Abstract
Expanded hemodialysis (HDx) provides increased clearance of conventional and large middle molecules through innovative medium cutoff (MCO) membranes. However, there is a paucity of real‐world data regarding the benefits and safety of HDx. This large observational study evaluated outcomes among patients in Colombia undergoing HDx at a extended dialysis clinical services provider. This was a prospective single cohort study of prevalent patients who were treated with HDx; baseline information was collected from the most recent data before patients were started on HDx. Patients were followed prospectively for 1 year for changes in serum albumin and other laboratory parameters compared with the baseline. Survival, hospitalization and safety were assessed from the start of HDx. A total of 1000 patients were invited to enroll; 992 patients met the inclusion criteria for data analysis and 638 patients completed the year of follow‐up. Seventy‐four (8%) patients died during 866 patient‐years (PY) of follow‐up; the mortality rate was 8.54 deaths/100 PY (95% confidence interval [CI], 6.8‐10.7). There were 673 hospitalization events with a rate of 0.79 events/PY (95% CI, 0.73‐0.85) with 6.91 hospital days/PY (95% CI, 6.74‐7.09). The observed variability from baseline and maximum average change in mean serum albumin levels were −1.8% and −3.5%, respectively. No adverse events were related to the MCO membrane. HDx using an MCO membrane maintains stable serum albumin levels and is safe in terms of nonoccurrence of dialyzer related adverse events.
Keywords: expanded hemodialysis, hemodialysis, medium cutoff membranes, real world evidence, Colombia
1. INTRODUCTION
Since the valiant years in Seattle during the early 1960s, 1 the care and treatment of patients with end‐stage renal disease (ESRD) have undergone momentous changes. Advances in technology have improved safety, the monitoring profile of hemodialysis (HD) machines, and the capacity of dialyzers to remove uremic toxins. 2 , 3 Advancement in biomaterials ushered in the development of high‐flux membranes with improved clearance of molecules such as beta 2 microglobulin. 4 More recent advances have introduced expanded hemodialysis (HDx) utilizing medium cutoff (MCO) membranes with high retention onset membranes. Because of nanodesign, 4 MCO membranes easily clear conventional and large middle molecules 5 , 6 with clinically acceptable levels of albumin removal (2‐4 g/session), which maintains serum albumin levels within the normal range. 7
Although advanced age, smoking, dyslipidemia, hypertension, and diabetes mellitus contribute to cardiovascular disease (CVD) in patients with ESRD undergoing HD, the increased risk for CVD and mortality in these patients cannot be explained by just these factors. 8 Persistent inflammation, demonstrated by elevated levels of high sensitivity C‐reactive protein (hsCRP) and other inflammatory biomarkers, also contributes to the increased risk of CVD, protein‐energy wasting, and mortality in patients undergoing HD. 9 Middle molecules, which accumulate during HD, are considered to be inflammatory mediators. Inflammation also contributes to decreased serum albumin levels. 10 , 11 Importantly, a lower serum albumin level in incident HD patients is associated with increased mortality. 12 The higher mortality rate associated with low serum albumin has been reported to be dependent on inflammation as assessed by hsCRP levels. 13 Indeed, as a marker of nutritional and inflammatory status, the level of serum albumin is an important measurement in patients undergoing chronic HD. The removal of albumin by dialysis may vary depending on the type of membrane and operating conditions (eg, degree of flux across the membrane, use of predilution, etc.). Albumin loss as high as 5 g per dialysis session has been reported when high‐flux membranes are used in online hemodiafiltration. 5 , 12 , 13 , 14
Renal Therapy Services (RTS) is a large nationwide provider in Colombia that serves over 9000 patients who are undergoing HD or peritoneal dialysis. RTS dialysis units provide HD to over 5500 patients, which accounts for approximately 29% of the patients in Colombia receiving HD. The RTS dialysis model constitutes an integrated and evidence‐based “best‐practice” approach to the delivery of care.
There is a paucity of longitudinal data regarding the clinical outcomes and safety of MCO membranes, especially in the current practice setting. The objective of our study was to leverage the large RTS patient registry database to describe the outcomes and trends in serum albumin levels among a large cohort of patients switched from conventional high‐flux HD to HDx utilizing an MCO membrane and document the long‐term safety.
2. PATIENTS AND METHODS
2.1. Study design and patients
Expanded Hemodialysis Registry Protocol in Colombia (COREXH) is a prospective observational, multicenter cohort study of patients undergoing HDx in Colombia (ISRCTN45211359). Between 4 September 2017 to 30 November 2017, prevalent HD patients (ie, receiving HD therapy for at least 90 days at an RTS network renal clinic that meets water quality standards established by the Association for the Advancement of Medical Instrumentation) were invited to participate in the registry. Patients were required to be at least 18 years of age and receiving HDx for a minimum of 4 hours 3 times per week using an MCO membrane (Theranova, Baxter, Deerfield, Illinois). The prescription of HDx was a medical indication. Patients with a life expectancy of less than 6 months or those with an active infection diagnosed within the previous 4 weeks were not invited to participate. Baseline data were obtained of the last seven days before the switching to HDx and represent the initial state of the patient´s health, serum albumin levels, and other laboratory parameters. Patients were prospectively followed for 1 year from enrollment into the registry. Analyses were performed using both an intention to treat (ITT) and a per‐protocol (PP) population. The population for the ITT group was defined as all patients who received HDx with the MCO membrane for at least one week and with at least three months of follow‐up within the study. The PP population was defined as patients who received all treatments with the MCO membrane during the follow‐up period or until an outcome occurred. All patients provided written informed consent. Because this was an observational study with no planned intervention, there was no risk to the patients to participate. The study protocol was approved by the clinical research ethics committee of RTS (1 August 2017, Minute, item number 007).
2.2. Data source and analysis
2.2.1. Baseline patient characteristics
Baseline demographic and disease characteristic variables including age, sex, weight, height, body mass index (BMI), cause of chronic kidney disease, past medical history of hypertension, diabetes mellitus and acute cardiovascular events, ESRD comorbidity index, Karnofsky performance status score, history of protein energetic wasting, malnutrition inflammation score, and date of initiating chronic renal replacement therapy were collected from patients' electronic medical record (Figure 1). HD treatment parameters, including session duration, number of sessions per week, HD machine model, blood‐flow rate, dialysate flow rate, vascular access type, and baseline values of hemoglobin (ie, hematocrit, serum phosphorus levels, serum calcium levels, and single‐pool Kt/V) before the switch to the MCO membrane (baseline data captured correspond to the one week prior to switching to MCO membrane).
FIGURE 1.
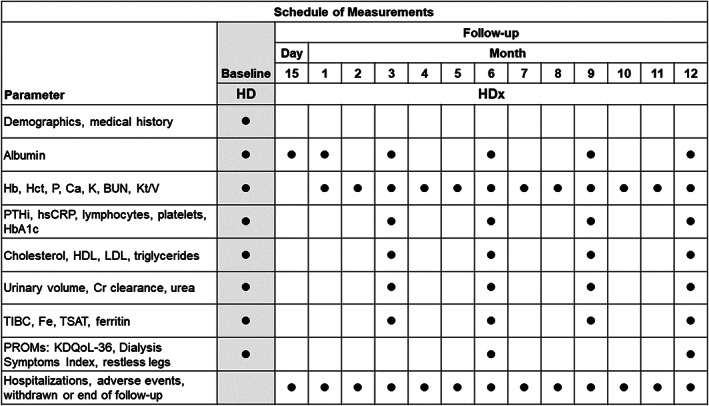
Schedule of assessments. BUN, blood urea nitrogen; Ca, calcium; Fe, iron; Hb, hemoglobin; Hct, hematocrit; HD, hemodialysis; HDL, high‐density lipoprotein; HDx, expanded hemodialysis therapy; K, potassium; KDQoL‐36; Kidney Disease Quality of Life‐36; hsCRP, high‐sensitivity C‐reactive protein; Kt/V, (dialyzer clearance of urea × dialysis time)/volume of distribution of urea; LDL, low‐density lipoprotein; P, phosphorus; PROMs, patient‐reported outcome measures; PTHi, parathyroid hormone, intact; TIBC, total iron binding capacity; TSAT, transferrin saturation
2.2.2. Prospective data collection
Primary outcome measures that were prospectively assessed included serum albumin levels, rate of hospitalization, number of hospital days, and mortality rate. The rate of nonfatal cardiovascular events was also prospectively analyzed. Hospitalization rate, number of hospital days, and mortality were estimated per patient year (PY). After patients started HDx, serum albumin levels were prospectively measured on day 15, and at 1, 3, 6, 9, and 12 months using a bromocresol green assay with reference value of 3.5 g/dL for lower limit of normal and 5.2 g/dL for upper limit of normal (Figure 1). Hemoglobin, hematocrit, and serum phosphorus levels; serum calcium levels; and volume of plasma cleared of urea (calculated by the single‐pool Kt/V ratio) were measured monthly. Serum hsCRP and parathyroid hormone levels were measured at 3, 6, 9, and 12 months. Other parameters not reported in this analysis, including hematology parameters, kidney function, iron status parameters, and patient‐reported outcome measures, were measured every month or every 3 months (Figure 1).
Adverse events (AEs) were monitored throughout the study. Serious AEs were defined as events that resulted in death; hospitalization; prolongation of a hospitalization; permanent or transient damage to an organ or system; abortion; or threatened the patient's life, in the opinion of the investigator. The occurrence of adverse events and causality were carefully evaluated by the local investigator, the medical monitor of the study, and a pharmacovigilance team, in accordance with the standard device vigilance practices established for this purpose. 15 , 16 Special surveillance was performed for events such as bacteremia of unclear origin, febrile syndromes, and type A, and type B reactions to dialysis membranes. The causality evaluation was categorized as not associated, improbably associated, possibly associated, probably associated, or impossible to determine. AEs and serious AEs were estimated per PY. Date of loss to follow‐up and the associated reasons, as well as outcome measures (including hospitalizations and death) were analyzed.
2.3. Statistical analysis
We used descriptive statistics to report the population characteristics; for continuous variables used mean and SD for normally distributed variables, and median and interquartile range (IQR) for non‐normal distributed variables. For categorical variables we used proportions reported as percentages. No data imputation procedure was performed. Only patients who had baseline and all six scheduled albumin measurements during HDx were included in the PP population serum albumin analysis.
One‐way repeated measures ANOVA using a “one‐within design” were performed to identify statistical differences for measurements repeated over time. P values were adjusted using the Box's conservative epsilon. Considering the large sample size of the study, we selected a conservative P value of .01 for hypotheses testing. We estimated the outcomes mortality rate, hospitalization rate, number of hospital days, and number of AEs per PY. STATA 14 (StataCorp LLC, College Station, Texas) and R (R Foundation for Statistical Computing, Vienna, Austria) were used in the statistical analyses.
Trial Registration: ISRCTN45211359 https://doi.org/10.1186/ISRCTN45211359.
3. RESULTS
3.1. Patients
A total of 1000 patients at 12 clinics across Colombia were invited to participate. A total of 992 patients met the participation criteria and were included in the ITT group (Figure 2). The majority (62%) of the patients were men and at enrollment the mean age was 60 years (Table 1). Over 90% of the patients had a history of hypertension and nearly 50% had a history of diabetes; 67% of the patients had chronic kidney disease (CKD) attributed to hypertension (28%) or diabetes (39%). Patients included in the analysis had been undergoing HD for a median of 3.86 years before switching to HDx. Overall, before HDx the cohort had good nutritional status; 93% of the patients had a low malnutrition inflammation score. A total of 638 patients were eligible for the 1‐year follow‐up assessment (Figure 2); the median (IQR) follow‐up was 1.0 (0.16) year.
FIGURE 2.
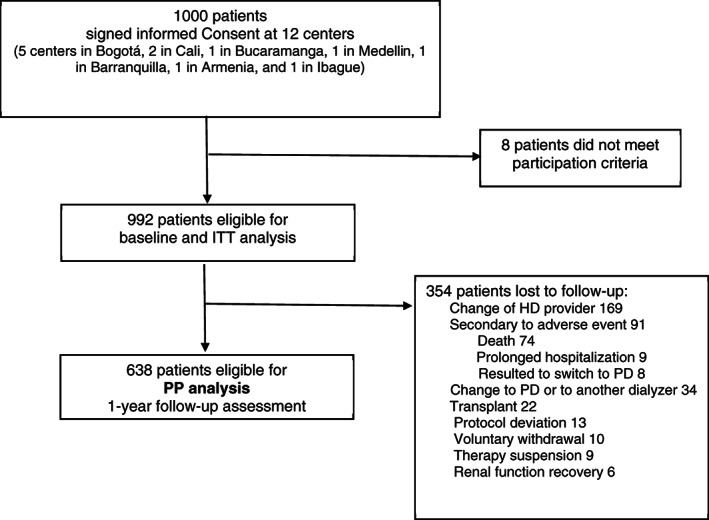
Patient Flow chart. HD, hemodialysis; ITT, intention to treat; PP, per‐protocol; PD, peritoneal dialysis
TABLE 1.
Baseline characteristics of the study population
| Baseline characteristic | Intention to treat, N = 992 |
|---|---|
| Age, mean (SD) (y) | 60 (15) |
| Sex (% [n]) | |
| Men | 62 (619) |
| Women | 38 (373) |
| BMI, mean (SD) (kg/m2) | 25.2 (4.61) |
| CKD cause (% [n]) | |
| Hypertension | 28 (276) |
| Diabetes | 39 (384) |
| Autoimmune | 8 (79) |
| Obstructive | 7 (66) |
| Unknown | 12 (121) |
| Other | 7 (66) |
| Medical history (% [n]) | |
| Hypertension | 92 (917) |
| Diabetes | 44 (436) |
| Cardiovascular disease | 37 (364) |
| Comorbidity index (% [n]) | |
| 0 to 3 | 81 (802) |
| 4 to 6 | 17 (170) |
| ≥7 | 2 (20) |
| Karnofsky PS score, median (IQR) | 80 (20) |
| Dialysis vintage, median (IQR) (y) | 3.86 (6.26) |
| PEW diagnosis (% [n]) | 17 (163) |
| Malnutrition inflammation score (% [n]) | |
| Normal | 2 (17) |
| Low | 93 (915) |
| Moderate | 5 (54) |
| Severe | 0.1 (1) |
| Vascular access (% [n]) | |
| AV fistula | 83 (824) |
| Catheter | 15 (144) |
| Graft | 2 (24) |
| No. of treatments/week (% [n]) | |
| 3 | 99 (984) |
| 4 | 0.7 (7) |
| 5 | 0.01 (1) |
| Duration of a dialysis session, median (IQR) (hr) | 4 (0) |
| Dialysate flow, median (IQR) (mL/min) | 500 (0) |
| Blood flow, median (IQR) (mL/min) | 350 (100) |
| Follow‐up time mean (SD) (months) | 10.62 (2.82) |
Abbreviations: BMI, body mass index; CKD, chronic kidney disease; IQR, interquartile range; PEW, protein‐energy wasting; PS, performance scale.
3.2. Albumin levels
The cumulative change in serum albumin levels in the ITT population during the follow‐up was −1.8% (Table 2). The lowest mean albumin level occurred in month 3 and represented a decrease of −3.5% with respect to baseline. The results of a one‐way repeated measures ANOVA showed a statistically significant differences in mean serum albumin concentration over the follow‐up (F [6, 4816] = 37.61, P < .0001). Although a decrease was noted, mean serum albumin levels remained within the normal range through 12 months.
TABLE 2.
Change of serum albumin levels over time (ITT population)
| Follow‐up | n | Marginal mean a (g/dL) | Change from baseline (%) | Change from previous b (%) | Cumulative change (%) |
|---|---|---|---|---|---|
| Baseline | 992 | 4.05 (4.04‐4.07) | — | — | — |
| 15 days | 938 | 3.98 (3.97‐4.00) | −1.7 | −1.7 | −1.7 |
| 1 month | 951 | 4.00 (3.98‐4.01) | −1.2 | 0.3 | −1.4 |
| 3 months | 883 | 3.91 (3.90‐3.93) | −3.5 | −2.0 | −3.5 |
| 6 months | 728 | 3.94 (3.92‐3.96) | −2.7 | 0.7 | −2.8 |
| 9 months | 735 | 3.94 (3.92‐3.96) | −2.7 | 0 | −2.8 |
| 12 months | 587 | 3.98 (3.96‐4.00) | −1.7 | 1.0 | −1.8 |
Abbreviation: ITT, intention to treat.
Marginal mean is the means estimation based on the fitted model in repeated measures and are presented as 95% confidence interval.
The percentual change from the last measurement value.
An analysis of the population that fulfilled all the measurements of serum albumin (PP approach) was also performed. A total of 468 patients in the PP population had all six scheduled serum albumin measurements taken during HDx. The changes in serum albumin levels among these patients were less pronounced. For the PP population, there was an accumulated change of −1.2% and a nadir compared with baseline of −2.7% (ANOVA, F [6, 2802] = 11.98, P = .0006) (Table 3).
TABLE 3.
Change of serum albumin levels over time (PP a )
| Follow‐up | n | Marginal mean b (g/dL) | Change from baseline (%) | Change from previous c (%) | Cumulative change (%) |
|---|---|---|---|---|---|
| Baseline | 468 | 4.03 (4.01‐4.05) | — | — | — |
| 15 days | 468 | 4.00 (3.98‐4.02) | −0.9 | −0.9 | −0.9 |
| 1 month | 468 | 3.98 (3.96‐4.00) | −1.3 | −0.4 | −1.3 |
| 3 months | 468 | 3.93 (3.91‐3.95) | −2.7 | −1.4 | −2.7 |
| 6 months | 468 | 3.95 (3.93‐3.97) | −2.0 | 0.7 | −2.0 |
| 9 months | 468 | 3.96 (3.94‐3.98) | −1.9 | 0.0 | −2.0 |
| 12 months | 468 | 3.99 (3.97‐4.01) | −1.2 | 0.8 | −1.2 |
Abbreviation: PP, per‐protocol defined as patients who received all treatments with the MCO membrane during the follow‐up period or until hospitalization that involved >12 dialysis sessions without MCO or death.
Only patients in the PP population who had baseline and all six scheduled serum albumin measurements during HDx were included in the analysis.
Marginal mean is the means estimation based on the fitted model in repeated measures and are presented as 95% confidence interval.
The percentual change from the last measurement value.
3.3. Outcomes
Seventy‐four (8%) of the 992 patients died during 866 PY of follow‐up; the mortality rate was 8.54 deaths per 100 PY (95% confidence interval [CI], 6.8‐10.7). Cardiovascular and cerebrovascular events were identified as the cause of death for 34 patients with a rate of 3.92 cardio‐cerebrovascular deaths per 100 PY (95% CI, 2.71‐5.48). Other common causes of death included infection (15 patients) and respiratory complications (six patients). There were 673 hospitalizations with a rate of 0.79 events per PY (95% CI, 0.73‐0.85) with 6.91 hospital days per PY (95% CI, 6.74‐7.09). There were 229 cardio‐cerebrovascular causes of admission, which represent a rate of 0.26 hospitalizations per PY (95% CI, 0.23‐0.30) with 2.32 hospital days per PY (95% CI, 2.22‐2.42) during HDx. There were 175 nonfatal cardiovascular events with a rate of 20.20 per 100 patient years (95% CI, 17.32‐23.43).
3.4. Dialysis parameters
During follow‐up, there were statistically significant fluctuations in calcium; parathyroid hormone, intact (PTHi); and spKt/V (Table 4). At month 12, there was a slight decrease in serum calcium levels and a decrease in PTHi. The greatest improvement in spKt/V compared with baseline occurred at month 1 with improvement in spKt/V sustained through month 12. There were no significant differences across time for hemoglobin, phosphorus, or hsCRP (P > .01).
TABLE 4.
Other laboratory and dialysis parameters during 1 year of follow‐up (ITT population)
| Marginal mean (n = 992) | ||||||
|---|---|---|---|---|---|---|
| Follow‐up | Hemoglobin (g/dL) | Phosphorous (mg/dL) | Calcium (mg/dL) | PTHi (pg/mL) | hsCRP (mg/L) | spKt/V |
| Baseline | 11.92 | 4.61 | 8.93 | 499.82 | 1.02 | 1.62 |
| 1 months | 11.85 | 4.54 | 8.85 | — | — | 1.67 |
| 2 months | 11.92 | 4.59 | 8.75 | — | — | 1.70 |
| 3 months | 11.83 | 4.58 | 8.75 | 533.75 | 1.37 | 1.69 |
| 4 months | 11.83 | 4.57 | 8.73 | — | — | 1.68 |
| 5 months | 11.89 | 4.53 | 8.79 | — | — | 1.67 |
| 6 months | 11.82 | 4.58 | 8.77 | 503.03 | 1.69 | 1.68 |
| 7 months | 11.75 | 4.63 | 8.73 | — | — | 1.68 |
| 8 months | 11.63 | 4.56 | 8.72 | — | — | 1.68 |
| 9 months | 11.68 | 4.61 | 8.74 | 480.82 | 1.37 | 1.68 |
| 10 months | 11.82 | 4.54 | 8.75 | — | — | 1.68 |
| 11 months | 11.84 | 4.61 | 8.83 | — | — | 1.69 |
| 12 months | 11.82 | 4.55 | 8.81 | 457.88 | 1.83 | 1.70 |
| Repeated measures ANOVA P value a | .033 | .29 | .001 | .001 | 0.015 | .001 |
Abbreviations: ITT, intention to treat; PTHi, parathyroid hormone, intact; spKt/V, single‐pool clearance of urea × dialysis time/volume of distribution of urea.
For hypothesis testing, type I error significance was set at P = .01.
3.5. Safety
During the follow‐up period, there were 1019 adverse events during 866 person‐years of follow‐up for a rate of 1.18 adverse events per PY (95% CI, 1.10‐1.25). The number of sessions performed with MCO membranes were 130 601. Six hundred and seventy‐seven (66.4%) AEs were serious, and of these, 91 (8.9%) resulted in withdrawal from the study. No AEs during HDx were deemed related to MCO membrane use, according to the investigator and techno‐surveillance evaluation. One hundred forty‐six AEs were deemed related to the dialytic procedure, which represents 0.17 events per PY (95% CI, 0.14‐0.20), equivalent to 1.12 events per 1000 HD sessions (95% CI, 0.90‐1.30). Details of the AEs and the causality relationship with the hemodialysis procedure are presented (Table 5).
TABLE 5.
Adverse events related to the hemodialysis procedure (causality assessment)
| Diagnostic group | AEs causality assessment | |||||
|---|---|---|---|---|---|---|
| Probably associated | Possibly associated | Unable to determine | Unlikely associated | Not associated | Total | |
| n (%) | n (%) | n (%) | n (%) | n (%) | n | |
| Drug hypersensitivity | 0 | 0 | 0 | 0 | 1 (100) | 1 |
| Pregnancy | 0 | 0 | 0 | 0 | 1 (100) | 1 |
| Malnutrition | 0 | 0 | 0 | 0 | 2 (100) | 2 |
| Pruritus | 0 | 0 | 0 | 0 | 5 (100) | 5 |
| Falls | 2 (20.0) | 0 | 0 | 0 | 8 (80.0) | 10 |
| Anemia | 0 | 0 | 0 | 0 | 12 (100) | 12 |
| Hypotension | 3 (20.0) | 4 (26.7) | 0 | 0 | 8 (53.3) | 15 |
| Fractures | 0 | 0 | 0 | 0 | 23 (100) | 23 |
| Clotted dialysis circuit | 30 (100) | 0 | 0 | 0 | 0 | 30 |
| Metabolic | 1 (3.3) | 0 | 4 (13.3) | 0 | 25 (83.4) | 30 |
| High blood pressure | 4 (12.1) | 1 (3.0) | 2 (6.1) | 0 | 26 (78.8) | 33 |
| Urinary tract infection | 0 | 0 | 0 | 0 | 37 (100) | 37 |
| Cerebrovascular disorders | 0 | 0 | 1 (2.4) | 1 (2.4) | 39 (95.2) | 41 |
| Respiratory disorders | 1 (2.0) | 0 | 0 | 0 | 49 (98.0) | 50 |
| Skin infections | 0 | 1 (1.8) | 0 | 0 | 54 (98.2) | 55 |
| Ischemic cardiovascular | 0 | 3 (5.0) | 0 | 0 | 57 (95.0) | 60 |
| Other infections | 0 | 0 | 1 (1.5) | 0 | 65 (98.5) | 66 |
| Infection of vascular access | 28 (42.4) | 7 (10.6) | 0 | 0 | 31 (47.0) | 66 |
| Cardiovascular nonischemic | 2 (2.7) | 7 (9.3) | 0 | 0 | 66 (88.0) | 75 |
| Gastrointestinal disorders | 0 | 0 | 1 (0.9) | 0 | 109 (99.1) | 110 |
| HD vascular access noninfectious | 27 (24.3) | 14 (12.6) | 1 (0.9) | 0 | 69 (62.2) | 111 |
| Other | 5 (2.7) | 6 (3.2) | 1 (0.5) | 0 | 174 (93.6) | 186 |
| Total n (%) | 103 (10.1) | 43 (4.2) | 11 (1.1) | 1 (0.1) | 861 (84.5) | 1019 |
4. DISCUSSION AND CONCLUSIONS
Given the strict inclusion and exclusion criteria, patient cohorts in most clinical trials do not fully represent the real‐world population of patients that may receive the treatment being investigated. Patients in current practice typically have more comorbidities and are less adherent to treatment than patients enrolled in a trial, and therefore may have poorer outcomes. 17 Further, treatment benefit in subgroups of patients, particularly in poorly resourced environments, are not well represented in clinical trials. 18 Therefore, it is important to collect evidence on the safety and effectiveness of new interventions. Existing data on the long‐term safety of the MCO membrane in the current practice setting is limited to a retrospective analysis of 10 patients after they switched from online hemodiafiltration to the use of an MCO membrane. 19 Additionally, an observational study in Korea compared 38 patients using an MCO membrane to 19 patients undergoing high‐flux, online HD. 20
We must highlight that most of the current evidence is about the efficacy of MCO membranes in the clearance of large middle molecules. 5 , 6 , 14 , 21
Our study, which to our knowledge is the largest analysis of an MCO membrane utilization to date (N = 992 with approximately 130 000 dialysis sessions), describes that HDx is a safe therapy in an inclusive patient population in Colombia. It is worth highlighting the finding that after a carefully techno‐surveillance process, no adverse events (serious or nonserious) related to the use of the dialyzer were observed, as has already been evidenced in other reports. 5 Of interest is the absence of type A dialyzer reactions (which begin almost immediately and occur within the first few minutes of dialysis) and type B reactions (which generally start within the first 15‐30 minutes). The absence of dialyzer reactions could be a marker of biocompatibility of the MCO membrane and indirect evidence of no translocation of bacterial degradation products through the membrane, as has already been seen in experimental designs. 22
The MCO is close to the albumin molecular weight 23 and, in comparison to conventional HD, increased albumin loss has been described in single sessions. 5 In this regard, the measurement of albumin level over time becomes valuable in a current practice context. We observed a slight decrease in albumin over 12 months of observation. Despite being statistically significant in our large cohort study, we believe this shall be considered as clinically insignificant. The largest change from basal pre‐HDx levels was 3.5%, and this nadir occurred at month 3. Thereafter albumin level tended to recover and the average cumulative change in serum albumin after 12 months in the ITT population was only −1.8%. At all times the observed variability was within 5% from baseline and the mean serum albumin concentration remained within the normal range (3.5‐5.5 g/dL). This statement considers extensive population studies, where the baseline level of risk for serum albumin is 3.9 g/dL, which coincides with the nadir of decreased albumin in our study. 12
The observed mortality rate, hospitalization rate, and number of hospital days in our analysis is somewhat lower than other reports 24 , 25 and also lower than our previous experiences from the RTS network in Colombia, where we have reported mortality rate of 14.6 events per 100 PY and hospitalization rates of 1.15 hospitalizations per PY. 26 This could be due to a number of predictive factors like population characteristics, the RTS model of care and the impact of the new dialyzer, among others. We must note that due to the study design of a single cohort without a comparison group, these observed outcomes cannot be causally attributed to the implementation of the MCO membrane, but only generate a hypothesis that must be contrasted with other types of designs.
The definitive goals in treating patients with ESRD is to prolong survival and improve quality of life, and although other aspects of patient care are important, adequacy of dialysis is crucial to achieving these goals. HDx efficacy, as measured by single‐pool Kt/V and serum phosphorus, was highlighted throughout follow‐up. Single‐pool Kt/V was 1.68, which is a very good level of adequacy for small‐molecule reduction and is well above the minimum 1.2 per HD session for patients treated thrice weekly, as is recommended by the National Kidney Foundations' Kidney Disease Outcomes Quality Initiative. 27 Other countries and regions have similar guidelines. 28 , 29 Interestingly, serum phosphorus levels remained relatively constant throughout the 12 months, with a mean of 4.55 mg/dL at month 12, which is below the recommended level of 5.5 mg/dL. 30
The link between inflammation and cardiovascular mortality in patients undergoing HD is well known 31 , 32 A chronic micro‐inflammatory state is common among patients undergoing HD; elevated levels of proinflammatory cytokines, an independent risk factor for CVD, is found in 30% to 60% of patients undergoing HD in North America and Europe. 33 There is an association between levels of large middle‐molecule uremic toxins and inflammation and immune dysfunction. 5 Dialysis membranes with larger pores providing more effective removal of large middle molecules are likely to have a positive impact on a patient's inflammatory state. The use of the MCO membrane reduces inflammation in patients undergoing long‐term HD to a greater extent than does high‐flux HD, 34 and significantly improves endothelial function compared with high‐flux HD. 35 These physiological mechanisms could explain in part the low rate of total and cardiovascular mortality observed in this HDx registry. Evidently, given the study design, we cannot definitively conclude a causal relationship between HDx and improvements in dialysis outcomes. Future studies are needed to evaluate these hypotheses.
Abe et al. 36 reported that the use of poorly biocompatible membranes, lower serum albumin levels, and higher hsCRP levels are all associated with higher mortality in patients undergoing HD. We observed a slight increase in hsCRP over the observation period, which could be related to various factors for this population having a high proportion of native arteriovenous fistula. The apparent increase in hsCRP could reflect a tendency of dialysis patients to increase their inflammation level over time. However, the hsCRP level was low also at end of the observation period compared to other reports. 37 In parallel, a slight but statistically significant decrease in PTH was observed at 12 months, which could be related to better control of phosphorus levels and better clearance of large uremic toxins, as has been proposed in the literature. 38 Further, there were no febrile episodes, which suggests that translocation of bacterial degradation products across the MCO membrane does not occur, as already described in the literature. 22 A serum albumin level maintained within the normal range, a relatively low hsCRP level, absence of dialyzer reactions and febrile episodes, all constitute a positive and effective dialysis picture.
Strengths of the present study include the prospective collection of current practice data, an analysis of nearly 1000 patients undergoing HDx, with baseline information and a follow‐up for 12 months. Therefore, this analysis reports on important practical experience on the effectiveness of the new MCO membrane. Limitations of the study include the absence of a comparison group, which diminishes the strength of adjudging causality to the observed effects. A positive selection bias cannot be excluded, although given the large number of patients and renal clinics involved, it does not appear that the population in this analysis differs much from the general prevalent HD population in the RTS network in Colombia. However, translating our findings to other dialysis populations and other practices should be done with caution.
In conclusion, based on this current practice large cohort study, the use of the MCO membrane is safe and preserves serum albumin levels within the normal range among patients undergoing expanded hemodialysis.
CONFLICT OF INTEREST
Mr. Bunch and Mr. Sanabria are full‐time employees of Renal Therapy Services‐Latin America, Bogotá, Colombia. Mr. Sanchez has received honorarium for consultancy from Renal Therapy Services‐Colombia. Mr. Nilsson, Dr. Bernardo, and Dr. Rivera are full‐time employees of Baxter Healthcare Corporation, Chicago, Illinois, USA. Ms. Vesga and Dr. Ardila are full‐time employees of Renal Therapy Services‐Colombia, Bogotá, Colombia. Dr. Guerrero is a full‐time employee of Renal Therapy Services‐Barranquilla, Barranquilla, Colombia.
AUTHOR CONTRIBUTIONS
Mr. Alfonso Bunch, Dr. Fredy Ardila, and Dr. Ivan M. Guerrero: Original research project conception and design, data acquisition, and data interpretation. Mr. Ricardo Sanchez: Original research project conception and design, statistical analysis, and data interpretation. Mr. Lars‐Göran Nilsson, Dr. Angelito A. Bernardo, and Dr. Angela S. Rivera: Original research project conception and design and data interpretation. Ms. Jasmin I. Vesga and Mr. Rafael M. Sanabria: Original research project conception and design, data acquisition, statistical analysis, and data interpretation. All authors have been involved in the drafting of the manuscript or revising it critically for important intellectual content and provided final approval of the version to be published. All authors verify that they have met all the journal's requirements for authorship. All authors agree to be accountable for all aspects of the work, ensuring the accuracy and integrity of the publication. All authors approved the final manuscript draft submitted for publication. The authors received no financial compensation for the development of this manuscript.
ACKNOWLEDGMENTS
The authors thank all the investigators who participated in the Colombian Registry of Expanded Hemodialysis study: Alexander Arias, MD (Medellín), Adolfo E. Capella, MD (Armenia), Carlos Y. Coronado, MD (Ibagué), Leyder Corzo, MD (Bogota), Maria P. Dazzarola, MD (Cali), Daniel A. Ducuara, MD (Bogota), Rafael A. Gómez, MD (Cali), Mario D. Munevar, MD (Barranquilla), Edward A. Martinez, MD (Bucaramanga), Anthony E. Martinez, MD (Bogota), Alejandra P. Molano, MD (Bogota), Sylvia C. Quiñonez, MD (Bogota), and Eduardo A. Zuñiga, MD (Bogota). All investigators are employees of RTS Ltd Colombia, an affiliate of Baxter Healthcare. The authors wish to express their gratitude to all the patients and nursing teams who participated in the study. We also thank Lamara D. Shrode, PhD, CMPP, who, on behalf of Baxter Healthcare Corporation, provided editorial support and assisted in implementing author revisions. All the activities of this study were conducted by Renal Therapy Services‐Colombia, an independent entity owned by Baxter International, Inc. Funding for this study was provided by Baxter Healthcare Corporation, Deerfield, Illinois.
Bunch A, Sanchez R, Nilsson L‐G, et al. Medium cut‐off dialyzers in a large population of hemodialysis patients in Colombia: COREXH registry. Ther Apher Dial. 2021;25:33–43. 10.1111/1744-9987.13506
Funding information Baxter Health Corporation; Baxter Healthcare Corporation
DATA AVAILABILITY STATEMENT
A secure database that meets the requirements of confidentiality that safeguards patient privacy is maintained as part of the study protocol (ISRCTN45211359). To ensure patient privacy is maintained, the data will not be made available. The protocol is available at http://www.isrctn.com/ISRCTN45211359.
REFERENCES
- 1. Blagg CR. The early history of dialysis for chronic renal failure in the United States. A view from Seattle. Am J Kidney Dis. 2007;49:482–496. [DOI] [PubMed] [Google Scholar]
- 2. Vanholder R, Van Laecke S, Glorieux G. What is new in uremic toxicity? Pediatr Nephrol. 2008;23:1211–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ward RA, Ronco C. Improvements in technology: A path to safer and more effective hemodialysis. Blood Purif. 2009;27:6–10. [DOI] [PubMed] [Google Scholar]
- 4. Ronco C. The rise of expanded hemodialysis. Blood Purif. 2017;44:I–VIII. [DOI] [PubMed] [Google Scholar]
- 5. Kirsch AH, Lyko R, Nilsson LG, et al. Performance of hemodialysis with novel medium cut‐off dialyzers. Nephrol Dial Transplant. 2017;32(1):165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirsch AH, Rosenkranz AR, Lyko R, Krieter DH. Effects of hemodialysis therapy using dialyzers with medium cut‐off membranes on middle molecules. Contrib Nephrol. 2017;191:158–167. [DOI] [PubMed] [Google Scholar]
- 7. Ronco C, Marchionna N, Brendolan A, Neri M, Lorenzin A, Martinez Rueda AJ. Expanded haemodialysis. From operational mechanism to clinical results. Nephrol Dial Transplant. 2018;33:iii41–iii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stenvinkel P, Carrero JJ, Axelsson J, Lindholm B, Heimburger O, Massy Z. Emerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: How do new pieces fit into the uremic puzzle? Clin J Am Soc Nephrol. 2008;3:505–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dai L, Golembiewska E, Lindholm B, Stenvinkel P. End‐stage renal disease, inflammation and cardiovascular outcomes. Contrib Nephrol. 2017;191:32–43. [DOI] [PubMed] [Google Scholar]
- 10. Carrero JJ, Stenvinkel P. Inflammation in end‐stage renal disease—What have we learned in 10 years? Semin Dial. 2010;23:498–509. [DOI] [PubMed] [Google Scholar]
- 11. Kaysen GA, Chertow GM, Adhikarla R, Young B, Ronco C, Levin NW. Inflammation and dietary protein intake exert competing effects on serum albumin and creatinine in hemodialysis patients. Kidney Int. 2001;60:333–340. [DOI] [PubMed] [Google Scholar]
- 12. Kaysen GA, Johansen KL, Cheng SC, Jin C, Chertow GM. Trends and outcomes associated with serum albumin concentration among incident dialysis patients in the United States. J Ren Nutr. 2008;18:323–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alves FC, Sun J, Qureshi AR, et al. The higher mortality associated with low serum albumin is dependent on systemic inflammation in end‐stage kidney disease. PLoS One. 2018;13:e0190410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maduell F, Rodas L, Broseta JJ, et al. Medium cut‐off dialyzer versus eight hemodiafiltration dialyzers: Comparison using a global removal score. Blood Purif. 2019;48(2):167–174. [DOI] [PubMed] [Google Scholar]
- 15. US Food and Drug Administration . Safety reporting requirements for INDs and BA/BE studies. Guidance for industry and investigators [cited 2020 Apr 4]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents.
- 16.European Medicines Agency and Heads of Medicines Agencies Module IV ‐ Management and reporting of adverse reactions to medicinal products. In: Guidelines on good pharmacovigilance practices (GVP). [cited 2020 Apr 4]. https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/good-pharmacovigilance-practices.
- 17. Sherman RE, Anderson SA, Dal Pan GJ, et al. Real‐world evidence ‐ What is it and what can it tell us? N Engl J Med. 2016;375:2293–2297. [DOI] [PubMed] [Google Scholar]
- 18. Hoque DM, Kumari V, Ruseckaite R, Romero L, Evans SM. Impact of clinical registries on quality of patient care and health outcomes: Protocol for a systematic review. BMJ Open. 2016;6:e010654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belmouaz M, Diolez J, Bauwens M, et al. Comparison of hemodialysis with medium cut‐off dialyzer and on‐line hemodiafiltration on the removal of small and middle‐sized molecules. Clin Nephrol. 2018;89:50–56. [PubMed] [Google Scholar]
- 20. Cho NJ, Park S, Islam MI, Song HY, Lee EY, Gil HW. Long‐term effect of medium cut‐off dialyzer on middle uremic toxins and cell‐free hemoglobin. PLoS One. 2019;14:e0220448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Belmouaz M, Bawens M, Hauet T, et al. Comparison of the removal of uraemic toxins with medium cut‐off and high‐flux dialysers: a randomized clinical trial. Nephrol Dial Transplant. 2020;35(2):328–335. [DOI] [PubMed] [Google Scholar]
- 22. Schepers E, Glorieux G, Eloot S, et al. Assessment of the association between increasing membrane pore size and endotoxin permeability using a novel experimental dialysis simulation set‐up. BMC Nephrol. 2018;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boschetti‐de‐Fierro A, Voigt M, Storr M, Krause B. MCO membranes: Enhanced selectivity in high‐flux class. Sci Rep. 2015;16(5):18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. United States Renal Data System . 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2018. [Google Scholar]
- 25. Molnar AO, Moist L, Klarenbach S, et al. Hospitalizations in dialysis patients in Canada: A national cohort study. Can J Kidney Health Dis. 2018;5:2054358118780372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bunch A, Tamer L, Ardila F, et al. Impact of a disease management model on a population undergoing dialysis in Colombia. Rev Fac Med. 2016;64(4):695–700. [Google Scholar]
- 27. National Kidney Foundation . KDOQI Clinical Practice Guideline for Hemodialysis Adequacy: 2015 update. Am J Kidney Dis. 2015;66:884–930. [DOI] [PubMed] [Google Scholar]
- 28. Kerr PG, Toussaint ND, Kidney Health A , Caring for Australasians with Renal I . KHA‐CARI guideline: Dialysis adequacy (haemodialysis): Dialysis membranes. Nephrology (Carlton). 2013;18:485–488. [DOI] [PubMed] [Google Scholar]
- 29. Mactier R, Hoenich N, Breen C. Renal association clinical practice guideline on haemodialysis. Nephron Clin Pract. 2011;118(Suppl.1):c241–c286. [DOI] [PubMed] [Google Scholar]
- 30. Uhlig K, Berns JS, Kestenbaum B, et al. KDOQI US commentary on the 2009 KDIGO clinical practice guideline for the diagnosis, evaluation, and treatment of CKD‐mineral and bone disorder (CKD‐MBD). Am J Kidney Dis. 2010;55(5):773–799. [DOI] [PubMed] [Google Scholar]
- 31. Stenvinkel P, Gillespie IA, Tunks J, et al. Inflammation modifies the paradoxical association between body mass index and mortality in hemodialysis patients. J Am Soc Nephrol. 2016;27:1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vanholder R, Massy Z, Argiles A, et al. Chronic kidney disease as cause of cardiovascular morbidity and mortality. Nephrol Dial Transplant. 2005;20:1048–1056. [DOI] [PubMed] [Google Scholar]
- 33. Kalantar‐Zadeh K, Ikizler TA, Block G, Avram MM, Kopple JD. Malnutrition‐inflammation complex syndrome in dialysis patients: Causes and consequences. Am J Kidney Dis. 2003;42:864–881. [DOI] [PubMed] [Google Scholar]
- 34. Zickler D, Schindler R, Willy K, et al. Medium cut‐off (MCO) membranes reduce inflammation in chronic dialysis patients‐a randomized controlled clinical trial. PLoS One. 2017;12:e0169024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitra S, Kharbanda K. Effects of expanded hemodialysis therapy on clinical outcomes. Contrib Nephrol. 2017;191:188–199. [DOI] [PubMed] [Google Scholar]
- 36. Abe M, Hamano T, Wada A, Nakai S, Masakane I. High‐performance membrane dialyzers and mortality in hemodialysis patients: A 2‐year cohort study from the annual survey of the Japanese Renal Data Registry. Am J Nephrol. 2017;46:82–92. [DOI] [PubMed] [Google Scholar]
- 37. Bazeley J, Bieber B, Li Y, et al. C‐reactive protein and prediction of 1‐year mortality in prevalent hemodialysis patients. Clin J Am Soc Nephrol. 2011;6(10):2452–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Florens N, Juillard L. Expanded haemodialysis: News from the field. Nephrol Dial Transplant. 2018;33(suppl. 3):iii48–iii52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
A secure database that meets the requirements of confidentiality that safeguards patient privacy is maintained as part of the study protocol (ISRCTN45211359). To ensure patient privacy is maintained, the data will not be made available. The protocol is available at http://www.isrctn.com/ISRCTN45211359.


