Abstract
Aim
Very preterm birth is associated with a high risk of enteropathies. Diagnosis is challenging, especially in mild forms, leading to unnecessary periods of cessation of enteral feeding. This study aimed at establishing a prognosis score of enteropathy combining clinical parameters and faecal calprotectin concentration.
Methods
This prospective multicentric study included preterm neonates born at a gestational age of 33 weeks or less. Stools were collected weekly until hospital discharge, and daily in case of digestive events for calprotectin measurement (ELISA and immunochromatography) and microbiota analyses (16S rRNA gene sequencing).
Results
Among the 121 neonates included, 21 experienced at least one episode of enteropathy, mainly mild forms. By ELISA testing, median faecal calprotectin was 88 (8‐798) µg/g faeces. No statistically significant association was found between the outset of enteropathy and maternal and neonatal characteristics, and calprotectin levels. The agreement between ELISA and immunochromatography assay was moderate (intra‐class correlation coefficient 0.58, 95%CI [0.47‐0.66]). Comparison of species diversity and relative bacterial abundance profiles between infants with or without enteropathy revealed no specific alterations associated with enteropathy.
Conclusion
The study failed to propose a prognostic score of enteropathy, probably due the large inter‐ and intra‐individual variability of faecal calprotectin in very preterm neonates.
Keywords: enteropathy, faecal calprotectin, gut microbiota, necrotising enterocolitis, preterm neonates
Abbreviations
- FC
faecal calprotectin
- NEC
necrotising enterocolitis
- IC
immunochromatography
- NICU
neonatal intensive care nit
- POCT
point‐of‐care testing
- GA
gestational age
- OTU
operational taxonomic unit
Key Notes.
Early diagnosis of enteropathies in very preterm neonates is challenging, leading to unnecessary enteral feeding discontinuation and prolonged courses of antibiotics.
Faecal calprotectin, a reliable marker of inflammation in children and adults, showed highly variable levels in very preterm neonates, limiting its use to predict enteropathy.
By contrast with necrotising enterocolitis, the most severe form of enteropathies, gut microbiota did not show a specific profile linked to milder forms.
1. INTRODUCTION
Very premature birth is associated with a high risk of serious complications, including gastrointestinal diseases, that is enteropathies, which the most severe form is necrotising enterocolitis (NEC), one of the leading cause of morbidity and mortality in neonatal intensive care units. 1 Enteropathies are classified according to Bell's stages from mild‐to‐severe forms. 2 The prevalence of the milder form of enteropathy (Bell's stages Ia and Ib, suspected NEC) can be as high as 44%. 3 The severe form, that is definite NEC (Bell's stages IIb to IIIb), has an incidence depending on the country, from 2% to about 10% in extremely preterm neonates, associated with a mortality up to 50%. 1 , 4
Diagnosis is mainly supported by a body of presumptions based on clinical and radiological signs according to Bell's stage. However, it can be challenging in numerous suspected NEC cases, especially in the early course of the disease or in mild forms. This diagnostic uncertainty leads to frequent unnecessary and extended periods of cessation of enteral feeding that could impair the intestinal maturation, and prolonged courses of parenteral nutrition and antibiotics. New tools are thus required to facilitate an early and precise diagnosis and to optimise the management of preterm neonates with intestinal symptoms.
Biomarkers are molecular indicators of disease process, diagnosis, and/or prognosis. There is currently a paucity of reliable and robust biomarkers for neonatal enteropathies, including NEC. 5 Noninvasive biomarkers have obvious advantages in preterm infants. Among the noninvasive faecal biomarkers, the most studied is faecal calprotectin (FC), a protein mainly expressed in the cytoplasm of neutrophils and highly resistant to proteolysis, which is now routinely measured to evaluate mucosal inflammation in patients with inflammatory bowel diseases. 6 , 7 , 8 Physiological values of neonatal FC are higher than adult ones with a large inter‐individual variability, reflecting the permeability of neonatal digestive tract, the immune stimulation induced by the microbiota settlement and the first contacts with feeding allergens. 9 Several studies have shown increased FC levels in infants with enterocolitis. However, there is still no consensus about cut‐off levels in the neonatal population and regarding its use in current clinical practice. 10
In a retrospective study, we previously proposed a FC threshold at 350 µg/g faeces (sensitivity 0.65, specificity 0.82) using enzyme‐linked immunosorbent assay (ELISA) for early diagnosis of neonatal enteropathy requiring strict enteral feeding interruption in preterm neonates. 11 The current prospective cohort study was designed to establish a prognosis and diagnosis score of enteropathy based on combined criteria including neonatal characteristics and FC concentration. Furthermore, the study aimed at establishing the agreement between the ELISA method considered as the reference assay performed in laboratories and a point of care testing (POCT), that is an immunochromatographic (IC) assay, more convenient for rapid diagnosis. In addition, faecal microbiota composition during the digestive event was analysed.
2. PATIENTS AND METHODS
2.1. Patients
This prospective multicentre study was conducted in three French Neonatal Intensive Care Units (NICUs). Eligible preterm neonates had a gestational age (GA) of 33 weeks or less, and did not present with any relevant malformation. They were enrolled at birth, and various perinatal parameters were recorded: sex, GA, mode of delivery, Apgar score, intra‐uterine growth retardation, acute foetal distress, respiratory distress, antenatal and per partum antibiotic therapy, and suspicion of maternofoetal infection. Neonates underwent a weekly clinical follow‐up until hospital discharge, including weight, height, head circumference, abdominal circumference, collateral venous abdominal circulation and mode of feeding. Digestive tolerance was evaluated through stool frequency and characteristics according to ‘Amsterdam’ stool form scale, 12 stool bleeding, bloating, type and volume of feeding, and residual gastric volume. Enteropathy was suspected when occurred an intestinal distress requiring the interruption of enteral feeding for more than 48 hours, the commonly used treatment for a suspected enterocolitis (Bell's stage ≥ Ia). When neonates experienced such events, a daily monitoring was set‐up including all relevant previously described parameters, and biological parameters (C‐reactive protein (CRP), procalcitonin). Medical files were further checked by both a gastropaediatrician and a public health specialist to ensure that the event had a digestive origin and was not secondary to another infectious disease. Cases, that is definite enteropathies, were defined by the concurrent presence of an abdominal distension (either clinically diagnosed by visual observation and/or measurement of the abdominal circumference, or radiologically diagnosed, or both) and either rectal bleeding or elevated gastric retention (the threshold was higher than one third of the volume of milk ingested per 24 hours) or both. All cases were classified according to the Bell's scale. 2
Stools were collected at enrolment, then weekly until discharge, for FC assays and gut microbiota analysis, and immediately stored at −20°C and −80°C, respectively. During the event, complementary stool samples were collected daily from the first digestive symptoms. The expected quantity of stools was over 2g to make it possible high‐quality grade assays.
This study was approved by an Ethic Committee (CPP Ile‐de‐France I, number 2012‐Janv.‐12813) and registered on https://clinicaltrials.gov/ (NCT02010268). Parental written consent was signed prior to any study procedure.
2.2. Calprotectin assays
Stool samples were assayed for FC by Calprest ELISA method (Eurospital, Italy) and Calfast IC assay (Eurospital, Italy)—as the POCT—according to manufacture recommendations. Assays were performed at the end of the sampling period and simultaneously for both methods to avoid any bias in the measurements. Hence, samples were stored at −20°C before the assay no more than 12 months, which is known to prevent from significant alteration of FC levels. 13
2.3. Gut microbiota analysis
Neonates who had a stool sample collected at the time of an enteropathy event (from −2 to + 5 days) were enrolled in the gut microbiota analysis. Two control neonates were selected per case and matched on the following criteria: absence of enteropathy, stool specimen collected in the same postnatal week, birth GA (± 1.5 weeks), birthweight (± 100g), mode of delivery and neonatal intensive care unit.
Total DNA was extracted from 0.2g of faecal samples as previously described. 14 Bacterial diversity and composition were assessed by 16S rRNA gene sequencing–based method. Variable regions V3‐V4 were amplified by polymerase chain reaction (PCR) and sequenced on an Illumina MiSeq platform. Next‐generation sequencing data set was analysed using the open‐source bioinformatics pipeline Find, Rapidly, OTUs with Galaxy Solution (FROGS). 15 After sequence processing, operational taxonomic units (OTUs) were assigned to different taxonomic levels using the NCBI reference databases and Ribosomal database Project Classifier. 16 , 17
2.4. Statistical analysis
Cases were defined as infants with intestinal distress leading to interruption of enteral feeding for more than 48 hours, associated with an abdominal distension and either rectal bleeding or elevated gastric retention or both. The sample size was calculated to show that a FC threshold (ELISA) higher than 350 µg/g faeces is a risk factor of feeding interruption in preterm neonates as previously demonstrated. 11 , 18 We hypothesised that the prevalence of enteropathy was 9% in neonates with FC < 350 µg/g. For a RR equal to 4 with alpha risk 5% and power 90%, it was needed to include forty‐seven infants per class group (FC ≥ 350 µg/g, FC < 350 µg/g). Ninety‐four infants were required, and the sample size was extended to 125 infants overall to account for other possible risk factors.
All statistical analyses were undertaken using R 2.3.3 software (https://cran.rproject.org/). Statistical tests were two‐sided with statistical significance of P < .05.
Characteristics of the neonates were described in the overall population. Mean ± SD or median [range] were reported for quantitative variables, and frequencies (%) for qualitative variables. Univariate analyses were carried out to determine the association between enteropathy and FC levels using ELISA, clinical parameters and the occurrence of an enteropathy. Since a prognosis score was seeking, this analysis was performed on neonates with at least one sample available before day 7 of life (D7) and before the event when it occurred before D7. The agreement between faecal calprotectin concentrations determined by ELISA and IC assay was evaluated by intra‐class correlation coefficient (ICC) and Bland and Altman plot. 19 Gut microbiota was analysed using Wilcoxon test and permutational multivariate analysis of variance (PERMANOVA).
3. RESULTS
3.1. Population
Between September 2013 and November 2014, 122 preterm neonates were included in the study with a sex ratio of 1.1. One neonate died before any follow‐up. One patient with a malformation (clubfoot) was maintained in the analysis population. One‐hundred and twenty‐one patients were included in the analysis population. Forty infants (33%) were prematurely transferred before the planned NICU discharge. Neonates’ characteristics, and pregnancy and delivery conditions are detailed in Table 1. The median GA was 30.9 weeks ranging from 24.9 to 32.9 weeks. The majority of the neonates were very premature, with only 20 extremely premature infants, that is born at a GA under 28 weeks, and among them only 4 under 27 weeks. All infants were included from 0 to 7 days old (median age: 2 days). All documented neonates had an enteral feeding, and for 95% (n = 114), it was exclusive. Exclusive banked breastfeeding was the main diet type (n = 90, 75%), and 11 neonates (9.2%) had exclusively preterm milk formula.
TABLE 1.
Characteristics of the 121 infants included
| Pregnancy's characteristics | ||
| Multiple birth (n, %) | 68 | 56.2 |
| Intrauterine retardation (n, %) | 15 | 12.4 |
| Maternal corticosteroid (n, %) | 112 | 92.6 |
| Maternal smoking (n, %) a | 18 | 17.8 |
| Maternal antibiotics (n, %) a | ||
| Antenatal (one week before delivery) | 31 | 26.5 |
| Intrapartum | 53 | 44.5 |
| Caesarean section (n, %) | 94 | 77.7 |
| Neonatal characteristics | ||
| Gestational age (weeks, median, range) | 30.9 | 24.9‐32.9 |
| APGAR score (median, range) 1 min | 8 | 0‐10 |
| 5 min | 9 | 2‐10 |
| 10 min | 10 | 3‐10 |
| Acute foetal distress (n, %) a , b | 4 | 3.4 |
| Maternofoetal infection (n, %) | 79 | 65.3 |
| Antibiotic therapy (n, %) | 77 | 97.5 |
| Confirmed infection (n, %) | 4 | 5 |
| Birthweight (g, mean, SD) | 1330 | 329 |
| Age at inclusion (day, median, range) | 2 | 0‐7 |
| Enteral feeding (n, %) a | 120 | 99.1 |
| Exclusive bank milk feeding | 90 | 74.4 |
| Exclusive premature milk feeding | 11 | 9.2 |
| Exclusive breastfeeding | 1 | 0.8 |
| Breast + bank milk feeding | 12 | 10.0 |
| Other mixed feeding | 6 | 5.0 |
Missing values: maternal smoking during pregnancy, 20; antenatal and intrapartum antibiotic therapy, 4 and 2, respectively; acute foetal distress, 3; enteral feeding, 1.
At least 2 critical criteria among at‐risk Apgar score (<4 at 1 min or < 7 at 5 min), foetal cardiac rhythm abnormality (type II dips,) umbilical artery pH < 7, meconium‐stained amniotic fluid.
3.2. Enteropathies
During their study follow‐up (median duration 41 days [1‐83]), 21 neonates (17%) experienced at least one episode of enteropathy (one event for 19 neonates and two events for 2 neonates. The first enteropathy occurred at a median age of 15 days [1‐38], and the second at 21 and 71 days, respectively. The enteropathy was diagnosed within 48h after the first digestive symptoms. The 23 digestive events were mainly mild enteropathies [Bell's stage: Ia‐‐Ib, 18 patients (78.3%); IIa, four patients (17.4%); IIb, one patient (4.3%)] (Table 2).
TABLE 2.
Characteristics of the enteropathies’ events (n = 23) in 21 patients (two patients had two events separated by more than 2 wk)
| Age at the event time (d) | 20 [1‐71] |
| Bell's stage (n, %) | |
| Ia | 12 (52.2) |
| Ib | 6 (26.1) |
| IIa | 4 (17.4) |
| Intestinal distension (n, %) | 23 (100.0) |
| Abdominal circumference (cm, mean ± SD) a | 25.2 ± 2.2 |
| Collateral venous circulation (n, %) a | 4 (18.2) |
| Gastric residual volume a | |
| Gastric residual volume over 24 h (mL, median [range]) | 13 [0‐49] |
| Ratio of gastric residual volume per ingested milk volume over 24 h (%,median [range]) | 7.5 [1.6‐118.8] |
| Pneumatosis intestinalis (n, %) a | 3 (13.6) |
| Stools within the 48 h prior to the event | 21 (91.3) |
| Number of stools within 24h prior to the event a | 5 [1‐11] |
| Blood in the stool (n, %) a | 7 (31.8) |
| Biological markers | |
| CRP (μg/L, median, range) a | 6 [0‐157] |
| PCT (μg/L, median, range) a | 0.4 [0.0‐9.7] |
Abbreviations: CRP, C‐reactive protein; PCT, procalcitonin.
Missing values: abdominal circumference, 10; collateral venous circulation, 1; gastric residual volume over 24 h, 2; gastric residual volume ratio, 8; number of stools within 24 h prior to the event, 2; pneumatosis intestinalis, 1; blood in the stools, 1; CRP, 2; PCT, 2.
3.3. Faecal calprotectin
Overall, 681 samples were assayed for FC levels. One sample was excluded for an aberrant value (5250 µg/g faeces), and 634 samples (93%) were studied by both ELISA and IC assay. The median FC‐ELISA was 88 µg/g faeces (range [8‐798], inter‐quartile range [44.3‐140]). FC‐ELISA ≥ 350 µg/g faeces were found only in 12 patients (10%) from the cohort. Among them, 7 had no enteropathies during hospital stay, 4 had a high FC‐ELISA during or after the event, and 1 before the event. Thus, the threshold for enteropathy of 350 µg/g faeces was not confirmed in the current cohort.
The agreement between ELISA and IC results was moderate (ICC = 0.58, 95% CI [0.47‐0.66] (Figure S1, the Bland and Altman plots corroborate the moderate agreement between FC measured using ELISA and IC). However, FC levels present a similar evolution with both methods, as shown by the individual plots overlaying the results of neonates who experienced enteropathy (Figure 1) or not (Figure S2, which shows FC dynamics using ELISA and IC in the 99 without enteropathy), with a high inter‐ and intra‐individual variability.
FIGURE 1.
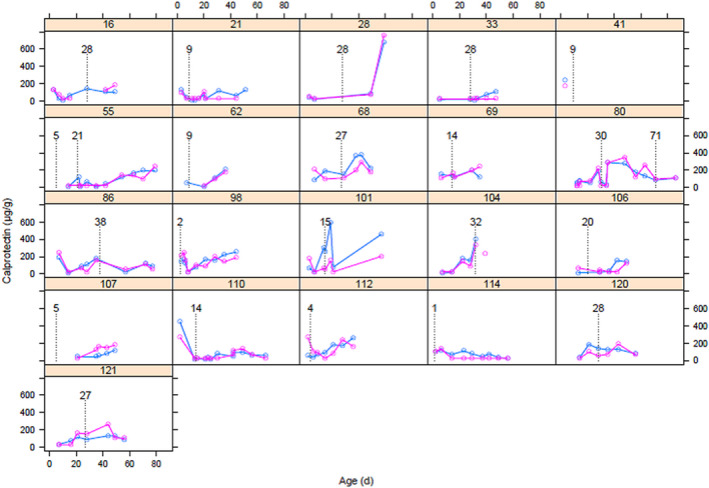
Individual evolution of faecal calprotectin in the 21 neonates suffering from enteropathy event(s). Events are identified by the first day (D) of enteral feeding discontinuation (vertical dotted line): 18 patients with mild enteropathy (suspected NEC, Bell stage Ia and Bell stage Ib), and 5 with severe enteropathy (definite NEC, patients 80 (D71), 86, 120 and 121: Bell stage IIA; patient 104: Bell stage IIb). Blue line: faecal calprotectin (FC) by ELISA; pink line: FC by immunochromatographic (IC) assay
3.4. Prognosis factors of enteropathy based on faecal calprotectin and clinical parameters
Prognostic value of FC‐ELISA was evaluated on the 84 neonates for whom we collected at least one sample before D7 and before the event (when it occurred before D7). Among these neonates, 14 (17%) presented with an enteropathy. Univariate analysis did not show any significant association between the onset of enteropathy, FC‐ELISA at inclusion and/or before the event, and the clinical parameters recorded prior inclusion, precluding the establishment of a prognostic score (supplementary file, Table S1, which demonstrates the absence of significant association between FC and clinical parameters).
Diagnostic score could not be evaluated due to the low occurrence of NEC cases in our cohort.
3.5. Gut microbiota characteristics
To identify microbiota signatures of enteropathy, microbiota composition was analysed at various phylogenetic levels. Two main profiles with either Proteobacteria or Firmicutes predominance were observed in both enteropathy and control groups (Figure 2A). Comparison of species diversity and relative bacterial abundance profiles between infants with or without enteropathy revealed no specific alterations associated with the onset of enteropathy, except a trend towards a higher diversity in infants without enteropathy (Figure 2B,C). No correlations with perinatal factors were identified.
FIGURE 2.
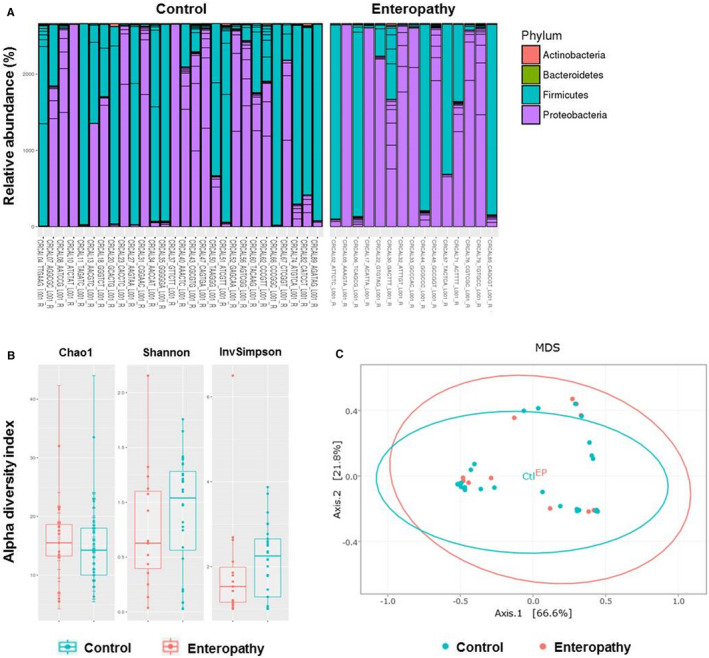
Microbiota profiles in neonates suffering from enteropathy and matching controls. A, Relative abundance of sequence reads at the phylum level assigned to different bacterial taxa in each neonate. B, Bacterial alpha‐diversity using the Chao‐1, Shannon and Inverse Simpson Diversity Indexes in control and neonates suffering from enteropathy. C, Beta‐diversity was analysed using multidimensional scaling (MDS) plot of samples according to disease status (enteropathy vs control) on the weighted‐UniFrac distance metrics
4. DISCUSSION
This multicentre prospective study on 121 very preterm neonates aimed at defining a combined prognostic and diagnostic score of enteropathy including perinatal parameters and FC level. Univariate analysis results did not make possible to propose neither a prognostic score nor a diagnostic score as FC‐ELISA did not differentiate neonates that will or will not have enteropathy.
Literature reported wide variations in FC levels in premature infants. 10 In our cohort, FC levels were of the same order than those described recently by MacQueen et al 20 and Ho et al 21 but lower than those of Van Zoonen et al 22 or Nakayuenyongsuk et al. 23 These discrepancies could be related to either neonates’ characteristics as a recent study indicated a relationship between FC and corrected GA, 24 or to the use of different methods of assay, which are known to give numerical values which differ substantially. 25 This outcome points out that results between cohorts are not interchangeable. Moreover, it should necessitate the use of adapted clinical cut‐off values in the purpose of help on clinical decision‐making. Interestingly, very recently, a calprotectin upper reference interval incorporating corrected GA has been shown to best predict NEC. 20
We failed to determine a prognosis score of enteropathy. Of note, the prevalence of enteropathies in our cohort (23 enteropathies in 21 patients, including only 5 with Bell's stage II) was lower than expected although our population included patients with the highest risk of NEC (low GA and low birthweight). This can be due to improvements in the care of very preterm neonates that may have led to a decrease in the risk of enteropathy and NEC, with an incidence of NEC in France of 3.2% in very preterm infants in a recent nationwide cohort. 26 Moreover, only 14 cases out of the 23 with enteropathy could be included in the prognosis score calculation. The hypothesised threshold of 350 µg/g faeces to characterise enteropathy requiring feeding interruption was reached in very few neonates with and without enteropathy. Several cut‐off values have been proposed, especially for prediction of severe form of NEC. 10 However, these cut‐off levels remain within the range of FC levels found in infants without digestive symptom enteropathies. Recent studies also failed to establish such relationship. Van Zoonen et al did not find any differences between control infants and the ten ones who further developed NEC (Bell’ stages 2 or 3). 22 Interestingly, MacQueen et al 20 found more frequently lower calprotectin levels in preterm infants with bloody stools (Bell's stage Ib), but who did not progress to NEC, suggesting that calprotectin level may be a marker of the severity of the enteropathy. However, this relationship was imperfect, with neonates with elevated FC levels and without NEC, and neonates with levels within the reference range and with NEC.
The usefulness of point‐of‐care test to determine FC in preterm infants is obvious, providing results rapidly available to the paediatricians. POCT assay was carried out centrally and compared with FC‐ELISA as the reference assay performed on the same samples. We have previously shown a correlation between the two techniques in adult patients with IBD. 27 The expected agreement was not observed in our very preterm neonate population, even if the individual plots overlaying the results of both techniques presented slightly similar evolution whatever the digestive status. A recent study using a different POCT confirmed the high calprotectin levels with major fluctuations, and nonspecific increase in some infants that developed or not NEC. 20
There are few information on microbiota in mild‐to‐moderate enteropathies by contrast to NEC. We found no specific microbiota profiles by comparison with infants without digestive events, by contrast with that we 26 and others previously reported for NEC. 28 However, the high heterogeneity of microbiota of very preterm infants that we and others described, and the low number of digestive events in this study may have limited the analysis power.
4.1. Strengths and limitations
This prospective study on preterm neonates born at less than 33 weeks of GA gave detailed information on their characteristics that may help to define population of further studies. Neonates with enteropathy leading to interruption of enteral feeding were fully described. The strength of our study also lies in the repeated measurements of the faecal calprotectin. However, in our cohort, the occurrence of enteropathy was lower than expected and reached only half of the one observed in our previous study used to support the hypothesis for sample size calculation, 9 thus precluding the power of the study to define a prognosis score.
5. CONCLUDING REMARKS
This study did not achieve its goal to propose a prognostic score of enteropathy mainly due to the large inter‐ and intra‐individual variability of faecal calprotectin levels that limit its use as a biomarker of enteropathy in very preterm infants. However, the interest of a prognostic score in this population at high risk of mild‐to‐severe enteropathy that will help to take appropriate management of the disease as early as possible deserves to design a larger multicentric study which would take into account the difficulties faced in this study.
CONFLICT OF INTEREST
None declared.
Supporting information
Fig S1
Fig S2
Table S1
ACKNOWLEDGEMENTS
The authors would like to thank the support of all the staff of the neonatal units participating in this project and the parents for their agreement in the study. They would also like to thank the staff Clinical Research Unit of Necker‐Enfants Malades for the monitoring of the clinical trial, Robert Campos Oriola from AUXESIA for the manuscript preparation, Julie Salomon, gastropaediatrician at Necker‐Enfants Malades hospital and Maria‐Elisabetta Baldassarre, neonatologist at Aldo Moro University, Bari, Italy, for their helpful advice. The sponsor was Assistance Publique—Hôpitaux de Paris (Clinical Research and Innovation Department).
Campeotto F, Elie C, Rousseau C, et al. Faecal calprotectin and gut microbiota do not predict enteropathy in very preterm infants. Acta Paediatr. 2021;110:109–116. 10.1111/apa.15354
Marie‐José Butel and Nathalie Kapel are contributed equally to this work.
Clinical trial registration number NCT02010268
Funding information
The study was funded by a grant from Programme Hospitalier de Recherche Clinique—PHRC 2012 (AOR12088, Ministry of Health).
Contributor Information
Marie‐José Butel, Email: florence.campeotto@aphp.fr.
Nathalie Kapel, Email: florence.campeotto@aphp.fr.
REFERENCES
- 1. Battersby C, Santhalingam T, Costeloe K, Modi N. Incidence of neonatal necrotising enterocolitis in high‐income countries: a systematic review. Arch Dis Child Fetal Neonatal Ed. 2018;103:F182‐F189. [DOI] [PubMed] [Google Scholar]
- 2. Kliegman RM, Walsh MC. Neonatal necrotizing enterocolitis: pathogenesis, classification, and spectrum of illness. Curr Probl Pediatr. 1987;17:219‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campeotto F, Kalach N, Lapillonne A, Butel MJ, Dupont C, Kapel N. Time course of faecal calprotectin in preterm newborns during the first month of life. Acta Paediatr. 2007;96:1531‐1533. [DOI] [PubMed] [Google Scholar]
- 4. Rich BS, Dolgin SE. Necrotizing Enterocolitis. Pediatr Rev. 2017;38:552‐559. [DOI] [PubMed] [Google Scholar]
- 5. Gephart SM, Gordon PV, Penn AH, et al. Changing the paradigm of defining, detecting, and diagnosing NEC: Perspectives on Bell's stages and biomarkers for NEC. Semin Pediatr Surg. 2018;27:3‐10. [DOI] [PubMed] [Google Scholar]
- 6. Fagerhol MK. Calprotectin, a faecal marker of organic gastrointestinal abnormality. Lancet. 2000;356:1783‐1784. [DOI] [PubMed] [Google Scholar]
- 7. van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: diagnostic meta‐analysis. BMJ. 2010;341:c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heida A, Van de Vijver E, van Ravenzwaaij D, et al. Predicting inflammatory bowel disease in children with abdominal pain and diarrhoea: calgranulin‐C versus calprotectin stool tests. Arch Dis Child. 2018;103:565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kapel N, Campeotto F, Kalach N, Baldassare M, Butel MJ, Dupont C. Faecal calprotectin in term and preterm neonates. J Pediatr Gastroenterol Nutr. 2010;51:542‐547. [DOI] [PubMed] [Google Scholar]
- 10. Pergialiotis V, Konstantopoulos P, Karampetsou N, et al. Calprotectin levels in necrotizing enterocolitis: a systematic review of the literature. Inflamm Res. 2016;65:847‐852. [DOI] [PubMed] [Google Scholar]
- 11. Campeotto F, Baldassarre M, Butel MJ, et al. Fecal calprotectin: cutoff values for identifying intestinal distress in preterm infants. J Pediatr Gastroenterol Nutr. 2009;48:507‐510. [DOI] [PubMed] [Google Scholar]
- 12. Bekkali N, Hamers SL, Reitsma JB, Van Toledo L, Benninga MA. Infant stool form scale: development and results. J Pediatr. 2009;154(521–6):e1. [DOI] [PubMed] [Google Scholar]
- 13. Ton H, Brandsnes DS, Holtlund J, et al. Improved assay for fecal calprotectin. Clin Chim Acta. 2000;292:41‐54. [DOI] [PubMed] [Google Scholar]
- 14. Rousseau C, Levenez F, Fouqueray C, Dore J, Collignon A, Lepage P Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J Clin Microbiol. 2011;49:858‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Escudie F, Auer L, Bernard M, et al. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics (Oxford, England). 2018;34:1287‐1294. [DOI] [PubMed] [Google Scholar]
- 16. Federhen S. The NCBI Taxonomy database. Nucleic Acids Res. 2012;40:D136‐D143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cole JR, Wang Q, Fish JA, et al. Ribosomal Database Project: data and tools for high throughput rRNA analysis. Nucleic Acids Res. 2014;42:D633‐D642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Q, Smith PB, Goldberg RN, Cotten CM. Dynamic change of fecal calprotectin in very low birth weight infants during the first month of life. Neonatology. 2008;94:267‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307‐310. [PubMed] [Google Scholar]
- 20. MacQueen BC, Christensen RD, Yost CC, et al. Reference intervals for stool calprotectin in preterm neonates and their utility for the diagnosis of necrotizing enterocolitis. J Perinatol. 2018;38:1379‐1385. [DOI] [PubMed] [Google Scholar]
- 21. Ho TTB, Groer MW, Kane B, et al. Enteric dysbiosis and fecal calprotectin expression in premature infants. Pediatr Res. 2019;85:361‐368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van Zoonen A, Hulzebos CV, Muller Kobold AC, Kooi EMW, Bos AF, Hulscher JBF. Serial fecal calprotectin in the prediction of necrotizing enterocolitis in preterm neonates. J Pediatr Surg. 2019;54:455‐459. [DOI] [PubMed] [Google Scholar]
- 23. Nakayuenyongsuk W, Christofferson M, Stevenson DK, Sylvester K, Lee HC, Park KT. Point‐of‐Care Fecal Calprotectin Monitoring in Preterm Infants at Risk for Necrotizing Enterocolitis. J Pediatr. 2018;196(98–103):e1. [DOI] [PubMed] [Google Scholar]
- 24. Yoon JM, Park JY, Ko KO, Lim JW, Cheon EJ, Kim HJ. Fecal calprotectin concentration in neonatal necrotizing enterocolitis. Korean J Pediatr. 2014;57:351‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Labaere D, Smismans A, Van Olmen A, et al. Comparison of six different calprotectin assays for the assessment of inflammatory bowel disease. United European Gastroenterol J. 2014;2:30‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roze JC, Ancel PY, Lepage P, et al. Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very‐preterm infants. Am J Clin Nutr. 2017;106:821‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Benahmed NA, Manene D, Barbot‐Trystram L, Kapel N. Evaluation of Calfast(R) immunochromatographic quantitative assay for the measurement of calprotectin in faeces. Clin Chem Lab Med. 2014;52:e143‐e145. [DOI] [PubMed] [Google Scholar]
- 28. Baldassarre ME, Di Mauro A, Capozza M, et al. Dysbiosis and prematurity: is there a role for probiotics? Nutrients. 2019;11(6):1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1
Fig S2
Table S1


