Abstract
Objectives
Emergency medicine in low‐ and middle‐income countries (LMICs) is hindered by lack of research into patient outcomes. Chief complaints (CCs) are fundamental to emergency care but have only recently been uniquely codified for an LMIC setting in Uganda. It is not known whether CCs independently predict emergency unit patient outcomes.
Methods
Patient data collected in a Ugandan emergency unit between 2009 and 2018 were randomized into validation and derivation data sets. A recursive partitioning algorithm stratified CCs by 3‐day mortality risk in each group. The process was repeated in 10,000 bootstrap samples to create an averaged risk ranking. Based on this ranking, CCs were categorized as “high‐risk” (>2× baseline mortality), “medium‐risk” (between 2 and 0.5× baseline mortality), and “low‐risk” (<0.5× baseline mortality). Risk categories were then included in a logistic regression model to determine if CCs independently predicted 3‐day mortality.
Results
Overall, the derivation data set included 21,953 individuals with 7,313 in the validation data set. In total, 43 complaints were categorized, and 12 CCs were identified as high‐risk. When controlled for triage data including age, sex, HIV status, vital signs, level of consciousness, and number of complaints, high‐risk CCs significantly increased 3‐day mortality odds ratio (OR = 2.39, 95% confidence interval [CI] = 1.95 to 2.93, p < 0.001) while low‐risk CCs significantly decreased 3‐day mortality odds (OR = 0.16, 95% CI = 0.09 to 0.29, p < 0.001).
Conclusions
High‐risk CCs were identified and found to predict increased 3‐day mortality independent of vital signs and other data available at triage. This list can be used to expand local triage systems and inform emergency training programs. The methodology can be reproduced in other LMIC settings to reflect their local disease patterns.
Emergency medicine continues to be underdeveloped and heterogeneous in low‐ and middle‐income countries (LMICs). 1 With recent estimates suggesting that high‐quality emergency care could address more than 50% of annual deaths in LMICs, the World Health Organization, World Bank, and African Federation for Emergency Medicine have called for a renewed focus on research and investment into emergency care. 2 , 3 , 4 However, local needs remain poorly characterized due to insufficient data in several areas including prehospital care, emergency unit triage, and patient outcomes. 5 , 6
The Working Group on Emergency Care Data Management at the 2013 Academic Emergency Medicine consensus conference generated a list of research priorities for global emergency medicine and emphasized the capture and analysis of chief complaint (CC) data. 7 The CC, or reason for visit, is the first piece of information collected from a patient and can be reliably captured in nearly all emergency unit environments. 8 Since definitive diagnoses are often unavailable to LMIC emergency care providers, the CC also serves as a syndromic label to guide triage, diagnostic, and therapeutic decisions. 7 , 9 Although few LMICs have the means to map CC data, a comprehensive understanding of CCs is postulated to enhance provider training and help optimize the allocation of scant financial, diagnostic, and human resources. 7
To date, few studies have examined emergency unit CCs in LMICs. These studies have expanded the understanding of acute care presentations, but have been limited by use of preexisting or nonlocal lexicons, a focus on nonemergent cases, small patient populations, specific subsets of patients (i.e., trauma), and a lack of outcomes data. 10 , 11 , 12 To the authors’ knowledge, a 2018 project within a Ugandan emergency unit was the first and only study to derive a unique CC ontology using locally captured data and a local lexicon. 13 This research reflects a larger trend in medicine, with increasing importance being placed on the role of ontologies to more precisely classify patients and support clinical reasoning. 14
Training programs in high‐income countries (HICs) often focus on high‐risk CCs, but definitions of riskiness tend to combine complex notions of patient safety, medical–legal landscapes, and challenging diagnoses. 15 Only a handful of studies have examined patient‐oriented outcomes, such as mortality, to define high‐risk CCs in HICs. 16 , 17 , 18 To date, to our knowledge, no identification of high‐risk CCs using patient‐oriented outcomes has been published from any LMIC. This study represents the first published work to attempt to identify high‐risk CCs that independently predict mortality in any LMIC using a Ugandan context‐specific ontology derived by Rice et al. in 2018. 13 The following analysis seeks to test the hypothesis that there are high‐risk CCs that predict increased mortality independent of other predictors available at triage. The ultimate goal of this list is to enrich local triage, rationalize resource utilization, and improve provider training by focusing on locally relevant, context‐specific patterns of disease and mortality.
METHODS
Global Emergency Care (GEC) is a U.S.‐ and‐Uganda‐based nongovernmental organization dedicated to providing emergency care training in Uganda. In collaboration with Karoli Lwanga Hospital, GEC developed a 2‐year training program through which nurses earn an emergency care practitioner (ECP) certification and independently provide emergency care in a dedicated emergency unit at the Hospital. The program has since been adopted by Mbarara University of Science and Technology (MUST), and MUST and GEC are working collaboratively with multiple Ugandan stakeholders to expand the training capacity. Karoli Lwanga Hospital is located in the rural Rukungiri District of southwest Uganda, has a 6‐bed emergency unit that is staffed by ECPs from 8am until midnight, and cares for patients with medical and surgical emergencies (maternal emergencies are triaged to a separate labor and delivery ward). The annual census of the emergency unit has been relatively stable since 2009, seeing approximately 3,000–3,500 adults and 1,500 children less than 18 years old every year. Karoli Lwanga Hospital is not a referral hospital so very few patients present with diagnoses from other facilities. Emergency unit care is provided by ECPs trained in medical management, resuscitation, trauma, and minor surgical and orthopedic procedures and who have access to a limited number of blood tests, intravenous and oral medications, and extremely limited and inconsistent medical imaging. They admit to a hospital with separate medical and surgical wards managed by nurses and physicians. Further details about the setting, resource availability, and outcomes of the training program are comprehensively described elsewhere. 19 , 20 , 21
Global Emergency Care has maintained a prospectively collected quality assurance database of all emergency unit visits since 2009. The database captures data including demographics, CCs, vital signs, laboratory and radiology results, treatments given, procedures, diagnoses, disposition, and patient outcomes. Data were collected by trained research assistants at the time patients presented for care. Follow‐up data were collected in person for admitted patients and by structured telephone interview within 3 days for patients who were discharged from the emergency unit. If a patient could not be reached on the initial attempt, calls were made daily until 10 days had elapsed since the initial visit. CCs were entered as unstructured free text with the ability to enter multiple CCs for each patient visit.
The study population included all adults 18 years of age and older who presented to the Karoli Lwanga Hospital emergency unit between November 2009 and December 2018. All patients who were “dead on arrival” were excluded. This exclusion applied only to patients who arrived at triage clearly dead; with no vital signs; who received no treatments; and who, by definition, were unable to provide any CC. Patients who arrived at triage with signs of life but died while in the emergency unit were included in analysis. All patients with “pregnancy‐related” complaints were also excluded. This exclusion applied only to patients who presented for emergencies associated with a known pregnancy. Hospital policy dictated that patients with pregnancy‐related emergencies were supposed to be evaluated and treated in a maternity ward housed in a separate building from the main emergency department. Since those patients were explicitly not intended to be seen at the study site they were excluded from analysis. Patients for whom a pregnancy was discovered during evaluation (e.g., for vaginal bleeding or abdominal pain) were included in analysis. Patient visit data were abstracted from the quality assurance database and randomly split into two data sets: one that included 75% of cases for derivation and one with 25% of cases for validation. The derivation data set was subsequently split into two groups: patients with a single CC and patients with multiple CCs.
Each shortlist CC from Rice et al. 13 was analyzed independently and ranked by mortality risk using a recursive partitioning algorithm. This was performed in both the single and the multiple CC groups. Crude mortality was calculated for each remaining CC and the highest mortality CC was recorded. Every patient record that included that highest‐risk complaint was then removed from the database for the next round of analysis. This analysis also expressly omitted interaction terms. The above procedure was repeated until all complaints with associated mortality had been ranked as a list. The remaining CCs were not associated with any mortality and were then preserved as a separate list. Finally, any CC that occurred in fewer than 0.5% of total patient visits was removed and not included in subsequent analysis. Both the “associated mortality” and the “no mortality” lists were subject to this exclusion criteria.
Since some CCs occurred relatively infrequently in the total data set, their distribution and inclusion for analysis was susceptible to the randomization process above. This variability was addressed by using bootstrap aggregation. The above randomization and risk ranking algorithm were run in 10,000 bootstrap samples with results averaged to generate risk lists for single and multiple CCs based on average risk categories. A final list was formed by combining the two lists, placing each CC into the highest‐risk category in which it appeared during at least 10% of the iterations of the algorithm. In this way, the final list categorized CCs as high (greater than twice baseline mortality), medium (half to twice baseline mortality), low (less than half baseline mortality), and zero risk (no associated mortality). Sensitivity analysis was performed using alternative thresholds for high‐risk (both one‐and‐a‐half and triple baseline mortality) with the aim of identifying at least 10% of the population as high‐risk.
Univariate analysis was conducted for all variables available at triage using Student’s t‐test for continuous variables and the chi‐square test for categorical variables. Physiologic variables were defined as follows: for blood pressure, hypotension was defined as systolic blood pressure ≤ 90 and hypertension as systolic blood pressure ≥180; for heart rate, bradycardia was defined as heart rate < 60 beats/min and tachycardia as heart rate ≥ 120 beats/min; for temperature, hypothermia was defined as ≤ 35°C and febrile as ≥ 37.5°C; altered mental status was defined as AVPU score of “verbal,” “pain,” or “unresponsive”; and hypoxia was defined as oxygen saturation on pulse oximeter (SpO2) < 92%. Missing values for physiologic variables were coded as “normal.” Patients missing age were excluded from analysis. Sensitivity analysis of patients discharged from the emergency unit showed that those who answered follow‐up phone calls and those who did not (including those who did not answer the phone, provided a wrong number or had no phone) were very similar in terms of demographics, CCs, and indicators of disease severity. These similarities supported an assumption that missingness was completely at random. Given the low mortality rate for patients discharged from the emergency unit, all missing mortality outcomes were coded as alive for analysis and multiple imputation was not used. Multivariable logistic regression, controlling for demographic and clinical factors, was performed to test the association of an ordered categorical variable of CC “riskiness” (high, medium, and low) with 3‐day mortality. This multivariable logistic regression model was applied to two data sets as a sensitivity analysis to verify the assumptions about the handling of missing outcome data: one assumed patients with missing outcomes to be alive and a second excluded all patients missing confirmed outcomes. All univariate variables were added in a stepwise manner, with CC riskiness added as the final variable. A likelihood ratio test was performed to test the significance for including CC in the model. The coefficients from the logistic regression model built in the derivation data set were then applied to the validation data set. The sensitivity and specificity obtained from those coefficients were used for a net benefit calculation. The area under the receiver operating characteristics curve (AUROC), Hosmer‐Lemeshow goodness of fit, Brier score, and mean bias were all calculated for this data set. All analyses were performed using Stata Statistical Software version 15.1.
Ethics Approval
Approval for the study was sought by local hospital administration in conjunction with U.S.‐based researchers and was granted by the institutional review board at the Makerere School of Public Health (Kampala, Uganda), the Uganda National Council of Science and Technology (Kampala, Uganda), and the University of Massachusetts (Amherst, MA).
Patient and Public Involvement
The ECP training program was originally developed in response to several years of clinical emergency medicine experience in Uganda. The positive response of patients, staff, and administrators at Karoli Lwanga Hospital to the training programs and their interest in improving patient care led to ongoing research and program evaluation. Patients and the public were not involved in the design of the study; however, outcome measures are explicitly patient oriented. Results will be disseminated through open‐access publication.
RESULTS
Using all visits for patients aged > 18 years from 2009 to 2018, a total of 21,953 individuals were included in the derivation data set and 7,313 in the validation data set. Overall, more patients presenting to the emergency unit were admitted (63.1%, n = 18,451) than discharged (34.5%, n = 10,089). Patients had an overall 3‐day mortality of 2.76% (95% confidence interval [CI] = 2.54 to 2.98), with admitted patients having a higher 3‐day mortality of 3.51% (95% CI = 3.24 to 3.77) compared to discharged patients at 0.06% (95% CI = 0.01–0.1). Of the admitted patients, 35.7% were still admitted at 3 days (n = 6,587), 55.9% were discharged alive (n = 10,304), 0.69% were referred (n = 122), and 4.3% were lost to follow‐up (n = 791). Examining patients discharged from the emergency unit, after 7 consecutive days of calling, 45.6% of patients had a confirmed outcome (n = 4,592 alive and n = 6 dead), 44.3% had no follow‐up outcome because they had no valid phone number (n = 4,476), and 10.1% had no follow‐up attempt recorded (n = 958). Only 17 patients were excluded for being dead on arrival and 11 patients for having pregnancy‐related complaints.
Derivation
The derivation data set was split into two groups: 12,371 patients that had a single CC and 9,582 that had multiple CCs. Table 1 describes the baseline characteristics of those two groups, with the multiple CC group having significantly more risk features (e.g., HIV positivity, abnormal vital signs) and a significantly higher 3‐day mortality. The combined results of the bootstrap aggregations of the recursive partitioning algorithm are presented in Table 2 below. Ultimately, 43 CCs occurred at least 0.5% of the time in either CC group. Complaints that were excluded from analysis because they occurred less than 0.5% of the time are presented in Data Supplement S1, Appendix S1 (available as supporting information in the online version of this paper, which is available at http://onlinelibrary.wiley.com/doi/10.1111/acem.14013/full). Using the highest‐risk ranking achieved in either single or multiple CC analysis, 12 were considered high‐risk, 20 were medium‐risk, and 11 were low‐risk. The high‐risk group accounted for 15.0% of patients in the aggregate dataset. Sensitivity analysis using the alternative threshold of triple baseline mortality reduced the high‐risk group to only six CCs and only identified 6.5% of the population as high‐risk. Using the threshold of one‐and‐a‐half times baseline mortality did not change the list. Detailed results from the bootstrap aggregations are presented in Data Supplement S1, Appendix S2. The risk ranking and average frequency of the CC in each group are summarized in Figure 1 and the final rankings are displayed as text in Table 2.
Table 1.
Demographics
|
Single Complaint (n = 12,371) |
Multiple Complaints (n = 9,582) |
p‐value | |
|---|---|---|---|
| Age (years) [95% CI] | 44.5 [44.0–44.9] | 43.7 [43.2–44.2] | 0.02 * , † |
| Age group | |||
| 18–64 years | 77.3 (9,556) | 79.7 (7,640) | <0.001 |
| 65 and older | 22.8 (2,815) | 20.3 (1,942) | |
| Female | 37.5 (4,670) | 52.5 (5,023) | <0.001 † |
| HIV positive | 5.27 (652) | 8.67 (831) | <0.001 † |
| Blood pressure | |||
| Hypotensive (sBP ≤ 90) | 7.86 (972) | 13.4 (1,282) | <0.001 † |
| Normal | 82.8 (10,248) | 79.9 (7,654) | |
| Hypertensive (sBP ≥ 180) | 9.30 (1,151) | 6.74 (646) | |
| Heart rate | |||
| Bradycardic (<60 beats/min) | 2.97 (368) | 2.18 (209) | <0.001 † |
| Normal | 88.8 (10,985) | 83.7 (8,022) | |
| Tachycardic (≥120 beats/min) | 8.23 (1,018) | 14.1 (1,351) | |
| Temperature | |||
| Hypothermic (≤35°C) | 10.4 (1,281) | 9.46 (906) | <0.001 † |
| Normal | 80.4 (9,942) | 69.2 (6,631) | |
| Febrile (≥37.5°C) | 9.28 (1,148) | 21.3 (2,045) | |
| AVPU abnormal | 3.02 (373) | 1.06 (102) | <0.001 † |
| Hypoxic | 7.78 (963) | 11.6 (1,112) | <0.001 † |
| Three‐day mortality | 2.26 (280) | 3.40 (326) | <0.001 † |
Data are reported as % (n) unless otherwise specified.
Indicates t‐test; all others use chi‐square.
Indicates statistically significant.
Table 2.
Listing of CCs by Risk Group
|
High‐risk (>2× baseline mortality) |
Medium‐risk (2×–0.5× baseline mortality) |
Low‐risk (<0.5× baseline mortality) |
Zero‐risk (No Associated Mortality) |
|---|---|---|---|
|
|
|
|
CC = chief complaint.
Figure 1.
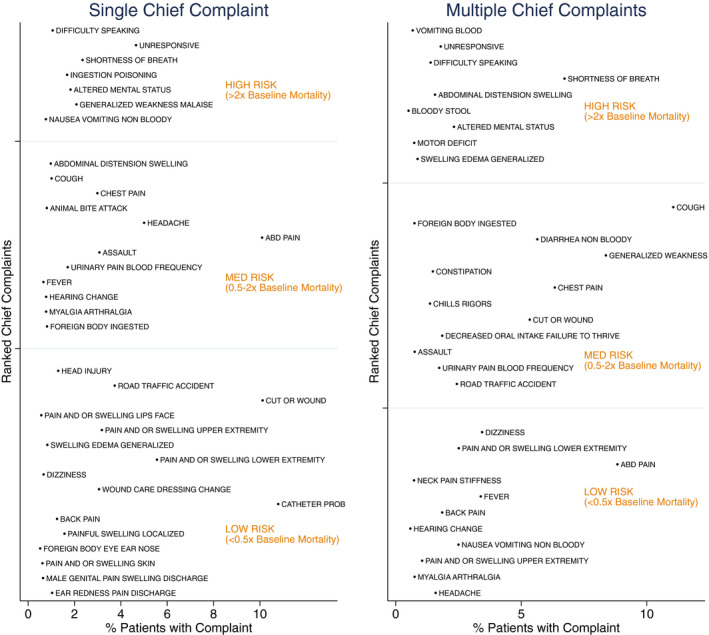
Ranked CC risk for single and multiple complaints by complaint frequency. CC = chief complaint.
Univariate logistic regression analysis was performed for all data available at triage. The results of this univariate analysis are listed in Table 3. Advanced age; male sex; HIV positivity; abnormal blood pressure, heart rate, temperature, and oxygen saturation; and altered level of consciousness were all associated with significantly higher mortality. In the final step before adding the ranked CC list, a binary variable for the presence of single or multiple CCs was introduced to the existing model. Having multiple CCs at triage was found to be significantly associated with increased mortality (odds ratio [OR] = 1.44, 95% CI = 1.20 to 1.72, p < 0.001). In the final model, the ranked CC list in Table 2 above was introduced as an ordered categorical variable, and results are displayed in Table 3. The adjusted OR for low‐risk CCs, controlling for all of the above variables, was 0.16 (95% CI = 0.09 to 0.29, p < 0.001), and the adjusted OR for high‐risk CCs was 2.39 (95% CI = 1.95 to 2.93, p < 0.001). When the ranked CC risk category was added to the model, the presence of multiple CCs lost its significant association with mortality (OR = 0.96, 95% CI = 0.80 to 1.16, p = 0.67). The p‐value for the likelihood ratio test for inclusion of ranked CC risk categories into the model was <0.001. The AUROC for the multivariable logistic regression model without CC risk categories was 0.82 (95% CI = 0.80 to 0.84), while the AUROC for model with CC risk categories was 0.85 (95% CI = 0.83 to 0.86; Figure 2). This difference in ROC was significant (p < 0.001). The p‐value for Hosmer‐Lemeshow goodness‐of‐fit test was 0.17, the Brier score was 0.025, and the mean absolute error was 0.049. To test assumptions about the handling of missingness in these data, the multivariable logistic regression model was applied to a subset of the derivation data set that included only patients with confirmed follow‐up outcomes (n = 15,146 with 10,903 admitted patients). In this subgroup analysis, the adjusted OR for mortality for the high‐risk CC group was 2.27 (95% CI= 1.85 to 2.79, p < 0.001). The AUROC for the multivariable logistic regression model without CC risk categories was 0.82 (95% CI = 0.80 to 0.83) while the AUROC for model with CC risk categories was 0.84 (95% CI = 0.82 to 0.85). This difference in ROC was significant (p < 0.001).
Table 3.
Multivariate Logistic Regression of the Association Between CC “Riskiness” and Mortality
| Crude OR | 95% CI | p‐value | Adjusted OR | 95% CI | p‐value | |
|---|---|---|---|---|---|---|
| Age group (years) | ||||||
| Adult (18‐65) | REF | REF | ||||
| Elderly (65+) | 1.68 | 1.41–2.01 | <0.001 * | 1.65 | 1.35–2.01 | <0.001 * |
| Sex | ||||||
| Male | REF | REF | ||||
| Female | 0.63 | 0.53–0.75 | <0.001 * | 0.53 | 0.44–0.64 | <0.001 * |
| HIV status | ||||||
| Negative | REF | REF | ||||
| Positive | 2.85 | 2.29–3.56 | <0.001 * | 1.98 | 1.54–2.55 | <0.001 * |
| Blood pressure | ||||||
| Hypotensive | 4.93 | 4.08–5.95 | <0.001 * | 2.32 | 1.88–2.86 | <0.001 * |
| Normotensive | REF | REF | ||||
| Hypertensive | 3.92 | 3.15–4.88 | <0.001 * | 3.93 | 3.07–5.02 | <0.001 * |
| Heart rate | ||||||
| Bradycardic | 3.91 | 2.84–5.39 | <0.001 * | 2.73 | 1.91–3.91 | <0.001 * |
| Normal | REF | REF | ||||
| Tachycardic | 3.33 | 2.76–4.02 | <0.001 * | 2.09 | 1.68–2.60 | <0.001 * |
| Temperature | ||||||
| Hypothermic | 4.11 | 3.39–4.97 | <0.001 * | 2.34 | 1.89–2.90 | <0.001 * |
| Normal | REF | REF | ||||
| Febrile | 1.68 | 1.35–2.10 | <0.001 * | 0.86 | 0.67–1.11 | 0.24 |
| AVPU | ||||||
| Normal/not recorded | REF | REF | EEE | |||
| Abnormal (P/V/U) | 5.09 | 3.80–6.81 | <0.001 * | 1.57 | 1.11–2.22 | 0.01 * |
| Oxygen saturation | ||||||
| Normal | REF | REF | ||||
| Hypoxic | 8.03 | 6.79–9.50 | <0.001 * | 3.25 | 2.68–3.94 | <0.001 * |
| Multiple CCs | ||||||
| No | REF | REF | ||||
| Yes | 1.52 | 1.29–1.79 | <0.001 * | 0.96 | 0.80–1.16 | 0.67 |
| CC risk group | ||||||
| Low risk | 0.21 | 0.12–0.36 | <0.001 * | 0.16 | 0.09–0.29 | <0.001 * |
| Medium risk | REF | REF | ||||
| High risk | 4.21 | 3.50–5.07 | <0.001 * | 2.39 | 1.95–2.93 | <0.001 * |
CC = chief complaint.
Indicates statistically significant.
Figure 2.
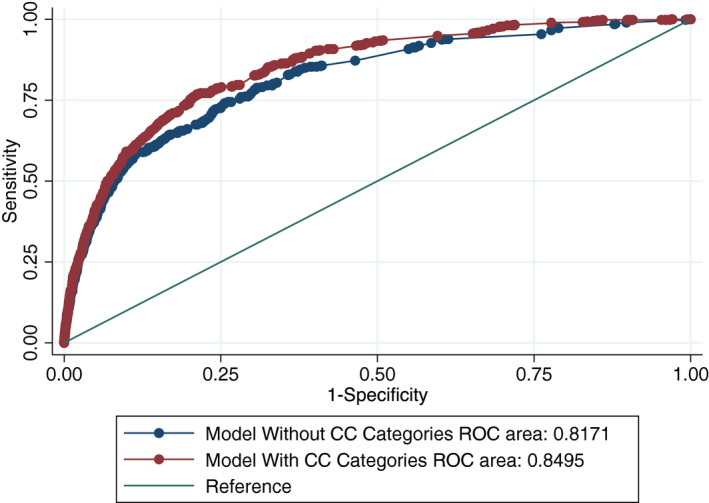
Difference in AUROC after incorporating CCs into the model. AUROC = area under the receiver operating characteristics curve; CC = chief complaint.
Validation
There were no statistically significant differences in mortality or baseline characteristics between the validation and derivation groups. Those characteristics are presented in Data Supplement S1, Appendix S3. Taking the coefficients derived from the model in the initial data set including the high‐risk CCs and applying them to the validation set yielded an AUROC of 0.84. The p‐value for Hosmer‐Lemeshow goodness‐of‐fit test was 0.74, the Brier score was 0.026, and the mean bias was 0.050. Put into practical terms, given the prevalence of mortality in the validation data set (2.75%), the model without CC risk categories had a sensitivity of 68.2% and a specificity of 80.3%, while the model with CC risk categories had a sensitivity of 77.4% and a specificity of 78.6%. When those differences were applied to the validation data set, the inclusion of CC risk categories in the model resulted in a net benefit of 18 true positives identified (high‐risk patients that died) in exchange for 118 false positives (high‐risk patients that lived). This additional at‐risk population identified had a mortality rate of 13.2%.
DISCUSSION
Taken in total, the above analysis suggests that there were 12 high‐risk CCs that independently and significantly predicted increased 3‐day mortality even when controlling for demographic and clinical characteristics. This finding was then successfully validated in a separate data set. The list of high‐risk CCs was generated a posteriori using mortality data, but it was not known if the excess mortality risk in this group was associated exclusively with the high‐risk features (e.g., advanced age, hypoxia) that cluster within certain CCs (e.g., shortness of breath) or if the CCs themselves were independently associated with increased mortality after controlling for those other high‐risk features. The final multivariable logistic regression model as applied to the validation data set strongly supports the assertion that high‐risk CCs independently and significantly conferred an increased risk of 3‐day mortality above and beyond what would be predicted from other available data. Moreover, although excess mortality was initially associated with a patient having more than one CC, the significance disappeared when the complaints themselves were included in the analysis. This strongly suggests that the nature of CCs outweighed their quantity. The strong performance of the mortality model in the validation data set further supports its strength. The model performed nearly identically in both the full data set and a subgroup analysis that excluded any patients with missing outcome data. This supported both assumptions about missingness made in the methods and the overall strength of the model.
To the authors’ knowledge, this article represents not only the first study to evaluate high‐risk CCs in an LMIC, but also the most rigorous assessment of CC mortality risk in any setting, including HICs. To date, HIC‐based studies have been published that examined dyspnea only or evaluated four “cardinal” high‐risk CCs that were determined a priori by the authors. 16 , 17 Neither used multivariable regression. In LMICs, the only study to examine the interaction between CC and triage examined only under‐ and overtriage as outcomes. 22 Other studies of CCs in LMICs have been exclusively descriptive. The above data and analysis therefore provide a novel and fundamental insight: in this population, the patient’s initial description of their disease process independently predicted their 3‐day mortality. This is the strongest finding published to date to support the focus that emergency medicine researchers and development groups have placed on CCs.
The model was designed not only to evaluate mortality outcomes but also to provide actionable clinical information for front‐line clinicians. Interaction terms were deliberately omitted so that providers could easily identify patients at higher risk of 3‐day mortality. Likewise, by analyzing only those CCs that comprised at least 0.5% of overall complaints, Type 1 errors were minimized while generating a comprehensive but not exhaustive list of CCs. The resultant list of high‐risk complaints can be digested and memorized by clinicians or posted in a triage area. If a clinician in rural Uganda hears any of the high‐risk complaints, she or he knows that the patient is at higher risk for mortality independent of other triage findings or CCs.
There was minimal novelty for several of these high‐risk CCs, such as unresponsive, altered mental status, and even shortness of breath, having already figured as high‐risk features in accepted triage systems such a South African Triage Scale 23 . However, the mortality risk associated with toxic ingestions, dysentery, generalized edema, hematemesis, and general weakness/malaise have not been captured in other triage systems. Also interesting is the presence of “motor vehicle accident” and “head injury” as low‐risk CCs. Any patients with one of these “mechanism‐only” complaints lacked any associated higher risk symptoms (e.g., unresponsive, “vomiting”), which would have been identified earlier in the algorithm. Because both of these traumatic injury mechanisms carry objective risk for mortality, this finding emphasizes the need to develop CC data collection systems that discriminate between mechanism of injury and associated symptoms. Ideally, both features can be maintained because both contribute to overall concepts of risk.
The methods were also largely automated and expressly designed to be portable to other data sets. However, care must be taken to build systems in LMICs that address local language and disease patterns. For instance, certain CCs that are often considered high‐risk in developed settings (e.g., chest pain, abdominal pain) were absent from this high‐risk list. 15 , 24 , 25 At the same time, certain CCs that are typically, albeit not universally, considered low‐risk in HICs (e.g., general weakness/malaise) were present in this high‐risk list. 18 Moreover, a case‐by‐case review of the mortality associated with “abdominal distension” showed the most common diagnoses to be bowel obstruction, peritonitis, and liver disease. These disease patterns and the language used to describe them may not exist in other settings and thus lead to a different risk ranking for abdominal distension elsewhere. This all underlines the impact that language and medical literacy have on presenting complaints and accentuates the risk inherent in transporting medical training from developed settings into LMICs. Overall, it emphasizes that care must be taken to build systems in LMICs that are data‐driven and locally appropriate.
In seeking to triage the severity of emergency unit patient presentations in LMICs, multiple other groups have developed and/or validated tools based on vital signs, functional or neurologic status, traumatic injury, and HIV status. These include scores such as the South African Triage Scale mentioned as well as the Kampala Trauma Risk Score, VitalPAC Early Warning Score, Universal Vital Assessment, and Modified Early Warning Score. 26 , 27 , 28 , 29 While these scores have provided critical insights into admission and mortality risk, all are predicated on physiologic assessment and none account for the patient’s subjective assessment of their disease. One model has combined CC with a basic assessment of stability to create a two‐step triage model for LMIC settings but does not include any outcome data. 30 If frontline clinicians can combine physiologic triage tools with a robust understanding of local CC data, they may become more adept at targeting those patients who require immediate lifesaving intervention. Future goals include prospectively enriching existing triage tools with CC rankings and evaluating how such approaches could impact patient outcomes. Additional next steps should also include external validation of the above methods.
LIMITATIONS
This study database was produced from patient visits at a single site. The overall volume of patients and mortality rate were lower than has been reported at some sites in sub‐Saharan Africa; however, neither was outside the median interquartile range for emergency units in rural sub‐Saharan Africa. 1 The CCs recorded as free text were impacted by local dialects that are culturally and linguistically specific. Furthermore, the recorded CCs were affected by the rural setting (e.g., more agricultural injuries or poisonings) as well as the presence of ancillary local health care services including an adjacent but physically separate emergency unit dedicated to maternal illness. Hospital policy dictated that all maternal emergencies in patients with known pregnancy were to be seen at this separate facility. Therefore, despite the fact that maternal mortality in LMICs is a critical problem warranting study, any maternal emergencies that came to the study site were, by definition, improperly triaged and thus excluded from analysis. Overall, missing data were a challenge in this data set—a commonly encountered problem in LMIC‐based research—especially surrounding outcomes for discharged populations. Because little data have been published regarding LMIC emergency unit discharges, there exists no standard methodology to employ. Therefore, to address missingness, this study incorporated sensitivity analysis at multiple levels including extensive comparison of groups missing data and regression models run with and without missing outcomes. However, even a rigorous handling of missingness is clearly a limitation and one that can hopefully be addressed in future confirmatory studies by inclusion of in‐person village visits to confirm outcomes for patients without phones.
CONCLUSIONS
This study demonstrates that locally contextualized chief complaints independently predict 3‐day mortality and are additive to other information available at triage. This more precise categorization of high‐ and low‐risk chief complaints can better inform emergency patient care, system‐strengthening efforts, and provider training programs in Uganda. Overall, further study into chief complaints, both inside and outside Uganda, can guide emergency care development to hopefully mitigate otherwise preventable death and disability across the globe.
Supporting information
Data Supplement S1. Supplemental material.
Acknowledgments
The authors acknowledge all of the emergency care practitioners who provided the essential care described above in addition to the program directors, research coordinators, and research assistants at Karoli Lwanga Hospital who made the data collection possible.
Academic Emergency Medicine 2020;27:1291–1301.
Author contributions: BR—literature search, study design, development of data analysis plan, data interpretation, figures, tables, writing, and critical review of manuscript; JL—literature search, data interpretation, writing, and critical review of manuscript; HM—contributed to conceptual design, writing, and critical review of manuscript; NTK—data collection, data interpretation, writing, and critical review of manuscript; EMM—data interpretation, writing, and critical review of manuscript; MB—contributed to conceptual design, data collection, data interpretation, writing, and critical review of manuscript; HK—data collection, data interpretation, writing, and critical review of manuscript; JAN—data interpretation, writing, and critical review of manuscript; MS—data interpretation, writing, and critical review of manuscript; KN—data analysis, data interpretation, writing, and critical review of manuscript; MK—development of data analysis plan, methodology, biostatistics, data interpretation, writing, and critical review of manuscript; and the Global Emergency Care Investigator Group (Members: Mark Bisanzo, Stacey Chamberlain, Bradley Dreifuss, Heather Hammerstedt, Sara Nelson)—conception and design of database and program, data collection, and critical review of manuscript.
A related article appears on page 1360.
REFERENCES
- 1. Obermeyer Z, Abujaber S, Makar M, et al. Emergency care in 59 low‐ and middle‐income countries: a systematic review. Bull World Health Organ 2015;93:577–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mathers C, Stevens G, Hogan D, Mahanani WR, Ho J. Disease Control Priorities, Third Edition (Volume 9): Improving Health and Reducing Poverty. Washington, DC: World Bank Group, 2018. [Google Scholar]
- 3. Calvello E, Reynolds T, Hirshon JM, et al. Emergency care in sub‐Saharan Africa: results of a consensus conference. Afr J Emerg Med 2013;3:42–8. [Google Scholar]
- 4. Global Emergency and Trauma Care Initiative. Geneva: World Health Organization, 2018. [Google Scholar]
- 5. Reynolds TA, Sawe H, Rubiano AM, Do SS, Wallis L, Mock CN. Strengthening Health Systems to Provide Emergency Care In: Disease Control Priorities, Third Edition (Volume 9): Improving Health and Reducing Poverty. Geneva: World Health Organization, 2018. p. 247–65. [Google Scholar]
- 6. Reynolds TA, Bisanzo M, Dworkis D, et al. Research priorities for data collection and management within global acute and emergency care systems. Acad Emerg Med 2013;20:1246–50. [DOI] [PubMed] [Google Scholar]
- 7. Mowafi H, Dworkis D, Bisanzo M, et al. Making recording and analysis of chief complaint a priority for global emergency care research in low‐income countries. Acad Emerg Med 2013;20:1241–5. [DOI] [PubMed] [Google Scholar]
- 8. Pollock DA, Adams DL, Bernardo LM, et al. Data elements for emergency department systems, release 1.0 (DEEDS): a summary report. Acad Emerg Med 1998;5:185–93. [PubMed] [Google Scholar]
- 9. Begier EM, Sockwell D, Branch LM, et al. The National Capitol Region’s emergency department syndromic surveillance system: do chief complaint and discharge diagnosis yield different results? Emerg Infect Dis 2003;9:393–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yan LD, Mahadevan SV, Yore M, et al. An observational study of adults seeking emergency care in Cambodia. Bull World Health Organ 2015;93:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oteng R, Whiteside L, Rominski S, et al. Individual and medical characteristics of adults presenting to an urban emergency department in Ghana. Ghana Med J 2015;49:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Becker J, Dell A, Jenkins L, Sayed R. Reasons why patients with primary health care problems access a secondary hospital emergency centre. South Afr Med J Suid‐Afr Tydskr Vir Geneeskd 2012;102:800–1. [DOI] [PubMed] [Google Scholar]
- 13. Rice BT, Bisanzo M, Maling S, Joseph R, Mowafi H. Derivation and validation of a chief complaint shortlist for unscheduled acute and emergency care in Uganda. BMJ Open 2018;8:e020188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Haendel MA, Chute CG, Robinson PN. Classification, ontology, and precision medicine. N Engl J Med 2018;379:1452–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Woo K, Schneider JI. High‐risk chief complaints I: chest pain‐the big three. Emerg Med Clin North Am 2009;27:685–712. [DOI] [PubMed] [Google Scholar]
- 16. Mockel M, Searle J, Muller R, et al. Chief complaints in medical emergencies: do they relate to underlying disease and outcome? The Charité Emergency Medicine Study (CHARITEM). Eur J Emerg Med 2013;20:103–8. [DOI] [PubMed] [Google Scholar]
- 17. Kelly AM, Keijzers G, Klim S, et al. An observational study of dyspnea in emergency departments: the Asia, Australia, and New Zealand Dyspnea in emergency departments study (AANZDEM). Acad Emerg Med 2017;24:328–36. [DOI] [PubMed] [Google Scholar]
- 18. Nemec M, Koller MT, Nickel CH, et al. Patients presenting to the emergency department with non‐specific complaints: the Basel Non‐specific Complaints (BANC) study. Acad Emerg Med 2010;17:284–92. [DOI] [PubMed] [Google Scholar]
- 19. Hammerstedt H, Maling S, Kasyaba R, et al. Addressing WHO resolution 60.22: a pilot project to create access to acute care services in Uganda. Ann Emerg Med 2014;64:461–8. [DOI] [PubMed] [Google Scholar]
- 20. Rice B, Periyanayagam U, Chamberlain S, et al. Mortality in children under five receiving nonphysician clinician emergency care in Uganda. Pediatrics 2016;137:e20153201. [DOI] [PubMed] [Google Scholar]
- 21. Chamberlain S, Stolz U, Dreifuss B, et al. Mortality related to acute illness and injury in rural Uganda: task shifting to improve outcomes. PLoS One 2015;10:e0122559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hinson JS, Martinez DA, Schmitz PS, et al. Accuracy of emergency department triage using the Emergency Severity Index and independent predictors of under‐triage and over‐triage in Brazil: a retrospective cohort analysis. Int J Emerg Med 2018;11:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gottschalk SB, Wood D, Devries S, Wallis LA, Bruijns S. The Cape Triage Score: a new triage system South Africa. Proposal from the Cape Triage Group. Emerg Med J 2006;23:149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nentwich L, Ulrich AS. High‐risk chief complaints II: disorders of the head and neck. Emerg Med Clin North Am 2009;27:713–46. [DOI] [PubMed] [Google Scholar]
- 25. Tekwani K, Sikka R. High‐risk chief complaints III: abdomen and extremities. Emerg Med Clin North Am 2009;27:747–65. [DOI] [PubMed] [Google Scholar]
- 26. Opio MO, Nansubuga G, Kellett J. Validation of the VitalPACTM Early Warning Score (ViEWS) in acutely ill medical patients attending a resource‐poor hospital in sub‐Saharan Africa. Resuscitation 2013;84:743–6. [DOI] [PubMed] [Google Scholar]
- 27. Moore CC, Hazard R, Saulters KJ, et al. Derivation and validation of a universal vital assessment (UVA) score: a tool for predicting mortality in adult hospitalised patients in sub‐Saharan Africa. BMJ Glob Health 2017;2:e000344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Haac B, Varela C, Geyer A, Cairns B, Charles A. The utility of the Kampala Trauma Score as a triage tool in a Sub‐Saharan African Trauma Cohort. World J Surg 2015;39:356–62. [DOI] [PubMed] [Google Scholar]
- 29. Wheeler I, Price C, Sitch A, et al. Early warning scores generated in developed healthcare settings are not sufficient at predicting early mortality in Blantyre, Malawi: a prospective cohort study. PLoS One 2013;8:e59830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Khan A, Mahadevan SV, Dreyfuss A, et al. One‐two‐triage: validation and reliability of a novel triage system for low‐resource settings. Emerg Med J 2016;33:709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Supplement S1. Supplemental material.


