Abstract
Objective
To investigate the learning curve of robot‐assisted laparoscopy in early‐stage cervical cancer and quantify impact on oncological outcomes.
Design
Observational cohort study.
Setting
Tertiary referral centre with one surgical team.
Population
All women with early‐stage cervical cancer treated consecutively with robot‐assisted laparoscopy between 2007 and 2017.
Methods
With multivariate risk‐adjusted cumulative sum analysis (RA‐CUSUM), we assessed the learning curve of robot‐assisted laparoscopy of a single surgical team based on cervical cancer recurrence. Subsequently, a survival analysis was conducted comparing oncological outcomes of women treated during different phases of the learning curve.
Main outcome measures
Surgical proficiency based on recurrence, survival rates in the different learning phases.
Results
One hundred and sixty‐five women with cervical cancer underwent robot‐assisted laparoscopy, with a median follow up of 57 months (range 3–132 months). The RA‐CUSUM analysis demonstrated two phases of the learning curve: a learning phase of 61 procedures (group 1) and an experienced phase representing the 104 procedures thereafter (group 2). The 5‐year disease‐free survival was 80.2% in group 1 and 91.1% in group 2 (P = 0.040). Both the 5‐year disease‐specific survival and overall survival significantly increased after the learning phase.
Conclusion
The learning phase of robot‐assisted laparoscopy in early‐stage cervical cancer in this institutional cohort is at least 61 procedures, with higher survival rates in the women treated thereafter. The learning curve of robot‐assisted laparoscopy affects oncological outcomes and warrants more attention in the design of future studies.
Tweetable abstract
The learning curve of robot‐assisted laparoscopy in early‐stage cervical cancer affects oncological outcomes and warrants more attention.
Keywords: Cervical cancer, learning curve, recurrence, risk‐adjusted cumulative sum analysis, robot‐assisted laparoscopy, survival
Tweetable abstract
The learning curve of robot‐assisted laparoscopy in early‐stage cervical cancer affects oncological outcomes and warrants more attention.
Introduction
The perioperative advantages of robot‐assisted laparoscopy have resulted in its widespread adoption in the treatment of early‐stage cervical cancer. Numerous observational studies and meta‐analyses report superior results in blood loss, hospital stay and complication rates compared with the laparotomic approach. 1 , 2 , 3 , 4 In addition, equal survival rates of robot‐assisted laparoscopy and laparotomy were suggested. 5 , 6 , 7 , 8 However, a recent trial by Ramirez et al., 9 which randomised between minimally invasive surgery (MIS) and laparotomy, showed a significantly increased recurrence rate and reduced overall survival in patients receiving MIS for International Federation of Obstetrics and Gynecology (FIGO) stage Ia1–Ib1 cervical cancer. The MIS arm of this trial consisted predominately of conventional laparoscopy with only 15.6% of the patients undergoing robot‐assisted surgery. Multiple subsequent observational studies have substantiated these results regarding MIS, 10 , 11 , 12 , 13 whereas others reported non‐inferiority of recurrence and survival rates after (specifically) robot‐assisted surgery. 14 , 15 , 16
A large population‐based cohort study in Sweden, where cervical cancer surgery is highly centralised, recently concluded it to be safe to continue with robot‐assisted surgery when performed by experienced, high‐volume surgeons. 14 In the previous studies, the learning‐curve effect on oncological outcomes in cervical cancer – specifically in robot‐assisted laparoscopy – is not yet reported. This could be a potential confounder and could offer a possible explanation for the conflicting reports. In addition, until today the literature available on learning curves has mainly focused on the duration of surgery only. 17 , 18 , 19 , 20 To analyse proficiency in robot‐assisted laparoscopy the oncological outcome should be considered the foremost relevant parameter. In other oncological diseases, for instance prostate cancer, it has been shown that oncological outcomes are associated with the learning curve of robot‐assisted surgery but the number of procedures needed to reach an accepted plateau of the learning curve varies widely and requires further research. 21 , 22
The objective of this study is to investigate the learning curve of a single surgical team and its effect on the risk of cervical cancer recurrence, and to quantify the impact on survival. By using a multivariate risk‐adjusted cumulative sum (RA‐CUSUM) analysis we aim to establish the number of surgeries needed to ascertain oncological proficiency in robot‐assisted laparoscopy in the treatment of early‐stage cervical cancer.
Methods
Design
Our institution is the only referral centre for the radical treatment (surgery and chemoradiation) of cervical cancer serving an adherence region of 800 000 women. We performed an observational cohort study reporting on all patients treated consecutively with robot‐assisted laparoscopy for early‐stage cervical cancer (FIGO stages Ia1, Ia2, Ib1 and IIa1 according to 2009 FIGO staging and guidelines 23 ) between 1 December 2007 and 1 April 2017. Inclusion criteria were a histopathologically proven carcinoma of the cervix and the intention to perform robot‐assisted radical surgery as primary treatment. Women were excluded in the case of an ongoing pregnancy or when treated with neoadjuvant chemotherapy. All procedures were part of standard clinical care, for which informed consent was obtained. Participants were not involved in the development of this analysis and no core outcome sets were used in this study.
The primary outcome of interest was surgical proficiency, based on cervical recurrence rate. Secondary outcomes were disease‐free survival (DFS), disease‐specific survival (DSS) and overall survival (OS) in the different learning phases. Survival was defined as the time interval between date of diagnosis or first visit and date of disease recurrence diagnosis (DFS) or death due to the sequelae of cervical cancer (DSS) or death from any cause (OS).
Surgical technique
All surgical procedures were performed at a tertiary referral centre by a surgical team consisting of two gynaecological oncologists (RV, RZ) with similar experience in robot‐assisted operative techniques. In all women with early‐stage cervical cancer and an indication for radical surgery, the da Vinci (S until 2010 and Si until 2017) Surgical System (Intuitive Surgical, Sunnyvale, CA, USA) was used. Robot‐assisted laparoscopy was the standard of care, with laparotomy only performed in ten cases during the inclusion period for those who had an absolute contraindication (e.g. advanced pregnancy, large uterus). Primary surgical treatment for early‐stage cervical cancer consisted typically of robot‐assisted pelvic sentinel lymph node evaluation and systematic lymph node dissection combined with radical hysterectomy or radical vaginal trachelectomy when fertility preservation was desired and the maximum tumour diameter was ≤2 cm. When sentinel nodes were found to be positive on frozen section, the intended radical uterine surgery was discontinued and chemoradiation was initiated. In case of an unexpected finding of cervical cancer after simple hysterectomy (for benign indication), primary treatment was expanded with robot‐assisted pelvic lymph node dissection and parametrectomy. During the study period no changes in surgical techniques or use of uterine manipulator (McCartney tube, LiNA Medical, Glostrup, Denmark) occurred. Details of the surgical techniques performed at our institution have been previously described. 5 , 24
Data collection
Parameters were extracted from the institutional medical records. The clinical, surgical, histopathological and follow‐up data collected from medical records included: age at diagnosis, body mass index (BMI, in kg/m2), history of abdominal surgery, American Society of Anesthesiologists classification, FIGO stage (2009), type of procedure, tumour histology and size, lymph vascular space invasion, nodal count and status, parametrial involvement, vaginal involvement, positive resection margins, adjuvant or adjusted treatment (intraoperative finding of positive lymph nodes leading to the abandonment of the radical procedure and replacing it with chemoradiation), disease recurrence, time and sites of recurrence and survival information. Disease recurrence was defined as the local and/or distant (outside the inner pelvis) presence of malignant tissue originating from the primary tumour, determined clinically, radiographically and/or histopathologically. Death records were verified using the municipal Personal Records Database.
Oncological follow up was performed for a total of 5 years according to national guidelines consisting of ambulant visits to a gynaecological oncologist at intermissions of 3 months (first year), 4 months (second year) and 6 months up to 5 years of follow up.
Statistical analysis
To evaluate the institutional learning curve of robot‐assisted laparoscopy, RA‐CUSUM statistical analysis was performed. The cumulative sum (CUSUM) procedure has been validated to monitor surgical outcomes and is able to detect small changes over time in the surgical performance. 25 , 26 The RA‐CUSUM is an extension of this statistical method by adjusting for each patient's individual risk of surgical failure through the use of a likelihood‐based scoring method. 25 We defined surgical failure as cervical cancer recurrence. As the estimated risk of recurrence varies among patients, a multivariate risk adjustment is essential. The probability of recurrence for each patient was modelled by a logistic regression analysis. The variables included in the risk model were based on literature on prognostic factors for recurrence. 27 We limited this model to three degrees of freedom to prevent overfitting. Sensitivity analyses with different models were tested in the RA‐CUSUM to ensure that the presented data are robust.
Following Steiner et al. 25 we used two RA‐CUSUM procedures. The first, here referred to as RA‐CUSUM+, is designed to detect a doubling of the odds of recurrence (R A = 2). The second, here referred to as RA‐CUSUM−, is designed to detect a halving of the odds of recurrence (R A = 0.5) (based on a similar analysis in robot‐assisted hemicolectomy by Parisi et al. 28 ). Details of the RA‐CUSUM functions are provided in the Supplementary material (Appendix S1).
To summarise, the RA‐CUSUM plots the difference between the cumulative expected occurrence of an event (here: recurrence) and the actual observation. In the upper RA‐CUSUM+ chart, the curve moves up for every case with recurrence and down for every case without recurrence. The magnitude by which the line ascends or descends is determined by the difference between the observed and expected probability of recurrence. For instance, if a patient modelled as having a high probability of recurrence subsequently develops a recurrence, the curve ascends less (i.e. small penalty) than it would if a recurrence is diagnosed in a low‐risk modelled patient (i.e. larger penalty). In the lower RA‐CUSUM− chart, surgical success is indicated by a negative drift of the curve.
Based on the RA‐CUSUM plot, the ‘learning phase’ of robot‐assisted laparoscopy at our institution was determined. The procedures performed during this first phase were compared with the rest of the procedures performed thereafter. The Statistical Package for the Social Sciences version 25.0.2 (SPSS; International Business Machines, Armonk, NY, USA) was used for modelling analysis and the RA‐CUSUM analyses were performed using microsoft excel 2010 for windows. As we performed an intention‐to‐treat analysis, cases where radical hysterectomy was aborted because of positive lymph nodes were included in the analysis.
Comparisons of continuous variables were conducted using the Mann–Whitney U test. Categorical data were reported as proportions and compared between groups using chi‐square test or Fisher’s exact test as appropriate.
Survival curves for both groups were estimated using Kaplan–Meier method and differences between the two groups were compared using log‐rank test. Statistical tests were two‐sided with significance set at P < 0.05, with confidence intervals (CI) at the 95% level.
Results
Population
The study population consisted of 165 women with a median age of 40 years (range 23–81 years) and median BMI was 24 kg/m2 (range 18–41 kg/m2). The majority (90.3%) were staged as FIGO Ib1. One robot‐assisted procedure was converted to conventional laparoscopy because of technical difficulties (case number 74). There were no conversions to laparotomy.
A total of 145 (87.9%) procedures were preceded by sentinel lymph node evaluation and in 84.1% of these cases the sentinel nodes were detected bilaterally. Due to lymphatic tumour involvement, radical hysterectomy was omitted in 13 women (7.9%) and subsequently primary treatment was adjusted to chemoradiation. Another 22 women (13.3%) received adjuvant radiotherapy or chemoradiation because of postoperative histopathological findings of positive lymph nodes (n = 5), parametrial invasion (n = 4), positive (n = 1) or narrow (<5 mm, n = 6) resection margins, extensive lymph vascular space invasion (n = 1) or a combination of any of these criteria (n = 5). One of the women with micrometastasis in one lymph node did not receive adjuvant chemoradiation because of contraindications for radiotherapy. The median follow‐up duration was 57 months (range 3–132 months).
Risk model and sensitivity analysis
To determine the learning curve with RA‐CUSUM, multiple risk models, obtained with logistic regression, were tested. The first risk‐of‐recurrence model containing three independent variables – age, parametrial involvement and lymph node status – was overall significant (P = 0.009). However, only age and lymph node status were unique significant variables (odds ratio 1.05, 95% CI 1.01–1.09 and odds ratio 4.23, 95% CI 1.32–13.57, respectively). As the majority of patients were staged Ib1, the FIGO stage was not included. Histology and fertility‐sparing surgery appeared as not significant covariates in both univariate and multivariate logistic regression analyses and were not included in the risk model. When replacing parametrial involvement and lymph node status with adjuvant or adjusted treatment, which summarises multiple prognostic factors, this variable yielded a more significant risk model (P = 0.001) and a strong association with recurrent disease (odds ratio 3.85, 95% CI 1.46–10.16). The final model used for the RA‐CUSUM chart (below) included age and adjuvant or adjusted treatment. Outcomes of the RA‐CUSUM did not change substantially for the various models.
Learning curve
The RA‐CUSUM chart is displayed in Figure 1. The RA‐CUSUM+ chart shows a peak at 61 procedures, after which a consistent decrease is observed and the RA‐CUSUM+ chart moves towards zero, indicating satisfactory results with respect to the predicted recurrence rates. Hence, the first 61 procedures comprise the learning phase for the surgical team. In the 20 procedures thereafter, representing the first part of the experienced phase, surgical performance is still improving as the chart moves further negatively, indicating a decrease of surgical failure as presented by the RA‐CUSUM− chart. From procedure 81 onwards the RA‐CUSUM− chart stabilises, indicating the plateau of the learning curve has been reached.
Figure 1.
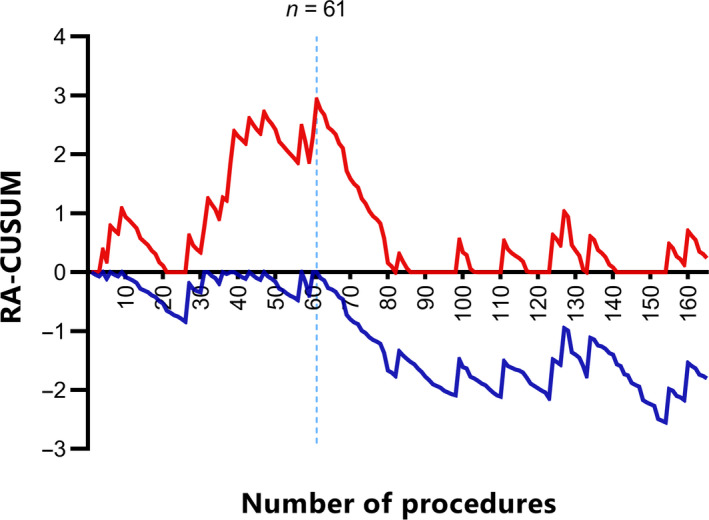
The learning curve of robot‐assisted laparoscopy for recurrent cervical cancer. The x‐axis indicates the number of procedures performed. The y‐axis indicates the cumulative sum of success and failure of the surgical team in terms of recurrence, adjusted for the probabilities from the risk model. The RA‐CUSUM+ (red) is designed to detect decrease in surgical performance while RA‐CUSUM− (blue) is designed to detect increase in the surgical performance. Both curves move upward for surgical failure and downward for surgical success.
As a sensitivity analysis, RA‐CUSUM plots were constructed for the different risk models as described earlier, all showing an identical learning phase of 61 procedures.
Based on the RA‐CUSUM plot the patients were divided into two groups: group 1 (the learning phase; procedure 1–61) and group 2 (the experienced phase; procedure 62–165).
Patient characteristics and survival by learning phases
Table 1 shows the baseline characteristics of the patients treated during the two phases. Apart from follow‐up duration no significant differences were found. The median follow‐up duration was 62 months (range 14–132 months) in group 1 (case 1–61) and 43 months (range 3–91 months) in group 2 (case 62–165) (P < 0.001). In both groups the majority of patients completed a 3‐year follow up (90.2% in group 1 versus 80.8% in group 2, P = 0.168).
Table 1.
Baseline characteristics of the two groups divided by the learning curve analysis
| Group 1 (n = 61) | Group 2 (n = 104) | P | |
|---|---|---|---|
| Age (years), median (range) | 39 (24–81) | 40 (23–81) | 0.94 |
| BMI (kg/m2), median (range) | 23.1 (19.3–31.6) | 24.2 (18.0–41.0) | 0.27 |
| History of abdominal surgery | 19 (31.1) | 33 (31.7) | 1.00 |
| ASA score a | 60 | 104 | 0.22 |
| 1 | 44 (73.3) | 86 (82.7) | |
| ≥2 | 16 (26.7) | 18 (17.3) | |
| FIGO stage | 1.00 b | ||
| Ia1 and Ia2 | 4 (6.6) | 7 (6.7) | |
| Ib1 and IIa | 57 (93.4) | 97 (93.3) | |
| Histology | 0.58 | ||
| Squamous cell | 41 (67.2) | 71 (68.3) | |
| Adenocarcinoma | 17 (27.9) | 24 (23.1) | |
| Other (adenosquamous, clear cell, villoglandular) | 3 (4.9) | 9 (8.7) | |
| Grade c | 60 | 102 | 0.26 |
| I | 10 (16.7) | 27 (26.5) | |
| II | 36 (60.0) | 49 (48.0) | |
| III | 14 (23.3) | 26 (25.5) | |
| Type of procedure | 0.38 | ||
| PLND and RH | 43 (70.5) | 59 (56.7) | |
| PLND and RVT | 8 (13.1) | 20 (19.2) | |
| PLND only | 4 (6.6) | 9 (8.7) | |
| Other d | 6 (9.8) | 16 (15.4) | |
| SN procedure added to procedure | 53 (86.9) | 92 (88.5) | 0.96 |
| LN harvested at PLND, median (range) | 27 (12–56) | 24 (10–61) | 0.31 |
| LN harvested at PLND | 0.13 b | ||
| <17 lymph nodes harvested e | 2 (3.3) | 11 (11.0) | |
| ≥17 lymph nodes harvested | 58 (96.7) | 89 (89.0) | |
| Positive LN status | 7 (11.5) | 14 (13.5) | 0.90 |
| Positive LVSI | 28 (45.9) | 47 (46.1) | 1.00 |
| Positive parametrial involvement | 4 (6.6) | 4 (3.8) | 0.47 b |
| Adjuvant or adjusted treatment | 15 (24.6) | 20 (19.2) | 0.54 |
| Radiotherapy | 10 (16.4) | 8 (7.7) | 0.22 |
| Chemoradiation | 5 (8.2) | 12 (11.5) | |
| Follow‐up duration (months), median (range) | 62 (14–132) | 43 (3–91) | <0.001 f |
ASA, American Society of Anesthesiologists; BMI, body mass index; FIGO, International Federation of Gynecology and Obstetrics; LN, lymph node; LVSI, lymph‐vascular space invasion; PLND, pelvic lymph node dissection; RH, radical hysterectomy; RVT, radical vaginal trachelectomy; SN, sentinel node.
Data are presented as n (%). Percentages may not total 100 because of rounding. Pearson's chi‐square test and Mann‐Whitney U test were used for categorical data and median values, respectively, unless otherwise specified.
One system missing.
Fisher's exact test (>20% expected count <5).
Three system missings (one villoglandular).
Robot parametrectomy with PLND (n = 10), robot RH only +/− SN (n = 3), robot PLND with simple hysterectomy (n = 1), laparoscopic PLND + SN expanded with robot RH (n = 1), robot LN sampling because of suspected nodes (n = 1), RVT + robot SN (n = 2), robot PLND + SN with conisation (n = 1), robot PLND + SN with radical cervical resection after supravaginal hysterectomy (n = 1), robot RH with laparoscopic PLND + SN in two tempi (n = 1), robot PLND + SN, RVT in another centre (n = 1).
Cut‐off based on earlier publication. 37
Statistically significant.
In total, 20 patients (12.1%) were diagnosed with recurrent disease within 5 years; 12 (19.7%) in group 1 and eight (7.7%) in group 2 (P = 0.028). In both group 1 and group 2 seven patients presented with locoregional recurrence (11.5 and 6.7%, respectively), four patients in group 1 (6.6%) and no patient in group 2 presented with distant recurrence and one patient in both group 1 and group 2 presented with a combination of locoregional and distant recurrence (1.6 and 1.0%, respectively). The 5‐year DFS was 80.2% in group 1 and 91.1% in group 2 (P = 0.040) (Figure 2A). The 3‐year DFS did not differ significantly between the groups (83.6% versus 92.8%, P = 0.064). Of the ten patients with recurrent disease who were alive at the time of analysis, five were treated curatively (three in group 1 and two in group 2) and five patients are currently on palliative treatment.
Figure 2.
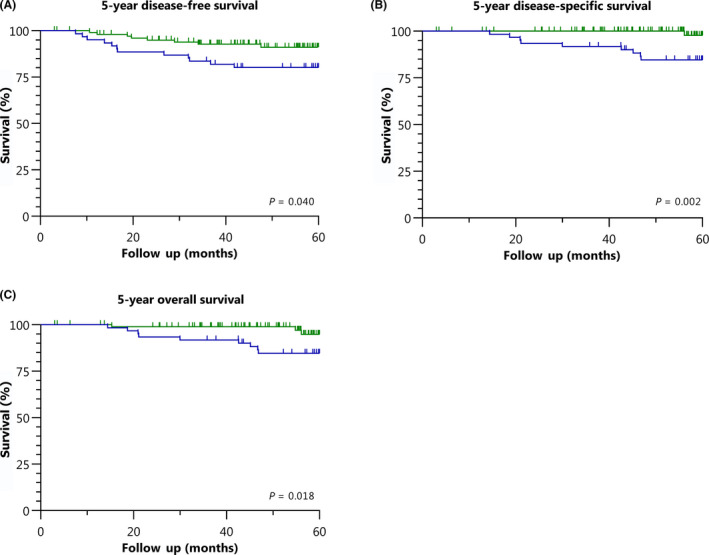
Kaplan–Meier survival curves for women treated during the learning phase (blue) or the experienced phase (green). (A) Five‐year disease‐free survival. (B) Five‐year disease‐specific survival. (C) Five‐year overall survival.
A total of 12 patients died within the 5 years of follow up, ten of whom died of cervical cancer; nine patients (14.8%) in group 1 and one patient (1.0%) in group 2. Both the DSS and OS at 5 years of follow up differed significantly between the two groups, in favour of group 2: 84.7% versus 97.7% (P = 0.002) (Figure 2B) and 84.7% versus 94.8% (P = 0.018) (Figure 2C), respectively.
Discussion
Main findings
This is the first study, using RA‐CUSUM analysis, to evaluate the effect of length of the learning period of robot‐assisted laparoscopy on oncological outcomes. Our results suggest an institutional learning phase of at least 61 procedures, showing a decreasing recurrence rate with increasing experience. The 5‐year DFS and DSS significantly improved in patients treated after this initial learning phase. The 5‐year DFS and OS in our cohort are comparable with other recent studies on robot‐assisted radical hysterectomy in the treatment of cervical cancer, 6 , 8 , 14 and are similar to the national recurrence and survival rates for early‐stage cervical cancer in the Netherlands. 29 When regarding the 4.5‐year DFS, the outcome parameter provided in the Laparoscopic Approach in Cervical Cancer (LACC) trial, we found that 93% of the patients treated during the experienced phase (group 2) were free of recurrence at 4.5 years of follow up. This is substantially higher than the 4.5‐year DFS of 86% reported in the MIS arm of the LACC trial. 9 Unfortunately, the exact MIS volume and (RA‐CUSUM based) institutional learning curves are unknown for the inclusion centres of the LACC trial. Furthermore, their surgical proficiency assurance was limited to providing data from (a minimum of) any ten laparoscopic surgeries and two procedural videos, which in light of our main findings could be considered insufficient.
Strengths and limitations
Since we started with robot‐assisted surgery at our institution at the end of 2007 it has been the standard of care for early‐stage cervical cancer, so minimising the risk of selection bias in our analysis. Another strength is that, in contrast to other studies on this subject, we performed a formal CUSUM analysis, which is considered the reference standard for studying surgical learning curves and recently emerged in other surgical fields. 30 , 31 Also, given the objective outcome parameters (i.e. mortality), misclassification of the outcome status (i.e. information bias) is unlikely to have occurred.
There were several limitations to this study. First, the shorter follow‐up time of the second group, inherent to the more recent surgery date in this group, could have led to overestimation of the learning‐curve effect. This effect is likely to be limited as the majority of the recurrences occurred in the first 3 years of follow up, which 80.8% of the patients in group 2 completed (not significantly different from the first group). Also, survival analysis with Kaplan–Meier plots corrects for differences in individual follow up through censoring, so still providing reliable data. Second, other robot‐assisted procedures were also performed in the period from December 2007 to April 2017 for high‐grade and serous endometrial cancer, which reinforces our finding that one needs at least 61 procedures before reaching surgical proficiency. The variety of robot‐assisted procedures is an inescapable reality in the daily practice of a high‐volume oncological centre and represents a practice comparable to other tertiary referral centres. This also applies to the diversity in the surgical treatments given to these relatively young patients with cervical cancer. Preservation of fertility is often desired and, if possible, radical surgery is performed without removal of the uterus. We chose to include all consecutive primary radical robot‐assisted laparoscopies in early‐stage cervical cancer as the robot‐assisted actions require equal surgical proficiency. Selecting only a subset of identical procedures, disregarding comparable surgeries during which transferrable skills are learned, could have led to biased underestimation of the learning curve. This approach, together with other subtle case‐mix differences (e.g. BMI), may contribute to different learning‐curve lengths in other centres.
Inevitably, individual learning curves may differ, but we did not carry out a per surgeon analysis. In any case, for daily practice, institutional performance is more important than individual performance. In the end, teams will consist of both experienced and less experienced surgeons, which should guarantee maintenance of team proficiency at an optimal level. Lastly, our analysis may have been affected by residual confounding, resulting from several factors contributing to the risk of recurrence, such as age, FIGO stage, parametrial involvement and lymph node status, all related to DFS. 27 By using RA‐CUSUM analysis we adjusted for these risk differences between patients but the limited number of events in some variables restricted the comprehensiveness of our model.
Interpretation
Until now, studies highlighting the impact of learning curve on oncological outcomes in gynaecology mainly focused on conventional laparoscopy. A recent retrospective cohort study by Liu et al. 32 showed that the adoption of conventional laparoscopy for the treatment of cervical cancer initially resulted in a significant reduction of DFS survival rates. In the years thereafter, the survival rates in that study gradually improved up to the level before the adoption of conventional laparoscopy, which strongly suggests an effect of a learning curve. 32
Compared with conventional laparoscopy, the learning process of robot‐assisted surgery was originally perceived as shorter. After the adoption of the robot‐assisted approach, it was stated that the three‐dimensional view of robotic laparoscopy allows for a significantly better performance and faster improvement in learning curve than conventional laparoscopy with two‐dimensional view. 33 Which specific part of robot‐assisted surgery contributes to the learning curve the most, remains to be established. From their data on the different parts of robot‐assisted surgery in endometrial cancer, Seamon et al. 34 concluded that hysterectomies – and in particular the closure of the vaginal cuff – had the longest learning curve. Other recent studies suggested an impact of the learning curve on oncological outcomes after robot‐assisted radical hysterectomy in women with cervical cancer. 35 , 36 Chong et al. 36 reported inferior OS after robot‐assisted radical hysterectomy during the learning period compared with conventional laparoscopic radical hysterectomy performed by experienced surgeons, though not significant (P = 0.07). Given the small study size (n = 65) and the absence of a CUSUM analysis, the exact length of the learning curve could not be defined in that study.
Based on our findings, we feel that the key issue is to avoid as much as possible putting women at risk when starting a new surgical procedure. Besides raising awareness of the impact of the learning curve, our results underscore the necessity of centralised health care combined with a validated learning curriculum to make the learning process of an innovative surgical technique as effective and short as possible. 20 , 33 Nowadays, simulation training should be mandatory and followed by robot‐assisted procedures using dual consoles. This allows for direct supervision of new trainees by a certified proctor. Further research in larger populations and other centres is needed to be able to determine to what extent the length of the initial learning phase will be universally applicable. With our results we aim to encourage others to assess their own institutional learning curve. Furthermore, we propose that the learning‐curve effect on oncological outcomes should be included in the design of future studies – including post hoc analyses of existing trials – comparing the safety of innovative and common surgical treatments.
Conclusion
This single‐institution study, with one surgical team over a period of 10 years, suggests the initial learning phase of robot‐assisted laparoscopy in early‐stage cervical cancer to be at least 61 procedures, with a significant increase in DFS and DSS after this initial learning phase. Overall, robot‐assisted laparoscopy in patients with early‐stage cervical cancer shows high survival rates, comparable with observational results both historically after open surgery as well as currently in national surveys of robot‐assisted radical hysterectomies, when performed by an experienced surgical team. The impact of the learning curve of robot‐assisted surgery on oncological outcomes warrants more attention and should be included in future studies on the safety of robot‐assisted surgery.
Disclosure of interests
RPZ is a proctor for robot‐assisted surgery in gynaecological oncology on behalf of Intuitive Surgical. RHMV reports grants and non‐financial support from Medtronic and grants from Intuitive Surgical Inc., all outside the submitted work. All other authors declare they have no conflicts of interests related to the presented research. Completed disclosure of interests forms are available to view online as supporting information.
Contribution to authorship
IGTB, JPH, CGG and RPZ designed the study. RHMV and RPZ performed all robotic surgeries. Data collection was carried out by JPH and IGTB. The statistical analysis was done by IGTB and the subsequent results were interpreted by IGTB, JPH, CGG and RPZ. The manuscript was drafted by IGTB, JPH and CGG, and critically revised and approved by HWRS, IMJS, RHMV and RPZ.
Details of ethics approval
This study was based on the departmental complication and treatment outcome register for robot‐assisted surgery, which is maintained as a part of standard clinical care and primarily aims to improve that care. The institutional review board was consulted and, in accordance to Dutch law, waived approval requirements for the use of the fully anonymised data.
Funding
None.
Supporting information
Appendix S1. RA‐CUSUM functions.
Supplementary Material
Baeten IGT, Hoogendam JP, Schreuder HWR, Jürgenliemk-Schulz IM, Verheijen RHM, Zweemer RP, Gerestein CG. The influence of learning curve of robot-assisted laparoscopy on oncological outcomes in early-stage cervical cancer: an observational cohort study. BJOG 2021; 128:563–571.
This article is commented on by H Falconer, p. 572 in this issue. To view this mini commentary visit https://doi.org/10.1111/1471-0528.16485.
References
- 1. Jin YM, Liu SS, Chen J, Chen YN, Ren CC. Robotic radical hysterectomy is superior to laparoscopic radical hysterectomy and open radical hysterectomy in the treatment of cervical cancer. PLoS One 2018;13:e0193033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang SS, Ding T, Cui ZH, Lv Y, Jiang RA. Efficacy of robotic radical hysterectomy for cervical cancer compared with that of open and laparoscopic surgery: a separate meta‐analysis of high‐quality studies. Medicine (Baltimore) 2019;98:e14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shazly SA, Murad MH, Dowdy SC, Gostout BS, Famuyide AO. Robotic radical hysterectomy in early stage cervical cancer: a systematic review and meta‐analysis. Gynecol Oncol 2015;138:457–71. [DOI] [PubMed] [Google Scholar]
- 4. Piedimonte S, Czuzoj‐Shulman N, Gotlieb W, Abenhaim HA. Robotic radical hysterectomy for cervical cancer: a population‐based study of adoption and immediate postoperative outcomes in the United States. J Minim Invasive Gynecol 2019;26:551–7. [DOI] [PubMed] [Google Scholar]
- 5. Hoogendam JP, Verheijen RH, Wegner I, Zweemer RP. Oncological outcome and long‐term complications in robot‐assisted radical surgery for early stage cervical cancer: an observational cohort study. BJOG 2014;121:1538–45. [DOI] [PubMed] [Google Scholar]
- 6. Shah CA, Beck T, Liao JB, Giannakopoulos NV, Veljovich D, Paley P. Surgical and oncologic outcomes after robotic radical hysterectomy as compared to open radical hysterectomy in the treatment of early cervical cancer. J Gynecol Oncol 2017;28:e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sert BM, Boggess JF, Ahmad S, Jackson AL, Stavitzski NM, Dahl AA, et al. Robot‐assisted versus open radical hysterectomy: a multi‐institutional experience for early‐stage cervical cancer. Eur J Surg Oncol 2016;42:513–22. [DOI] [PubMed] [Google Scholar]
- 8. Mendivil AA, Rettenmaier MA, Abaid LN, Brown JV III, Micha JP, Lopez KL, et al. Survival rate comparisons amongst cervical cancer patients treated with an open, robotic‐assisted or laparoscopic radical hysterectomy: a five year experience. Surg Oncol 2016;25:66–71. [DOI] [PubMed] [Google Scholar]
- 9. Ramirez PT, Frumovitz M, Pareja R, Lopez A, Vieira M, Ribeiro R, et al. Minimally invasive versus abdominal radical hysterectomy for cervical cancer. N Engl J Med 2018;379:1895–904. [DOI] [PubMed] [Google Scholar]
- 10. Melamed A, Margul DJ, Chen L, Keating NL, Del Carmen MG, Yang J, et al. Survival after minimally invasive radical hysterectomy for early‐stage cervical cancer. N Engl J Med 2018;379:1905–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cusimano MC, Baxter NN, Gien LT, Moineddin R, Liu N, Dossa F, et al. Impact of surgical approach on oncologic outcomes in women undergoing radical hysterectomy for cervical cancer. Am J Obstet Gynecol 2019;221:619.e1–24. [DOI] [PubMed] [Google Scholar]
- 12. Uppal S, Gehrig P, Vetter MH, Davidson BA, Lees BF, Brunette LL, et al. Recurrence rates in cervical cancer patients treated with abdominal versus minimally invasive radical hysterectomy: a multi‐institutional analysis of 700 cases. J Clin Oncol 2019;37 (15 Suppl):5504. [DOI] [PubMed] [Google Scholar]
- 13. National Cancer Registration and Analysis Service (NCRAS) . NCRAS cervical cancer radical hysterectomy analysis May 2019 final [Internet]. 2019. [www.bgcs.org.uk/ncras‐cervical‐cancer‐radical‐hysterectomy‐analysis/]. Accessed 27 January 2020.
- 14. Alfonzo E, Wallin E, Ekdahl L, Staf C, Radestad AF, Reynisson P, et al. No survival difference between robotic and open radical hysterectomy for women with early‐stage cervical cancer: results from a nationwide population‐based cohort study. Eur J Cancer 2019;116:169–77. [DOI] [PubMed] [Google Scholar]
- 15. Matanes E, Abitbol J, Kessous R, Kogan L, Octeau D, Lau S, et al. Oncologic and surgical outcomes of robotic versus open radical hysterectomy for cervical cancer. J Obstet Gynaecol Can 2019;41:450–8. [DOI] [PubMed] [Google Scholar]
- 16. Chen L, Liu LP, Wen N, Qiao X, Meng YG. Comparative analysis of robotic vs laparoscopic radical hysterectomy for cervical cancer. World J Clin Cases 2019;7:3185–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yim GW, Kim SW, Nam EJ, Kim S, Kim YT. Learning curve analysis of robot‐assisted radical hysterectomy for cervical cancer: initial experience at a single institution. J Gynecol Oncol 2013;24:303–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schreuder HW, Zweemer RP, van Baal WM, van de Lande J, Dijkstra JC, Verheijen RH. From open radical hysterectomy to robot‐assisted laparoscopic radical hysterectomy for early stage cervical cancer: aspects of a single institution learning curve. Gynecol Surg 2010;7:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Heo YJ, Kim S, Min KJ, Lee S, Hong JH, Lee JK, et al. The comparison of surgical outcomes and learning curves of radical hysterectomy by laparoscopy and robotic system for cervical cancer: an experience of a single surgeon. Obstet Gynecol Sci 2018;61:468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Schreuder HW, Wolswijk R, Zweemer RP, Schijven MP, Verheijen RH. Training and learning robotic surgery, time for a more structured approach: a systematic review. BJOG 2012;119:137–49. [DOI] [PubMed] [Google Scholar]
- 21. Abboudi H, Khan MS, Guru KA, Froghi S, de Win G, Van Poppel H, et al. Learning curves for urological procedures: a systematic review. BJU Int 2014;114:617–29. [DOI] [PubMed] [Google Scholar]
- 22. Doumerc N, Yuen C, Savdie R, Rahman MB, Rasiah KK, Pe Benito R, et al. Should experienced open prostatic surgeons convert to robotic surgery? The real learning curve for one surgeon over 3 years. BJU Int 2010;106:378–84. [DOI] [PubMed] [Google Scholar]
- 23. Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103–4. [DOI] [PubMed] [Google Scholar]
- 24. Hoogendam JP, Hobbelink MG, Veldhuis WB, Verheijen RH, van Diest PJ, Zweemer RP. Preoperative sentinel node mapping with (99m)Tc‐nanocolloid SPECT‐CT significantly reduces the intraoperative sentinel node retrieval time in robot assisted laparoscopic cervical cancer surgery. Gynecol Oncol 2013;129:389–94. [DOI] [PubMed] [Google Scholar]
- 25. Steiner SH, Cook RJ, Farewell VT, Treasure T. Monitoring surgical performance using risk‐adjusted cumulative sum charts. Biostatistics 2000;1:441–52. [DOI] [PubMed] [Google Scholar]
- 26. Bolsin S, Colson M. The use of the Cusum technique in the assessment of trainee competence in new procedures. Int J Qual Health Care 2000;12:433–8. [DOI] [PubMed] [Google Scholar]
- 27. Cibula D, Abu‐Rustum NR, Dusek L, Zikan M, Zaal A, Sevcik L, et al. Prognostic significance of low volume sentinel lymph node disease in early‐stage cervical cancer. Gynecol Oncol 2012;124:496–501. [DOI] [PubMed] [Google Scholar]
- 28. Parisi A, Scrucca L, Desiderio J, Gemini A, Guarino S, Ricci F, et al. Robotic right hemicolectomy: analysis of 108 consecutive procedures and multidimensional assessment of the learning curve. Surg Oncol 2017;26:28–36. [DOI] [PubMed] [Google Scholar]
- 29. Wenzel HHB, Smolders RGV, Beltman JJ, Lambrechts S, Trum HW, Yigit R, et al. Survival of patients with early‐stage cervical cancer after abdominal or laparoscopic radical hysterectomy: a nationwide cohort study and literature review. Eur J Cancer 2020;133:14–21. [DOI] [PubMed] [Google Scholar]
- 30. Steiner SH, Woodall WH. Debate: what is the best method to monitor surgical performance? BMC Surg 2016;16:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van Zanten F, Schraffordt Koops SE, Pasker‐de Jong PCM, Lenters E, Schreuder HWR. Learning curve of robot‐assisted laparoscopic sacrocolpo(recto)pexy: a cumulative sum analysis. Am J Obstet Gynecol 2019;221:483.e1–11. [DOI] [PubMed] [Google Scholar]
- 32. Liu Y, Li L, Wu M, Ma S, Tan X, Zhong S, et al. The impact of the surgical routes and learning curve of radical hysterectomy on the survival outcomes in stage IB cervical cancer: a retrospective cohort study. Int J Surg 2019;68:72–7. [DOI] [PubMed] [Google Scholar]
- 33. Blavier A, Gaudissart Q, Cadiere GB, Nyssen AS. Comparison of learning curves and skill transfer between classical and robotic laparoscopy according to the viewing conditions: implications for training. Am J Surg 2007;194:115–21. [DOI] [PubMed] [Google Scholar]
- 34. Seamon LG, Fowler JM, Richardson DL, Carlson MJ, Valmadre S, Phillips GS, et al. A detailed analysis of the learning curve: robotic hysterectomy and pelvic‐aortic lymphadenectomy for endometrial cancer. Gynecol Oncol 2009;114:162–7. [DOI] [PubMed] [Google Scholar]
- 35. Wallin E, Floter Radestad A, Falconer H. Introduction of robot‐assisted radical hysterectomy for early stage cervical cancer: impact on complications, costs and oncologic outcome. Acta Obstet Gynecol Scand 2017;96:536–42. [DOI] [PubMed] [Google Scholar]
- 36. Chong GO, Lee YH, Lee HJ, Hong DG, Lee YS. Comparison of the long‐term oncological outcomes between the initial learning period of robotic and the experienced period of laparoscopic radical hysterectomy for early‐stage cervical cancer. Int J Gynecol Cancer 2018;28:226–32. [DOI] [PubMed] [Google Scholar]
- 37. Zaal A, Zweemer RP, Zikan M, Dusek L, Querleu D, Lecuru F, et al. Pelvic lymphadenectomy improves survival in patients with cervical cancer with low‐volume disease in the sentinel node: a retrospective multicenter cohort study. Int J Gynecol Cancer 2014;24:303–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. RA‐CUSUM functions.
Supplementary Material


