Abstract
Background
The deterioration of the skin accentuates over time, affecting its aesthetic appearance. This is characterized by the weakening of the mechanisms involved in the regeneration and repair of the dermal matrix. Consequently, the skin losses elasticity and smoothness resulting in the formation of wrinkles. The alternatives for facial rejuvenation include surgery, injection of botulinum toxin, and the application of masks. Topic products are less invasive, can be self‐applied, and have an increased benefit/risk relationship.
Aim
We developed a liquid formulation containing collagen hydrolyzed and evaluated the product by cutting‐edge technology in order to define proper its quality attributes.
Methods
We employed nuclear magnetic resonance (NMR), size‐exclusion chromatography (SEC), and mass spectrometry (MS). Additionally, we analyzed its cosmetical effect in five volunteers and we demonstrate the product safety.
Results
Our results demonstrate the following: (a) a stable secondary structure identity associated to the known triple helix arrangement in liquid and solid states; (b) a typical conformational flexibility depending on its hydration state; (c) thermal stability confirmed by liquid‐ and solid‐state nuclear magnetic resonance schemes; and (d) a molecular mass distribution of peptides between 0.5 and 19.5 kDa. The product faded wrinkles in the forehead, an effect that remained after removing the mask. The formula was non‐irritating and hypoallergenic.
Conclusion
We characterized, using state‐of‐the‐art methodologies, the quality attributes that are critical for the safety and beneficial effect of a new collagen‐containing formula.
Keywords: hydrolyzed collagen, liquid formulation, quality attributes cosmetic, safety cosmetic product, skin aging
1. INTRODUCTION
Advances in medicine have increased the global life expectancy at birth. The natural aging produces progressive loss of functionality of all organs and tissues, including the skin (the body's largest organ). In the face, aging produces wrinkles and expression lines, mainly in the forehead region, rictus, frown, and around the eyes. 1 Several factors are involved in the aging process of the skin, which include exposure to the ultraviolet light and pollution, repetitive facial expressions, gravity, sleeping position, loss of subcutaneous support, tooth loss, and smoking. 2 The mechanisms involved in skin aging include the following: (a) The oxidative stress, which has the greatest impact, caused by reactive oxygen species (ROS) produced by UV‐radiation, xenobiotics, and environmental influences (pollution) 3 ; (b) Mitochondrial DNA (mtDNA) mutations in genes encoding key enzymes in the respiratory chain, thus disrupting oxidative phosphorylation and increasing ROS 4 ; (c) Telomere shortening that occurs during DNA replication and prevents aberrant cell proliferation in the skin could be affected by UV‐radiation; and (d) Hormonal changes during menopause and andropause decline the level of testosterone, resulting in decreased elasticity, density, and collagen content of the skin. 5
To date, there are several treatment options available for the rejuvenation of skin, being the most common: (a) Surgery, which despite presenting significant advances in the field, requires a long recovery period and still remains invasive and painful. Further, it can produce a temporary or permanent injury to the peripheral nerves resulting in negative functional and psychological consequences for patients. 6 (b) Injection of botulinum toxin, where a broad spectrum of adverse effects may occur, from the lack of effect, local reactions in the site of injection, ptosis, or even deaths associated to allergic reactions. 7 (c) Non‐invasive mask‐based treatments, performed with formulations containing complex mixtures of components (plasticizers, moisturizers, preservatives, surfactants, fragrances, and active substances) in the same application. Although the formulations are commonly safe, their beneficial effect on the skin is variable and commonly it is unclear which component is most important in the effect. 8
The resistant solid state of tissues and joints is partially provided by collagen; thus, it is a common component of mask‐based treatments. Collagen administration using liquid formulations requires its solubilization since it naturally occurs in a fibrillar structure. 9 The most effective treatment to solubilize collagen is achieved by chemical or enzymatic partial hydrolysis, a critical process that generates a distribution of collagen fragments. The physicochemical characteristics of those fragments are key to obtain the desired physicochemical properties for its intended use and to increase their preservation in the formulation. Thus, it is necessary to have an analytical platform capable to properly determine their distribution profile. Currently, there are advanced analytical methodologies suitable for this purpose such as mass spectrometry (MS), nuclear magnetic resonance (NMR), and size‐exclusion chromatography (SEC), which have been previously employed for establishing the quality attributes of collagen hydrolysates in liquid formulations. 10
Furthermore, defining the physicochemical characteristics of the collagen‐derived peptides allows the production of batches with hypoallergenicity, as is required for any cosmetic product. 11
Herein, we report the development of a partially hydrolyzed collagen in a liquid formulation to be employed as a mask for skin care. The quality of the mask was evaluated by cutting‐edge technology, including NMR (nuclear magnetic resonance), size‐exclusion chromatography (SEC), and mass spectrometry (MS). The results allowed to define the physicochemical properties of the product as quality attributes. The safety of its formulation was tested on rabbits and healthy volunteers, and its effectiveness was demonstrated by applying the product to five healthy volunteers and evaluating the beneficial effect on the skin.
2. MATERIALS AND METHODS
2.1. Materials
Colagenart Beauty Mask® was acquired from LEMAR SAPI de CV. Sodium chloride, and monobasic/dibasic sodium phosphate were acquired from J. T. Baker. Water, formic acid, and acetonitrile (MS grade) and dimethyl sulfoxide (DMSO) (analytical grade) were acquired from Sigma‐Aldrich. Deuterated water solution (99.98%) was acquired from Cambridge Isotope Laboratories, Inc.
2.2. Quality attributes
2.2.1. Appearance
The appearance was evaluated by visual inspection according to international pharmacopoeias. 12
2.2.2. Structural properties and thermostability by NMR
Both liquid‐ and solid‐state NMR (ls‐NMR and ss‐NMR) spectroscopy were carried out in an NMR spectrometer at 14.1 T of magnetic field (equivalent to 600 MHz of proton frequency). CPMG acquisition parameters comprises a pseudo‐2D experiment constructed with TD (F1) points equal to the number of t delays (26 points). T2 calculations were done with the program Bruker Biospin Dynamics Center®.
ls‐NMR: Sample treatment and data acquisition were performed according to our previous study. 10 The following set of NMR experiments were conducted:
{1Hwater_presat NMR}: 1D single pulse NOESY was done as previously described. 10
{1H‐1H} short‐range homonuclear correlation spectra with a water presaturation module [COSYwater_presat] NMR. 10
the experimental details of {1H‐13C} HMBC and 1H Carr‐Purcell‐Meiboom‐Gill (CPMG) pulse sequence are described in Appendix S1.
ss‐NMR: About 5mL of Colagenart Beauty Mask® was dried in an opened Petri dish under a laminar flow cabinet during 24 hours, after that 60 mg of them were mixed with inert powder (KBr) as bulk agent and packed in a 4 mm ss‐NMR zirconium rotor. All spectra were recorded on a Bruker 600 AVANCE NEO equipped with a 4 mm MAS double‐resonance probehead.
Experimental details of standard 1H‐one dimensional NMR, 13C‐direct irradiation, {1H‐13C} Cross‐polarization and 1H Carr‐Purcell‐Meiboom‐Gill spin‐echo trains in the solid state are described in the SI.
2.2.3. Size distribution by SEC and MS
The size exclusion analysis was performed using ultraperformance liquid chromatography (SE‐UPLC), and intact mass of Colagenart Beauty Mask® was determined on a Vion ESI‐IMS‐Q‐TOF spectrometer (Waters®) coupled to an Acquity UPLC Class‐I system, as previously described for the characterization of collagen hydrolysates. 10
2.3. Safety test
2.3.1. Skin and ocular irritability
The skin irritability and ocular irritability test was performed on six New Zealand rabbits of 2‐3.5 kg, maintained under controlled conditions at 16‐26°C, and 40%‐70% of relative humidity according to NOM‐062‐ZOO‐1999.
The back area of each rabbit was shaved and divided into four quadrants, two as intact skin and two as abraded skin. About 0.5 mL of Colagenart Beauty Mask® was applied on the designed areas and covered with a sterile gauze secured with adhesive tape. After 24 hours, the patches were removed. The skin reactions were observed and scored at 24 and 72 hours. 13 About 0.1 mL of Colagenart Beauty Mask® was applied in one eye, keeping another eye untreated as control. Irritability was evaluated at 24, 48, and 72 hours.
2.3.2. Skin irritation and partial sensitization tests
Skin irritation test in humans was performed in ten volunteers who met the requirements established in NOM‐039‐SSA1‐1993. The sample of Colagenart Beauty Mask® (0.3 mL) was applied in three areas on the lateral surface of the arm cleaned with 95% of ethanol, between the shoulder and elbow and removed after 24 hours. The skin irritation was evaluated between 48 and 72 hours post‐application, as well as every seven days, while partial sensitization was assessed until day 28.
2.4. Effectiveness
The test to evaluate the tensor effect in facial skin was performed in five volunteers (one male and four females between 35 and 55 years old). Before the application of Colagenart Beauty Mask®, the face, neck, and neckline were cleaned with soap (pH 4.5‐5.5), using disposable cotton pads and physiological saline solution to remove the soap. The mask was applied using two fingers with circular movements. After 8 minutes, the mask was removed with physiological saline solution.
3. RESULTS AND DISCUSSION
3.1. Quality attributes
3.1.1. Appearance
The liquid formulation is free of visible particles containing a mixture of stabilized peptides in a citrate buffer solution with glycerol (10%), pH 5.0, in congruence with previous studies that demonstrated solubility favoring at low pH. 14 After its application on the face skin, this formula changes from the liquid state to solid state. This change is owed to a modification of the environment of collagen peptides, resulting in structural rearrangements due to electrostatic and hydrophobic interactions between inter‐ and intra‐molecular peptides. 15 The peptides are exposed to a hydrophobic environment by the generation of a thin film on the face skin, which increases the surface area, promoting the exposure of the hydrophobic continuous repeating amino acid sequence constituted by Gly‐X‐Y‐, in where X (Pro) is mostly proline and Y is mostly trans‐4‐hydroxyproline (Hyp). 16 Similarly as those sequences containing hydrophobic amino acids presented in collagen sequence, such as phenylalanine, valine, leucine, isoleucine, and methionine, 17 this hydrophobic environment is also promoted by water evaporation contributing in structural changes that allow the formation of the mask, as was demonstrated by NMR.
3.1.2. Structural properties by NMR
Previous magnetic resonance studies comprising collagen have aware that (a) secondary structure associated to a triple helix arrangement is conservative, but rigid in dry samples and mobile as collagen's hydration is increased; (b) hydration level in collagen is intrinsically linked to its local backbone and side‐chain dynamics, 18 whereas an increase of hydration will promote faster local dynamics; (c) slowly diffusing and highly mobile water molecules are made up of H2O “bounded” moieties, which pass through the collagen fibers, to be linked in turn to hydroxyl functional groups from Hyp residues; (d) said H2O‐4‐hydroxy‐Hyp interaction by hydrogen bridges 19 promotes an important increase on the ensemble molecular mobility of collagen in the solid state.
The present study includes liquid and solid state analyses that reveal structural properties of collagen peptides contained in the Colagenart Beauty Mask® formulation with respect to our previous analysis in liquid state. 10 Assignment strategy first comprised the acquisition of {1H‐1H} short‐range intra‐residual correlation spectrum (COSY) which showed Gly, Pro, Hyp, Phe, Ala, Arg, Asp, Glu, Val, Leu, and Ile spin systems (Figure SI.1). In turn, 13C resonances were assigned with the aid of {1H‐13C} scalar long‐range intra‐residual HMBC correlation spectrum in liquid state (Figure SI.2, Table SI.1). 13C NMR fingerprints in the liquid state were compared with those corresponding to solid‐state NMR studies. 20 While 13C‐MAS direct irradiation produced a spectrum with narrow signals corresponding to mobile glycerol matrix, the 13C‐CPMAS revealed a spectrum that roughly matches its liquid‐state counterpart (Figure 1). Deeper analysis of 13C stacked plots in both hydrated (liquid) and dehydrated (solid) collagen peptides revealed that glycine's Cα frequency region (39.37‐44.8 ppm Figure 1) is showed as a set of visible resonances in the solid‐state, despite its dehydrated condition. These results confirm the approximately 30% of glycine as major content in collagen species 18 and even, its rigid property mainly in the backbone regions with lack of water “bounded” moieties, in agreement with previous works in collagen fibrils. 21
FIGURE 1.

13C one‐dimensional spectra of Colagenart Beauty Mask® in the liquid (red) and solid state (black) regimes. Solid‐state NMR 13C spectra comprise, respectively, a carbon direct excitation with 1H decoupling scheme (top) and {1H‐13C} CPMAS experiment (middle), both at 10 kHz MAS. Carbon spin systems were assigned from {1H‐13C} correlation spectra and reference in the solid state was done by matching the 13C resonance at 72.0 ppm with its liquid‐state counterpart that corresponds to a sharp glycerol buffer signal (upper spectrum)
The hydration‐dependent aggregation of collagen species from triple helix tropocollagen microfibrils, larger fibrils up to fibers, has been associated to the local dynamics of both water and collagen macromolecular species and experimentally observed by both T2 transverse relaxation times and translational diffusions, 22 with the following typical trend: Shorter T2 values are related to slower translational diffusion at different collagen aggregation states. The assigned resonances in liquid state of Colagenart Beauty Mask® to Phe, Pro, and glycerol buffer showed a T2 values of 197 ± 1.1, 111 ± 1.0 and 118 ± 7.2 ms, respectively; while in solid state the T2 values were 7.66 ± 0.42, 1.1 ± 0.24 and 34.8 ± 0.25 ms, respectively (Figure 2). In the solid state, an expected order of magnitude of T2 decreased as a function of its change of the physical state associated to dehydration, mainly a fast T2 relaxation value of Pro moieties indicates the key role of these amino acids during gelation. Additionally, as the T2 value signal of glycerol buffer is slower with respect to Phe and Pro moieties of collagen, demonstrates that the excipients contained in the formulation do not participate in the faster translational dynamics of collagen peptides (Figure 2). Unlike, T2 relaxation values of Phe, Pro and glycerol citrate buffer solution showed the same order of magnitude in liquid state, suggesting a co‐participation of glycerol in the stability of collagen in liquid formulation.
FIGURE 2.

Proton spin‐spin relaxation times (T2) of Colagenart Beauty Mask® in the liquid (left curves) and solid state (right curves) regimes of three assigned resonances: Phe aromatic proton signal at 7.13 ppm (top), Pro NH residual signals at 8.51 ppm (middle) and glycerol signal at 3.3 ppm (bottom). T2 values were obtained from the signal attenuation decay, fitted with the expression M(t) = M0[exp(−t/T2)], and calculated T2 values (in milliseconds) are highlighted at each decay curve
3.1.3. Thermostability by NMR
In order to obtain site‐specific information related to the thermostability of the Colagenart Beauty Mask® liquid collagen formulation, a set of temperature‐dependent proton NMR schemes were carried out.
The standard one dimensional 1H NMR spectra of Colagenart Beauty Mask® in the range 25‐75°C with increments of 10°C revealed a systematic shift of resonances of glycerol and side‐chain residues of partially hydrated moieties (Figure 3D, 3.8‐0.5 ppm). However, residual NH backbone resonances does not suffer a significant change (Figure 3C, 9.0‐7.7 ppm), which showed a conservation of the secondary structure that shall confer stability in the present proposed liquid formulation (Figure 3).
FIGURE 3.
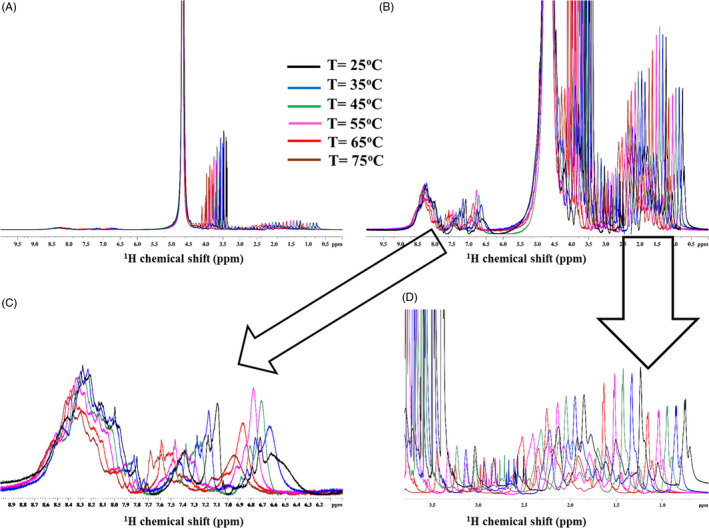
1H one‐dimensional spectra of Colagenart Beauty Mask® at different temperatures T: 25°C (black), 35°C (blue), 45°C (green), 55°C (magenta), 65°C (red), and 75°C (brown) and with the following projection: (A) Full spectra with absolute signal intensity at the level of water and glycerol resonances; (B) Full spectra with absolute signal intensity at the level of amino acid resonances; (C) Expansion at the NH backbone and aromatic signals (9.0‐7.7 ppm); and (D) Expansion at the protons’ side‐chain resonances (3.8‐0.5 ppm)
3.1.4. Size distribution by SEC and MS
According to SEC, the size distribution of a complex mixture of peptides was found in the range 1.35‐17 kDa (Figure 4). It was confirmed using a cutting‐edge MS analytic technique, which showed a mass range 0.5‐19.5 KDa (Figure 5). The size distribution determined by SEC is a routine analytical method used as release test to demonstrate the consistency of the product between batches, similar as was done for oral collagen hydrolyzed. 10
FIGURE 4.

Peptide polydispertion distribution by size‐exclusion chromatography. Molecular weight of Colagenart Beauty Mask® peptides (black) in the range 1.35‐17 kDa according to the molecular weight standards (gray) thyroglobulin/gamma‐globulin (>80 kDa), ovalbumin (44.2 kDa), equine myoglobin (17.0 kDa), and B12 vitamin (1.35 kDa)
FIGURE 5.

Exact mass analysis. Deconvoluted mass spectrum of the peptide distribution of Beauty Mask® shows a peptide distribution from 0.5 to 19.5 kDa
3.2. Safety
Skin irritability results showed collagen mask product safety, it was based on the observed average score of 0.06 and 0.17 after 72 hours for intact and abraded skin, respectively (Table 1); where the minimum grade is 0 which means no erythema or no edema and the maximum grade is 4 which means severe erythema or edema. 23 Equally, the results obtained from ocular irritability showed non‐positive reaction for all of the observed times at 24, 48, and 72 hours (data not shown). These results reduce the uncertainty of an unexpected reaction when the mask is used in humans, and also provide information to demonstrate product safety. It is well known that collagen is well tolerated when is used orally. 24 Here, we demonstrated that also it is well tolerated when is used topically.
TABLE 1.
Skin irritability tested in rabbits
| Rabbit | Intact skin | Abraded skin | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | ||||||
| Edema | Erythema | Edema | Erythema | Edema | Erythema | Edema | Erythema | ||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 2 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||
| 4 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||
| 5 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 6 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | |
| 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | ||
| ΣX | 3 | 0 | 0 | 0 | 8 | 0 | 0 | 0 | |
|
|
0.25 | 0 | 0 | 0 | 0.67 | 0 | 0 | 0 | |
| Σ /n | 0.06 | 0.17 | |||||||
Mask safety results in healthy human volunteers showed to be hypoallergenic and non‐irritating (Table SI.2). Equally, partial sensitization results showed non‐positive reaction for all of the observed times (data not shown)
3.3. Effectiveness
The non‐invasive treatment of the collagen mask for routinely use in the face skin care showed a high performance, since revealed an effective reduction of wrinkles and expression lines after 8 minutes of application in five volunteers. In all cases, Colagenart Beauty Mask® provided a tensor effect, a radiant and luminous appearance and improvement of the quality and softness of skin, similarly to another products which have shown an immediate positive effect and improvements in the appearance of fine and coarse wrinkles. 25
4. CONCLUSIONS
According to results of liquid‐ and solid‐state NMR, SEC and MS results, Colagenart Beauty Mask® showed a set of physicochemical properties that support their quality attributes, which can be related to its appearance and formulation stability in liquid state. Further, the Colagenart Beauty Mask® effectiveness as a cosmetic product was demonstrated in volunteers, as an alternative to reduce the natural effects of skin aging in face, neck and neck line. In accordance with international guidelines for cosmetics, irritability and hypoallergenicity tests demonstrated that Colagenart Beauty Mask® safe for its topical administration in healthy users.
Supporting information
Appendix S1
ACKNOWLEDGMENTS
Authors thank CONACyT who granted resources for our laboratory through Laboratorio Nacional para Servicios Especializados de Investigación, Desarrollo e Innovación (I+D+i) para Farmoquimicos y Biotecnológicos (LANSEIDI‐FarBiotec‐ CONACyT). Luis Gerardo Zepeda‐Vallejo thanks CONACyT (Grant INFRA 269012) and IPN (FIDEICOMISO) for the adquisition of the 600 MHz NMR instrument.
Mejía‐Calvo I, López‐Juárez LE, Vázquez‐Leyva S, et al. Quality attributes of partially hydrolyzed collagen in a liquid formulation used for skin care. J Cosmet Dermatol. 2021;20:150–158. 10.1111/jocd.13439
Contributor Information
Sonia M. Pérez‐Tapia, Email: sperezt@ipn.mx.
Emilio Medina‐Rivero, Email: emilio.medina@udibi.com.mx.
REFERENCES
- 1. Kligman M, Zheng P, Lavker RM. The anatomy and pathogenesis of wrinkles. Br J Dermatol. 1985;113(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 2. Jenkins G. Molecular mechanisms of skin ageing. Mech Ageing Dev. 2002;123(7):801‐810. [DOI] [PubMed] [Google Scholar]
- 3. Rinnerthaler M, Bischof J, Streubel MK, Trost A, Richter K. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545‐589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stout R, Birch‐Machin M, Stout R, Birch‐Machin M. Mitochondria's role in skin ageing. Biology (Basel). 2019;8(2):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Thornton MJ. Estrogens and aging skin. Dermatoendocrinol. 2013;5(2):264‐270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Azizzadeh B, Mashkevich G. Injuries and treatment in facial cosmetic surgery. Oral Maxillofac Surg Clin North America. 2009;21(1):23‐29. [DOI] [PubMed] [Google Scholar]
- 7. Coté TR, Mohan AK, Polder JA, Walton MK, Braun MM. Botulinum toxin type A injections: adverse events reported to the US Food and Drug Administration in therapeutic and cosmetic cases. J Am Acad Dermatol. 2005;53(3):407‐415. [DOI] [PubMed] [Google Scholar]
- 8. Velasco MVR, Vieira RP, Fernandes AR,et al. Short‐term clinical of peel‐off facial mask moisturizers. Int J Cosmet Sci. 2014;36(4):355‐360. [DOI] [PubMed] [Google Scholar]
- 9. Friess W. Collagen – biomaterial for drug delivery. Eur J Pharm Biopharm. 1998;45(2):113‐136. [DOI] [PubMed] [Google Scholar]
- 10. López‐Morales A, Vázquez‐Leyva S, Vallejo‐Castillo L, et al. Determination of peptide profile consistency and safety of collagen hydrolysates as quality attributes. J Food Sci. 2019;84(3):430‐439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nawanopparatsakul S, Euasathien J, Eamtawecharum C, et al. Skin irritation test of curcuminoids facial mask containing chitosan as a binder skin irritation test of curcuminoids facial mask containing chitosan as a binder. Silpakorn Univ Int J. 2005;5:140‐147. [Google Scholar]
- 12. USP Chapter <1790> Visual Inspection of Injections. Rockville, MD: USP Pharmacopeical Convention; 2017. [Google Scholar]
- 13. Mexican Pharmacopeia . MGA 0515 Irritabilidad en piel. Mexico City, Mexico: Comisión Permanente de la Farmacopea de los Estados Unidos Mexicanos; 2018. [Google Scholar]
- 14. Li Y, Asadi A, Monroe MR, Douglas EP. pH effects on collagen fibrillogenesis in vitro: electrostatic interactions and phosphate binding. Mater Sci Eng C. 2009;29(5):1643‐1649. [Google Scholar]
- 15. Achilli M, Mantovani D. Tailoring mechanical properties of collagen‐based scaffolds for vascular tissue engineering: the effects of pH, temperature and ionic strength on gelation. Polymers (Basel). 2010;2(4):664‐680. [Google Scholar]
- 16. Gómez‐Guillén MC, Giménez B, López‐Caballero ME, Montero MP. Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll. 2011;25(8):1813‐1827. [Google Scholar]
- 17. Shoulders MD, Raines RT. Collagen structure and stability. Annu Rev Biochem. 2009;78(1):929‐958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reichert O, Pascui ER, DeAzevedo TJ, Bonagamba KA, Huster D. A solid‐state NMR study of the fast and slow dynamics of collagen fibrils at varying hydration levels. Magn Reson Chem. 2004;42(2):276‐284. [DOI] [PubMed] [Google Scholar]
- 19. Knauss R, Fleischer G, Gründer W, Kärger J, Werner A. Pulsed field gradient NMR and nuclear magnetic relaxation studies of water mobility in hydrated collagen II. Magn Reson Med. 1996;36(2):241‐248. [DOI] [PubMed] [Google Scholar]
- 20. Mroue KH, Xu J, Zhu P, Morris MD, Ramamoorthy A. Selective detection and complete identification of triglycerides in cortical bone by high‐resolution 1 H MAS NMR spectroscopy. Phys Chem Chem Phys. 2016;18(28):18687‐18691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Traore A, Foucat L, Renou JP. 1H‐NMR study of water dynamics in hydrated collagen: transverse relaxation‐time and diffusion analysis. Biopolymers. 2000;53(6):476‐483. [DOI] [PubMed] [Google Scholar]
- 22. Peto S, Gillis P, Henri VP. Structure and dynamics of water in tendon from NMR relaxation measurements. Biophys J. 1990;57(1):71‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. OECD . OECD guideline 431 test no. 404: acute dermal irritation/corrosion. Paris, France: OECD; 2015. [Google Scholar]
- 24. Kim D‐U, Chung H‐C, Choi J, Sakai Y, Lee B‐Y. Oral intake of low‐molecular‐weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: a randomized, double‐blind, placebo‐controlled study. Nutrients. 2018;10(7):826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trookman NS, Rizer RL, Ford R, Ho E, Gotz V. Immediate and long‐term clinical benefits of a topical treatment for facial lines and wrinkles. J Clin Aesthet Dermatol. 2009;2(3):38‐43. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1


