured by diffusion tensor imaging (DTI) of the pyramidal tract, and retinal nerve fiber layer (RNFL) as measured by optical coherence tomography) were analyzed using a three‐way interaction model. A similar analysis was conducted with a different whole brain atrophy measure SIENA. 7 , 8 All statistical analyses were performed in SAS 9.4 Software (SAS Institute, Cary, NC). Since these were exploratory analyses, adjustment for multiplicity was not conducted.
Results
Baseline demographics and disease characteristics
The randomized population included 134 participants with PPMS and 121 with SPMS. Their demographic and disease characteristics are shown in Table 1. Notably, there were significant differences in the following baseline characteristics: Expanded Disability Status Scale (EDSS); 25‐foot walk; T2 lesion volume; retinal nerve fiber layer (RNFL) thickness; pyramidal tract diffusion tensor imaging longitudinal diffusivity (LD); and BPF.
Table 1.
Baseline demographic and disease characteristics by disease phenotype.
| Characteristic |
PPMS (n = 134) |
SPMS (n = 121) |
P‐value 1 |
|---|---|---|---|
| Age (yrs), mean (SD) | 55.24 (6.93) | 56.17 (7.64) | 0.31 |
| Females, n (%) | 57 (42.54%) | 79 (65.29%) | <0.01 |
| Race | |||
| Caucasian, n (%) | 124 (92.54%) | 112 (92.56%) | 1.00 2 |
| Black/ African American, n (%) | 5 (3.73%) | 6 (4.96%) | |
| Other, n (%) | 3 (2.24%) | 1 (0.83%) | |
| Unknown/Not Reported, n (%) | 2 (1.49%) | 2 (1.65%) | |
| Hispanic/Latino, n (%) | 3 (2.24%) | 4 (3.31%) | 0.71 3 |
| Ibudilast, n (%) | 68 (50.75%) | 61 (50.41%) | 0.96 |
| use of im therapy, n (%) | 31 (23.13%) | 49 (40.50%) | <0.01 4 |
| Glatiramer Acetate, n (%) | 21 (15.67%) | 22 (18.18%) | |
| Interferon‐beta, n (%) | 10 (7.46%) | 27 (22.31%) | |
| disease duration (yrs), median (min, max) | 6 (0, 34) | 16 (1, 41) | <0.01 6 |
| Expanded Disability Status Scale, median (min, max) 5 | 6.0 (2.5, 6.5) | 6.0 (3.0, 7.0) | <0.01 6 |
| 25‐foot walk (sec), median (min, max) | 8.13 (3.60, 75.30) | 11.60 (4.05, 180.00) | <0.01 6 |
| 9‐hole peg test (sec), median (min, max) | 28.73 (16.58, 167.75) | 30.75 (17.83, 201.88) | 0.08 6 |
| Symbol Digit Modality Test (number correct), mean (SD) | 42.41 (13.88) | 42.71 (14.88) | 0.87 |
| Low contrast visual acuity test (number correct), mean (SD) 7 | 29.53 (12.21) | 26.27 (13.02) | 0.04 |
| Brain parenchymal fraction (unitless), mean (SD) | 0.8087 (0.0262) | 0.7988 (0.0323) | 0.01 |
| T2 Lesion volume (cm 3 ), mean (SD) | 8.46 (10.80) | 12.44 (11.17) | <0.01 |
| median (min, max) | 4.05 (0.03, 55.80) | 9.28 (0.11, 47.47) | <0.01 6 |
| Magnetization transfer ratio in normal‐appearing brain tissue (normalized units), mean (SD) 8 | 0.34 (0.25) | 0.26 (0.30) | 0.03 |
| Cortical thickness (mm), mean (SD) | 3.0846 (0.1951) | 2.9759 (0.2450) | <0.01 |
|
Diffusion tensor imaging, longitudinal diffusivity (10‐3 mm2/sec), mean (SD) |
1.23 (0.05) | 1.25 (0.05) | 0.01 |
|
Diffusion tensor imaging, transverse diffusivity (10‐3 mm2/sec), mean (SD) |
0.55 (0.04) | 0.56 (0.05) | 0.10 |
| Retinal nerve fiber layer thickness (µm), mean (SD) 9 | 85.31 (12.03) | 78.50 (11.03) | <0.01 |
The P‐value for continuous variables was based on Student’s t‐test, unless specified otherwise. The P‐value for nominal variables was based on a chi‐square test, unless specified otherwise.
The P‐value for Caucasians vs. Non‐Caucasians was based on Fishers exact test.
The P‐value for Hispanics vs. Non‐Hispanics was based on Fishers exact test.
The P‐value compares use of IM therapy vs untreated.
Scores on the Expanded Disability Status scale range from 0.0 to 10.0, with higher scores indicating worse disability. For study eligibility, scores had to be within 3.0‐6.5 (inclusive). One subject was enrolled with a score below the required level. A protocol deviation was entered and the subject was kept in the study.
The P‐value was based on a Wilcoxon Rank Sum Test.
Data were not available for 1 patient in the PPMS group and 1 patient in the SPMS group.
Data were not available for 3 patients in the PPMS group.
Data were not available for 2 patients in the PPMS group and 7 patients in the SPMS group.
Differential effect of ibudilast on whole brain atrophy
Our analysis showed that there was a three‐way interaction between the time, treatment effect, and disease phenotype (P < 0.06). The overall ibudilast treatment effect was primarily driven by patients with PPMS (P < 0.01) [estimated difference between ibudilast and placebo within PPMS, 0.00166, 95% CI (0.0005, 0.00281)], and not by patients with SPMS (P = 0.97) [estimated difference between ibudilast and placebo within SPMS, 0.00002, 95% CI (−0.00121, 0.00125)] (Table 2).
Table 2.
Annualized rates of change in BPF estimates by disease phenotype with 95% CI according to PPMS vs. SPMS . (1 study year is defined as 48 weeks).
| Disease Phenotype |
Estimated rate of BPF change (95% CI) |
Estimated difference in rate of change (95% CI) |
P‐value for difference in rate of change | |
|---|---|---|---|---|
| Ibudilast | Placebo | |||
| Overall | −0.00097 | −0.00186 | 0.000890 | <0.04 |
| (−0.00157, −0.00036) | (−0.00245, −0.00126) | (0.000041, 0.001738) | ||
| PPMS 1 | −0.00088 | −0.00254 | 0.00166 | <0.01 |
| (−0.00171, 0.00005) | (−0.00334, −0.00173) | (0.00050, 0.00281) | ||
| SPMS 1 | −0.00106 | −0.00108 | 0.00002 | 0.97 |
| (−0.00193, −0.00019) | (−0.00195, −0.00022) | (−0.00121, 0.00125) | ||
Estimated rates of change by disease type based on model that included a three‐way interaction of time, treatment, and disease type using all modified intention‐to‐treat subjects.
Baseline covariates were evaluated to see whether they accounted for the apparent lack of efficacy on BPF in SPMS versus PPMS. In a backward selection model (P < 0.05) retaining age, T2 lesion volume, RNFL, and LD as significant baseline covariates, the test of differing treatment effect between PPMS and SPMS was P = 0.07 [estimated differing treatment effect, 0.0016, 95% CI (−0.00014, 0.0034) (Table 3)]. Therefore, after accounting for these covariates, the adjusted overall treatment effect was still driven principally by the PPMS cohort (P < 0.01) [estimated difference between ibudilast and placebo within PPMS, 0.00165, 95% CI (0.000459, 0.00283)]. Similar results were obtained from models that adjusted for baseline covariates one at a time.
Table 3.
Annualized rates of change in BPF estimates by disease phenotype adjusted for baseline covariates with 95% CI according to PPMS vs. SPMS. (1 study year is defined as 48 weeks).
| Disease Phenotype |
Estimated rate of BPF change (95% CI) |
Estimated difference in rate of change (95% CI) |
P‐value for difference in rate of change | |
|---|---|---|---|---|
| Ibudilast | Placebo | |||
| −0.00079 | −0.00243 | 0.001646 | <0.01 | |
| PPMS 1 | (−0.00164, 0.000067) | (−0.00326, −0.00161) | (0.000459, 0.002833) | |
|
SPMS 1 |
−0.00112 | −0.00115 | 0.000037 | 0.95 |
| (−0.00204, −0.00019) | (−0.00205, −0.00026) | (−0.00125, 0.001322) | ||
Estimated rates of change by disease phenotype from a model adjusted for baseline T2 lesion volume, retinal nerve fiber thickness, longitudinal diffusivity, and age.
Differential rate of cerebral atrophy (BPF) by disease phenotype
PPMS placebo patients had a significantly faster rate of BPF decline than SPMS placebo patients over 48 weeks (p < 0.02) (Table 4; Figure 1).
Table 4.
Differential rate of cerebral atrophy (BPF) by disease phenotype in placebo group.
|
Estimated rate of BPF decline over 48 weeks (95% CI) |
P‐value | |
|---|---|---|
| PPMS placebo patients | −0.00254 (−0.00334, −0.00173) | |
| SPMS placebo patients | −0.00108 (−0.00195, −0.00022) | |
| Difference (PPMS – SPMS) | −0.00146 (−0.00264, −0.00027) | <0.02 |
Figure 1.
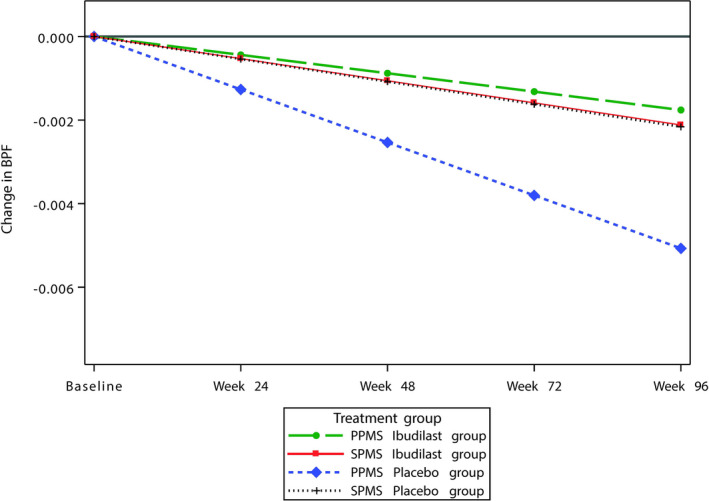
The estimated rate of change in cerebral atrophy (assessed by BPF) in patients receiving ibudilast or placebo by disease phenotype.
Effect of baseline brain size
The SPMS group had evidence of more parenchymal tissue loss at baseline. Because we were not able to control for a baseline BPF, when BPF was the outcome measure, we sought to evaluate a potential effect of baseline brain size by analyzing small versus large brains. Subjects were categorized into small or large brains based on the study‐wide median baseline BPF (Figure 2). Separate linear‐mixed models’s for BPF over time among those with small or large brains at baseline were fit for PPMS and SPMS subjects. Within each disease phenotype model, we included a three‐way interaction of time, treatment, and brain size along with adjustment for immunotherapy. We tested whether there was a differential treatment effect (ibudilast versus placebo) for large versus small brain sizes within each disease phenotype.
Figure 2.
Baseline brain atrophy as measured by BPF, according to brain size. Blue = placebo; red = ibudilast treatment.
There was not a significantly different treatment effect for large versus small brains within either disease phenotype (primary progressive: estimate of difference over 1 year = −0.00012, 95% CI [−0.00255, 0.00232], P‐value = 0.92; for secondary progressive: estimate of difference over 1 year = 0.00089, 95% CI [−0.00151, 0.00329], P‐value = 0.47).
In a second analysis, we fit separate linear‐mixed models in those above versus below median BPF and evaluated for three‐way interaction (time, treatment, disease phenotype). There was no differential treatment effect for disease phenotype within either brain size (P = 0.13 for patients below median BPF, and P = 0.31 for patients above median BPF). Within each brain size, the difference in rate of BPF decline between treatment groups was larger in the PPMS patients than in the SPMS patients. In patients whose baseline BPF was below the median value, the treatment effect was not significant in either disease phenotype. In patients whose baseline BPF was above the median value the treatment effect was significant in PPMS patients (P = 0.02) but not in SPMS patients (P = 0.5).
In a third analysis, we evaluated the effect of short vs long disease duration. For PPMS patients, the estimated treatment effect for long disease duration (n = 31, or 24.41%) was 0.00265, 95% CI [0.00028, 0.00501], P = 0.0282 and short disease duration (n = 31, or 26.50%) was 0.00124, 95% CI [−0.00014, 0.00263], P‐value = 0.0785, P = 0.0785. There was no difference in the treatment effect for long vs short disease duration: 0.00141, 95% CI [−0.00133, 0.00415], P = 0.3137. For SPMS patients, the estimated treatment effect for long disease duration was 0.00065, 95% CI [−0.00072, 0.00201], P = 0.3546 and short disease duration was −0.00153, 95% CI [−0.00386, 0.00080], P = 0.1978. There was no difference in the treatment effect for long vs short disease duration: 0.00218, 95% CI [−0.00053, 0.00488], P = 0.1146.
The original trial’s analysis model assumed an unstructured covariance matrix for the random effects (subject‐specific random intercepts and slopes) while maintaining independent errors within a subject given the random effects. To keep alignment with that original analysis model we used the same specifications for this report’s primary analysis. In an exploratory analysis, we fit a more parsimonious three‐way interaction model. For a model with only a random intercept, the estimated interaction effect of this model was 0.0016, 95% CI (0.0004, 0.0028), P = 0.0115. The estimated treatment effect of ibudilast versus placebo within PPMS was 0.0017, 95% CI (0.0009, 0.0025), P < 0.0001 and within SPMS was 0.0001, 95% CI (−0.0008, 0.0010), P = 0.7938. These results were similar to the primary analysis.
Differential effect of ibudilast on major secondary outcome
Cortical thickness declined by 0.00861 mm more per year in the placebo group relative to the ibudilast group, P < 0.01. The three‐way test for interaction indicated treatment effect was not differentiated by disease phenotype (P = 0.37), although was directionally consistent with that seen with BPF. For completeness, we estimated the treatment effect within each disease phenotype as we did for BPF. Results were similar to the BPF analyses: the treatment effect appeared more pronounced in the PPMS group than in the SPMS group (Table 5). Similarly, the three‐way test for interaction was directionally consistent but not statistically significant for normal‐appearing brain tissue MTR (P = 0.44), pyramidal tract longitudinal diffusivity (P = 0.70), and retinal nerve fiber layer (p = 0.77), but not for pyramidal tract transverse diffusivity (data not shown).
Table 5.
Annualized 1 rates of change in CTh (mm) estimates by disease phenotype.
| Disease Phenotype |
Estimated rate of CTh change (95% CI) |
Estimated difference in rate of change (95% CI) |
P‐value for difference in rate of change | |
|---|---|---|---|---|
| Ibudilast | Placebo | |||
| Overall | −0.00192 | −0.01053 | 0.00861 | <0.01 |
| (−0.00607, 0.00223) | (−0.01459, −0.00648) | (0.00283, 0.01440) | ||
| PPMS | 0.000193 | −0.01094 | 0.01114 | <0.01 |
| (−0.00554, 0.005925) | (−0.01649, −0.00540) | (0.003178, 0.01910) | ||
| SPMS | −0.00424 | −0.01003 | 0.005790 | 0.18 |
| (−0.01026, 0.001784) | (−0.01598, −0.00408) | (−0.00266, 0.01424) | ||
1 study year is defined as 48 weeks.
Discussion
The impetus to study ibudilast in progressive MS derived from the trial of ibudilast in relapsing MS. 9 Although that study failed to demonstrate an effect on the focal inflammatory pathology characteristic of relapsing disease, the favorable effects observed on brain atrophy suggested a neuroprotective effect.
Prior to the publication of standardized definitions of the MS phenotypes that described SPMS and PPMS in 1996, 3 no distinction was recognized between MS with gradually worsening neurological function, whether there had been relapsing activity at the outset of the disease (SPMS) or not (PPMS). More recently, some reports have suggested biological differences between these clinically determined disease phenotypes, and others have not. 4 Examples of therapies demonstrating beneficial responses in brain atrophy in progressive MS phenotypes include siponimod 10 and simvastatin 11 (not a registered therapy for MS) in SPMS and ocrelizumab 12 in PPMS. In contrast, fingolimod was not found to slow the progression of brain atrophy in PPMS. 13 Since these trials assessed treatment effect in separate clinical phenotypes, it remains unclear whether differential treatment effects occur between these phenotypes. 5
We performed a post hoc analysis of SPRINT‐MS data to assess whether the previously reported 1 treatment effect of ibudilast was similar in PPMS and SPMS. The results of this analysis suggest that the response to treatment observed in the BPF atrophy outcome within the PPMS group and not the SPMS group is driving the overall ibudilast‐related treatment difference. This difference held when the variables that were significantly different between the groups at baseline (age, LD, RNFL, T2 lesion volume, EDSS and 25‐foot walk) were accounted for in the model. Baseline brain size did not account for the PPMS versus SPMS difference, nor did it have a measurable influence on treatment effect.
We also evaluated the effect of disease duration (short vs. long). This model’s estimate suggests that disease duration does not explain the differential effect in treatment by disease phenotype. If disease duration was a driving factor, we would expect that PPMS subjects with longer disease duration would exhibit a lesser or no treatment effect of ibudilast and SPMS subjects with shorter disease duration would have a greater treatment effect. The results suggested the opposite: the estimated treatment effect for PPMS patients with long disease duration did not differ from short disease duration. In fact, the estimated effect was larger, albeit not statistically significantly. The estimated treatment effect for SPMS patients with short disease duration did not differ from long disease duration: the estimated effect for short disease duration suggested ibudilast had a lower effect, but was not statistically significant.
We also explored an alternative methodology for the assessment of cerebral atrophy using the SIENA method to assess change in total brain size over the course of the trial. We found a similar pattern of differential effect although this did not reach statistical significance. In addition, the initial trial results showed an overall reduction in the rate of cortical thickness loss, 1 and we again found a similar pattern according to phenotype: this effect was principally driven by the PPMS subjects. Results of conventional imaging outcomes are reported in a separate publication. 2
To our knowledge, the differential treatment effect on brain atrophy that we observed between SPMS and PPMS has not been described previously. Interestingly, there was a significantly higher rate of atrophy progression in PP versus SP MS seen in the placebo arm similar to the pattern observed in the literature. 14 We believe that this could account, at least in part, for our observations. Another possible explanation is that the PPMS phenotype was more susceptible to a specific treatment effect of ibudilast because of a more active disease process manifest as an approximately double rate of atrophy progression compared to SPMS. Since this was a post hoc exploratory analysis, additional investigation is warranted to assess the reproducibility and generalizability of these results. Furthermore, the clinical relevance of these imaging findings needs to be evaluated. If confirmed and found to be clinically relevant, these findings will need to be considered in future trial designs for progressive MS.
Authors’ Contributions
JKF, JY, and EAK have contributed to the statistical data analysis, interpreting data, and revising the manuscript. RTN, RAB, and ECK have contributed to collecting data, interpreting data, and drafting/revising the manuscript. CSC contributed as the NeuroNEXT network Data Coordinating Center PI and Statistician and has contributed to the statistical data analysis, interpreting data, and revising the manuscript. MC has contributed to the study design on behalf of the NeuroNEXT network, study‐related management, and revising the manuscript. CVG has contributed to study related management and revising the manuscript. RJF has contributed to the study as the protocol PI to include study design, collecting data, interpreting data, and drafting/revising the manuscript.
Conflicts of Interest
The authors have nothing to report as a relevant conflict of interest to report with respect to this study.
Supporting information
Appendix S1. NN102/ SPRINT‐MS Clinical Site Personnel, Investigator listing as of July 2016.
Acknowledgments
This study was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (U01NS082329) and the National Multiple Sclerosis Society (RG 4778‐A‐6) and by MediciNova through a contract with the National Institutes of Health (NIH). The NeuroNEXT Network is supported by the NINDS (Central Coordinating Center, U01NS077179; Data Coordinating Center, U01NS077352; and individual grants to each trial site). All study data are available from the NINDS Archived Clinical Research Dataset. We thank the persons with multiple sclerosis who made this trial possible by volunteering to participate.
Funding InformationThis study was supported by grants from the National Institute of Neurological Disorders and Stroke (NINDS) (U01NS082329) and the National Multiple Sclerosis Society (RG 4778‐A‐6) and by MediciNova through a contract with the National Institutes of Health (NIH). The NeuroNEXT Network is supported by the NINDS (Central Coordinating Center, U01NS077179; Data Coordinating Center, U01NS077352; and individual grants to each trial site). All study data are available from the NINDS Archived Clinical Research Dataset.
Funding Statement
This work was funded by National Institute of Neurological Disorders and Stroke (NINDS) grant U01NS082329; National Multiple Sclerosis Society grant RG 4778‐A‐6; MediciNova grant .
References
- 1. Fox RJ, Coffey CS, Conwit R, et al. Phase 2 Trial of ibudilast in progressive multiple sclerosis. N Engl J Med 2018;379:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Naismith RT, Coffey CS, Goodman AD,et al. Effects of Ibudilast on MRI measures in the Phase 2 SPRINT‐MS study. Neurology 2020;in press. [DOI] [PMC free article] [PubMed]
- 3. Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 2014;83:278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mahad DH, Trapp BD, Lassmann H. Progressive multiple sclerosis 1 Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 2015;14:183–193. [DOI] [PubMed] [Google Scholar]
- 5. Ontaneda D, Fox RJ, Chataway J. Clinical trials in progressive multiple sclerosis: lessons learned and future perspectives. Lancet Neurol 2015;14:208–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fox RJ, Coffey CS, Cudkowicz ME, et al. Design, rationale, and baseline characteristics of the randomized double‐blind phase II clinical trial of ibudilast in progressive multiple sclerosis. Contemp Clin Trials 2016;50:166–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Takao H, Abe O, Hayashi N, et al. Effects of gradient non‐linearity correction and intensity non‐uniformity correction in longitudinal studies using structural image evaluation using normalization of atrophy (SIENA). J Magn Reson Imaging 2010;32:489–492. [DOI] [PubMed] [Google Scholar]
- 8. Smith SM, De Stefano N, Jenkinson M, Matthews PM. Normalized accurate measurement of longitudinal brain change. J Comput Assist Tomo 2001;25:466–475. [DOI] [PubMed] [Google Scholar]
- 9. Barkhof F, Hulst HE, Drulovic J, et al. Ibudilast in relapsing‐remitting multiple sclerosis A neuroprotectant? Neurology 2010;74:1033–1040. [DOI] [PubMed] [Google Scholar]
- 10. Kappos L, Bar‐Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double‐blind, randomised, phase 3 study. Lancet 2018;391:1263–1273. [DOI] [PubMed] [Google Scholar]
- 11. Chataway J, Schuerer N, Alsanousi A, et al. Effect of high‐dose simvastatin on brain atrophy and disability in secondary progressive multiple sclerosis (MS‐STAT): a randomised, placebo‐controlled, phase 2 trial. Lancet 2014;383:2213–2221. [DOI] [PubMed] [Google Scholar]
- 12. Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. New Engl J Med 2017;376:209–220. [DOI] [PubMed] [Google Scholar]
- 13. Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double‐blind, placebo‐controlled trial. Lancet 2016. [DOI] [PubMed] [Google Scholar]
- 14. Eshaghi A, Prados F, Brownlee WJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol 2018;83:210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. NN102/ SPRINT‐MS Clinical Site Personnel, Investigator listing as of July 2016.


