Abstract
In vegetation stands, plants receive red to far‐red ratio (R:FR) signals of varying strength from all directions. However, plant responses to variations in R:FR reflected from below have been largely ignored despite their potential consequences for plant performance. Using a heterogeneous rose canopy, which consists of bent shoots down in the canopy and vertically growing upright shoots, we quantified upward far‐red reflection by bent shoots and its consequences for upright shoot architecture. With a three‐dimensional plant model, we assessed consequences of responses to R:FR from below for plant photosynthesis. Bent shoots reflected substantially more far‐red than red light, causing reduced R:FR in light reflected upwards. Leaf inclination angles increased in upright shoots which received low R:FR reflected from below. The increased leaf angle led to an increase in simulated plant photosynthesis only when this low R:FR was reflected off their own bent shoots and not when it reflected off neighbour bent shoots. We conclude that plant response to R:FR from below is an under‐explored phenomenon which may have contrasting consequences for plant performance depending on the type of vegetation or crop system. The responses are beneficial for performance only when R:FR is reflected by lower foliage of the same plants.
Keywords: heterogeneous canopy, light absorption, photosynthesis, red to far‐red ratio, reflection, rose (Rosa hybrida), shade‐avoidance, three‐dimensional plant modelling
Short abstract
In vegetation stands, plants receive light signals of varying strength from all directions. The presence of lower canopy decreased the red to far‐red ratio (R:FR) in light reflected upwards, which induced a steeper leaf angle in the upper canopy. Such a response to R:FR from below, when reflecting from the lower part of the same canopy, increased whole canopy photosynthesis by allowing more light to penetrate to the lower canopy.
1. INTRODUCTION
Plants have limited options to escape competitive environments during their lifetime. To optimize competitiveness and ensure survival and reproduction, plants growing in vegetation stands need to show appropriate growth responses to neighbour presence by perceiving and interpreting environmental signals. In this regard, the low red to far‐red ratio (R:FR) of light reflected by neighbouring vegetation (i.e., because plant tissues preferentially absorb red‐ and reflect far‐red light) is a well‐studied signal of neighbour proximity (Ballaré & Pierik, 2017; Casal, 2013; Pierik & De Wit, 2014; Smith, 2000). Plants respond typically to reductions in R:FR through set of responses, for example, internode elongation, suppression of branching and tillering and increases in leaf inclination angles, collectively known as the shade‐avoidance syndrome (Casal, 2013; Holmes & Smith, 1977; Smith, 2000).
Although the actual amounts of red and far‐red light determine the photoequilibrium, the directions that the R:FR ratios come from could also potentially contain competitive information. In vegetation stands, a plant receives R:FR signals coming from all directions (e.g., from above, the side and below). These signals are transmitted or reflected by surrounding foliage that can belong either to neighbouring plants or the plant itself. This origin of the R:FR signal may be associated with the direction that R:FR signals come from. In general, vertically propagating R:FR signals are more likely to originate from foliage of the same plant (self‐signalling), whereas horizontally propagating R:FR signals are more likely to come from neighbours (non‐self‐signalling). For non‐self‐signals, the directions of R:FR may to some extent indicate the type of the neighbour, as the angle of the incoming signal is likely to be correlated with the size difference between the neighbour and the target plant. Competitiveness and size of the neighbour are linked, as for instance small neighbours may not pose a direct threat, but similar‐sized neighbours might. Thus, plant responses to R:FR light signals may depend on angle of incidence of these signals (Dudley & Schmitt, 1995). Many studies focused on plant responses to R:FR signals in incident light or horizontally reflected light, as R:FR ratios from these directions are likely coming from large or similar‐sized neighbours (Evers, Vos, Andrieu, & Struik, 2006; Héraut‐Bron, Robin, Varlet‐Grancher, & Guckert, 2001; Smith, Casal, & Jackson, 1990; Van Hinsberg & Van Tienderen, 1997). However, plant responses to R:FR reflected from below have been largely ignored.
Although shade‐avoidance responses are largely confirmed to be beneficial for plant performance when growing in dense canopies (Bell & Galloway, 2007; Dudley & Schmitt, 1996; Keuskamp, Sasidharan, & Pierik, 2010), low R:FR may not always be a reliable indicator of the competitive strength of neighbours. Coloured (e.g., red and green) soil mulches and small weeds were found to increase shoot‐root ratio and stem length and decrease biomass allocated to reproductive organs in crop plants, indicating that low R:FR in the light reflected from below by soil mulches and weeds also induces shade‐avoidance responses (Green‐Tracewicz, Page, & Swanton, 2012; Hunt, Kasperbauer, & Matheny, 1989; Kasperbauer, 1994; Page, Tollenaar, Lee, Lukens, & Swanton, 2010; Rajcan, Chandler, & Swanton, 2004). In many vegetation types (e.g., mixed natural vegetation, crops inter‐planted with mulches or agroforestry systems), taller plants grow together with much shorter ones, and thus receive such non‐self low R:FR signals from below. Additionally, R:FR signals from below could be self‐signals, generated by foliage of the same plant. In either case, R:FR from below being a self‐signal or coming from a smaller non‐self neighbour, light competition is unlikely to happen. No studies have investigated the consequences of responses to R:FR signals reflected from below for plant performance.
The objectives of this study were to quantify: (a) plant architectural responses to R:FR signals reflected from below and (b) the effects of these architectural responses on plant light absorption and photosynthesis. We explicitly included the consequences of plant responses to both R:FR reflected by the lower part of the same plant canopy (self‐signal) and R:FR reflected by foliage from other plants (non‐self‐signal). We chose a rose crop (Rosa hybrida) as our study system, which has a heterogeneous canopy structure with distinctly different crop parts. These crop parts consist of alternating strips of bent shoots and upright shoots, which occupy lower and upper parts of the canopy, respectively (see Figure 1). Such a canopy structure makes it possible to cause R:FR signals in light reflected upwards by the lower part of the canopy. First, a greenhouse experiment was conducted to investigate the effect of bent‐shoot presence on the distribution of R:FR ratios as perceived by the upper canopy (i.e., the upright shoots) and quantify upright shoot architectural responses to R:FR ratios reflected from below. Subsequently, a three‐dimensional (3D) plant simulation model was used to quantify the consequences of shoot architectural responses in upper canopy on plant light absorption and photosynthesis.
FIGURE 1.
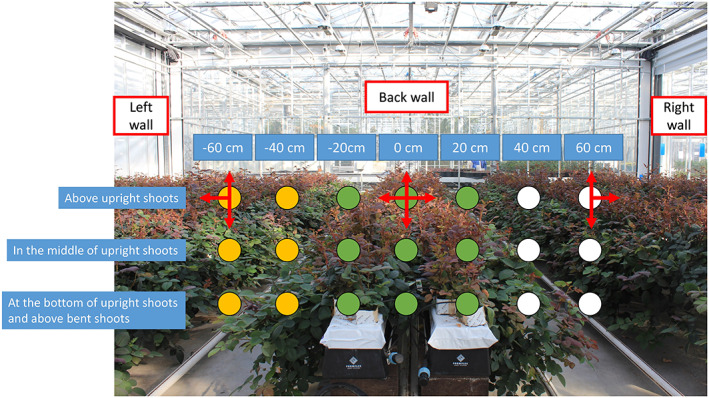
The red to far‐red ratio (R:FR) measurement plan. Measurements were conducted at three heights (above, in the middle and at the bottom of upright shoots but still above bent shoots) and seven positions with spectrometer sensor facing up, down, right and left. The seven positions include one position at the centre of the canopy (0 cm), and 20, 40 and 60 cm from the centre, respectively, to the right (20, 40 and 60 cm) and left (−20, −40 and −60 cm) of the canopy. Arrows represent the directions that the spectrometer sensor was facing. In all yellow circles (−40 and −60 cm) three measurements were taken, with the spectrometer sensor facing up, down and left; in all green circles (−20, 0 and 20 cm) four measurements were taken, with the sensor facing up, down, right and left; finally in all white circles (40 and 60 cm), again three measurements were taken, now with the sensor facing up, down and right [Colour figure can be viewed at wileyonlinelibrary.com]
2. MATERIALS AND METHODS
2.1. Experimentation
2.1.1. Plant growth conditions
Rose plants (Rosa hybrida cv. ‘Red Naomi!’) were grown in a compartment (12 m × 12 m) of a Venlo‐type glasshouse located in Wageningen, the Netherlands (52°N, 6°E). On January 4, 2017, one‐node cuttings, bearing a shoot grown in rockwool cubes, were transplanted in the compartment at a density of 7.5 plants m−2 (Figure S1). Plants were first grown for four growth cycles (one growth cycle is defined as the time duration from one harvest of flowering shoots to the next, approximately 6–8 weeks) to allow the basal part to grow thick enough to support multiple axillary buds growing simultaneously on the plant. Then plants were pruned to keep four axillary buds sprouting on each plant. When 80% of the shoots were flowering, the flower buds were removed, and all the four shoots were bent downwards, resulting in four bent shoots but no upright shoots on each plant. After bending, plants were allowed to form new branches, of which four were kept to develop further. This resulted in four upright shoots and four bent shoots on each plant. Treatments started on September 1, 2017 (the next day after the pruning) and lasted for 4 weeks.
During the experiment, assimilation lighting (600 W high‐pressure sodium lamps, Philips, Eindhoven, The Netherlands) was turned on for approximately 13 hr per day with a light intensity of ca. 150 μmol m−2 s−1 at the canopy level. Average day and night temperatures during the experiment were 21.7 and 18.6°C, respectively. Average day and night relative humidities during the experiment were 75 and 86%, respectively. Average ambient CO2 concentration at light period during the experiment was 461 ppm. Plants were irrigated hourly between 7:00 and 19:00 with standard nutrient solution (EC = 2.2 mS cm−1; pH = 5.8; Table S1) for rose crop used in practice.
2.1.2. Treatments
In total four treatments were established in a randomized block design with three blocks, four plots (treatments) per block and 72 plants in each plot (Figure S1). In each treatment, plants chosen for measurements were considered as focal plants. All other plants were considered as neighbour plants. The four treatments were (a) focal plants (F) without bent shoots (−; all bent shoots were removed from the plant) and neighbour plants (N) with bent shoots (+; all bent shoots were kept on the plant) (F−N+), (b) focal plants with bent shoots and neighbour plants with bent shoots (F+N+), (c) focal plants without bent shoots and neighbour plants without bent shoots (F−N−) and (d) focal plants with bent shoots and neighbour plants without bent shoots (F+N−) (Figure S2). Note that in treatments F−N+ and F−N−, focal plants first experienced shoot bending and then experienced shoot pruning, whereas in treatments F+N+ and F+N−, focal plants only experienced shoot bending. Therefore, these four treatments enabled us to do pairwise comparisons. The comparisons between F−N+ versus F−N− and between F+N+ versus F+N− allowed us to study the effect of bent‐shoot presence on upright shoots that either experienced both bending and pruning or experienced only bending. In total four focal plants per plot were randomly chosen on the condition that at least three neighbour plants were in between two focal plants (Figure S1). The gaps between focal plants were to ensure that: (a) bent shoots of neighbour plants in F−N+ could cover the gaps caused by the removal of bent shoots from the focal plants, thus resulting in a similar light reflection by bent shoots in treatments F−N+ and F+N+; (b) bent shoots of focal plants in F+N− do not cause substantial light reflection in the treatment plot, thus resulting in a similar light reflection by the concrete floor in treatments F−N− and F+N−.
2.1.3. Red, far‐red and full light spectral measurements
Red (R, 660 ± 20 nm) and far‐red (FR, 730 ± 20 nm) light intensities were measured using a spectrometer (SpectroSense2 system, Skye Instruments Ltd, UK) on Day 4 (early developmental stage), Day 13 (shoot elongation stage) and Day 21 (flowering stage) after start of treatments. The spectrometer sensor measured the amount of red and far‐red light coming from all directions within 180°. Measurements were conducted at three locations (front, middle and back) in each plot (Figure S1). These measuring locations were not necessarily at the locations of focal plants. At each location, R and FR were measured at three heights: above, in the middle and at the bottom of upright shoots but still being above the bent shoots (Figure 1). At each height, R and FR were measured at seven positions: in the centre of the canopy (0 cm), and 20, 40 and 60 cm from the centre to the right side (20, 40 and 60 cm) and left side (−20, −40 and −60 cm) of the canopy (Figure 1). R and FR measurements inside the upright shoots (at −20, 0 and 20 cm) were done with the sensor facing up, down, right and left (Figure 1). R and FR measurements outside the upright shoots (at −40, −60, 40 and 60 cm) were done with the sensor facing all the aforementioned directions except for the directions facing the upright shoots (Figure 1), as light from such directions was propagating away from the upright shoots instead of towards them, rendering those signals irrelevant for focal plant responses. As the spectrometer sensor measures light coming from all directions within 180° (i.e., a hemisphere), R or FR light intensities measured with sensor facing up and down were summed up (resulting in a whole sphere) to represent the total light intensity at a given position in the canopy. At flowering stage (day 24), full light spectra (400–800 nm) were also measured using a spectrometer (USB 2000 + UV–VIS, Ocean Optics, Duiven, the Netherlands) according to the same measurement approach of R and FR measurement (Figure 1).
2.1.4. Light measurements
Incident light intensities were measured on Day 14 and Day 22 after start of treatments, using a line quantum sensor (ACCUPAR LP‐80, METER Group, Inc.). The scheme of light measurements was very similar to the R:FR measurements (Figure S3). Light intensities were measured at three locations in each plot (front, middle and back, Figure S1). At each location, measurements were conducted at four heights (above, in the middle and at the bottom of upright shoots, and below bent shoots, Figure S3). At each height, measurements were conducted at six positions (at 20, 40 and 60 cm from the centre of the canopy, respectively, to the right and left, Figure S3).
2.1.5. Plant architecture and biomass measurements
For each focal plant, one upright shoot was subjected to detailed measurements of plant architectural traits. Internode length and leaf length at each rank of the shoot were measured non‐destructively at Day 6 and 12 after start of treatments. When flower buds started to open (Day 25), the shoots were harvested to measure length and diameter of all internodes, leaf length, width, area, leaflet number and leaf inclination angle of all leaves, peduncle length and diameter, and flower bud diameter. The final harvest lasted for 3 days (Day 25, 26 and 27). On each day, plants in one block were harvested plot by plot for measurements. After these architectural measurements, individual organs were put in the oven for 48 hr at 105°C to measure organ dry weight. After the detailed shoot measurements, the other three upright shoots on each focal plant, and the four bent shoots of focal plants in F+N+ and F+N− were harvested to measure total leaf area of upright shoots and bent shoots. Length and width measurements were conducted using a ruler. Diameter was measured using a calliper. Leaf area was measured using a leaf area meter (LICOR‐3100, Lincoln, NE). Leaf inclination angle was measured as the insertion angle of the leaf relative to the horizontal level using a protractor.
2.1.6. Statistical analysis
A one‐way ANOVA (p < .05) of R (version R 3.6.1, R Core Team) was used to test for treatment effects on R:FR ratios from each direction (with sensor facing up, down, left and right), relative light intensities, plant architectural traits and organ dry weight. When a significant treatment effect was detected, a post‐hoc test was further conducted for pairwise comparisons of the four treatments using Fisher's Protected Least Significant Difference (LSD) test (p < .05) in R.
2.2. Model simulations
2.2.1. Model development
A 3D rose model was constructed in the plant modelling software GroIMP (Hemmerling, Kniemeyer, Lanwert, Kurth, & Buck‐Sorlin, 2008). The model includes (a) a 3D representation of rose plants at flowering stage, (b) a radiation (including photosynthetically active radiation and far‐red light) and photosynthesis model to simulate light absorption and photosynthesis of rose plants and (c) virtual sensors to measure R:FR from different directions and at various positions in the canopy.
3D rose plants
Each plant consisted of four upright shoots, either with or without bent shoots. Upright shoots were constructed using basic plant units representing internodes with compound rose leaves that together make up the shoot. Architectural parameters used for constructing an individual upright shoot included length and diameter of all internodes, length, width, area and leaflet number of all leaves, peduncle length and diameter, and flower width. Architectural measurements of focal plants (measured at final harvest) were used to build a database (i.e., a set of individual architectural parameters obtained from measurements on each shoot) for each treatment. In each simulation, architecture parameters of individual upright shoots were randomly selected from the database. Due to the architectural complexity of bent shoots, they were constructed by randomly distributing a number of leaves in the area occupied by bent shoots. Total leaf area of bent shoots was obtained from the experiment. Bent‐shoot presence could be switched on or off in the model according to the type of treatment to be simulated. Row distance and plant density were set to the same values as in the experiment.
The radiation and photosynthesis model
The light environment was modelled using a diffuse light dome with moderate gradation towards zenith and azimuthal uniformity (Evers et al., 2010). The light dome started at 60° above the horizontal plane considering that most of the light inside the glasshouse compartment (including sunlight and light from the assimilation lamps) comes from the top. Light was simulated for individual wavebands of photosynthetically active radiation (PAR), red and far‐red. In each simulation, two rows of plants were simulated, with nine plants in each row. To eliminate the border effects in the light environment, the simulated plant population (18 plants in total) was replicated 10 times in the x and y directions, resulting in average light conditions as experienced by 100 copies of each individual plant population (1,800 plants in total; de Vries, Poelman, Anten, & Evers, 2018). The amount of PAR, red and far‐red light reaching the plant organs was calculated using a Monte‐Carlo ray tracer embedded in GroIMP (Hemmerling et al., 2008). Leaf reflectance and transmittance of PAR, red and far‐red were obtained from spectrophotometric measurements on rose leaves (Figure S4). Internodes were assumed to have the same reflectance of PAR, red and far‐red as leaves, but without transmission. Plant net photosynthesis was calculated as the sum of net photosynthesis of individual leaves, which was in turn calculated based on the light absorption and photosynthetic parameters (including leaf photosynthetic capacity, dark respiration rate and quantum efficiency) of individual leaves (Method S1). The light‐saturated photosynthesis (photosynthetic capacity, A max) of individual leaves in both upright and bent shoots was assumed:
| (1) |
where Q/Q 0 is the relative light intensity on a given leaf, A 0 is the photosynthetic capacity of an unshaded leaf in upright shoots (A 0,upright) or bent shoots (A 0,bent) and k is the exponent that determines the steepness of A max decreasing with Q/Q 0 (Niinemets & Anten, 2009). Dark respiration rate of individual leaves was assumed to be proportional to photosynthetic capacity of that leaf (Hikosaka, Kumagai, & Ito, 2016). All leaves were assumed to have the same quantum efficiency. Photosynthetic parameters of rose leaves (i.e., values of A 0,upright, A 0,bent, k, dark respiration rate and quantum efficiency) were obtained from another experiment with the same rose cultivar (Zhang, van Westreenen, Evers, Anten, & Marcelis, 2019; Figure S5) and can be found in Method S1.
Virtual sensors
The virtual sensors were constructed such that the amount of red and far‐red light coming from different directions within 180° was measured, similar to the spectrometer used in the experiment. The virtual sensors were rotated and located to mimic the actual measurement plan used in the experiment (Figure 1; Figure S6).
2.2.2. Model evaluation
The simulated distributions of R:FR ratios in the canopy at flowering stage were compared with the measurements for the four treatments. Virtual sensors were put at the same virtual locations as where the actual R:FR measurements were performed, with sensor facing up, down, right and left. The R:FR ratios measured by virtual sensors were compared with R:FR ratios measured by the spectrometer in the experiment by calculating the coefficient of determination (r 2) and the relative root‐mean‐square error (rRMSE):
| (2) |
where y i is the simulated value, x i is the measured value, n is the number of data points and is the mean of the measured values.
2.2.3. Scenarios
The model simulations were conducted to quantify the effects of different measured upright shoot architectures (resulting from R:FR reflected from below) on plant light absorption and photosynthesis. In the simulations, incoming light intensity was kept at 200 μmol m−2 s−1 which represented the average light intensity inside the glasshouse compartment during the experiment. Only PAR waveband in the model was used to calculate light absorption and photosynthesis in the simulations, and red and far‐red wavebands were not used in scenario studies. Since we found in the experiment that leaf inclination angle of upright shoots increased when R:FR from below decreased by the presence of bent shoots, we specifically evaluated the consequences of increasing leaf angle for light absorption and photosynthesis of the upright shoots, the bent shoots and the whole plant. In all simulations, canopies contained both upright shoots and bent shoots (Table 1). In Scenarios (i), (iii) and (v), upright shoots and bent shoots together made up the whole plant (Table 1). So, in these scenarios bent shoots contributed to whole‐plant photosynthesis. In Scenarios (ii), (iv) and (vi), bent shoots were considered to be part of a neighbour plant, and thus the simulated plants were only consisting of upright shoots (Table 1). So, in this case bent shoots did not contribute to whole‐plant photosynthesis of the simulated plants, but were considered as independent foliage.
TABLE 1.
Descriptions for the simulation scenarios which studied the effects of upright shoot responses to the red to far‐red ratio (R:FR) reflected from below on light absorption and photosynthesis of bent shoots and upright shoots
| Scenario type | Experimental treatment used for building upright shoots | Attribute of the bent shoots |
|---|---|---|
| (i) | F+N− | Part of the simulated plants |
| (ii) | F−N− | Independent foliage |
| (iii) | F+N−, leaf angles increase by 10–40% | Part of the simulated plants |
| (iv) | F−N−, leaf angles increase by 10–40% | Independent foliage |
| (v) | F+N+ | Part of the simulated plants |
| (vi) | F−N+ | Independent foliage |
Note: Architectural parameters of upright shoots were taken as measured in four treatments of the experiment (Scenarios i, ii, v and vi). In Scenario iii and iv, leaf angles obtained from the experiment were, respectively, increased by 10, 20, 30 and 40%. The column ‘Experimental treatment used for building upright shoots’ gives the treatment from which the upright shoot architecture was simulated in each scenario. ‘F’ represents focal plant. ‘N’ represents neighbour plant. ‘−’ indicates plant with no bent shoots. ‘+’ indicates plant with bent shoots.
The measurements of upright shoot architecture from the four treatments were used to build the upright canopy in the model. First, in Scenarios (i) and (ii), we constructed upright shoots using architectural parameters obtained from treatments in which neighbour plants did not have bent shoots, that is, treatments F+N− (i) and F−N− (ii) (Table 1). Thus, simulations combining such upright shoot architecture in the presence of bent shoots represented the case that upright shoots receive low R:FR from below but do not show any responses to the presence of bent shoots. Then, in Scenarios (iii) and (iv), leaf angle in upright shoots was progressively increased by 10%, 20%, 30% and finally 40%, to test situations in which upright shoot leaf angles respond with different strengths to low R:FR signalling from below. The percentages chosen covered the range of changes in leaf angle between treatments observed in the experiment. Finally, in Scenarios (v) and (vi), we constructed upright shoots using architectural parameters obtained from treatments in which neighbour plants had bent shoots, that is, treatments F+N+ (v) and F−N+ (vi), which represented a full phenotype including all measured architectural responses to low R:FR from below. In Scenarios (i), (iii) and (v) in which plants had both upright and bent shoots, whole‐plant light absorption and photosynthesis were calculated as the sum of upright shoots and bent shoots. In scenarios (ii), (iv) and (vi) in which bent shoots were independent foliage of neighbours, plant light absorption and photosynthesis equalled to that of upright shoots. The relative changes of light absorption and photosynthesis (ϕ) were calculated (Equation 3) to evaluate the effect of different canopy structure on plant performance.
| (3) |
where Y ref is the light absorption or photosynthesis calculated in simulation Scenarios (i) or (ii); Y is the light absorption or photosynthesis calculated in simulation Scenarios (iii)–(vi). ϕ is calculated either between Scenarios (i), (iii) and (v) (bent shoots as part of the plants) or between Scenarios (ii), (iv) and (vi) (bent shoots representing independent foliage). Note that the differences between Scenarios (i) versus (v) and (ii) versus (vi) could result from not only the differences in architectural traits, but also in shoot arrangements and leaf area distribution. The former may be due to random selection of individual shoots from a different treatment database and the latter may be caused by simultaneous changes of individual internode length and leaf area.
3. RESULTS
3.1. The presence of neighbour bent shoots decreased r:FR reflected from below
At all three developmental stages of the upright shoots, R:FR ratios in light reflected from below (with sensor facing down) were lower when neighbour plants had bent shoots (F−N+ and F+N+) than when neighbour plants did not (F−N− and F+N−), especially for ratios measured in the middle and at bottom of upright shoots (Figure 2; Figure S7 and S8). Such trends were not affected by the presence of bent shoots in focal plants (Figure 2; Figures S7 and S8). The R:FR from below, especially at the bottom of the upright shoots, were slightly lower in F+N− than in F−N− (Figure 2; Figures S7 and S8), but the difference was not significant in most cases (Table S2). The R:FR of incident light (measured with sensor facing up) and of horizontally travelling light (with sensor facing right and left) were hardly affected by treatments at all developmental stages (Figures S7–S9). The total R:FR ratios calculated using total red and far‐red light intensities at different positions in the canopy tended to be lower when neighbour plants had bent shoots (F−N+ and F+N+), but the differences were not always significant (Figure S10 and Table S3). The far‐red light reflected upwards (i.e., reflected from below) accounted for about 20–30% of the total far‐red light intensity at a given position in the canopy, whereas the red light reflected upwards accounted for about 5–10% of the total red light intensity (Figure S11). Actual intensities of red and far‐red light measured for each treatment (Table S4) and full light spectra measured at final harvest (Figure S12) were given in the Supporting Information. The fraction of light intercepted by upright shoots was hardly affected by treatments, and the fraction of light intercepted by the whole‐plant canopy was significantly higher at the presence of neighbour bent shoots (Figure S13 and Table S5).
FIGURE 2.
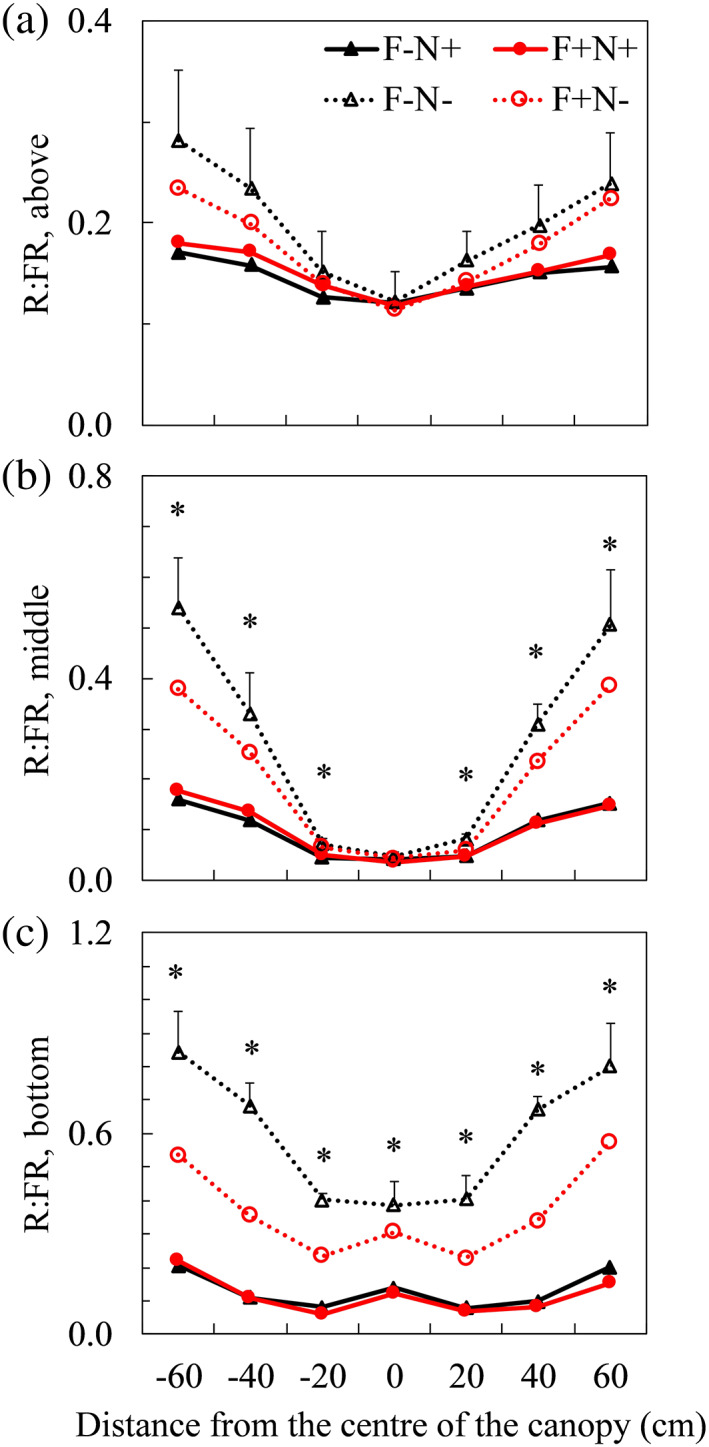
Measured red to far‐red (R:FR) ratios above (a), in the middle (b) and at the bottom (c) of upright shoots at flowering stage (Day 21 after start of treatments) with spectrometer sensor facing down. In x‐axis, positive and negative values are horizontal distances from the centre of the canopy, respectively, to the right and to the left of the canopy. Details on measurement positions can be found in Figure 1. Positive error bars (only given in the highest line in each panel) are standard errors of means. * indicates significant treatment effects when comparing at the same measurement positions (p < .05). Pairwise comparisons results of R:FR in different treatments can be found in Table S2. Triangles indicate focal plants (F) without bent shoots (−). Circles indicate focal plants with bent shoots (+). Closed symbols with solid lines indicate neighbour plants (N) with bent shoots. Open symbols with dotted lines indicate neighbour plants without bent shoots [Colour figure can be viewed at wileyonlinelibrary.com]
3.2. Leaf inclination angle increased in upright shoots that experienced low R:FR from below
As we were interested in plant responses that are relevant to light competition, we specifically investigated treatment effects on internode length, leaf length and leaf inclination angle. At all developmental stages, internode length and leaf length of upright shoots were larger when focal plants had bent shoots (F+N+ and F+N−) than when they did not (F−N+ and F−N−) (Figure 3a–d; Figure S14; Tables S6 and S7). These trends were not affected by the presence of bent shoots in neighbour plants (Figure 3a–d; Figure S14; Tables S6 and S7). The inclination angles of individual leaves and the average leaf inclination angle in upright shoots were increased in treatments in which neighbour plants had bent shoots (F−N+ and F+N+), regardless of whether or not shoots of the focal plant had been bent (Figure 3e,f; Table S6). This suggested that leaf angle was affected by R:FR from below. Results of other plant architectural traits (e.g., leaf area and internode diameter) measured for developing the 3D model, and plant biomass measurements can be found in the Supporting Information (Figures S15–S18 and Table S6).
FIGURE 3.
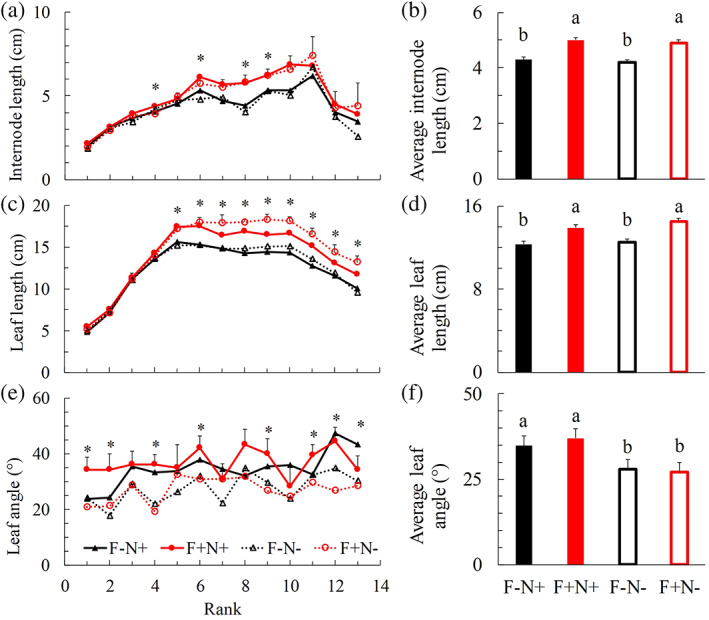
Measurements of individual organ length at each rank and the average organ length for internodes (a and b) and leaves (c and d), leaf inclination angle at each rank (e) and the average leaf angle (f) in upright shoots at final harvest (Day 25 after start of treatments). Positive error bars are standard errors of means (only given in the highest line in panels a, c, e). * (in panels a, c, e) and different letters (in panels b, d, f) indicate significant difference when comparing different treatments at the same rank and for the same trait (p < .05). Pairwise comparison results of panels (a), (c) and (e) can be found in Table S6. ‘F’ represents focal plant. ‘N’ represents neighbour plant. ‘−’ indicates plant without bent shoots. ‘+’ indicates plant with bent shoots [Colour figure can be viewed at wileyonlinelibrary.com]
3.3. The consequences of responding to R:FR from below depended on the type of plants (i.e., with or without bent shoots)
Our model gave sufficiently accurate simulations of R:FR ratios from different directions (up, horizontal and down) and at different heights (above, middle and bottom) in upright shoots (Figure S19). The overall rRMSE between measured and simulated R:FR values from all directions and at all heights was 0.23, and the overall r 2 was .88. This result indicates that the 3D architecture of rose plants both with and without bent shoots was accurately represented in the model, which allowed us to do simulations to explore the consequences of responding to low R:FR from below for plant performance.
When plants respond to low R:FR by increasing the leaf angle of upright shoots, the simulated light absorption and photosynthesis of upright shoots decreased (Figure 4). This occurred in plants both with and without bent shoots (Figure 4). For plants with bent shoots, increasing leaf angle of upright shoots resulted in an unchanged whole‐plant light absorption, as the light not intercepted by upright shoots was intercepted by bent shoots (Figure 4a). Whole‐plant photosynthesis was increased by allowing more light to penetrate to bent shoots (Figure 4b). For plants without bent shoots, increasing leaf angle decreased plant light absorption and photosynthesis (Figure 4). Light response curves that we measured and fitted with the non‐rectangular hyperbola equation (Equation S1 in Method S1) were significantly non‐linear in the trajectory 50–200 μmol m−2 s−1; hence, the increment in photosynthesis with increasing PAR declined notably over this trajectory (Figure S5). These relationships were used in the model, and therefore, in the scenarios, changes in light absorption were not linearly correlated with changes in photosynthesis even though the incoming light intensity in the model was set to 200 μmol m−2 s−1. When full responses to R:FR from below (including leaf inclination angle and other trait responses that were not statistically significant) were considered, plants with bent shoots increased light absorption and photosynthesis in their bent shoots and the whole plant (Figure 5). Upright shoot light absorption and photosynthesis were also increased when upright shoots showed full responses to R:FR from below (Figure 5). For plants without bent shoots, full responses to R:FR from below slightly decreased plant light absorption (1–2%), while plant photosynthesis was not affected (Figure 5).
FIGURE 4.
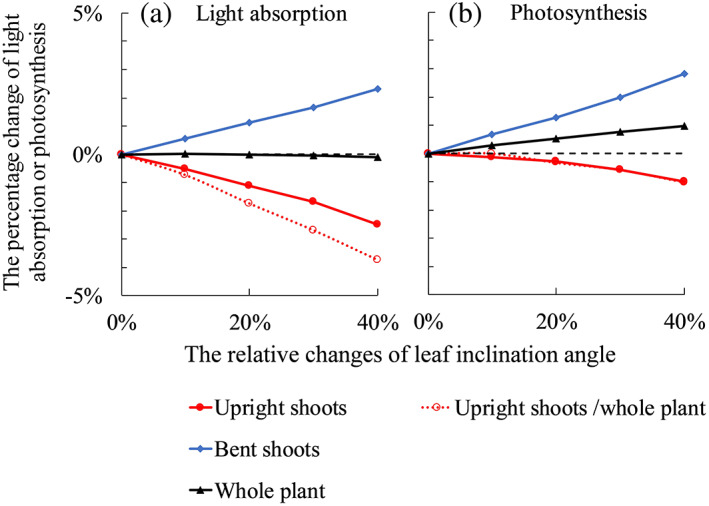
The relative changes of simulated plant light absorption (a) and photosynthesis (b) with increasing leaf angles on upright shoots by 10, 20, 30 and 40%. Closed symbols with solid lines in each panel are results of comparing Scenario (iii) to Scenario (i) (in Table 1), where upright shoot architecture was obtained from the treatment in which focal plants had bent shoots and neighbour plants did not have bent shoots (F+N−). Open symbols with dotted lines in each panel are results of comparing Scenario (iv) to Scenario (ii) (in Table 1), where upright shoot architecture was obtained from the treatment in which both focal and neighbour plants did not have bent shoots (F−N−). In all panels, bent shoots are present in the simulation. Bent shoots are considered attached to the plants in simulations showing by closed symbols with solid lines. Bent shoots are considered independent of the plant in simulations showing by open symbols with dotted lines. The black dashed line in each panel indicates the level of 0% [Colour figure can be viewed at wileyonlinelibrary.com]
FIGURE 5.
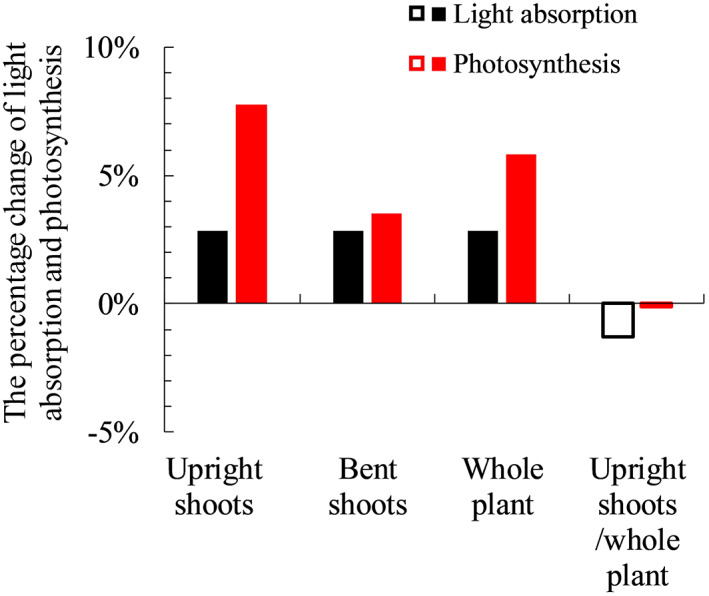
The relative changes of simulated plant light absorption and photosynthesis when comparing Scenario (v) to scenario (i) (closed columns), and when comparing Scenario (vi) to Scenario (ii) (open columns). Detailed descriptions of all scenarios can be found in Table 1. For results showing by closed columns, upright shoot architecture obtained from treatment in which both focal and neighbour plants had bent shoots (F+N+) is compared to upright shoot architecture obtained from treatment in which focal plants had bent shoots and neighbour plants did not have bent shoots (F+N−). For results showing by open columns, upright shoot architecture obtained from treatment in which focal plants did not have bent shoots and neighbour plants had bent shoots (F−N+) is compared to upright shoot architecture obtained from treatment in which both focal and neighbour plants did not have bent shoots (F−N−). Bent shoots are present in all simulations. In simulations showing by closed columns, bent shoots are considered attached to the plants. In simulations showing by open columns, bent shoots are considered independent of the plants [Colour figure can be viewed at wileyonlinelibrary.com]
4. DISCUSSION
4.1. A ‘bottom‐up’ effect of far‐red on shoot architecture in heterogeneous canopies
Leaves preferentially absorb red light and transmit and reflect a large fraction of far‐red light. As a result the R:FR ratio in a plant canopy shows a gradient with the lowest values at the bottom (Holmes & Smith, 1977). Here, we showed that in a heterogeneous canopy, the lower part of a canopy (in this case bent shoots) also reflects substantially more far‐red light and less red light to the upper part of the canopy (in this case upright shoots), and by consequence the upper canopy perceives low R:FR from below (Figure 2; Figures S7 and S8; Table S2). The decrease in R:FR from below caused by the presence of a lower canopy is the likely cause of an increase of leaf inclination angle (i.e., insertion angle of the leaf relative to horizontal level) in upper shoots (Figure 3e,f; Table S6), as low R:FR is found to induce steeper leaf angles in rose (Zhang, van Westreenen, Anten, Evers, & Marcelis, 2019) and in other species (Ballaré & Pierik, 2017). In previous studies, the low R:FR reflected from green mulches or weeds below the plants induced longer stems and higher shoot‐root ratio in plants (Kasperbauer, 1994; Rajcan et al., 2004). However, we did not find any significant effects of low R:FR from below on either internode length or leaf length, as these traits were not affected by the presence of a lower canopy which induced low R:FR reflection from below (Figure 3a–d; Figure S14; Tables S6 and S7). Nevertheless, specific internode length (cm g−1) and specific leaf area (cm2 g−1) were higher in upright shoots that experienced low R:FR from below (Figure S17 and Table S6), indicating a shift in allometry whereby these shoots invested relatively more assimilates into their length growth. Overall, our results indicate that lower vegetation may substantially affect R:FR perception in higher layers, eliciting shade‐avoidance responses.
R:FR could also potentially function as a signal for optimizing canopy performance. Plant canopies are characterized by dramatic gradients of light within the canopy, resulting in leaves in the upper canopy experiencing saturating light conditions, whereas leaves in the lower canopy being heavily shaded (Hara, 1985). To optimize canopy photosynthesis, plants tend to distribute their leaf photosynthetic capacities according to the light gradient in the canopy by regulating photosynthetic nitrogen distribution among individual leaves (Niinemets & Anten, 2009). While this phenomenon has been extensively studied (see review by Hikosaka et al., 2016), there is still a debate on what exactly drives leaf nitrogen distribution in the canopy (Boonman et al., 2007; Pons & De Jong‐Van Berkel, 2004). Light gradient in the canopy has been proposed as an important mechanism determining the leaf nitrogen distribution, possibly through a transpiration gradient induced by light gradient (Boonman et al., 2007). However the gradient in R:FR has been proposed as an additional signal for plants to redistribute nitrogen from lower to upper leaves in the canopy (Pons & De Jong‐Van Berkel, 2004; Pons, van Rijnberk, Scheurwater, & van der Werf, 1993). More generally however there might be physiological and selective constraints on the extent to which plants can fully optimize their nitrogen distribution (Anten & During, 2011). Alternatively plants can also optimize light distribution within the canopy by altering canopy structure (Hara, 1985). Here, we showed that the R:FR reflected from the lower canopy (a ‘bottom‐up’ R:FR gradient) may function as a signal to optimize canopy structure, as a steeper leaf angle in the upper canopy (likely induced by the low R:FR reflected from below) allows more light to penetrate to the lower canopy (Figure 4a). Nevertheless, the increase of leaf angle in the upper canopy only led to a marginal increase (1–2%) in plant photosynthesis (Figure 4b).
The ‘bottom‐up’ R:FR effect, however, has been ignored in both shade‐avoidance studies and canopy performance optimizations. Most shade‐avoidance studies have focussed on horizontal or top‐down light gradients, thus implicitly assuming that only R:FR signals from similarly sized or larger neighbours matter (Bongers, Pierik, Anten, & Evers, 2018; Dudley & Schmitt, 1995, 1996; Evers et al., 2006; Weijschedé, Martínková, De Kroon, & Huber, 2006). Similarly, only the top‐down R:FR gradient has received some attention in studies on optimizing canopy photosynthesis (Pons et al., 1993; Pons & De Jong‐Van Berkel, 2004). However, in most natural systems and some crop production systems that feature spatially heterogeneous canopies, such as the rose crop in our experiment but also strip intercropping systems (Brooker et al., 2015), far‐red light is transmitted or reflected not only from above and the side, but also from below. As we showed that the fraction of reflected far‐red light at any given position in the canopy was not negligible (Figure S11), and such R:FR signals from below may elicit plant responses affecting their performance, the relevance of R:FR signals coming from below should not be ignored.
4.2. R:FR from below: to respond or not?
Both lower leaves of a plant canopy and lower vegetation of neighbours located below the plants could induce low R:FR reflection from below. Our simulations showed that the consequences of responding to R:FR from below in terms of leaf inclination angles depend on whether far‐red is reflected by lower parts of the same plants or by foliage of other plants (Figure 4). The responses are only beneficial for plant photosynthesis when R:FR from below is reflected by the lower part of the same plant canopy. Increased angle of leaves in the upper part of the canopy allows more light to penetrate to the lower canopy, that is, light penetrates deeper in the canopy without affecting total canopy light absorption. This increases whole‐plant photosynthesis (Figure 4). Steeper leaf angle is advantageous only if the light penetrating through upper canopy can be utilized by lower canopy of the same plant (Hikosaka & Hirose, 1997). These results suggest that for a heterogeneous plant canopy consisting of distinctly different parts, responses of the upper canopy to R:FR from below decrease the level of competition within the plant, being that the upper canopy elements ‘give away’ part of their light absorption to the lower canopy, and such responses increase whole‐plant performance. In this case, a plant avoids self‐competition between different parts of its own canopy. Such a response seems to be a form of self‐organization of canopy structure. R:FR‐mediated self‐organization was characterized before in sunflower in which individual stands of a dense population inclined away from their neighbours, resulting in an increase of oil yield (López Pereira, Sadras, Batista, Casal, & Hall, 2017). In our case, self‐organization seems to happen between different parts of the same plant canopy and leads to a balance between facilitation and competition. Our results support the idea that plants optimize their performance by avoiding wasteful competition (Novoplansky, 2009; Pierik, Mommer, & Voesenek, 2013). While in previous studies, this idea is mostly exemplified by results on roots belowground (Falik, de Kroon, & Novoplansky, 2006; Falik, Reides, Gersani, & Novoplansky, 2003), we extend this idea by results on light absorption and photosynthesis of shoots aboveground.
When low R:FR is reflecting from lower canopy of independent foliage that pertains to a different plant, responses to R:FR from below decrease plant light absorption and photosynthesis (Figure 4). This result suggests that for plants growing with independent lower vegetation, as is the case in vegetation in which species of different stature co‐exist (as is usually the case in natural forest or grassland stands but also in agroforestry systems), responses to R:FR from below decrease plant performance. This is in line with our previous study which shows that when shade‐avoidance responses (induced by low R:FR) do not lead to greater light acquisition, these responses are not beneficial to plant photosynthesis (Zhang, van Westreenen, Anten, et al., 2019). In the current case, increasing leaf inclination angle is not beneficial because those leaves are already present above their unthreatening smaller neighbours.
To optimize competitive responses and ‘pick battles wisely’ (Novoplansky, 2009), ideally, plants should only respond to R:FR signals from below when such signals come from lower canopy of the same plant, as such responses lead to self‐organization of canopy structure. This requires plants to be able to discriminate between R:FR neighbour signals and self‐signals. There is evidence that belowground plants can discriminate between roots of themselves and roots of non‐self neighbours (see review by Chen, During, & Anten, 2012). But no studies have shown whether and how plants can distinguish between self‐ and non‐self‐light signals aboveground. While Crepy and Casal (2015) showed that plants are able to identify their kin neighbours based on the vertical profile of R:FR ratios, light profiles do not seem enough for plants to discriminate between self and non‐self‐light signals: bent shoots of both focal and neighbour plants entail R:FR reflection from below with a similar spatial distribution (Figure 2; Figures S7 and S8). Plants may need to combine light profile with other information to distinguish self‐ from non‐self‐signals, for example, physical connection between upper and lower plant parts or perception site of the signal.
In regard to R:FR perception at the leaf scale, no study has ever investigated whether the upper and lower sides of a leaf have a different share in the perception of R:FR. Two possible scenarios could be that (a) leaves integrate R:FR signals perceived by their lower and upper leaf sides as an average signal experienced by the whole leaf, which then drives responses, and (b) the asymmetrical leaf anatomy in vertical direction may cause a heterogeneous distribution of phytochrome inside the leaf; this may enable the leaf to independently perceive R:FR at lower side or upper side based on R:FR perceived by phytochromes in different cells (e.g., upper epidermal cells vs. spongy mesophyll cells). Previous studies have shown that the distribution of phytochrome is highly specific with respect to both organs and cell types within an organ, and the distribution is different in different species (Pratt & Coleman, 1971, 1974).Our results suggest that the increase of leaf angle may not be induced by the total R:FR perceived by a leaf, as total R:FR was hardly affected by the presence of a lower canopy (Figure S10). Leaf angle was correlated with R:FR reflected from below but not with total R:FR (Figure S20), suggesting that the increased leaf angle may be caused by R:FR perceived by part of the leaf but not the whole leaf, as R:FR reflected from below likely represents a signal that is largely perceived by lower side of the leaf. Further studies are needed to investigate whether or not plants can discriminate between self‐ and non‐self‐signals aboveground and how plants use self‐signals aboveground to optimize canopy structure to increase plant performance.
4.3. Future perspectives
Understanding the mechanisms of plant responses to far‐red reflected from below may provide new insights in breeding of ideotypes for different crop systems. In mixed‐species systems, shade‐avoidance responses of the large crop induced by R:FR signals reflected by the small crop may allow more light to penetrate to the small crop, reducing the competition for light by the large crop and thus increase productivity of the whole system. In such a case, R:FR signals from below can be regarded as a ‘self‐signal’ for the system as a whole. Responses to this signal could reduce ‘self‐competition’ within the system and may improve overall light capture and productivity (similar to our simulation results in Figure 4). Therefore, if possible, trait selection could focus on crop phenotypes with responses in some traits (e.g., increasing leaf angle) to R:FR from below. In contrast, in crop‐weed systems, shade‐avoidance responses of the crop plants induced by the low R:FR reflected by weeds, especially in early crop developmental stages, could be relevant to crop yield loss (Page et al., 2010). This is possibly because low R:FR reflected by weeds (a) induces an increase of biomass allocation to stem at the expense of other organs and decreases leaf area and harvest index of the crop and (b) increases the shoot‐root ratio of the crop and may hamper the belowground competition for water and nutrients with weeds (Ballaré & Casal, 2000; Page et al., 2010; Rajcan & Swanton, 2001). Our simulations also suggested that responses to R:FR from independent foliage of neighbours below the plants decreased plant light absorption and photosynthesis (Figure 4). Hence, for higher crop productivity in weed‐infested systems, crop genotypes with less or no shade‐avoidance responses to R:FR signals from below could be selected for. However, trait selection should also consider the growth light conditions for the crop. Our further simulation showed that under extreme light conditions (incoming light intensity = 2,000 μmol m−2 s−1, with 100% direct light, which is not uncommon in field situations), increasing leaf angle decreased light absorption but increased plant photosynthesis, even when the increased leaf inclination angle was caused by low R:FR reflected from independent foliage (Figure S21). This is because under such high light, top leaves in the canopy are light saturated. Therefore, although a steeper leaf angle leads to a reduction in canopy light absorption, this would not affect canopy photosynthesis; on the other hand, the steeper leaf angle allows more light to penetrate to lower leaves in the canopy, which leads to higher canopy photosynthesis. These examples show the relevance of further research on how and by which plant parts R:FR signals are perceived (Pantazopoulou et al., 2017) and how this information can be used to optimize plant responses through breeding, taking into account the potential growth environment of the crop (e.g., in the greenhouse vs. in the field).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Figure S1 Block design in the glasshouse compartment.
Figure S2 Pictures of the four treatments in the experiment.
Figure S3 Scheme of light measurements.
Figure S4 Optical properties of a rose leaf.
Figure S5 Responses of rose leaf photosynthetic rate to light intensity between 0–200 μmol m−2 s−1.
Figure S6 Examples of simulated rose plants with bent shoots, with virtual sensors locating at different positions and facing different directions.
Figure S7 Measured red to far‐red (R:FR) ratios above, in the middle and at the bottom of upright shoots on day 4 after start of treatments with spectrometer sensor facing up, horizontal and down.
Figure S8 Measured R:FR ratios above, in the middle and at the bottom of upright shoots on day 13 after start of treatments with spectrometer sensor facing up, horizontal and down.
Figure S9 Measured R:FR ratios above, in the middle and at the bottom of upright shoots on day 21 after start of treatments with spectrometer sensor facing up and horizontal.
Figure S10 Total R:FR ratios above, in the middle and at the bottom of upright shoots measured on day 4, day 13 and day 21.
Figure S11 The percentage of red or far‐red light reflected from below that accounts for the total red or far‐red light intensities at different positions in the canopy on day 4, day 13 and day 21.
Figure S12 Light spectral (wavelength 400–800 nm) measurements above, in the middle and at the bottom of upright shoots on day 24 after start of treatments with spectrometer sensor facing up, horizontal and down.
Figure S13 Relative light intensities measured in the middle and at bottom of upright shoots, and below bent shoots on day 14 and 22 after start of treatments.
Figure S14 Measurements of internode length and leaf length at each rank of upright shoots on day 6 and 12 after start of treatments.
Figure S15 Measurements of internode diameter, leaf width, leaf area and leaflet number at each rank, peduncle length and diameter, and flower diameter at final harvest.
Figure S16 Measurements on dry weight of individual internodes and leaves, and peduncle and flower dry weight.
Figure S17 Specific internode length and specific leaf area at each rank, and average specific internode length and average specific leaf area.
Figure S18 Total leaf area of upright shoots and bent shoots of a focal plant in each treatment, and leaf area index of each treatment.
Figure S19 Comparisons between measured and simulated R:FR ratios at flowering stage with sensor facing up, horizontal and down.
Figure S20 Relationships between R:FR ratios vs. leaf angle, leaf length or internode length.
Figure S21 The relative changes of simulated plant light absorption and photosynthesis with increasing leaf angle on upright shoots at a light intensity of 2000 μmol m−2 s−1 with 100% direct light.
Table S1 Ion concentration of the nutrient solution used in the experiment.
Table S2 Pairwise comparison results of R:FR ratios measured at different positions in the canopy with spectrometer sensor facing down.
Table S3 Pairwise comparison results of total R:FR measured at different positions in the canopy.
Table S4 Measured red and far‐red light intensities above, in the middle and at the bottom of upright shoots on day 4, day 13 and day 21, with sensor facing up, horizontal and down.
Table S5 Pairwise comparison results of relative light intensities at different positions in the canopy.
Table S6 Pairwise comparisons results of plant traits measured at final harvest.
Table S7 Pairwise comparison results of internode length and leaf length measured on day 6 and day 12.
Method S1 The methodology of calculating plant photosynthesis.
Excel S1 Measured red and far‐red light intensities with spectrometer sensor facing up, horizontal and down, and the total red and far‐red light intensities at different positions in the canopy on day 4, day 13 and day 21.
ACKNOWLEDGMENTS
We thank Unifarm staff, Ms. Qianxixi Min, Mr. Wenqing Jin and Mr. Dick M. van der Sar for valuable help and contributions to the experiment, and the financial support from the China Scholarship Council (CSC) (No. 201406850003) and from the project ‘More roses for less’ (No. 870.15.040) funded by the Netherlands Organisation for Scientific Research (NWO), Signify and Glastuinbouw Nederland.
Zhang N, van Westreenen A, He L, Evers JB, Anten NPR, Marcelis LFM. Light from below matters: Quantifying the consequences of responses to far‐red light reflected upwards for plant performance in heterogeneous canopies. Plant Cell Environ. 2021;44:102–113. 10.1111/pce.13812
Present address Lizhong He, Shanghai Key Lab of Protected Horticulture Technology, Horticultural Research Institute, Shanghai Academy of Agricultural Science, Shanghai, China
Funding information China Scholarship Council (CSC), Grant/Award Number: 201406850003; Glastuinbouw Nederland; Netherlands Organisation for Scientific Research (NWO), Grant/Award Number: 870.15.040; Signify
Contributor Information
Ningyi Zhang, Email: ningyi.zhang@wur.nl.
Lizhong He, Email: leo.marcelis@wur.nl.
Leo F. M. Marcelis, Email: leo.marcelis@wur.nl.
REFERENCES
- Anten, N. P. R. , & During, H. J. (2011). Is analysing the nitrogen use at the plant canopy level a matter of choosing the right optimization criterion? Oecologia, 167, 293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré, C. L. , & Casal, J. J. (2000). Light signals perceived by crop and weed plants. Field Crops Research, 67(2), 149–160. [Google Scholar]
- Ballaré, C. L. , & Pierik, R. (2017). The shade‐avoidance syndrome: Multiple signals and ecological consequences. Plant Cell and Environment, 40, 2530–2543. [DOI] [PubMed] [Google Scholar]
- Bell, D. L. , & Galloway, L. F. (2007). Plasticity to neighbour shade: Fitness consequences and allometry. Functional Ecology, 21(6), 1146–1153. [Google Scholar]
- Bongers, F. J. , Pierik, R. , Anten, N. P. R. , & Evers, J. B. (2018). Subtle variation in shade avoidance responses may have profound consequences for plant competitiveness. Annals of Botany, 121(5), 863–873. 10.1093/aob/mcx151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonman, A. , Prinsen, E. , Gilmer, F. , Schurr, U. , Peeters, A. J. M. , Voesenek, L. A. C. J. , & Pons, T. L. (2007). Cytokinin import rate as a signal for photosynthetic acclimation to canopy light gradients. Plant Physiology, 143(4), 1841–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooker, R. W. , Bennett, A. E. , Cong, W. , Daniell, T. J. , George, T. S. , Hallett, P. D. , et al. (2015). Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytologist, 206, 107–117. [DOI] [PubMed] [Google Scholar]
- Casal, J. J. (2013). Photoreceptor signaling networks in plant responses to shade. Annual Review of Plant Biology, 64, 403–427. [DOI] [PubMed] [Google Scholar]
- Chen, B. J. W. , During, H. J. , & Anten, N. P. R. (2012). Detect thy neighbor: Identity recognition at the root level in plants. Plant Science, 195, 157–167. [DOI] [PubMed] [Google Scholar]
- Crepy, M. A. , & Casal, J. J. (2015). Photoreceptor‐mediated kin recognition in plants. New Phytologist, 205(1), 329–338. [DOI] [PubMed] [Google Scholar]
- Dudley, S. A. , & Schmitt, J. (1995). Genetic differentiation in morphological responses to simulated foliage shade between populations of Impatiens capensis from open and woodland sites. Functional Ecology, 9(4), 655–666. [Google Scholar]
- Dudley, S. A. , & Schmitt, J. (1996). Testing the adaptive plasticity hypothesis: Density‐dependent selection on manipulated stem length in Impatiens capensis . The American Naturalist, 147(3), 445–465. [Google Scholar]
- Evers, J. B. , Vos, J. , Yin, X. , Romero, P. , Van Der Putten, P. E. L. , & Struik, P. C. (2010). Simulation of wheat growth and development based on organ‐level photosynthesis and assimilate allocation. Journal of Experimental Botany, 61(8), 2203–2216. [DOI] [PubMed] [Google Scholar]
- Evers, J. B. , Vos, J. , Andrieu, B. , & Struik, P. C. (2006). Cessation of tillering in spring wheat in relation to light interception and red:far‐red ratio. Annals of Botany, 97(4), 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falik, O. , de Kroon, H. , & Novoplansky, A. (2006). Physiologically‐mediated self/nonself root discrimination. Plant Signaling & Behavior, 1(3), 116–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falik, O. , Reides, P. , Gersani, M. , & Novoplansky, A. (2003). Self/non‐self discrimination in roots. Journal of Ecology, 91(91), 525–531. [Google Scholar]
- Green‐Tracewicz, E. , Page, E. R. , & Swanton, C. J. (2012). Light quality and the critical period for weed control in soybean. Weed Science, 60(1), 86–91. [Google Scholar]
- Hara, T. (1985). Mathematical analysis on the optimal foliage structure in plant communities. Journal of Inferential and Deductive Biology, 1, 17–29. [Google Scholar]
- Hemmerling, R. , Kniemeyer, O. , Lanwert, D. , Kurth, W. , & Buck‐Sorlin, G. (2008). The rule‐based language XL and the modelling environment GroIMP illustrated with simulated tree competition. Functional Plant Biology, 35, 739–750. [DOI] [PubMed] [Google Scholar]
- Héraut‐Bron, V. , Robin, C. , Varlet‐Grancher, C. , & Guckert, A. (2001). Phytochrome mediated effects on leaves of white clover: Consequences for light interception by the plant under competition for light. Annals of Botany, 88, 737–743. [Google Scholar]
- Hikosaka, K. , & Hirose, T. (1997). Leaf angle as a strategy for light competition: Optimal and evolutionarily stable light‐extinction coefficient within a leaf canopy. Ecoscience, 4(4), 501–507. [Google Scholar]
- Hikosaka, K. , Kumagai, T. , & Ito, A. (2016). Modeling canopy photosynthesis In Canopy photosynthesis: From basics to applications (pp. 239–268). Dordrecht, The Netherlands: Springer. [Google Scholar]
- Van Hinsberg, A. , & Van Tienderen, P. (1997). Variation in growth form in relation to spectral light quality (red/far‐red ratio) in Plantago lanceolata L. in sun and shade populations. Oecologia, 111(4), 452–459. [DOI] [PubMed] [Google Scholar]
- Holmes, M. G. , & Smith, H. (1977). The function of phytochrome in the natural environment—II. The influence of vegetation canopies on the spectral energy distribution of natural daylight. Photochemistry and Photobiology, 25(6), 539–545. [Google Scholar]
- Hunt, P. G. , Kasperbauer, M. J. , & Matheny, T. A. (1989). Soybean seedling growth responses to light reflected from different colored soil surfaces. Crop Science, 29(1), 130–133. [Google Scholar]
- Kasperbauer, M. J. (1994). Cotton plant size and fiber developmental responses to FR/R ratio reflected from the soil surface. Physiologia Plantarum, 91(2), 317–321. [Google Scholar]
- Keuskamp, D. H. , Sasidharan, R. , & Pierik, R. (2010). Physiological regulation and functional significance of shade avoidance responses to neighbors. Plant Signaling & Behavior, 5(6), 655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Pereira, M. , Sadras, V. O. , Batista, W. , Casal, J. J. , & Hall, A. J. (2017). Light‐mediated self‐organization of sunflower stands increases oil yield in the field. Proceedings of the National Academy of Sciences of the United States of America, 114(30), 201618990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niinemets, Ü. , & Anten, N. P. R. (2009). Packing the photosynthetic machinery: From leaf to canopy In Photosynthesis in silico (pp. 363–399). Netherland: Springer. [Google Scholar]
- Novoplansky, A. (2009). Picking battles wisely: Plant behaviour under competition. Plant, Cell and Environment, 32(6), 726–741. [DOI] [PubMed] [Google Scholar]
- Page, E. R. , Tollenaar, M. , Lee, E. A. , Lukens, L. , & Swanton, C. J. (2010). Shade avoidance: An integral component of crop‐weed competition. Weed Research, 50(4), 281–288. [Google Scholar]
- Pantazopoulou, C. K. , Bongers, F. J. , Küpers, J. J. , Reinen, E. , Das, D. , Evers, J. B. , … Pierik, R. (2017). Neighbor detection at the leaf tip adaptively regulates upward leaf movement through spatial auxin dynamics. Proceedings of the National Academy of Sciences of the United States of America, 114(28), 7450–7455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierik, R. , Mommer, L. , & Voesenek, L. A. (2013). Molecular mechanisms of plant competition: Neighbour detection and response strategies. Functional Ecology, 27(4), 841–853. [Google Scholar]
- Pierik, R. , & De Wit, M. (2014). Shade avoidance: Phytochrome signalling and other aboveground neighbour detection cues. Journal of Experimental Botany, 65(11), 2815–2824. [DOI] [PubMed] [Google Scholar]
- Pons, T. L. , & De Jong‐Van Berkel, Y. E. M. (2004). Species‐specific variation in the importance of the spectral quality gradient in canopies as a signal for photosynthetic resource partitioning. Annals of Botany, 94(5), 725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons, T. L. , van Rijnberk, H. , Scheurwater, I. , & van der Werf, A. (1993). Importance of the gradient in photosynthetically active radiation in a vegetation stand for leaf nitrogen allocation in two monocotyledons. Oecologia, 95(3), 416–424. [DOI] [PubMed] [Google Scholar]
- Pratt, L. H. , & Coleman, R. A. (1971). Immunocytochemical localization of phytochrome. Proceedings of the National Academy of Sciences of the United States of America, 68(10), 2431–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt, L. H. , & Coleman, R. A. (1974). Phytochrome distribution in etiolated grass seedlings as assayed by an indirect antibody‐labelling method. American Journal of Botany, 61(2), 195–202. [Google Scholar]
- Rajcan, I. , & Swanton, C. J. (2001). Understanding maize‐wheat competition: Resource competition, light quality and the whole plant. Field Crops Research, 71, 139–150. [Google Scholar]
- Rajcan, I. , Chandler, K. J. , & Swanton, C. J. (2004). Red‐far‐red ratio of reflected light: A hypothesis of why early‐season weed control is important in corn. Weed Science, 52(5), 774–778. [Google Scholar]
- Smith, H. , Casal, J. J. , & Jackson, G. M. (1990). Reflection signals and the perception by phytochrome of the proximity of neighbouring vegetation. Plant, Cell & Environment, 13(1), 73–78. [Google Scholar]
- Smith, H. (2000). Phytochromes and light signal perception by plants—An emerging synthesis. Nature, 407(6804), 585–591. [DOI] [PubMed] [Google Scholar]
- de Vries, J. , Poelman, E. H. , Anten, N. P. R. , & Evers, J. B. (2018). Elucidating the interaction between light competition and herbivore feeding patterns using functional–structural plant modelling. Annals of Botany, 121(5), 1019–1031. 10.1093/aob/mcx212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijschedé, J. , Martínková, J. , De Kroon, H. , & Huber, H. (2006). Shade avoidance in Trifolium repens: Costs and benefits of plasticity in petiole length and leaf size. New Phytologist, 172(4), 655–666. [DOI] [PubMed] [Google Scholar]
- Zhang, N. , van Westreenen, A. , Anten, N. P. R. , Evers, J. B. , & Marcelis, L. F. M. (2019). Disentangling the effects of photosynthetically active radiation and red to far‐red ratio on plant photosynthesis under canopy shading. A simulation study using a functional‐structural plant model. Annals of Botany. 10.1093/aob/mcz197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, N. , van Westreenen, A. , Evers, J. B. , Anten, N. P. R. , & Marcelis, L. F. M. (2019). Quantifying the contribution of bent shoots to plant photosynthesis and biomass production of flower shoots in rose (Rosa hybrida) using a functional–structural plant model. Annals of Botany. 10.1093/aob/mcz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Block design in the glasshouse compartment.
Figure S2 Pictures of the four treatments in the experiment.
Figure S3 Scheme of light measurements.
Figure S4 Optical properties of a rose leaf.
Figure S5 Responses of rose leaf photosynthetic rate to light intensity between 0–200 μmol m−2 s−1.
Figure S6 Examples of simulated rose plants with bent shoots, with virtual sensors locating at different positions and facing different directions.
Figure S7 Measured red to far‐red (R:FR) ratios above, in the middle and at the bottom of upright shoots on day 4 after start of treatments with spectrometer sensor facing up, horizontal and down.
Figure S8 Measured R:FR ratios above, in the middle and at the bottom of upright shoots on day 13 after start of treatments with spectrometer sensor facing up, horizontal and down.
Figure S9 Measured R:FR ratios above, in the middle and at the bottom of upright shoots on day 21 after start of treatments with spectrometer sensor facing up and horizontal.
Figure S10 Total R:FR ratios above, in the middle and at the bottom of upright shoots measured on day 4, day 13 and day 21.
Figure S11 The percentage of red or far‐red light reflected from below that accounts for the total red or far‐red light intensities at different positions in the canopy on day 4, day 13 and day 21.
Figure S12 Light spectral (wavelength 400–800 nm) measurements above, in the middle and at the bottom of upright shoots on day 24 after start of treatments with spectrometer sensor facing up, horizontal and down.
Figure S13 Relative light intensities measured in the middle and at bottom of upright shoots, and below bent shoots on day 14 and 22 after start of treatments.
Figure S14 Measurements of internode length and leaf length at each rank of upright shoots on day 6 and 12 after start of treatments.
Figure S15 Measurements of internode diameter, leaf width, leaf area and leaflet number at each rank, peduncle length and diameter, and flower diameter at final harvest.
Figure S16 Measurements on dry weight of individual internodes and leaves, and peduncle and flower dry weight.
Figure S17 Specific internode length and specific leaf area at each rank, and average specific internode length and average specific leaf area.
Figure S18 Total leaf area of upright shoots and bent shoots of a focal plant in each treatment, and leaf area index of each treatment.
Figure S19 Comparisons between measured and simulated R:FR ratios at flowering stage with sensor facing up, horizontal and down.
Figure S20 Relationships between R:FR ratios vs. leaf angle, leaf length or internode length.
Figure S21 The relative changes of simulated plant light absorption and photosynthesis with increasing leaf angle on upright shoots at a light intensity of 2000 μmol m−2 s−1 with 100% direct light.
Table S1 Ion concentration of the nutrient solution used in the experiment.
Table S2 Pairwise comparison results of R:FR ratios measured at different positions in the canopy with spectrometer sensor facing down.
Table S3 Pairwise comparison results of total R:FR measured at different positions in the canopy.
Table S4 Measured red and far‐red light intensities above, in the middle and at the bottom of upright shoots on day 4, day 13 and day 21, with sensor facing up, horizontal and down.
Table S5 Pairwise comparison results of relative light intensities at different positions in the canopy.
Table S6 Pairwise comparisons results of plant traits measured at final harvest.
Table S7 Pairwise comparison results of internode length and leaf length measured on day 6 and day 12.
Method S1 The methodology of calculating plant photosynthesis.
Excel S1 Measured red and far‐red light intensities with spectrometer sensor facing up, horizontal and down, and the total red and far‐red light intensities at different positions in the canopy on day 4, day 13 and day 21.


