Abstract
Objectives
Acquiring tool‐assisted foraging skills can potentially improve dietary quality and increase fitness for wild chimpanzees (Pan troglodytes). In contrast to chimpanzees in East and West Africa, chimpanzees in the Congo Basin use tool sets and brush‐tipped fishing probes to gather termites. We investigated the ontogeny of these tool skills in chimpanzees of the Goualougo Triangle, Republic of Congo, and compared it to that for chimpanzees at Gombe, Tanzania. We assessed whether chimpanzees acquired simple tool behaviors and single tool use before more complex actions and sequential use of multiple tool types.
Materials and Methods
Using a longitudinal approach, we scored remote video footage to document the acquisition of termite‐gathering critical elements for 25 immature chimpanzees at Goualougo.
Results
All chimpanzees termite fished by 2.9 years but did not manufacture brush‐tipped probes until an average of 4.3 years. Acquisition of sequential tool use extended into juvenility and adolescence. While we did not detect significant sex differences, most critical elements except tool manufacture were acquired slightly earlier by females.
Discussion
These findings contrast with Gombe, where chimpanzees learn to both use and make fishing probes between ages 1.5–3.5 and acquire the complete task by age 5.5. Differences between sites could reflect tool material selectivity and design complexity, the challenge of sequential tool behaviors, and strength requirements of puncturing subterranean termite nests at Goualougo. These results illustrate how task complexity may influence the timing and sequence of skill acquisition, improving models of the ontogeny of tool behavior among early hominins who likely used complex, perishable technologies.
Keywords: chimpanzee, termite fishing, tool manufacture, tool use, sex differences

Chimpanzees at Goualougo learned tool use before brush‐tipped probe manufacture.
Simple actions involving single tools were acquired before more complex sequences.
The sequence and timing of skill acquisition differed between Goualougo and Gombe.
1. INTRODUCTION
Tool use has been documented in a range of animal species, but it is relatively rare. The habitual and flexible use of tools is most prevalent within the Primates and Passeriformes orders; among nonhuman primates, it occurs in all wild chimpanzee (Pan troglodytes) populations, and some populations of orangutans (Pongo abelli and P. pygmaeus), macaques (Macaca fascicularis), and capuchins (Sapajus spp.) (Smith & Bentley‐Condit, 2010; Shumaker, Walkup, & Beck, 2011). Multiple factors such as the assimilation of sensorimotor knowledge, the development of mechanical reasoning ability, and social and ecological influences intersect to support the emergence of tool skills. Studies into the ontogeny of tool behavior can help illuminate potential reasons for differences in tool behavior between and within taxa and add to our understanding of the adaptive basis for tool skills (Meulman, Seed, & Mann, 2013).
Perception‐action theory (Lockman, 2000) posits that early exploratory actions with objects scaffold the maturation of tool behaviors. This theory predicts that over the course of development, simple actions involving single objects will precede combinatory actions involving multiple objects, or an object and a surface, and that an individual's manipulative actions will become increasingly effective over time as individuals gain experience with object properties. The specific tool behaviors that emerge across species may reflect phylogenetic biases for particular types of object manipulation. For example, human and capuchin infants hit objects against substrates (Fragaszy & Adams‐Curtis, 1991; Kahrs & Lockman, 2014), and these behaviors are later refined into percussive tool use for humans and some populations of capuchins (Resende, Ottoni, & Fragaszy, 2008). In several populations of macaques, stone handling behavior during infancy may scaffold the development of stone tool use under certain conditions (Tan, 2017). Chimpanzees are highly motivated to insert objects into holes or hollows (Hayashi & Matsuzawa, 2003), and stick tool use is prevalent across many wild chimpanzee populations (McGrew, 1992; Sanz & Morgan, 2007).
The development of mechanical reasoning skills may also be necessary for the acquisition of tool skills, particularly for complex tasks involving the flexible use of tools. Flexible tool use is characterized by the ability to use tools across contexts, to attribute multiple functions to a single tool, and to combine tools creatively (Call, 2013). Flexible tool users can adjust their behavior as needed during a tool‐using sequence by including, repeating, or excluding actions in order to achieve a goal (Byrne, Sanz, & Morgan, 2013). Complex tool behaviors are also defined by the presence of cumulative elements, such as the use of multiple different objects concurrently or in sequence (Pradhan, Tennie, & van Schaik, 2012). For example, using a hammer and anvil concurrently during nut cracking requires managing multiple, dynamic relations among objects (Visalberghi & Fragaszy, 2012). In addition, individual task components must be integrated into the correct order. In Loango, Gabon, chimpanzees use highly flexible action sequences to extract honey from underground nests (Estienne, Stephens, & Boesch, 2017), and immatures do not exhibit the complete, adult behavioral repertoire until age six or older (Estienne, Robira, Mundry, Deschner, & Boesch, 2019). The use of multiple tool types in sequence poses additional demands in that it requires a tool user to manage different causal relationships among objects in a specific order, often with a time delay between identifying a goal and achieving success (Boesch, 2013). In captive experiments with chimpanzees, sequential tasks are typically acquired after age three; at this age, chimpanzees may become more capable of socially learning sequential behaviors (Marshall‐Pescini & Whiten, 2008). The age at which basic tool‐using competency is reached differs between primate species and populations as well as across tasks (Table 1).
TABLE 1.
Developmental studies of different tool tasks observed in wild nonhuman primates
| Taxon | Study site | Task | Acquisition age (yrs.) a | Sample size male:female |
|---|---|---|---|---|
| Pan troglodytes | Bossou, Guinea | Leaf to drink water (Biro, Sousa, & Matsuzawa, 2006) | > 1.5 | 5:3 |
| Bossou, Guinea | Ant dip (Humle, Snowdon, & Matsuzawa, 2009) | 2–3 | 2:3 | |
| Bossou, Guinea | Elaeis guineensis Nut crack (Biro, Sousa, & Matsuzawa, 2006; Inoue‐Nakamura & Matsuzawa, 1997; Matsuzawa, 1994) | > 3.5 | 1:2 | |
| Taï, Ivory Coast | Pandaoleosa nut crack (Boesch & Boesch‐Achermann, 2000) | ≥ 5 | 16:14 b | |
| Taï, Ivory Coast | Coulaedulis nut crack (Boesch & Boesch‐Achermann, 2000; Estienne, Cohen, et al., 2019) | 3–4 | 6:5; 23:30 b , c | |
| Loango, Gabon | Honey extract (Estienne, Robira, et al., 2019) | ≥ 6 | 10:6 | |
| Gombe, Tanzania | Termite fish (Lonsdorf, 2005) | 5.5 | 5:3 b | |
| Goualougo, Rep. Congo | Termite fish (this study) | 2.9 | 10:15 b | |
| Goualougo, Rep. Congo | Perforate + termite fish (this study) | 10.5 | 4:3 | |
| Pongo abelii | Suaq Balimbing, Sumatra | Tree hole probe (Meulman et al., 2013) | 5 | 1:0 |
| Suaq Balimbing, Sumatra | Neesia seed extract (Meulman et al., 2013) | 9 | 2:3 | |
| Macaca fascicularis | Koram Island, Thailand | Shellfish crack (Tan, 2017) | 2.5–3.5 | 37:32 b |
| Sapajus libidinosus | Fazenda Boa Vista, Brazil | Nut crack (Eshchar, Izar, Visalberghi, Resende, & Fragaszy, 2016) | > 5 | 7:9 b |
| Sapajus apella | Tietê Ecological Park, Brazil | Nut crack (Resende et al., 2008) | > 2 | 2:0 |
Values are the age or age range by which most individuals acquire basic competency.
Sample sizes reflect the entire data set; ages of acquisition are derived from a subset of these individuals for whom acquisition was documented.
Chimpanzees exhibit substantial intraspecific diversity in tool‐assisted foraging behaviors, including the resources gathered and techniques used (McGrew, 1992; Sanz & Morgan, 2007; Whiten et al., 2001). Across their geographic range, chimpanzees use a variety of tool types to gather insects and insect products (McGrew, 2014), which comprise a valuable source of fat and protein as well as specific fatty acids, vitamins, and amino acids (O'Malley & Power, 2012, 2014). Termites and other social insects offer particular nutritional payoff because of their high collective biomass (Deblauwe & Janssens, 2008), and termite fishing has been documented in multiple populations (Boesch et al., 2020; Bogart & Pruetz, 2008; Goodall, 1986; McGrew & Collins, 1985; McGrew, Tutin, & Baldwin, 1979; Nishida & Uehara, 1980; Sanz, Morgan, & Gulick, 2004). In East and West Africa, chimpanzees use a single tool type, a fishing probe, to gather termites. These tools are manufactured from a range of materials such as grass, twigs, vines, bark, or palm fronds (Goodall, 1968; McGrew et al., 1979; Pascual‐Garrido, 2019).
In Central Africa, in contrast, chimpanzees gather invertebrate resources with the use of tool sets (Bermejo & Illera, 1999; Boesch, Head, & Robbins, 2009; Deblauwe, Guislain, Dupain, & Van Elsacker, 2006; Fay & Carroll, 1994; Muroyama, 1991; Sabater Pí, 1974; Sanz et al., 2004; Sanz, Schöning, & Morgan, 2010; Sugiyama, 1985; Suzuki, Kuroda, & Nishihara, 1995). A tool set is defined as the use of two or more types of tools sequentially to achieve a goal (Brewer & McGrew, 1990). In the termite‐gathering context, chimpanzees in this region use two tool sets to gather termites of the genus Macrotermes from epigeal (above‐ground) and subterranean nests. The use and manufacture of these different tool types has been observed across different chimpanzee communities living in the Goualougo Triangle, Republic of Congo (Sanz et al., 2004; Sanz & Morgan, 2007). At epigeal nests, chimpanzees first use their fingers or a perforating twig to open sealed termite exit holes on the nest surface before using an herbaceous probe, the end of which chimpanzees have modified to a brush tip, to termite fish. In the subterranean nest setting, termites reside in underground chambers at an average depth of 50.6 cm from the nest surface (Sanz, Deblauwe, Tagg, & Morgan, 2014), and chimpanzees use a durable, woody puncturing stick to tunnel into these chambers before using a fishing probe to extract termites (Sanz et al., 2004). These chimpanzees are highly selective in plant species chosen to manufacture both puncturing sticks and fishing probes, and this is not an artifact of plant species abundance. Ninety‐eight percent of puncturing sticks are manufactured from Thomandersia hensii, which has straight, rigid, and durable branches. More than 96% of the fishing probes are manufactured from two species of herb from the Marantaceae family, which is smooth, pliable, and of ideal length and diameter for use as a probe; in addition, its fibers can be effectively frayed to a brush tip (Sanz & Morgan, 2007). The production of brush tips onto the herb stems is an intentional modification that improves the efficiency of the tool at gathering insects (Sanz, Call, & Morgan, 2009). The complex tool behaviors of chimpanzees in this region comprise some of the clearest evidence for cumulative technology in animals (Sanz et al., 2009), so examining how they are acquired offers unique comparative insights for understanding the emergence of cumulative culture during human evolution.
To master the termite‐gathering task, young chimpanzees must acquire each of the components of tool manufacture and tool use and integrate them into the correct sequence. “Critical elements” are the individual, component steps that are necessary to extract termites and that characterize the adult form of this behavior (Lonsdorf, 2005). These steps differ between populations and between tasks depending on whether termite gathering involves fishing for termites with a single tool type versus using a perforating or a puncturing tool set (Figure 1). For infants, manipulation of tools is another important critical element of tool skill acquisition. Developmentally, critical elements are acquired in the following order for all Gombe chimpanzees: identify a hole; manipulate tool; make a tool; insert a tool into the hole; and successfully extract termites. All individuals make tools in the same year, or in the year prior to when they first insert tools (Lonsdorf, 2005).
FIGURE 1.
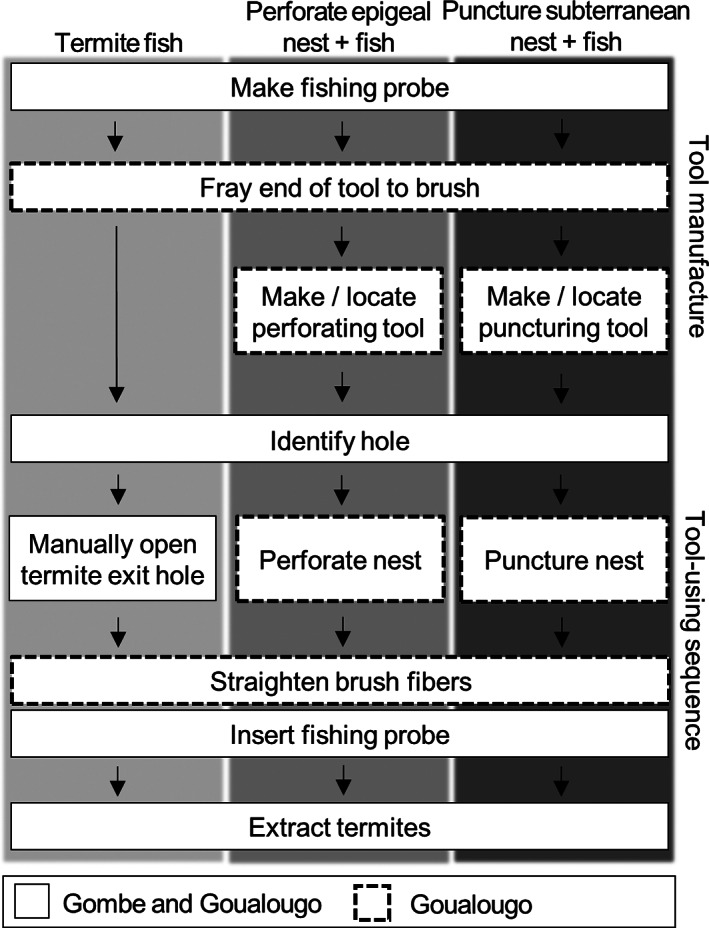
Termite‐gathering critical elements. Elements are listed from top to bottom according to the typical sequence of tool manufacture and tool use, which at Goualougo differs from the sequence in which these elements are acquired. At both sites, identifying termite exit holes sometimes precedes tool manufacture, though at Goualougo chimpanzees often gather tools in advance of arriving at termite nests. Termite fishing occurs at both Goualougo and Gombe, while perforating and puncturing occur only at Goualougo
Females at Gombe learned to termite fish at a mean age of 31 ± 4 months, an average of 27 months earlier than males, who learned at a mean age of 58 ± 6 months (Lonsdorf, Eberly, & Pusey, 2004). Females spent more time watching their mothers termite fish and were more likely to insert tools to similar depths as their mothers, suggesting that they relied more on imitative learning than did males (Lonsdorf, 2005). The socio‐ecological model predicts that females will engage in behavior that maximizes food intake, and several studies have shown that among chimpanzees, adult females compared to adult males use tools more often to acquire termites (Goodall, 1986; McGrew, 1979), nuts (Boesch & Boesch, 1984) and vertebrates (Pruetz & Bertolani, 2007). Tool use among captive bonobos (Pan paniscus) is also female‐biased (Boose, White, & Meinelt, 2013; Gruber, Clay, & Zuberbühler, 2010; but see Herrmann, Hare, Call, & Tomasello, 2010). No sex differences were detected for ant dipping at Bossou, however, for immatures or adults (Humle et al., 2009). Among macaques and capuchins, no sex differences have been reported in the acquisition of tool skills, but there are sex differences in adult tool use (Falótico & Ottoni, 2014; Gumert, Hoong, & Malaivijitnond, 2011; Moura & Lee, 2010; Spagnoletti, Visalberghi, Ottoni, Izar, & Fragaszy, 2011). Some of these differences may be attributable to body size dimorphism, as percussive tool use is likely more energetically demanding for smaller‐bodied females (Gumert et al., 2011; Spagnoletti et al., 2011; Visalberghi & Fragaszy, 2013). Other factors are required to explain some differences, such as male bias in use of lightweight probe tools in capuchins (Falótico & Ottoni, 2014). By documenting when and how sex differences emerge, developmental studies of skill acquisition can help identify the contribution of ecological, morphological, and social factors that may contribute to the variable pattern of sex differences observed across tool‐using taxa.
In the present study, we investigated how wild chimpanzees acquire a complex tool task involving the sequential use of different tool types, selectivity for raw materials, and tool design modifications. Using a longitudinal approach, we examined the age and sequence in which chimpanzees at Goualougo acquired critical elements of termite gathering. We predicted that chimpanzees would first perform simple manipulations of tools before manipulating tools in combination with the termite mound. We also predicted that chimpanzees would learn tool use before tool manufacture, due to the raw material selectivity and design complexity involved in probe manufacture. We further predicted that use of single tools would precede use of tool sets, and that puncturing tool use would be acquired last due to the physical difficulty of puncturing subterranean termite nests. We also examined whether there were sex differences in the acquisition of termite fishing. Finally, we compared the development of termite gathering among chimpanzees at Goualougo to those at Gombe.
2. MATERIALS AND METHODS
2.1. Study site and subjects
Observations of chimpanzee were carried out in the Goualougo Triangle, which is located along the southern boundary of the Nouabalé‐Ndoki National Park (N 2°05–3°03; E 16°51–16°56) in the Republic of Congo. The study region encompasses 380 km2 of evergreen and semi‐deciduous lowland forest, with altitudes ranging between 330 and 600 m. There is a primary rainy season from August to November and a short rainy season in May. Subjects included 25 immature chimpanzees of known birthdate (15 females, 10 males).
2.2. Data collection
We placed remote video cameras with passive infrared sensors at termite nests to record chimpanzee visitation and tool‐using behaviors (Sanz et al., 2004). All video footage was archived on hard drives and scored using INTERACT (Mangold, 2017). Approximately 662 hours of footage of chimpanzee visitation to termite nests collected between 2003–2018 were screened for the presence of focal chimpanzees. All footage of focal individuals was then screened and coded for the first observed occurrences of critical elements of termite‐gathering (Table 2) adapted for this study from Lonsdorf (2005) and Sanz and Morgan (2011). In addition to coding for the critical elements characterizing the adult form of these tasks, we also screened for first occurrences of “Manipulate fishing probe” and “Mound plus tool”, which aid in indexing the acquisition of tool competence.
TABLE 2.
Critical elements of termite‐gathering
| Critical element | Definition |
|---|---|
| Identify hole a | Probes with finger, mops, sniffs, or looks into termite exit hole on nest. |
| Manually open termite exit hole a | Attempts to open termite exit hole by picking at soil with fingers. |
| Manipulate fishing probe a | Possesses tool with any body part and may hold, carry, or play with tool. |
| Mound plus tool a | Actively contacts termite nest with probe but does not insert tool. |
| Insert fishing probe a | Inserts probe into hole on surface of the termite nest. |
| Straighten brush fibers | Pulls tool through mouth, hands or fingers to straighten brush fibers. |
| Extract termites a | Successfully acquires termites on a minimum of three different attempts to insert and extract fishing probe during the same visit to a nest. |
| Fray end of tool to brush | Uses teeth or hand to fray the end of tool into a brush. |
| Manufacture brush‐tipped fishing probe | Detaches raw material; uses teeth or hands to fray the end of the tool; and inserts or attempts to insert tool into termite nest. |
| Perforate epigeal nest | Presses the tip of a woody twig tool into the sealed tunnels of a termite nest, often rotating wrist to drill the tip into the nest. |
| Tool set: Perforate + fish b | Perforates termite nest, then inserts and extracts fishing probe. |
| Puncture subterranean nest | Pushes woody puncturing stick through the ground into a subterranean termite nest and successfully creates a new fishing tunnel. |
| Tool set: Puncture + fish b | Punctures subterranean termite nest, then inserts and extracts fishing probe. |
Indicates that elements are also observed at Gombe.
Tool set use was scored even if chimpanzees did not have success fishing on the first occasion the behavior was observed.
Remote cameras record the dates of observation, enabling calculation of the ages at which behaviors were first observed. In order for an observation to be included in the data set, the focal individual must have been observed visiting a termite nest and have had the opportunity to engage in tool use at least once in the nine‐month period prior to the visit in which they were first observed engaging in the behavior of interest. This ensured that individuals' behaviors were detected with comparable precision to Gombe, where data were collected over four years during three‐month termite‐fishing seasons and individuals could have acquired skills in the nine‐month period between field seasons. Differing sample sizes between elements reflect these selection criteria. Within‐subjects analyses comparing acquisition of multiple elements included the subset of subjects for whom both of the relevant critical elements were observed in accordance with these criteria. For example, 12/25 subjects could be included for the within‐subjects comparison of acquisition ages for manipulation of a fishing probe versus use of a fishing probe in combination with a termite mound.
2.3. Reliability and replicability
To ensure that operational definitions were consistently applied, an inter‐observer reliability test was conducted with an expert observer (familiar with primate behavior) who was not involved in this study. The test comprised 20% of the critical element first occurrences, with examples from both subterranean and epigeal contexts. Inter‐observer reliability was 100%. In addition, two coauthors reviewed all the critical elements for each chimpanzee to ensure consensus in how all critical elements were assigned (sensu Humle & Matsuzawa, 2002). In the case that two coauthors were unable to reach agreement, additional coauthors were consulted. For examples of each critical element, see supplemental videos S1 (epigeal context) and S2 (subterranean context).
2.4. Analysis
Prior to conducting analyses, we visually inspected raw data and used Shapiro–Wilk tests to determine whether data were normally distributed. All tests were two‐tailed and the significance threshold was set at .05. Analyses were conducted in R (version 3.4.4) (R Core Team, 2018).
We first examined whether the ages at which individuals first learned to insert probes and to extract termites were comparable between epigeal and subterranean nest types. We assessed a subset of individuals observed in both epigeal and subterranean settings during early infancy, using paired t‐tests to compare their ages of acquisition of the critical elements “Insert fishing probe” and “Extract termites” in the epigeal versus subterranean settings. These two elements in particular were assessed because structural differences between nest types could place difference technical demands on the tool user.
To test our prediction that simple actions would precede combinatory manipulations, we compared ages at which chimpanzees exhibited the critical elements “Manipulate fishing probe” and “Mound plus tool”. To assess whether tool use would precede the manufacture of brush‐tipped probes, we compared the ages of acquisition of “Extract termites” and “Manufacture brush‐tipped fishing probe”. These tests were within‐subjects and so we conducted paired t‐tests. We report descriptive statistics comparing the ages of acquisition of “Extract termites” to “Tool set: perforate + fish” to evaluate whether use of single tools would precede use of multiple tools.
To test for sex differences in the acquisition of termite fishing skills, we compared females and males with respect to ages of acquisition of “Extract termites” using a Wilcoxon‐Mann–Whitney test, and “Manufacture brush‐tipped fishing probe” using an independent‐samples t‐test. Means are reported with standard deviation.
2.5. Ethical note
This was a strictly observational study involving remote video monitoring of wild chimpanzees. Remote video monitoring of chimpanzee tool use sites was initiated in the Goualougo Triangle in 2003 to complement direct observations of chimpanzees, who are well habituated to these devices. Remote cameras vastly increase the sample size of individuals observed while minimizing impact on the forest and the apes. For more detail, see Sanz et al. (2004). All research reported in this manuscript complied with the protocols approved by the Washington University in St. Louis Institutional Animal Care and Use Committee (Protocol Number 20160081) and the legal requirements of the Republic of Congo and adheres to the ASAB/ABS Guidelines for the Use of Animals in Research.
3. RESULTS
3.1. Comparison of epigeal and subterranean nest settings
We did not detect significant differences in the age at which chimpanzees learned to insert fishing probes in epigeal (M = 2.2 ± 0.7 years) versus subterranean (M = 1.9 ± 0.4 years) nest contexts (paired t‐test, t6 = 1.05, N = 7, p = 0.33, 95% CI [−0.3, 0.7]). We also did not detect significant differences in the ages at which immature chimpanzees were successful extracting termites in epigeal (M = 2.6 ± 0.7 years) versus subterranean (M = 2.3 ± 0.7 years) nest contexts (paired t‐test, t4 = 0.66, N = 5, p = .55, 95% CI [−0.9, 1.5]).
3.2. Simple versus combinatory actions
The majority of individuals (9/12) were observed manipulating tools at earlier visits than they were observed using a tool in combination with the mound, while three individuals were first observed manipulating a tool and using it in conjunction with the mound during the same visit. There was a significant difference in the age at which chimpanzees first began manipulating fishing probes (M = 1.2 ± 0.5 years) and the age at which they first used a fishing probe in combination with a termite mound (M = 1.6 ± 0.4 years) (paired t‐test: t11 = −4.01, N = 12, p = .002, 95% CI [−0.6, −0.2]).
3.3. Tool use versus tool manufacture
All infants successfully fished for termites by age 2.9 (Table 3). At this age, infants typically used discarded tools, or they received tools from conspecifics. Most chimpanzees (10/12 infants) inserted fishing probes and also succeeded at acquiring termites (9/12 infants) before they detached any type of raw material themselves and attempted to use those materials as a tool. Three individuals were observed detaching raw material near the nest to fish, but they were not successful with these tools.
TABLE 3.
Mean age of acquisition of critical elements for males and females at Goualougo
| Critical element | Female | Male |
|---|---|---|
| Termite fishing | ||
| Identify hole | 0.8 (0.4–1.3), N = 7 | 1.0 (0.6–1.7), N = 5 |
| Manipulate fishing probe | 1.2 (0.3–1.7), N = 7 | 1.2 (0.5–2.1), N = 5 |
| Manually open termite exit hole | 1.3 (0.6–2.1), N = 7 | 1.8 (0.8–2.5), N = 8 |
| Mound plus tool | 1.6 (1.0–2.3), N = 8 | 1.8 (1.0–2.7), N = 8 |
| Insert fishing probe | 1.8 (1.2–2.5), N = 5 | 1.9 (1.5–2.7), N = 8 |
| Straighten brush fibers | 1.9 (1.2–2.3), N = 5 | 2.1 (1.7–3.0), N = 7 |
| Extract termites | 2.1 (1.3–2.9), N = 4 | 2.3 (2.0–2.7), N = 7 |
| Fray end of tool to brush | 3.4 (1.4–4.8), N = 8 | 3.6 (2.4–4.7), N = 7 |
| Manufacture a brush‐tipped fishing probe | 4.6 (2.5–5.8), N = 4 | 4.1 (3.3–5.5), N = 6 |
| Perforating at epigeal termite nests | ||
| Perforate | 7.0 (3.9–9.7), N = 4 | 7.5 (4.3–10.5), N = 4 |
| Tool set: Perforate + fish | 7.1 (3.9–9.8), N = 4 | 9.0 (7.6–10.5), N = 3 |
| Puncturing at subterranean termite nests | ||
| Puncture | 11.0, N = 1 | 11.7, N = 1 |
| Tool set: Puncture and fish | 11.0, N = 1 | 11.7, N = 1 |
Note: Values are the mean age of acquisition for the critical element. Age ranges of the earliest and latest appearances of the behaviors are listed in parentheses, followed by sample size of individuals (N).
All individuals were observed successfully fishing for termites before they were observed independently gathering the specific herbaceous raw material adults typically select for this task and manufacturing brush‐tipped fishing probes. Manufacture of brush‐tipped fishing probes was first observed in chimpanzees at an average age of 4.3 ± 1.1 years (N = 10). There was a significant difference in the age of first successfully extracting termites (M = 2.2 ± 0.5 years) and brush‐tipped probe manufacture (M = 4 ± 1 year) (paired t‐test: t8 = 5.58, N = 8, p < .001, 95% CI [1.0, 2.5]).
3.4. Use of single versus multiple tools
Eight individuals were observed using perforating tools at epigeal nests. The youngest individual was a female at 3.9 years old, while other chimpanzees were observed using perforating tools for the first time between ages four and 11. All individuals began using fishing probes and were successful extracting termites before first using a perforating tool set (the perforating twig plus the fishing probe in sequence). Relative to the similarity in ages at which fishing probe insertion and extraction of termites were first observed, the age at which individuals were first observed perforating was more variable between individuals (Figure 2). One individual was also observed using his probe not only to fish but also to perforate. This involved reversing his fishing probe and using the unmodified end to clear a fishing tunnel, a behavior which has been observed among multiple individuals in this population (Sanz & Morgan, 2011). This was observed during the same visit where he was first observed using a perforating tool set, at age 10.5 years.
FIGURE 2.
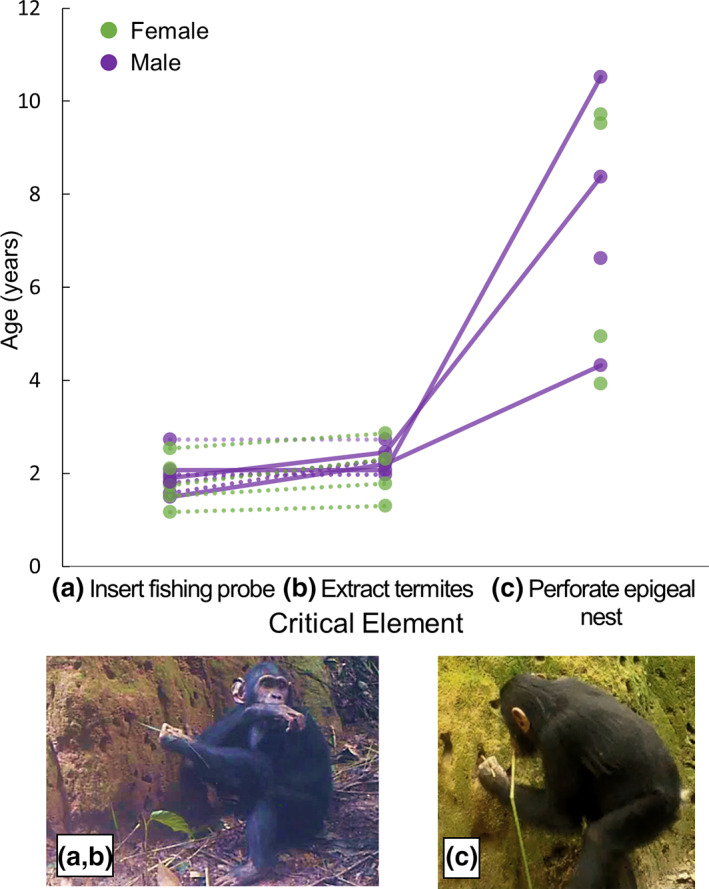
Ages of perforating tool use relative to fishing probe tool use. Dots represent individuals. Dotted lines connect observations for immature chimpanzees observed for both “Insert fishing probe” and “Extract termites”; solid lines connect observations for three individuals for whom we could document ages for these elements as well as for the age at which they first exhibited perforating tool use. While all three of these individuals could extract termites by age 2.4, the ages at which they were first observed perforating an epigeal nest (4.3, 8.4, and 10.5 years) varied widely. At left, a juvenile male inserts a fishing probe (a) and feeds on termites he has swept from the fishing probe after a successful extraction (b). At right (c), he uses a twig to perforate an epigeal nest, while holding a fishing probe in his mouth
In the subterranean termite nest setting, infant and juvenile chimpanzees frequently manipulated puncturing sticks, inserted these tools into existing or partially cleared tunnels created by older conspecifics, and attempted to puncture new holes into subterranean nests. We observed five individuals (four females, one male) exhibit the sequence of puncturing tool set use (M = 3.7 ± 1.6 years). This involved inserting a puncturing tool into an existing hole and then fishing or attempting to fish from the tunnel with a fishing probe. All of these individuals were observed inserting fishing probes at earlier visits than they were observed engaging in the sequence of puncturing tool set use. Only two subadult individuals (one male, 11.7 years, and one female, 11 years) were observed successfully puncturing a new hole into a subterranean termite nest.
3.5. Sex differences in termite gathering
The developmental trajectories of termite‐gathering were similar for female and male chimpanzees at Goualougo (Table 3; Figure 3). We did not detect a significant difference in the age at which females (M = 2.1 ± 0.7 years, N = 4) versus males (M = 2.3 ± 0.3 years, N = 7) learned to extract termites (Wilcoxon‐Mann–Whitney test: W = 12, N 1 = 4, N 2 = 7, p = .249). We also did not detect a significant difference between the ages at which females (M = 4.6 ± 1.5 years, N = 4) versus males (M = 4.1 ± 0.8, N = 6) first manufactured a brush‐tipped probe (independent samples t‐test: t8 = 0.67, p = .52; 95% CI [−1.2, 2.2]). Females did acquire most critical elements slightly before males, with the exception of tool manufacture. Acquisition ages for tool manufacture were more variable than those for fishing (Figure 4). The mean ages at which females and males first used tool sets were comparable and showed similar ranges (Table 3).
FIGURE 3.
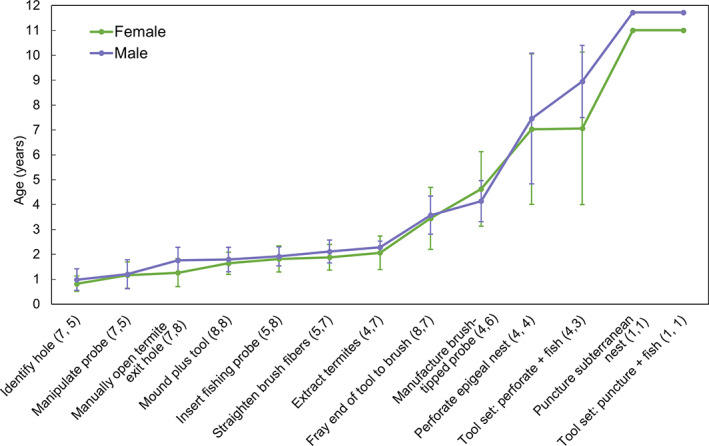
Ages of acquisition of critical elements for chimpanzees at Goualougo. Values are means and error bars represent standard deviation. Sample sizes are given for each sex in parentheses (female, male). Females and males acquired critical elements at comparable ages, though females acquired all critical elements except “Manufacture brush‐tipped fishing probe” before males. Compared to acquisition ages for single tool use, ages at which the use of tool sets were first observed were more variable
FIGURE 4.
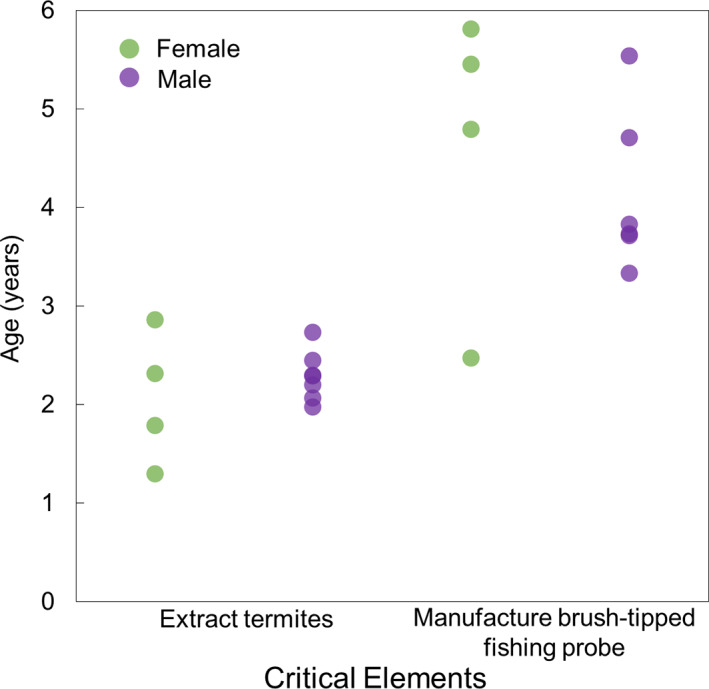
Ages of successful termite extraction versus tool manufacture for females and males. Dots represent individuals. The ages ranges for acquisition of both elements overlapped for males and females, though for both elements, the youngest observation was for a female. We observed that on average, females were observed successfully extracting termites at slightly younger ages than males, while males were observed making tools at younger ages than were females
3.6. Development of termite gathering at Goualougo compared to Gombe
Most infant chimpanzees at both Goualougo and Gombe begin interacting with tools and termite mounds within the first one to 2 years of life. There are differences, however, with respect to the timeframe in which infants first insert fishing probes, become capable of termite fishing, and independently manufacture tools (Table 4). In addition, the developmental period over which skills are acquired is longer at Goualougo. At Gombe, all individuals mastered the critical elements necessary for termite fishing by age 5.5. At Goualougo, individuals learned to termite fish during infancy, but several individuals were not observed perforating until they were juveniles or subadults. Only subadults were observed independently puncturing new tunnels into subterranean nests.
TABLE 4.
Maximum ages of acquisition of termite‐gathering critical elements in the Goualougo Triangle, Republic of Congo, and at Gombe, Tanzania
| Critical element | Goualougo | Gombe |
|---|---|---|
| Termite fishing | ||
| Identify hole | 1.7 (0.4–1.7), N = 12 | 1.5 (0.5–1.5), N = 8 |
| Manipulate fishing probe | 2.1 (0.3–2.1), N = 12 | 1.5 (0.5–1.5), N = 8 |
| Insert fishing probe | 2.7 (1.2–2.7), N = 13 | 4.5 (2.5–4.5), N = 8 |
| Extract termites | 2.9 (1.3–2.9), N = 11 | 5.5 (2.5–5.5), N = 6 |
| Manufacture fishing probe without brush tip | 3.0 (1.2–3.0), N = 6 | 3.5 (1.5–3.5), N = 6 |
| Manufacture brush‐tipped fishing probe | 5.8 (2.5–5.8), N = 10 | — |
| Perforating at epigeal termite nests | ||
| Perforate | 10.5 (3.9–10.5), N = 8 | — |
| Tool set: Perforate + fish | 10.5 (3.9–10.5), N = 7 | — |
| Puncturing at subterranean termite nests | ||
| Puncture | 11.7 (11.0–11.7), N = 2 | — |
| Tool set: Puncture and fish | 11.7 (11.0–11.7), N = 2 | — |
Note: Values are the age in years by which all individuals in the sample acquired the critical element. Age ranges of the earliest and latest appearances of the behaviors are listed in parentheses, followed by sample size of individuals (N). “—” indicates that the behavior does not occur at Gombe.
4. DISCUSSION
Tool‐assisted foraging traditions may emerge when they are profitable relative to other feeding strategies (Rutz & St. Clair, 2012; Sanz & Morgan, 2013a), so learning these behaviors could have important adaptive benefits. Examining how novices acquire tool skills can provide insight into the perceptuo‐motor and cognitive requisites of these skills as well as the way ecological factors, social input, and task characteristics affect acquisition. In this study, we took a longitudinal approach to investigate the acquisition of termite‐gathering critical elements among Goualougo Triangle chimpanzees. We found that these chimpanzees learn to termite fish before they manufacture brush‐tipped probes and that they become competent with single tools before they use multiple tool types sequentially. We also documented differences between Goualougo and Gombe in the sequence of skill acquisition, as well as the ages at which particular elements were acquired. In addition, in contrast to Gombe, we did not detect significant sex differences in the acquisition of termite fishing.
The onset of manipulative behaviors and tool use among chimpanzees at Goualougo is consistent with predictions of perception‐action theory (Lockman, 2000), which anticipates that simpler behaviors and single tool use will be acquired before more complex sequences. Within the first year of life, most chimpanzees manipulated objects and investigated termite mounds. Between ages one and three, they progressed to goal‐directed efforts to fish for termites, which involved locating a tool, manually opening a termite exit hole or using an exit hole opened by another chimpanzee, inserting a fishing probe, and successfully extracting termites. Probe insertion and fishing occurred at slightly earlier ages on average in the subterranean setting, and in future research we will examine whether there are differences in the specific skilled motor actions required to gather termites from each nest type. Nonetheless, once chimpanzees learned to use fishing probes in either the epigeal or subterranean context, they transferred their skills to the other setting. This ability to generalize skills from one context to another is a hallmark of flexible tool behavior. After becoming competent with single tools and learning to termite fish, chimpanzees then began manufacturing their own tools. Some individuals also began engaging in sequential tool use, involving a perforating twig plus a fishing probe in the epigeal context, and a puncturing stick plus a fishing probe in the subterranean context.
Our findings were also generally consistent with prior research showing that chimpanzees typically learn sequential behaviors after 3 years of age (Marshall‐Pescini & Whiten, 2008). As with use of tool sets, the behavioral sequence associated with manufacture and use of brush‐tipped fishing probes occurred on average after 3 years of age, and the component actions were acquired before they were combined into the correct order. Integration of actions into the correct sequence is hypothesized to be linked to the capacity for program‐level imitation (Hayashi & Inoue‐Nakamura, 2011; Marshall‐Pescini & Whiten, 2008). This process involves an individual perceiving the hierarchical organization of a task that emerges from statistical regularities in a model's behavior and parsing that behavior into meaningful units, enabling reproduction of the structure of the behavior (Byrne, 1994; Byrne & Russon, 1998). It has also been hypothesized that there is a critical period during development for acquiring hierarchically structured, sequential behaviors, after which such acquisition cannot occur (Biro et al., 2003). While we documented general patterns in the acquisition of sequential behaviors after age three, there were two infants who exhibited the use of a puncturing stick and a fishing probe in sequence before age three. Additionally, only some individuals used perforating tool sets. Increased opportunity to practice tool‐using skills and increased observation of conspecifics is associated with accelerated skill acquisition of termite fishing (Lonsdorf, 2006) and ant‐dipping (Humle et al., 2009). Further research is required to identify how differing opportunity for social learning may contribute to inter‐individual variation in the acquisition of complex, sequential skills at Goualougo.
As we predicted, puncturing subterranean nests was observed latest in development. In addition to cognitive factors, physical strength and body size are important constraints on the use of puncturing tool sets. Subadult and adult chimpanzees often grip puncturing sticks with their hands and a foot, using the weight of their bodies to forcefully push puncturing sticks down through the ground. Despite repeated attempts, infants and juveniles could not push the puncturing stick through the soil. Nonetheless, young chimpanzees do attempt to puncture throughout the infant and juvenile period, often focusing their efforts on existing or partially cleared tunnels that have been created by other chimpanzees. We are presently examining what contributes to the persistent efforts of young chimpanzees in this context.
We observed that both the timing and sequence of termite‐gathering skill acquisition differed between Goualougo and Gombe chimpanzees. At Goualougo, infants inserted fishing probes and learned to extract termites at younger ages than at Gombe, particularly when compared to male infants at Gombe. One possibility for these differences is that year‐round termite gathering at Goualougo (Sanz & Morgan, 2013a) provides greater opportunity for immature chimpanzees to develop skills relative to Gombe, where termite‐gathering efforts are concentrated during the rainy reason from October to December (Goodall, 1986; McGrew et al., 1979). The ages at which Goualougo chimpanzees began showing combinatory behaviors (“Mound plus tool” and “Insert fishing probe”) and learned to successfully extract termites are more comparable to patterns of acquisition documented in some captive experiments, where combinatory manipulation was observed frequently by 21 months of age (Takeshita et al., 2005), and infants could successfully “fish” for honey at just under two years of age (Hirata & Celli, 2003). At Goualougo, several infants learned to successfully extract termites before or around two years of age. In the captive study of honey fishing, infants had monthly opportunities to develop these skills (Hirata & Celli, 2003). Thus the opportunity to practice skills year round may result in faster acquisition than a shorter period of concentrated practice (Lonsdorf, 2006). An additional possibility is that opportunities for social learning differ between sites. At Gombe, the presence of multiple models does not accelerate offspring acquisition of skill (Lonsdorf, 2006), and at Goualougo, average party size at termite nests is relatively small, 2.23 ± 1.57 individuals (Sanz & Morgan, 2013b). Thus, other aspects of social learning opportunity, such as tool sharing (Musgrave et al., 2019; Musgrave, Morgan, Lonsdorf, Mundry, & Sanz, 2016) may be more influential.
We also documented that there are differences between populations with respect to the sequence in which skills of tool use versus tool manufacture are acquired. At Gombe (Lonsdorf, 2006) and Bossou (Humle, 2006), infants learn to make tools before or at the same time they learn to use them. At Goualougo, infants rarely attempted to manufacture their own tools before they were capable of fishing; instead, they appear to rely on discarded herb tools or tools that conspecifics, typically their mother, transfer to them. They learned to fish effectively with these tools and to maintain the brush tip before moving on to gather herb stems independently and manufacture brush‐tipped probes. Thus the manufacture of adult‐like tools in this population occurred after learning to termite fish. In other populations and species where tool characteristics and raw material impact tool performance, youngsters also tend to first rely on others' tools rather than manufacturing or selecting their own (e.g., leaf‐folding to gather water in chimpanzees, Sousa, Biro, & Matsuzawa, 2009; Tonooka, 2001; or probing for insects by New Caledonian crows, Holzhaider, Gray, & Hunt, 2010), which can improve immatures' tool‐using efficiency (Estienne, Cohen, et al., 2019).
The population differences we observed between Goualougo and Gombe chimpanzees could be related to cognitive challenges associated with identifying and locating suitable raw material in the environment, linking behaviors in the appropriate sequence, and producing a tool of suitable dimensions and with a functional brush tip at Goualougo. At Gombe, chimpanzees manufacture tools from varied materials and they do not engage in the brush‐tip modification, so probe manufacture is a simpler undertaking. We have documented significant differences in tool transfer behavior between these two populations: at Goualougo compared to Gombe, transfers occur at a higher rate, and mothers are more likely to respond positively to offspring requests for tools. These findings suggest that the acquisition of more complex tasks is associated with an enhanced role for social learning (Musgrave et al., 2019). The later age at which tool manufacture is acquired at Goualougo may also be associated with the fact that mature chimpanzees often gather raw material in advance of arriving at a termite nest (Byrne et al., 2013; Sanz et al., 2004). Young chimpanzees continue to dorsally ride on their mothers through age 4–5 and remain in constant association through age 8–10 (Boesch & Boesch‐Achermann, 2000; Goodall, 1968; Lonsdorf et al., 2014). If infants are traveling on their mother's body, they may be hesitant to dismount to independently acquire tool material on the way to the nest, or, similarly, to leave their mother's immediate vicinity upon arrival at the nest in order to acquire raw material.
Similar constraints as apply to fishing probes may help explain why we did not observe infants or juveniles manufacture puncturing sticks. In addition, these durable tools are conserved at subterranean termite nests over weeks or months, mitigating the need to manufacture a new tool. Given the inability of young individuals to puncture, there may also be little incentive to manufacture a new puncturing stick. We did observe youngsters manufacture perforating tools; unlike fishing probes and puncturing sticks, these tools were procured by gathering or detaching a twig from the immediate vicinity of the nest. The development of tool use and manufacture by immature chimpanzees in this population thus reflects the raw material demands and design features of the different tool types and highlights the importance of access to others' tools in enabling the opportunity to practice tool skills, particularly for fishing and puncturing.
With respect to sex differences, the youngest individual observed engaging in each critical element was female, and on average, females acquired most critical elements of termite fishing slightly before males did. The exception to this was tool manufacture, which was observed on average 6 months earlier in males than females. We did not observe significant sex differences in the ages of skill acquisition such as have been documented for termite fishing at Gombe, where female infants fish significantly more, and at earlier ages than do males (Lonsdorf, 2005). However, our analytical approach here was to compare the acquisition ages of critical elements between the sexes, rather than the percent of time allocated to termite‐fishing behaviors as was done at Gombe. It is also possible that there are sex differences in the acquisition of termite gathering at Goualougo that we did not have sufficient statistical power to detect. In addition, differences that are not statistically significant may nonetheless reflect meaningful variation in development that merits further study, particularly given the relatively small sample sizes that often characterize developmental studies. The differences we observed could reflect subtle variation between males and females, for example with respect to propensity for object manipulation (Koops, Furuichi, Hashimoto, & van Schaik, 2015) or in spatial independence (Lonsdorf et al., 2014). At Kalinzu, Uganda, immature female chimpanzees show more diverse types of object manipulation, potentially in preparation for adult tool use (Koops et al., 2015). At Gombe, male compared to female infant chimpanzees begin traveling independently at earlier ages and show increased distance from their mothers by age three. These differences may index earlier gross motor development in males (Lonsdorf et al., 2014). The slightly younger ages of manufacture we observed in males at Goualougo could reflect earlier ages of spatial independence from mothers, which is necessary for raw material procurement. Relatively little is known about manual, fine motor control in great apes (Bardo, Cornette, Borel, & Pouydebat, 2017), though there is some evidence for superior performance by human female infants in fine motor tasks (e.g., Kokštejn, Musálek, & Tufano, 2017). The impact of these factors, and of sex differences in social learning strategies (Lonsdorf, 2005) on tool skill acquisition may also be accentuated at Gombe by the highly seasonal nature of termite gathering in this population (Goodall, 1986; McGrew et al., 1979). If males do not learn to termite fish in a given termite fishing season, they may not have an opportunity to develop this skill until the subsequent season. At Goualougo, in contrast, year‐round termite gathering (Sanz & Morgan, 2013a) provides continuous opportunity to develop tool skills.
Further research is required to investigate how sex differences in infancy relate to adult sex differences in tool use skill or frequency. At Gombe, the sex difference in how much time females versus males spent termite fishing when they were present at the mound disappeared after age 5.5, once all male infants had acquired the skill. This sex difference in time allocation is present again in adulthood, when females fish more frequently and for longer periods of time than males (McGrew, 1979; Pandolfi, van Schaik, & Pusey, 2003). Data for adult tool use are not yet available from Kalinzu. At Goualougo, adult females visit termite nests more frequently on average, though the average time spent in termite‐gathering tool use per day is similar between adult females and males (Ellison, Musgrave, Morgan, & Sanz, 2016). Females and males also do not differ in their mean dipping latencies, a measure of performance, when termite fishing (Sanz, Morgan, & Hopkins, 2016). We have observed that in both populations, immature females compared to males are more successful acquiring tools via transfer, but it is not yet clear to what extent this results from differential treatment by mothers (Musgrave et al., 2019). Continued investigation of this topic will add to our understanding of how the ontogeny of tool skills is related to adult patterns of sexually differentiated foraging in this population and for chimpanzees more broadly.
Comparative investigations of the ontogeny of tool behavior across tool‐using taxa, and within species between tasks, provide unique insights into the adaptive basis of tool skills and the factors supporting the maintenance of tool traditions over time. The present study offers the first assessment of the acquisition of termite gathering among chimpanzees in Central Africa. While the earliest stone tools date to 3.3 Mya (Harmand et al., 2015), indirect evidence suggests that the capacity for complex, flexible tool use likely evolved earlier, in the common ancestor of humans and the other great apes (Panger, Brooks, Richmond, & Wood, 2002). The rich, perishable tool repertoire of Central chimpanzees could provide clues to the tool skills of this common ancestor, evidence for which may not have been preserved in the archeological record (Haslam, 2014). We suggest that in addition to influencing the timing and sequence of skill acquisition, the complexity of the termite‐gathering task in this population is likely associated with an important role for social input in the acquisition of tool skills. Continued research on the ontogeny of complex elements in this context will further illuminate how the technology of chimpanzees in this region persists over generations.
CONFLICT OF INTEREST
The authors declare no conflict of interests.
AUTHOR CONTRIBUTIONS
Stephanie Musgrave: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; software; validation; writing‐original draft; writing‐review and editing. Elizabeth Lonsdorf: Conceptualization; methodology; supervision; validation; writing‐review and editing. David Morgan: Conceptualization; data curation; funding acquisition; investigation; methodology; project administration; resources; software; supervision; validation; writing‐review and editing. Crickette Sanz: Conceptualization; data curation; formal analysis; funding acquisition; investigation; methodology; resources; software; supervision; validation; writing‐review and editing.
Supporting information
Video S1 Critical Elements of Termite Gathering at Epigeal Nests. This video shows the critical elements of termite gathering in the context of above‐ground termite nests, in the order in which they are typically observed among immature chimpanzees. The behaviors “Manipulate fishing probe” and “Mound plus tool” are also shown, as they represent important indices of skill development. Young chimpanzees learn to fish for termites from epigeal nests during infancy at comparable ages, but the use of perforating tool sets occurs later and at more variable ages.
Video S2 Critical Elements for Termite Gathering at Subterranean Nests. This video shows the critical elements of termite gathering in the context of subterranean termite nests, in the order in which they are typically observed among immature chimpanzees. The behaviors “Manipulate fishing probe” and “Mound plus tool” are also shown, as they represent important indices of skill development. Young chimpanzees learn to extract termites from both subterranean and epigeal nests before they independently manufacture brush‐tipped fishing probes. While young chimpanzees sometimes insert puncturing tools into existing tunnels, the ability to puncture new tunnels into subterranean termite nests requires great physical strength and has only been observed among mature individuals.
ACKNOWLEDGMENTS
We are deeply appreciative of the opportunity to work in the Nouabalé‐Ndoki National Park and especially the Goualougo Triangle. This research would not have been possible without the continued support of the Ministère de l'Economie Forestière du gouvernement de la République du Congo and the Agence Congolaise de la Faune et des Aires Protégées (ACFAP). The Wildlife Conservation Society's Congo Program and the Nouabalé‐Ndoki Foundation are integral partners in this continuing research. Special thanks are due to M. Gately, E. Stokes, T. Breuer, P. Ngouembe, D. Dos Santos, E. Arnhem and M. Ngangoue. We would also like to recognize the tireless dedication of J. R. Onononga, C. Eyana‐Ayina, S. Ndolo Ebika, A. Nzeheke, W. Mayoukou, S. Kialiema, J. Wawa, F. Ebombi, J. M. Massamba, J. Ortega, D. Koni, M. Meguessa, I. Singono, and the Goualougo tracking team. We also thank J. Funkhouser and E. Bell for discussions and their contributions to this research. In addition, we thank the Editors and anonymous reviewers for their helpful comments on this manuscript. Grateful acknowledgment of funding is due to the National Science Foundation (Award ID 1613596), the Wenner‐Gren Foundation (Grant Number 9201), the Leakey Foundation, Lambda Alpha National Anthropology Honor Society, the Arcus Foundation, the Indianapolis Zoo, the Cincinnati Zoo and Botanical Garden, the Saint Louis Zoo, and the Columbus Zoo and Aquarium.
Musgrave S, Lonsdorf E, Morgan D, Sanz C. The ontogeny of termite gathering among chimpanzees in the Goualougo Triangle, Republic of Congo. Am J Phys Anthropol. 2021;174:187–200. 10.1002/ajpa.24125
Funding information Arcus Foundation; Cincinnati Zoo and Botanical Garden; Columbus Zoo and Aquarium; National Science Foundation, Grant/Award Number: 1613596; Indianapolis Zoo; Lambda Alpha National Anthropology Honor Society; Leakey Foundation; Saint Louis Zoo; Wenner‐Gren Foundation, Grant/Award Number: 9201
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Bardo, A. , Cornette, R. , Borel, A. , & Pouydebat, E. (2017). Manual function and performance in humans, gorillas, and orangutans during the same tool use task. American Journal of Physical Anthropology, 164(4), 821–836. 10.1002/ajpa.23323 [DOI] [PubMed] [Google Scholar]
- Bermejo, M. , & Illera, G. (1999). Tool‐set for termite‐fishing and honey extraction by wild chimpanzees in the Lossi Forest, Congo. Primates, 40(4), 619–627. 10.1007/BF02574837 [DOI] [Google Scholar]
- Biro, D. , Inoue‐Nakamura, N. , Tonooka, R. , Yamakoshi, G. , Sousa, C. , & Matsuzawa, T. (2003). Cultural innovation and transmission of tool use in wild chimpanzees: Evidence from field experiments. Animal Cognition, 6(4), 213–223. 10.1007/s10071-003-0183-x [DOI] [PubMed] [Google Scholar]
- Biro, D. , Sousa, C. , & Matsuzawa, T. (2006). Ontogeny and cultural propagation of tool use by wild chimpanzees at Bossou, Guinea: Case studies in nut cracking and leaf folding In Matsuzawa T., Tomonaga M., & Tanaka M. (Eds.), Cognitive development in chimpanzees (pp. 476–508). Tokyo: Springer‐Verlag. [Google Scholar]
- Boesch, C. (2013). Ecology and cognition of tool use in chimpanzees In Sanz C., Call J., & Boesch C. (Eds.), Tool use in animals: Cognition and ecology (pp. 21–47). Cambridge: Cambridge University Press; 10.1017/CBO9780511894800.003 [DOI] [Google Scholar]
- Boesch, C. , & Boesch‐Achermann, H. (2000). The chimpanzees of the Taï Forest: Behavioural ecology and evolution. Oxford: Oxford University Press. [Google Scholar]
- Boesch, C. , & Boesch, H. (1984). Possible causes of sex differences in the use of natural hammers by wild chimpanzees. Journal of Human Evolution, 13(5), 415–440. 10.1016/S0047-2484(84)80055-X [DOI] [Google Scholar]
- Boesch, C. , Head, J. , & Robbins, M. (2009). Complex tool sets for honey extraction among chimpanzees in Loango National Park, Gabon. Journal of Human Evolution, 56(6), 560–569. 10.1016/j.jhevol.2009.04.001 [DOI] [PubMed] [Google Scholar]
- Boesch, C. , Kalan A. K., Mundry, R. , Arandjelovic, M. , Pika, S. , Dieguez, P. , Ayimisin, E. A. , Barciela, A. , Coupland, C. , Egbe, V. E. , Eno‐Nku, M. , Michael F. J., Fine, D. , Adriana Hernandez‐Aguilar, R. , Hermans, V. , Kadam, P. , Kambi, M. , Llana, M. , Maretti, G. , Morgan, D. , Murai, M. , Neil, E. , Nicholl, S. , Ormsby, L. J. , Orume, R. , Pacheco, L. , Piel, A. , Sanz, C. , Sciaky, L. , Stewart, F. A. , Tagg, N. , Wessling, E. G. , Willie, J. , & Kühl, H. S. (2020). Chimpanzee ethnography reveals unexpected cultural diversity. Nature Human Behaviour, 10.1038/s41562-020-0890-1. [DOI] [PubMed] [Google Scholar]
- Bogart, S. , & Pruetz, J. (2008). Ecological context of savanna chimpanzee (Pan troglodytes verus) termite fishing at Fongoli, Senegal. American Journal of Primatology, 70(6), 605–612. 10.1002/ajp.20530 [DOI] [PubMed] [Google Scholar]
- Boose, K. J. , White, F. J. , & Meinelt, A. (2013). Sex differences in tool use acquisition in bonobos (Pan paniscus). American Journal of Primatology, 75(9), 917–926. 10.1002/ajp.22155 [DOI] [PubMed] [Google Scholar]
- Brewer, S. M. , & McGrew, W. C. (1990). Chimpanzee use of a tool‐set to get honey. Folia Primatologica, 54(1–2), 100–104. 10.1159/000156429 [DOI] [PubMed] [Google Scholar]
- Byrne, R. (1994). The evolution of intelligence In Slater P. J. B. & Halliday T. R. (Eds.), Behaviour and evolution (pp. 223–265). New York: Cambridge University Press. [Google Scholar]
- Byrne, R. , Sanz, C. , & Morgan, D. (2013). Chimpanzees plan their tool use In Sanz C., Call J., & Boesch C. (Eds.), Tool use in animals: Cognition and ecology (pp. 48–64). Cambridge: Cambridge University Press; 10.1017/CBO9780511894800.004 [DOI] [Google Scholar]
- Byrne, R. W. , & Russon, A. E. (1998). Learning by imitation: A hierarchical approach. Behavioral and Brain Sciences, 21(5), 667–721. 10.1017/S0140525X98001745 [DOI] [PubMed] [Google Scholar]
- Call, J. (2013). Three ingredients for becoming a creative tool user In Sanz C., Call J., & Boesch C. (Eds.), Tool use in animals: Cognition and ecology (pp. 3–20). Cambridge: Cambridge University Press; 10.1017/CBO9780511894800.002 [DOI] [Google Scholar]
- Deblauwe, I. , Guislain, P. , Dupain, J. , & Van Elsacker, L. (2006). Use of a tool‐set by Pan troglodytes troglodytes to obtain termites (Macrotermes) in the periphery of the Dja biosphere reserve, Southeast Cameroon. American Journal of Primatology, 68(12), 1191–1196. 10.1002/ajp.20318 [DOI] [PubMed] [Google Scholar]
- Deblauwe, I. , & Janssens, G. P. J. (2008). New insights in insect prey choice by chimpanzees and gorillas in Southeast Cameroon: The role of nutritional value. American Journal of Physical Anthropology, 135(1), 42–55. 10.1002/ajpa.20703 [DOI] [PubMed] [Google Scholar]
- Ellison, E. , Musgrave, S. , Morgan, D. , & Sanz, C. (2016). Sex differences in tool use during termite gathering among chimpanzees (Pan Troglodytes troglodytes) of the Goualougo Triangle, Republic of Congo. Presented at poster session, meeting of the American Association of Physical Anthropology, Atlanta, GA.
- Eshchar, Y. , Izar, P. , Visalberghi, E. , Resende, B. , & Fragaszy, D. (2016). When and where to practice: Social influences on the development of nut‐cracking in bearded capuchins (Sapajus libidinosus). Animal Cognition, 19(3), 605–618. 10.1007/s10071-016-0965-6 [DOI] [PubMed] [Google Scholar]
- Estienne, V. , Cohen, H. , Wittig, R. M. , & Boesch, C. (2019). Maternal influence on the development of nut‐cracking skills in the chimpanzees of the Taï forest, Côte d'Ivoire (Pan troglodytes verus). American Journal of Primatology, 81(7), e23022 10.1002/ajp.23022 [DOI] [PubMed] [Google Scholar]
- Estienne, V. , Robira, B. , Mundry, R. , Deschner, T. , & Boesch, C. (2019). Acquisition of a complex extractive technique by the immature chimpanzees of Loango National Park, Gabon. Animal Behaviour, 147, 61–76. 10.1016/J.ANBEHAV.2018.11.002 [DOI] [Google Scholar]
- Estienne, V. , Stephens, C. , & Boesch, C. (2017). Extraction of honey from underground bee nests by central African chimpanzees (Pan troglodytes troglodytes) in Loango National Park, Gabon: Techniques and individual differences. American Journal of Primatology, 79(8), e22672 10.1002/ajp.22672 [DOI] [PubMed] [Google Scholar]
- Falótico, T. , & Ottoni, E. B. (2014). Sexual bias in probe tool manufacture and use by wild bearded capuchin monkeys. Behavioural Processes, 108, 117–122. [DOI] [PubMed] [Google Scholar]
- Fay, J. M. , & Carroll, R. W. (1994). Chimpanzee tool use for honey and termite extraction in Central Africa. American Journal of Primatology, 34(4), 309–317. 10.1002/ajp.1350340403 [DOI] [PubMed] [Google Scholar]
- Fragaszy, D. M. , & Adams‐Curtis, L. E. (1991). Generative aspects of manipulation in tufted capuchin monkeys (Cebus apella). Journal of Comparative Psychology, 105(4), 387–397. 10.1037/0735-7036.105.4.387 [DOI] [PubMed] [Google Scholar]
- Goodall, J. (1968). The behaviour of free‐living chimpanzees in the Gombe stream reserve. Animal Behaviour Monographs, 1, 161–311. 10.1016/S0066-1856(68)80003-2 [DOI] [Google Scholar]
- Goodall, J. (1986). The chimpanzees of Gombe. Patterns of behavior (Vol. 73). Cambridge: Belknap Press; 10.1002/ajpa.1330730313 [DOI] [Google Scholar]
- Gruber, T. , Clay, Z. , & Zuberbühler, K. (2010). A comparison of bonobo and chimpanzee tool use: Evidence for a female bias in the Pan lineage. Animal Behaviour, 80(6), 1023–1033. 10.1016/j.anbehav.2010.09.005 [DOI] [Google Scholar]
- Gumert, M. D. , Hoong, L. K. , & Malaivijitnond, S. (2011). Sex differences in the stone tool‐use behavior of a wild population of burmese long‐tailed macaques (Macaca fascicularis aurea). American Journal of Primatology, 73(12), 1239–1249. 10.1002/ajp.20996 [DOI] [PubMed] [Google Scholar]
- Harmand, S. , Lewis, J. E. , Feibel, C. S. , Lepre, C. J. , Prat, S. , Lenoble, A. , … Roche, H. (2015). 3.3‐million‐year‐old stone tools from Lomekwi 3, West Turkana, Kenya. Nature, 521(7552), 310–315. 10.1038/nature14464 [DOI] [PubMed] [Google Scholar]
- Haslam, M. (2014). On the tool use behavior of the bonobo‐chimpanzee last common ancestor, and the origins of hominine stone tool use: Tool use in the bonobo‐chimpanzee LCA. American Journal of Primatology, 76(10), 910–918. 10.1002/ajp.22284 [DOI] [PubMed] [Google Scholar]
- Hayashi, M. , & Inoue‐Nakamura, N. (2011). From handling stones and nuts to tool‐use In Matsuzawa T., Humle T., & Sugiyama Y. (Eds.), The chimpanzees of Bossou and Nimba (pp. 175–182). Tokyo: Springer Japan. [Google Scholar]
- Hayashi, M. , & Matsuzawa, T. (2003). Cognitive development in object manipulation by infant chimpanzees. Animal Cognition, 6(4), 225–233. 10.1007/s10071-003-0185-8 [DOI] [PubMed] [Google Scholar]
- Herrmann, E. , Hare, B. , Call, J. , & Tomasello, M. (2010). Differences in the cognitive skills of bonobos and chimpanzees. PLoS One, 5(8), 2–5. 10.1371/journal.pone.0012438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, S. , & Celli, M. L. (2003). Role of mothers in the acquisition of tool‐use behaviours by captive infant chimpanzees. Animal Cognition, 6(4), 235–244. 10.1007/s10071-003-0187-6 [DOI] [PubMed] [Google Scholar]
- Holzhaider, J. , Gray, R. , & Hunt, G. (2010). The development of pandanus tool manufacture in wild new Caledonian crows. Behaviour, 147(5), 553–586. 10.1163/000579510X12629536366284 [DOI] [Google Scholar]
- Humle, T. (2006). Ant dipping in chimpanzees: An example of how microecological variables, tool use, and culture reflect the cognitive abilities of chimpanzees In Matsuzawa T., Tomonaga M., & Tanaka M. (Eds.), Cognitive development in chimpanzees (pp. 452–475). Japan: Springer. [Google Scholar]
- Humle, T. , & Matsuzawa, T. (2002). Ant‐dipping among the chimpanzees of Bossou, Guinea, and some comparisons with other sites. American Journal of Primatology, 58(3), 133–148. [DOI] [PubMed] [Google Scholar]
- Humle, T. , Snowdon, C. T. , & Matsuzawa, T. (2009). Social influences on ant‐dipping acquisition in the wild chimpanzees (Pan troglodytes verus) of Bossou, Guinea, West Africa. Animal Cognition, 12(Suppl 1), 37–48. 10.1007/s10071-009-0272-6 [DOI] [PubMed] [Google Scholar]
- Inoue‐Nakamura, N. , & Matsuzawa, T. (1997). Development of stone tool use by wild chimpanzees (Pan troglodytes). Journal of Comparative Psychology, 111(2), 159–173. 10.1037/0735-7036.111.2.159 [DOI] [PubMed] [Google Scholar]
- Kahrs, B. A. , & Lockman, J. J. (2014). Building tool use from object manipulation: A perception–action perspective. Ecological Psychology, 26(1–2), 88–97. 10.1080/10407413.2014.874908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokštejn, J. , Musálek, M. , & Tufano, J. J. (2017). Are sex differences in fundamental motor skills uniform throughout the entire preschool period? PLoS One, 12(4), 1–10. 10.1371/journal.pone.0176556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koops, K. , Furuichi, T. , Hashimoto, C. , & van Schaik, C. (2015). Sex differences in object manipulation in wild immature chimpanzees (Pan troglodytes schweinfurthii) and bonobos (Pan paniscus): Preparation for tool use? PLoS One, 10(10), 1–15. 10.1371/journal.pone.0139909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockman, J. J. (2000). A perception‐action perspective on tool use development. Child Development, 71(1), 137–144. 10.1111/1467-8624.00127 [DOI] [PubMed] [Google Scholar]
- Lonsdorf, E. (2005). Sex differences in the development of termite‐fishing skills in the wild chimpanzees, Pan troglodytes schweinfurthii, of Gombe National Park, Tanzania. Animal Behaviour, 70(3), 673–683. 10.1016/j.anbehav.2004.12.014 [DOI] [Google Scholar]
- Lonsdorf, E. (2006). What is the role of mothers in the acquisition of termite‐fishing behaviors in wild chimpanzees (Pan troglodytes schweinfurthii)? Animal Cognition, 9(1), 36–46. 10.1007/s10071-005-0002-7 [DOI] [PubMed] [Google Scholar]
- Lonsdorf, E. , Eberly, L. E. , & Pusey, A. E. (2004). Sex differences in learning in chimpanzees. Nature, 428(6984), 715–716. 10.1038/428715a [DOI] [PubMed] [Google Scholar]
- Lonsdorf, E. , Markham, A. C. , Heintz, M. R. , Anderson, K. E. , Ciuk, D. J. , Goodall, J. , & Murray, C. M. (2014). Sex differences in wild chimpanzee behavior emerge during infancy. PLoS One, 9(6), e99099 10.1371/journal.pone.0099099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangold (2017). INTERACT User Guide. Mangold International GmbH (Ed.) www.mangold-international.com
- Marshall‐Pescini, S. , & Whiten, A. (2008). Social learning of nut‐cracking behavior in east African sanctuary‐living chimpanzees (Pan troglodytes schweinfurthii). Journal of Comparative Psychology, 122(2), 186–194. 10.1037/0735-7036.122.2.186 [DOI] [PubMed] [Google Scholar]
- Matsuzawa, T. (1994). Field experiments of tool‐use In Wrangham R., McGrew W., de Waal F. B. M., & Heltne P. (Eds.), Chimpanzee cultures (pp. 351–370). Cambridge: Harvard University Press; 10.1007/978-4-431-53921-6 [DOI] [Google Scholar]
- McGrew, W. (1979). Evolutionary implications of sex differences in chimpanzee predation and tool use In Hamburg D. A. & McCown E. R. (Eds.), The great apes (pp. 441–463). Menlo Park, CA: Benjamin/Cummings. [Google Scholar]
- McGrew, W. (1992). Summary for policymakers In Chimpanzee material culture: Implications for human evolution. Cambridge: Cambridge University Press; 10.1017/CBO9781107415324.004 [DOI] [Google Scholar]
- McGrew, W. (2014). The “other faunivory” revisited: Insectivory in human and non‐human primates and the evolution of human diet. Journal of Human Evolution, 71, 4–11. 10.1016/j.jhevol.2013.07.016 [DOI] [PubMed] [Google Scholar]
- McGrew, W. , & Collins, D. A. (1985). Tool use by wild chimpanzees (Pan troglodytes) to obtain termites (Macrotermes herus) in the Mahale Mountains, Tanzania. American Journal of Primatology, 9(1), 47–62. 10.1002/ajp.1350090106 [DOI] [PubMed] [Google Scholar]
- McGrew, W. , Tutin, C. , & Baldwin, P. J. (1979). Chimpanzees, tools, and termites: Cross‐cultural comparisons of Senegal, Tanzania, and Rio muni. Man, 14(2), 185–214. 10.2307/2801563 [DOI] [Google Scholar]
- Meulman, E. , Seed, A. M. , & Mann, J. (2013). If at first you don't succeed… Studies of ontogeny shed light on the cognitive demands of habitual tool use. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1630), 20130050 10.1098/rstb.2013.0050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura, A. , & Lee, P. (2010). Wild capuchins show male‐biased feeding tool use. International Journal of Primatology, 31(3), 457–470. 10.1007/s10764-010-9406-6 [DOI] [Google Scholar]
- Muroyama, Y. (1991). Chimpanzees' choices of prey between two sympatric species of Macrotermes in the Campo Animal Reserve, Cameroon. Human Evolution, 6(2), 143–151. 10.1007/BF02435614 [DOI] [Google Scholar]
- Musgrave, S. , Lonsdorf, E. , Morgan, D. , Prestipino, M. , Bernstein‐Kurtycz, L. , Mundry, R. , & Sanz, C. (2019). Teaching varies with task complexity in wild chimpanzees. Proceedings of the National Academy of Sciences, 117(2), 969–976. 10.1073/PNAS.1907476116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrave, S. , Morgan, D. , Lonsdorf, E. , Mundry, R. , & Sanz, C. (2016). Tool transfers are a form of teaching among chimpanzees. Scientific Reports, 6, 34783 10.1038/srep34783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida, T. , & Uehara, S. (1980). Chimpanzees, tools, and termites: Another example from Tanzania. Current Anthropology, 21(5), 671–672. 10.1525/aa.1952.54.1.02a00460 [DOI] [Google Scholar]
- O'Malley, R. C. , & Power, M. L. (2012). Nutritional composition of actual and potential insect prey for the Kasekela chimpanzees of Gombe National Park, Tanzania. American Journal of Physical Anthropology, 149(4), 493–503. 10.1002/ajpa.22151 [DOI] [PubMed] [Google Scholar]
- O'Malley, R. C. , & Power, M. L. (2014). The energetic and nutritional yields from insectivory for Kasekela chimpanzees. Journal of Human Evolution, 71, 46–58. 10.1016/j.jhevol.2013.09.014 [DOI] [PubMed] [Google Scholar]
- Pandolfi, S. , van Schaik, C. P. , & Pusey, A. (2003). Sex differences in termite fishing among Gombe chimpanzees In de Waal F. B. M. & Tyack P. L. (Eds.), Animal social complexity: Intelligence, culture and individualized societies (pp. 414–418). Cambridge: Harvard University Press. [Google Scholar]
- Panger, M. A. , Brooks, A. S. , Richmond, B. G. , & Wood, B. (2002). Older than the Oldowan? Rethinking the emergence of hominin tool use. Evolutionary Anthropology, 11(6), 235–245. 10.1002/evan.10094 [DOI] [Google Scholar]
- Pascual‐Garrido, A. (2019). Cultural variation between neighboring communities of chimpanzees at Gombe, Tanzania. Scientific Reports, 9, 8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan, G. R. , Tennie, C. , & van Schaik, C. P. (2012). Social organization and the evolution of cumulative technology in apes and hominins. Journal of Human Evolution, 63(1), 180–190. 10.1016/j.jhevol.2012.04.008 [DOI] [PubMed] [Google Scholar]
- Pruetz, J. D. , & Bertolani, P. (2007). Savanna chimpanzees, Pan troglodytes verus, hunt with tools. Current Biology, 17(5), 412–417. 10.1016/j.cub.2006.12.042 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2018). R: A language and environment for statistical computing, Vienna, Austria: R Foundation for Statistical Computing; http://www.R-project.org/. [Google Scholar]
- Resende, B. D. , Ottoni, E. , & Fragaszy, D. (2008). Ontogeny of manipulative behavior and nut‐cracking in young tufted capuchin monkeys (Cebus apella): A perception‐action perspective. Developmental Science, 11(6), 828–840. 10.1111/j.1467-7687.2008.00731.x [DOI] [PubMed] [Google Scholar]
- Rutz, C. , & St. Clair, J. J. H. (2012). The evolutionary origins and ecological context of tool use in new Caledonian crows. Behavioural Processes, 89(2), 153–165. 10.1016/j.beproc.2011.11.005 [DOI] [PubMed] [Google Scholar]
- Sabater Pí, J. (1974). An elementary industry of the chimpanzees in the Okorobikó mountains, Rio muni (Republic of Equatorial Guinea), West Africa. Primates, 15(4), 351–364. 10.1007/BF01791672 [DOI] [Google Scholar]
- Sanz, C. , Call, J. , & Morgan, D. (2009). Design complexity in termite‐fishing tools of chimpanzees (Pan troglodytes). Biology Letters, 5(3), 293–296. 10.1098/rsbl.2008.0786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, C. , Deblauwe, I. , Tagg, N. , & Morgan, D. (2014). Insect prey characteristics affecting regional variation in chimpanzee tool use. Journal of Human Evolution, 71, 28–37. 10.1016/j.jhevol.2013.07.017 [DOI] [PubMed] [Google Scholar]
- Sanz, C. , & Morgan, D. (2007). Chimpanzee tool technology in the Goualougo triangle, republic of Congo. Journal of Human Evolution, 52(4), 420–433. 10.1016/j.jhevol.2006.11.001 [DOI] [PubMed] [Google Scholar]
- Sanz, C. , & Morgan, D. (2011). Elemental variation in the termite fishing of wild chimpanzees (Pan troglodytes). Biology Letters, 7(4), 634–637. 10.1098/rsbl.2011.0088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, C. , & Morgan, D. (2013a). Ecological and social correlates of chimpanzee tool use. Philosophical Transactions of the Royal Society B: Biological Sciences, 368(1630), 20120416 10.1098/rstb.2012.0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz, C. , & Morgan, D. (2013b). The social context of chimpanzee tool use In Sanz C., Call J., & Boesch C. (Eds.), Tool use in animals: Cognition and ecology (pp. 161–175). Cambridge: Cambridge University Press; 10.1017/CBO9780511894800.011 [DOI] [Google Scholar]
- Sanz, C. , Morgan, D. , & Gulick, S. (2004). New insights into chimpanzees, tools, and termites from The Congo Basin. The American Naturalist, 164(5), 567–581. 10.1086/424803 [DOI] [PubMed] [Google Scholar]
- Sanz, C. , Morgan, D. , & Hopkins, W. (2016). Lateralization and performance asymmetries in the termite fishing of wild chimpanzees in the Goualougo triangle, republic of Congo: Handedness and performance in wild chimpanzees. American Journal of Primatology, 78(11), 1190–1200. 10.1002/ajp.22574 [DOI] [PubMed] [Google Scholar]
- Sanz, C. , Schöning, C. , & Morgan, D. (2010). Chimpanzees prey on army ants with specialized tool set. American Journal of Primatology, 72(1), 17–24. 10.1002/ajp.20744 [DOI] [PubMed] [Google Scholar]
- Shumaker, R. W. , Walkup, K. R. , & Beck, B. B. (2011). Animal tool behavior: the use and manufacture of tools by animals, Baltimore, MD: The Johns Hopkins University Press. [Google Scholar]
- Smith, & Bentley‐Condit, V . (2010). Animal tool use: Current definitions and an updated comprehensive catalog. Behaviour, 147(2), 185–32A. 10.1163/000579509X12512865686555 [DOI] [Google Scholar]
- Sousa, C. , Biro, D. , & Matsuzawa, T. (2009). Leaf‐tool use for drinking water by wild chimpanzees (Pan troglodytes): Acquisition patterns and handedness. Animal Cognition, 12(S1), 115–125. 10.1007/s10071-009-0278-0 [DOI] [PubMed] [Google Scholar]
- Spagnoletti, N. , Visalberghi, E. , Ottoni, E. , Izar, P. , & Fragaszy, D. (2011). Stone tool use by adult wild bearded capuchin monkeys (Cebus libidinosus). Frequency, efficiency and tool selectivity. Journal of Human Evolution, 61(1), 97–107. 10.1016/j.jhevol.2011.02.010 [DOI] [PubMed] [Google Scholar]
- Sugiyama, Y. (1985). The brush‐stick of chimpanzees found in south‐West Cameroon and their cultural characteristics. Primates, 26(4), 361–374. 10.1007/BF02382453 [DOI] [Google Scholar]
- Suzuki, S. , Kuroda, S. , & Nishihara, T. (1995). Tool‐set for termite‐fishing by chimpanzees in the Ndoki Forest. Congo. Behaviour, 132(3/4), 219–235. [Google Scholar]
- Takeshita, H. , Fragaszy, D. , Mizuno, Y. , Matsuzawa, T. , Tomonaga, M. , & Tanaka, M. (2005). Exploring by doing: How young chimpanzees discover surfaces through actions with objects. Infant Behavior and Development, 28(3), 316–328. 10.1016/j.infbeh.2005.05.009 [DOI] [Google Scholar]
- Tan, A. W. Y. (2017). From play to proficiency: The ontogeny of stone‐tool use in coastal‐foraging long‐tailed macaques (Macaca fascicularis) from a comparative perception‐action perspective. Journal of Comparative Psychology, 131(2), 89–114. 10.1037/com0000068 [DOI] [PubMed] [Google Scholar]
- Tonooka, R . (2001). Leaf‐folding behavior for drinking water by wild chimpanzees (Pan troglodytes verus) at Bossou, Guinea. Animal Cognition, 4(3–4), 325–334 10.1007/s100710100110, 334 [DOI] [PubMed] [Google Scholar]
- Visalberghi, E. , & Fragaszy, D. (2012). What is challenging about tool use? The capuchin's perspective In Zentall T. R. & Wasserman E. A. (Eds.), Comparative cognition: Experimental explorations of animal intelligence (pp. 777–799). New York, NY: Oxford University Press; 10.1093/acprof [DOI] [Google Scholar]
- Visalberghi, E. , & Fragaszy, D. (2013). The etho‐cebus project: Stone‐tool use by wild capuchin monkeys In Sanz C., Call J., & Boesch C. (Eds.), Tool use in animals: Cognition and ecology (pp. 203–222). Cambridge: Cambridge University Press; 10.1017/CBO9780511894800.013 [DOI] [Google Scholar]
- Whiten, A. , Goodall, J. , McGrew, W. , Nishida, T. , Reynolds, V. , Sugiyama, Y. , … Boesch, C. (2001). Charting cultural variation in chimpanzees. Behaviour, 138(11–12), 1481–1516. 10.1163/156853901317367717 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1 Critical Elements of Termite Gathering at Epigeal Nests. This video shows the critical elements of termite gathering in the context of above‐ground termite nests, in the order in which they are typically observed among immature chimpanzees. The behaviors “Manipulate fishing probe” and “Mound plus tool” are also shown, as they represent important indices of skill development. Young chimpanzees learn to fish for termites from epigeal nests during infancy at comparable ages, but the use of perforating tool sets occurs later and at more variable ages.
Video S2 Critical Elements for Termite Gathering at Subterranean Nests. This video shows the critical elements of termite gathering in the context of subterranean termite nests, in the order in which they are typically observed among immature chimpanzees. The behaviors “Manipulate fishing probe” and “Mound plus tool” are also shown, as they represent important indices of skill development. Young chimpanzees learn to extract termites from both subterranean and epigeal nests before they independently manufacture brush‐tipped fishing probes. While young chimpanzees sometimes insert puncturing tools into existing tunnels, the ability to puncture new tunnels into subterranean termite nests requires great physical strength and has only been observed among mature individuals.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


