Abstract
Background
Risankizumab is a humanized IgG monoclonal antibody that selectively inhibits interleukin‐23 through binding the p19 subunit. In Phase 3 trials, risankizumab demonstrated superior efficacy compared with adalimumab and ustekinumab in patients with moderate‐to‐severe plaque psoriasis. Here, we evaluated the impact of baseline characteristics on efficacy of risankizumab compared with ustekinumab in patients with moderate‐to‐severe plaque psoriasis.
Methods
This analysis included all patients initially randomized to risankizumab or ustekinumab from the replicate, double‐blinded, randomized, placebo‐controlled phase 3 trials, UltIMMa‐1 (NCT02684370) and UltIMMa‐2 (NCT02684357). Patients received either risankizumab (150 mg) or ustekinumab (weight‐based; 45 or 90 mg per label) at weeks 0, 4, 16, 28 and 40. Efficacy was assessed as the proportion of patients achieving ≥90% improvement in Psoriasis Area and Severity Index (PASI 90) at weeks 16 and 52 by baseline patient demographics, disease characteristics and prior biologic exposure. Mean per cent improvement in PASI was calculated by body weight and body mass index at week 52. Missing efficacy data were imputed as non‐responders for categorical variables and last observation carried forward for continuous variables. Logistic regression analyses assessed for interactions between treatment and five independent variables (age, sex, weight, baseline PASI score and presence of psoriatic arthritis) at both weeks 16 and 52.
Results
Baseline patient demographics, disease characteristics and prior biologic exposure were similar between patients randomized to risankizumab (n = 598) and ustekinumab (n = 199). At weeks 16 and 52, risankizumab demonstrated superior efficacy compared with ustekinumab across these patient characteristics (P < 0.01). Logistic regression analyses demonstrated that risankizumab was superior to ustekinumab at weeks 16 and 52 in all models tested (P < 0.0001 for all).
Conclusions
Risankizumab demonstrated consistent and superior efficacy compared with ustekinumab regardless of patient demographics, disease characteristics or prior biologic exposure.
Introduction
Psoriasis is a chronic immune‐mediated disease that affects an estimated 100 million people worldwide. 1 , 2 Patients with moderate‐to‐severe psoriasis are at increased risk of comorbidities, including obesity. 3 , 4 Although recent scientific advances have led to highly efficacious treatments for moderate‐to‐severe psoriasis, lower efficacy has been reported with biologic treatments in patients with comorbid obesity or in those with prior biologic exposure and/or failure. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 Thus, there is a need for psoriasis treatments to demonstrate consistently high efficacy regardless of patient demographics or prior biologic therapy.
Recent preclinical and clinical findings demonstrated that interleukin‐23 (IL‐23) is a regulatory cytokine critical for the development and maintenance of psoriatic inflammation. 17 Preclinical research established that IL‐23 activates T helper and other cells to produce key cytokines and drive psoriatic inflammation. In clinical trials of patients with moderate‐to‐severe plaque psoriasis, efficacy has been demonstrated by an IL‐12/IL‐23 inhibitor, ustekinumab and, more recently, by the selective IL‐23p19 inhibitors, guselkumab, tildrakizumab and risankizumab, further supporting the role of IL‐23 in psoriasis. 18 , 19 , 20 , 21 , 22 , 23
Risankizumab is a humanized IgG monoclonal antibody that binds with high affinity and specificity to the p19 subunit and selectively inhibits IL‐23. 24 , 25 Exposure‐response analyses of Phase 2 and 3 trials demonstrated that, following initial doses at weeks 0 and 4, the every 12‐week dosing of risankizumab (150 mg) led to maximal efficacy at weeks 16 and 52. 26 In phase 3 trials (IMMhance, UltIMMa‐1, UltIMMa‐2, and IMMvent), risankizumab demonstrated superior efficacy compared with placebo, ustekinumab and adalimumab at week 16 that was sustained compared with adalimumab and ustekinumab at weeks 44 and 52, respectively. 22 , 23 , 27 Overall, risankizumab has demonstrated early and sustained high skin clearance with 12‐week dosing in patients with moderate‐to‐severe psoriasis. 22 , 23 To date, however, the association of baseline disease or prior treatment variables on risankizumab’s efficacy has not been reported.
Here, using integrated data from UltIMMa‐1 and UltIMMa‐2, we assessed the impact of baseline patient demographics, disease characteristics and prior biologic exposure on the efficacy of risankizumab compared with ustekinumab in patients with moderate‐to‐severe plaque psoriasis.
Methods
Patients
This integrated analysis included all patients initially randomized to risankizumab or ustekinumab in UltIMMa‐1 or UltIMMa‐2. In both trials, adult patients (≥18 years of age) were eligible if they had stable moderate‐to‐severe chronic plaque psoriasis for the preceding ≥6 months (with or without psoriatic arthritis) with body surface area (BSA) involvement of 10% or greater, Psoriasis Area and Severity Index (PASI) 12 or greater, and static Physician’s Global Assessment (sPGA) score 3 or greater. Patients were required to be candidates for systemic therapy, phototherapy and treatment with ustekinumab (according to local label). Prior treatment exposure to IL‐17 and tumour necrosis factor (TNF) inhibitors was allowed depending on timing prior to randomization; prior treatment exposure to ustekinumab or other IL‐23 inhibitors was not permitted. Prior biologic exposure was self‐reported; prior biologic failure was defined as any patient with prior biologic exposure who failed to respond to biologic treatment (primary failure) or failed due to loss of response or lack of tolerability (secondary failures). A complete list of inclusion and exclusion criteria is included in Table S1, Supporting Information).
The trials were conducted in accord with the Good Clinical Practice Guideline, as defined by the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, the Declaration of Helsinki and applicable local regulations. All study‐related documents (including the study protocol) were approved by an institutional review board or institutional ethics committee at each trial site, and all patients provided written informed consent before participation.
Study design
Detailed methodology has been reported previously for the replicate, multi‐part phase 3, multinational, multicenter, randomized, double‐blinded, placebo‐ and active comparator‐controlled trials, UltIMMa‐1 and UltIMMa‐2. 22 Here, we report on patients with moderate‐to‐severe plaque psoriasis who were initially randomized to either risankizumab (150 mg) or ustekinumab (weight‐based; 45 or 90 mg) in Part A (Fig. 1). Randomization was stratified by baseline weight (≤100 kg vs. >100 kg) and prior exposure to TNF inhibitor (yes vs. no). Patients initially randomized to receive risankizumab or ustekinumab received 150 mg risankizumab or weight‐based ustekinumab (45 mg for patients with body weight ≤100 kg or 90 mg for patients with body weight >100 kg) subcutaneously at weeks 0, 4, 16, 28 and 40.
Figure 1.
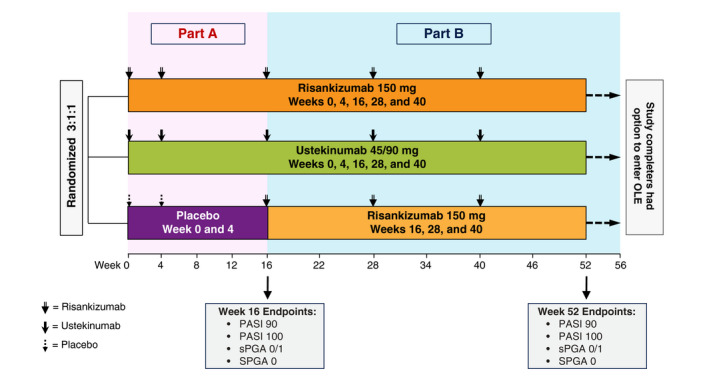
Study design for UltIMMa‐1 and UltIMMa‐2. OLE, open‐label extension; PASI, Psoriasis Area and Severity Index; sPGA, static Physician’s Global Assessment.
Assessments
Efficacy was analysed using pooled data from UltIMMa‐1 and UltIMMa‐2 in all patients initially randomized to either risankizumab or ustekinumab using an intention‐to‐treat analysis. Efficacy was assessed by demographics and baseline characteristics [age, sex, body mass index (BMI), weight, baseline PASI score, baseline sPGA score and presence of psoriatic arthritis] as well as prior treatment experience (prior biologic exposure, TNF inhibitor exposure, IL‐17 inhibitor exposure and prior biologic failure, including failure of 1 or ≥2 biologics and primary or secondary failure). Efficacy outcomes included the proportion of patients achieving ≥90% improvement in PASI compared to baseline (PASI 90) at weeks 16 and 52. Additional efficacy outcomes were the proportion of patients achieving sPGA score of clear or almost clear (sPGA 0 or 1), or sPGA score of clear (sPGA 0), and PASI 100.
Further efficacy analyses were conducted in subgroups at week 52 using the categories of BMI (<25, 25–<30, ≥30), weight (≤100 kg and >100 kg), weight quartiles, and weight deciles in order to fully explore the effect of weight on efficacy. Mean per cent improvement in PASI score from baseline to week 52 was analysed for risankizumab and ustekinumab using last observation carried forward.
Statistical analysis
Patients with missing efficacy data for categorical variables were handled by non‐responder imputation and for continuous variables with last observation carried forward. Categorical variables were tested using the Cochran‐Mantel‐Haenszel risk difference estimate. Per cent change in PASI scores were compared between risankizumab and ustekinumab using analysis of covariance with study, stratum, baseline value and treatment in the model. Logistic regression analyses were conducted for each efficacy endpoint at weeks 16 and 52 to assess the impact on risankizumab efficacy compared with ustekinumab of five independent variables (age, sex, baseline weight, baseline PASI and presence of psoriatic arthritis) and potential interactions between these five independent variables (Table S2, Supporting Information). The Bonferroni correction was used to adjust the critical value of 0.05 for statistical significance in order to account for the multiple comparisons. All statistical analyses were conducted using SAS® version 9.4 (SAS Institute, Inc., Cary, NC, USA) or higher using the UNIX operating system.
Results
Patient demographics and baseline disease characteristics
Overall, 797 patients were randomized to receive either 150 mg risankizumab (n = 598) or 45/90 mg ustekinumab (n = 199) at the start of UltIMMa‐1 or UltIMMa‐2, and were included in this integrated analysis. Demographics and baseline disease characteristics were similar between groups (Table 1). Overall, most were male (69.1%); mean age was 47.3 years [standard deviation (SD): 13.7 years] and mean weight was 90.1 kg (SD: 22.3 kg). Mean PASI score was 20.2 (SD: 7.5) and mean BSA involvement was 25.4% (SD: 15.2%). Prior systemic non‐biologic and biologic therapy exposure was reported in 398 (49.9%) and 295 (37.0%) patients, respectively.
Table 1.
Baseline demographics and disease characteristics of the intention‐to‐treat population
| Baseline characteristics |
Risankizumab (N = 598) |
Ustekinumab† (N = 199) |
|---|---|---|
| Age, years, mean (SD) | 47.2 (13.6) | 47.5 (14.1) |
| Male, n (%) | 415 (69.4%) | 136 (68.3%) |
| Race, white, n (%) | 455 (76.1%) | 165 (82.9%) |
| Weight, kg, mean (SD) | 90.0 (22.4) | 90.4 (22.2) |
| Weight >100 kg, n (%)† | 169 (28.3%) | 56 (28.1%) |
| BMI, kg/m2, mean (SD) | 30.5 (7.0) | 30.4 (6.8) |
| PASI, mean (SD) | 20.6 (7.7) | 19.2 (6.4) |
| sPGA of severe, n (%) | 114 (19.1%) | 33 (16.6%) |
| BSA involvement, %, mean (SD) | 26.2 (15.6) | 23.0 (13.6) |
| Psoriatic arthritis status, n (%)‡ | 159 (26.6%) | 50 (25.1%) |
| Any prior biologic therapy, n (%) | 222 (37.1%) | 73 (36.7%) |
| Prior TNFi exposure, n (%) § | 134 (22.4%) | 43 (21.6%) |
| Prior IL‐17i exposure, n (%) | 111 (18.6%) | 35 (17.6%) |
BMI, body mass index; BSA, body surface area; IL‐17i, interleukin‐17 inhibitor; PASI, Psoriasis Area Severity Index; SD, standard deviation; sPGA, static Physician’s Global Assessment; TNFi, tumour necrosis factor inhibitor.
Weight‐based dose per label.
Stratification factors at randomization.
Diagnosed or suspected.
Efficacy outcomes
Risankizumab demonstrated superior efficacy compared with ustekinumab regardless of patient subgroup (baseline demographics, disease characteristics or prior biologic exposure). Across all patient subgroups analysed, a significantly greater proportion of patients receiving risankizumab achieved PASI 90 responses at week 16 (70.3–82.2%) and week 52 (77.6–85.9%) compared with those receiving ustekinumab (34.6–55.6% and 30.8–56.3%, respectively, all P < 0.01; Fig. 2). Similar results were observed with sPGA 0 or 1, sPGA 0 (data not shown) and PASI 100 responses at week 16 and 52 (Figs S1 and S2, Supporting Information). The per cent improvement in PASI for individual patients taking risankizumab overall and subgroups of patients with PASI of 18 or above at baseline, BMI of 30 or higher, weight of 100 kg or greater, and who failed one or more biologics can be observed in time‐lapse videos provided as Videos [Link], [Link], [Link], [Link], [Link] (Supporting Information).
Figure 2.
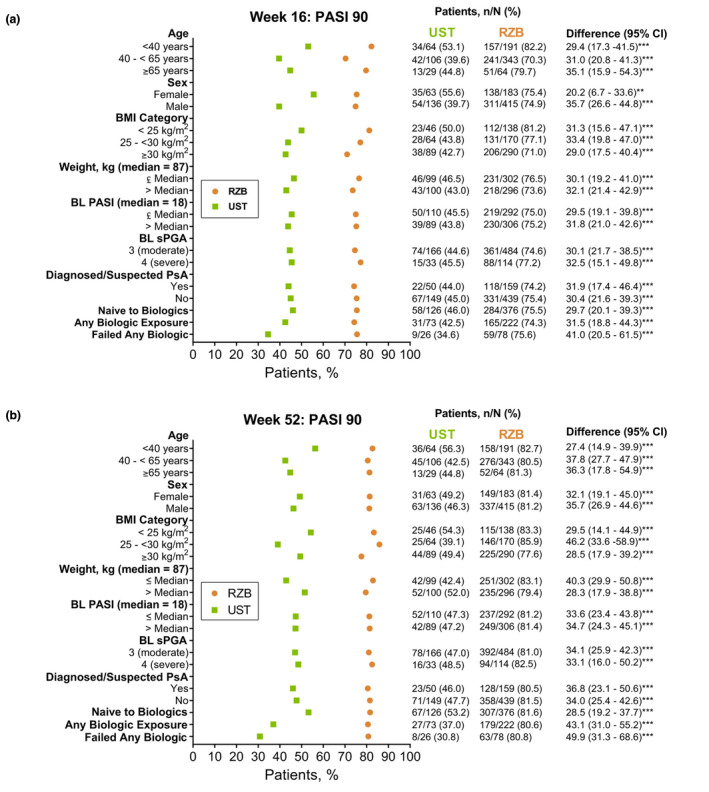
Proportion of patients (non‐responder imputation) achieving PASI 90 responses at week 16 (a) and week 52 (b) by baseline patient demographics, disease characteristics and prior biologic therapy (NRI). BL, baseline; NRI, non‐responder imputation; PASI, Psoriasis Area and Severity Index; PsA, psoriatic arthritis; RZB, risankizumab; sPGA, static Physician’s Global Assessment; UST, ustekinumab. **P < 0.01, ***P < 0.001 compared with ustekinumab.
Patients taking risankizumab achieved statistically greater mean improvement in PASI scores (per cent change from baseline) at week 52 compared with patients receiving ustekinumab by BMI (<25, 25–<30, ≥30), weight (≤100 kg or >100 kg) and weight quartiles (Fig. 3). Mean PASI improvement (per cent change from baseline) among patients receiving risankizumab remained high (88–98%) across all weight deciles at week 52 (Fig. S3, Supporting Information). Representative images of heavier patients with different baseline disease severity (PASI 15, PASI 26 and PASI 33) show substantial PASI improvement in patients treated with risankizumab from baseline to week 52 (Fig. S4, Supporting Information).
Figure 3.
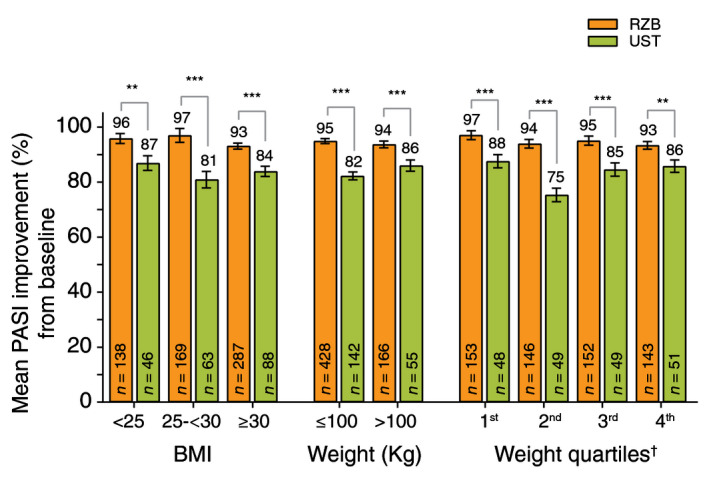
Mean PASI improvement (%) from baseline to week 52 for patients treated with risankizumab and/or ustekinumab by BMI and body weight quartiles (LOCF)†. BMI, body mass index; LOCF, last observation carried forward; PASI, Psoriasis Area and Severity Index; RZB, risankizumab; UST, ustekinumab. **P < 0.01, ***P < 0.001 compared with ustekinumab. Data expressed as mean per cent PASI improvement ± standard error (SE). †Weight quartiles (minimum – maximum) were defined in kg as follows: 1st (43.5–74.0), 2nd (74.2–86.8), 3rd (87.0–103.0) and 4th (103.2–170.0).
A significantly greater proportion of patients receiving risankizumab achieved PASI 90 response at week 16 (73.1–80.2%) and week 52 (78.4–81.6%) compared with those receiving ustekinumab (41.9–46.0% and 32.6–53.2%, respectively, all P < 0.001) regardless of the prior biologic exposure (Fig. 4). For patients with prior biologic failure, a numerically greater proportion of patients receiving risankizumab achieved PASI 90 response at week 16 (70.0–77.6%) and week 52 (70.0–84.5%) compared with those receiving ustekinumab (31.6–37.5% and 15.8–50.0%, respectively) regardless of the number or type of prior failures (Fig. 4). Similar results were observed with sPGA 0 or 1, sPGA 0 (data not shown) and PASI 100 responses (Figs S5 and S6, Supporting Information).
Figure 4.
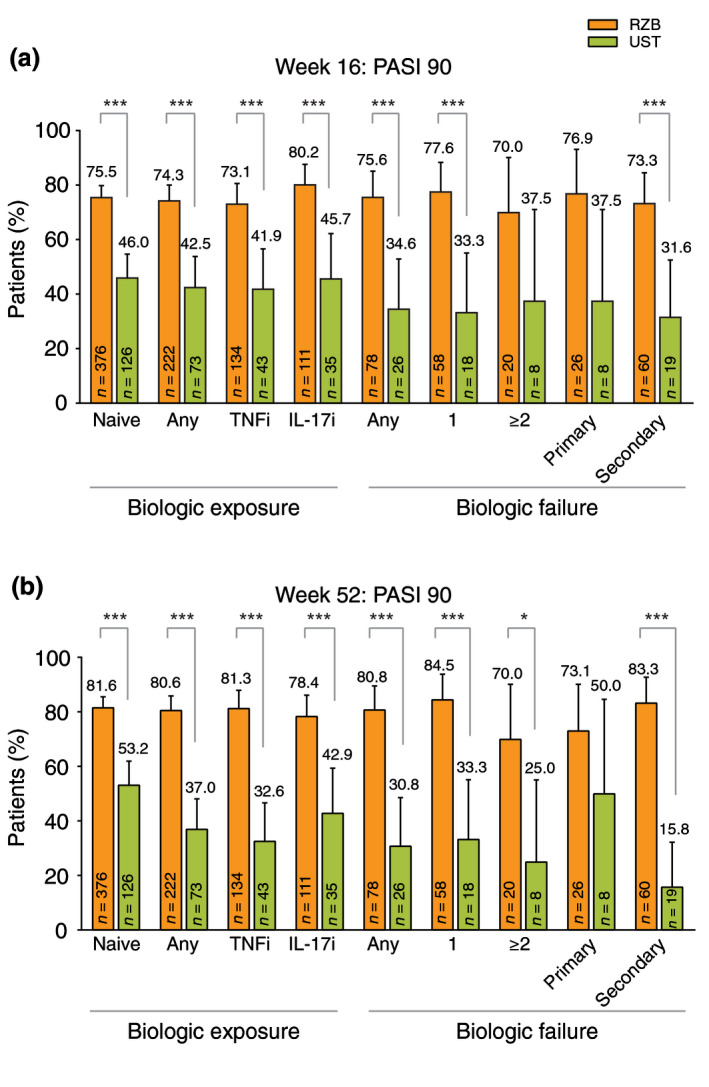
Proportion of patients (non‐responder imputation) achieving PASI 90 responses at week 16 (a) and week 52 (b) by prior biologic treatment experience (NRI). IL‐17i, interleukin‐17 inhibitors; NRI, non‐responder imputation; PASI, Psoriasis Area and Severity Index; RZB, risankizumab; TNFi, tumour necrosis factor inhibitor; UST, ustekinumab. Data expressed as per cent of patients with 95% confidence intervals (upper bounds shown). *P < 0.05, ***P < 0.001 compared with ustekinumab.
Multivariate logistic regression analyses
Logistic regression analyses were conducted in order to assess whether risankizumab demonstrated superior efficacy compared with ustekinumab at either week 16 or 52 regardless of patient characteristics (age, sex, weight, baseline PASI score and presence of psoriatic arthritis). All logistic regression models at week 16 and week 52 demonstrated that risankizumab treatment led to and maintained superior efficacy, respectively (all P < 0.001), compared with ustekinumab for PASI 90, PASI 100 and sPGA 0 or 1 (Table S3, Supporting Information); this analysis yielded similar results for sPGA 0 (data not shown) as for PASI 100.
Discussion
In this integrated analysis, risankizumab treatment resulted in statistically greater proportions of patients achieving high skin clearance compared with ustekinumab at both weeks 16 and 52, regardless of baseline patient demographics, disease characteristics, or prior biologic therapy exposure. Response rates with risankizumab treatment were consistently high across all subgroups analysed at both weeks 16 and 52. The proportion achieving PASI 90 or sPGA 0 or 1 on risankizumab treatment remained relatively stable across subgroups from week 16 to week 52, while risankizumab treatment showed an increase in the proportion of patients achieving complete clearance (PASI 100) from week 16 to week 52 across subgroups analysed. Logistic regression analyses revealed that risankizumab demonstrated superior efficacy compared with ustekinumab at weeks 16 and 52 even when accounting for effects of five independent covariates typically associated with reduced efficacy responses with other commonly used biologics.
Despite the availability of multiple classes of biologics with different treatment targets, psoriasis remains undertreated partially due to inadequate efficacy or loss of efficacy over time with current therapies. 28 In clinical practice, this has resulted in dose optimization in order to increase or maintain clinical responses. 29 , 30 , 31 In particular, patients with higher BMI or body weight have demonstrated lower response rates with many fixed‐dose biologic treatments, 5 , 6 , 8 , 9 , 10 , 11 , 12 leading some biologics to be used with weight‐based dosing or more frequent dosing schedules in order to achieve comparable efficacy in these patients. 7 , 32 , 33 Another factor that adversely affects efficacy with some biologics is prior exposure to or failure on a biologic. 13 , 14 , 15 , 16 In this analysis, risankizumab achieved superior efficacy compared with ustekinumab in patients with higher BMI or body weight and any prior biologic exposure or failure. Thus, these data support the use of risankizumab without dose adjustment to treat all patients, including those with higher BMI or body weight and in patients with prior biologic exposure or failure.
There are limitations to this study inherent to its design. This was a post hoc subgroup analysis of two replicate phase 3 trials. The self‐report of prior biologic exposure could be subject to recall errors and biases. Another limitation is the lack of information on disease duration for patients enrolled in these trials. Psoriasis disease duration has been shown to negatively impact efficacy of other biologic treatments for psoriasis. 18 Further clinical or real‐world studies are warranted to assess the impact of psoriasis disease duration on the efficacy of risankizumab. Some strengths are the inclusion of an active comparator through 52 weeks, inclusion of a high percentage of historically difficult‐to‐treat patients (prior biologic therapy exposure, high disease severity and high average BMI), and the inclusion of patients across the globe allowing for generalizability of the findings. An additional strength is the inclusion of the logistic regression analyses to control for the effect of five independent covariates (age, sex, weight, baseline PASI score and presence of psoriatic arthritis) and their potential interactions on the efficacy of risankizumab compared with ustekinumab. These multivariate analyses aimed to better represent patients routinely seen in clinical practice where the presence of multiple factors could affect efficacy. Risankizumab demonstrated superior efficacy compared with ustekinumab in all models tested, which included the covariate of weight (>100 kg) known to adversely affect efficacy of other biologic treatments. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12
In summary, risankizumab demonstrated superior efficacy compared with ustekinumab through 52 weeks of treatment regardless of patient baseline demographics, disease characteristics or prior biologic therapy exposure. These results support risankizumab as a psoriasis treatment suitable for a wide variety of patients given its high and durable skin clearance across all subgroups analysed.
Supporting information
Figure S1. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and PASI 100 (b) responses at week 16 by baseline patient demographics, disease characteristics and prior biologic therapy.
Figure S2. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and PASI 100 (b) responses at week 52 by baseline patient demographics, disease characteristics and prior biologic therapy.
Figure S3. Mean PASI improvement from baseline to week 52 by weight deciles in patients treated with risankizumab (LOCF)*.
Figure S4. Representative images from 3 patients (a–c) treated with risankizumab at baseline, week 4, week 16, and week 52.
Figure S5. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and PASI 100 (b) responses at week 16 by prior treatment experience.
Figure S6. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and sPGA 0 (b) responses at week 52 by prior treatment experience.
Table S1. Inclusion and exclusion criteria.
Table S2. Independent variables and their interactions analyzed for their effects on risankizumab compared with ustekinumab using logistic regression.
Table S3. Logistic regression models tested comparing proportion of patients achieving efficacy thresholds on risankizumab compared with ustekinumab at weeks 16 and 52.
Video S1. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment.
Video S2. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients with PASI 18 or above.
Video S3. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients with body mass index of 30 or more.
Video S4. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients with weight of 100 kg and above.
Video S5. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients who failed 1 or more biologics.
Acknowledgements
Medical writing support was provided by Daniel O’Brien, Ph.D., of AbbVie. Writing support was also provided by Janet Matsuura, Ph.D., of Complete Publication Solutions, LLC; this support was funded by AbbVie.
Conflict of interest
Bruce Strober has served as an investigator (no direct payments made to Bruce Strober, MD, PhD) for Dermavant, AbbVie, the CORRONA Psoriasis Registry, and Dermira; has served as Scientific Director (consulting fee) for the CORRONA Psoriasis Registry; has served as a consultant (honoraria) for AbbVie, Almirall, Amgen, Arena, Aristea, Boehringer Ingelheim, Bristol‐MyersSquibb, Celgene, Dermavant, Dermira, Janssen, Leo, Eli Lilly, Kyowa Hakko Kirin, Meiji Seika Pharma, Novartis, Pfizer, GlaxoSmithKline, UCB Pharma, Sun Pharma, Ortho Dermatologics, Regeneron, and Sanofi‐Genzyme; and has served as a speaker for AbbVie, Lilly, Janssen, and Ortho Dermatologics. Alan Menter has received grant support from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen Biotech, Inc., LEO Pharma, Merck, and Sienna; honoraria from AbbVie, Amgen, Boehringer Ingelheim, Eli Lilly, Janssen Biotech, Inc., LEO Pharma, Novartis, Sienna, and UCB; has served as an investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Biotech, Inc., LEO Pharma, Merck, Novartis, Sienna, and UCB; has served on the advisory board of AbbVie, Amgen, Boehringer Ingelheim, Janssen Biotech, Inc., LEO Pharma, and Sienna; has served as a consultant for AbbVie, Amgen, Eli Lilly, Janssen Biotech, Inc., LEO Pharma, Novartis, Sienna, and UCB; and has served as a speaker for AbbVie, Amgen, Janssen Biotech, Inc., LEO Pharma, Sienna, and UCB. Craig L. Leonardi, MD, FAAD, served as a consultant/advisory board member for AbbVie, Amgen, Boehringer Ingelheim, Celgene Corporation, Dermira, Eli Lilly and Company, Janssen Pharmaceuticals, Inc., Leo Pharma A/S, Ortho Dermatologics, Pfizer, Inc., Sandoz (a Novartis Company), UCB and Vitae receiving honoraria; as a speaker for AbbVie, Amgen, Celgene Corporation, Eli Lilly and Company, Novartis, Sun Pharmaceuticals, Ltd and UCB receiving honoraria; as a principal investigator for Actavis, Amgen, Boehringer Ingelheim, Celgene Corporation, Cellceutix, Coherus Biosciences, Corrona, Dermira, Eli Lilly and Company, Galderma Laboratories, L.P., Glenmark Generics, Inc., Janssen Pharmaceuticals, Inc., Leo Pharma, Inc., Merck, Novartis, Novella, Pfizer, Inc., Sandoz (a Novartis Company), Sienna Biopharmaceuticals, Stiefel a GSK company, UCB, and Warner Chillcott receiving other financial benefits (fee for service). Kenneth Gordon has received research grants from AbbVie, Bristol‐Myers Squibb, Celgene, Janssen, and Novartis, and has served as a consultant for AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol‐Myers Squibb, Celgene, Dermira, Eli Lilly, Janssen, Novartis, Pfizer, Sun, and UCB Pharma. Jo Lambert has received research grants from and has been an advisor/speaker for AbbVie, Celgene, Janssen, LEO Pharma, Eli Lilly, and Novartis. Lluís Puig has received grants/research support (paid to the institution) from or participated in clinical trials for AbbVie, Amgen, Boehringer Ingelheim, Janssen, Leo Pharma, Lilly, Novartis, Pfizer, Regeneron, Roche, Sanofi, and UCB; received honoraria or consultation fees from AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro, Janssen, Leo Pharma, Lilly, Merck‐Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Sanofi, and UCB; and has participated in company‐sponsored speakers' bureaus for Celgene, Janssen, Lilly, MSD, Novartis, and Pfizer. Huzefa Photowala, Michelle Longcore, and Tianyu Zhan are employees of AbbVie, Inc. and may hold stock or stock options. Peter Foley has received grant support from AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, and Sun Pharma. He has served as an investigator for AbbVie, Amgen, Boehringer Ingelheim, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB Pharma, Valeant, BMS, Celtaxsys, CSL, Cutanea, Dermira, Galderma, Genentech, GSK, Leo Pharma, Regeneron Pharmaceuticals Inc, Roche, and Sanofi. He has also served on advisory boards for AbbVie, Amgen, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Sun Pharma, UCB Pharma, Valeant, Galderma, GSK, Leo Pharma, and Sanofi. He has served as a consultant for Janssen, Lilly, Novartis, Pfizer, UCB Pharma, BMS, Galderma, Leo Pharma, and Roche. He has received travel grants from AbbVie, Janssen, Lilly, Merck, Novartis, Pfizer, Galderma, Leo Pharma, Roche, Sun Pharma, and Sanofi, and has served as a speaker for or received honoraria from AbbVie, Celgene, Janssen, Lilly, Merck, Novartis, Pfizer, Valeant, Galderma, GSK, Leo Pharma, and Roche.
Funding source
AbbVie sponsored the study, contributed to its design, data collection, analysis, and interpretation of the data, and participated in the writing, review, and approval of the manuscript. All authors had access to relevant data.
References
- 1. Harden JL, Krueger JG, Bowcock AM. The immunogenetics of psoriasis: a comprehensive review. J Autoimmun 2015; 64: 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Report on Psoriasis. World Health Organization, Geneva, 2016. [Google Scholar]
- 3. Rapp SR, Feldman SR, Exum L, Fleischer AB, Jr Reboussin DM. Psoriasis causes as much disability as other major medical diseases. J Am Acad Dermatol 1999; 41: 401–407. [DOI] [PubMed] [Google Scholar]
- 4. Lonnberg AS, Skov L, Skytthe A, Kyvik KO, Pedersen OB, Thomsen SF. Association of psoriasis with the risk for type 2 diabetes mellitus and obesity. JAMA Dermatol 2016; 152: 761–767. [DOI] [PubMed] [Google Scholar]
- 5. Clark L, Lebwohl M. The effect of weight on the efficacy of biologic therapy in patients with psoriasis. J Am Acad Dermatol 2008; 58: 443–446. [DOI] [PubMed] [Google Scholar]
- 6. Hsu S, Green LJ, Lebwohl MG, Wu JJ, Blauvelt A, Jacobson AA. Comparable efficacy and safety of brodalumab in obese and non‐obese patients with psoriasis: analysis of 2 randomized controlled trials. Br J Dermatol 2020182: 880–888. [DOI] [PubMed] [Google Scholar]
- 7. Lebwohl M, Yeilding N, Szapary P et al Impact of weight on the efficacy and safety of ustekinumab in patients with moderate to severe psoriasis: rationale for dosing recommendations. J Am Acad Dermatol 2010; 63: 571–579. [DOI] [PubMed] [Google Scholar]
- 8. Lee JE, Wang J, Florian J et al Effect of body weight on risk‐benefit and dosing regimen recommendation of secukinumab for the treatment of moderate to severe plaque psoriasis. Clin Pharmacol Ther 2019; 106: 78–80. [DOI] [PubMed] [Google Scholar]
- 9. Naldi L, Addis A, Chimenti S et al Impact of body mass index and obesity on clinical response to systemic treatment for psoriasis. Evidence from the Psocare project. Dermatology 2008; 217: 365–373. [DOI] [PubMed] [Google Scholar]
- 10. Papp KA, Reich K, Blauvelt A et al Efficacy of tildrakizumab for moderate‐to‐severe plaque psoriasis: pooled analysis of three randomized controlled trials at weeks 12 and 28. J Eur Acad Dermatol Venereol 2019; 33: 1098–1106. [DOI] [PubMed] [Google Scholar]
- 11. Prussick R, Unnebrink K, Valdecantos WC. Efficacy of adalimumab compared with methotrexate or placebo stratified by baseline BMI in a randomized placebo‐controlled trial in patients with psoriasis. J Drugs Dermatol 2015; 14: 864–868. [PubMed] [Google Scholar]
- 12. Reich K, Puig L, Mallbris L, Zhang L, Osuntokun O, Leonardi C. The effect of bodyweight on the efficacy and safety of ixekizumab: results from an integrated database of three randomised, controlled Phase 3 studies of patients with moderate‐to‐severe plaque psoriasis. J Eur Acad Dermatol Venereol 2017; 31: 1196–1207. [DOI] [PubMed] [Google Scholar]
- 13. Mazzotta A, Esposito M, Costanzo A, Chimenti S. Efficacy and safety of etanercept in psoriasis after switching from other treatments: an observational study. Am J Clin Dermatol 2009; 10: 319–324. [DOI] [PubMed] [Google Scholar]
- 14. Papp KA, Gordon KB, Langley RG et al Impact of previous biologic use on the efficacy and safety of brodalumab and ustekinumab in patients with moderate‐to‐severe plaque psoriasis: integrated analysis of the randomized controlled trials AMAGINE‐2 and AMAGINE‐3. Br J Dermatol 2018; 179: 320–328. [DOI] [PubMed] [Google Scholar]
- 15. Cassano N, Galluccio A, De Simone C et al Influence of body mass index, comorbidities and prior systemic therapies on the response of psoriasis to adalimumab: an exploratory analysis from the APHRODITE data. J Biol Regul Homeost Agents 2008; 22: 233–237. [PubMed] [Google Scholar]
- 16. Gottlieb AB, Lacour JP, Korman N et al Treatment outcomes with ixekizumab in patients with moderate‐to‐severe psoriasis who have or have not received prior biological therapies: an integrated analysis of two Phase III randomized studies. J Eur Acad Dermatol Venereol 2017; 31: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hawkes JE, Yan BY, Chan TC, Krueger JG. Discovery of the IL‐23/IL‐17 signaling pathway and the treatment of psoriasis. J Immunol 2018; 201: 1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 19. Reich K, Papp KA, Blauvelt A et al Tildrakizumab versus placebo or etanercept for chronic plaque psoriasis (reSURFACE 1 and reSURFACE 2): results from two randomised controlled, phase 3 trials. Lancet 2017; 390: 276–288. [DOI] [PubMed] [Google Scholar]
- 20. Blauvelt A, Papp KA, Griffiths CE et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the continuous treatment of patients with moderate to severe psoriasis: results from the phase III, double‐blinded, placebo‐ and active comparator‐controlled VOYAGE 1 trial. J Am Acad Dermatol 2017; 76: 405–417. [DOI] [PubMed] [Google Scholar]
- 21. Reich K, Armstrong AW, Foley P et al Efficacy and safety of guselkumab, an anti‐interleukin‐23 monoclonal antibody, compared with adalimumab for the treatment of patients with moderate to severe psoriasis with randomized withdrawal and retreatment: results from the phase III, double‐blind, placebo‐ and active comparator‐controlled VOYAGE 2 trial. J Am Acad Dermatol 2017; 76: 418–431. [DOI] [PubMed] [Google Scholar]
- 22. Gordon KB, Strober B, Lebwohl M et al Efficacy and safety of risankizumab in moderate‐to‐severe plaque psoriasis (UltIMMa‐1 and UltIMMa‐2): results from two double‐blind, randomised, placebo‐controlled and ustekinumab‐controlled phase 3 trials. Lancet 2018; 392: 650–661. [DOI] [PubMed] [Google Scholar]
- 23. Reich K, Gooderham M, Thaci D et al Risankizumab compared with adalimumab in patients with moderate‐to‐severe plaque psoriasis (IMMvent): a randomised, double‐blind, active‐comparator‐controlled phase 3 trial. Lancet 2019; 394: 576–586. [DOI] [PubMed] [Google Scholar]
- 24. Krueger JG, Ferris LK, Menter A et al Anti‐IL‐23A mAb BI 655066 for treatment of moderate‐to‐severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single‐rising‐dose, randomized, double‐blind, placebo‐controlled trial. J Allergy Clin Immunol 2015; 136: 116–124.e7. [DOI] [PubMed] [Google Scholar]
- 25. McKeage K, Duggan S. Risankizumab: first global approval. Drugs 2019; 79: 893–900. [DOI] [PubMed] [Google Scholar]
- 26. Khatri A, Suleiman AA, Polepally AR, Othman AA. Exposure‐response Relationship for Efficacy and Safety of Risankizumab in Patients with Moderate‐to‐severe Plaque Psoriasis: Integrated Analyses of Phase 2 and 3 Clinical Trials. Presented at the American Academy of Dermatology (AAD) meeting, Washington, DC, 2019. [Google Scholar]
- 27. Blauvelt A, Papp KA, Gooderham M et al Efficacy and safety of risankizumab, an interleukin‐23 inhibitor, in patients with moderate‐to‐severe chronic plaque psoriasis: 16‐week results from the phase III IMMhance trial. Psoriasis Gene to Clinic, 8th International Congress; December 2, London, UK, 2017. [Google Scholar]
- 28. Menter A, Strober BE, Kaplan DH et al Joint AAD‐NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol 2019; 80: 1029–1072. [DOI] [PubMed] [Google Scholar]
- 29. Brezinski EA, Armstrong AW. Off‐label biologic regimens in psoriasis: a systematic review of efficacy and safety of dose escalation, reduction, and interrupted biologic therapy. PLoS ONE 2012; 7: e33486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Carrascosa JM, Garcia‐Doval I, Perez‐Zafrilla B et al Use of off‐label doses is frequent in biologic therapy for moderate to severe psoriasis: a cross‐sectional study in clinical practice. J Dermatolog Treat 2015; 26: 502–506. [DOI] [PubMed] [Google Scholar]
- 31. Esposito M, Gisondi P, Conti A et al Dose adjustment of biologic therapies for psoriasis in dermatological practice: a retrospective study. J Eur Acad Dermatol Venereol 2017; 31: 863–869. [DOI] [PubMed] [Google Scholar]
- 32. Reich K, Puig L, Szepietowski JC et al Secukinumab dosing optimization in patients with moderate to severe plaque psoriasis: results from the randomised, open‐label OPTIMISE study. Br J Dermatol 2020182: 304–315. [DOI] [PubMed] [Google Scholar]
- 33. Langley RG, Lebwohl M, Krueger GG et al Long‐term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 2 study through 5 years of follow‐up. Br J Dermatol 2015; 172: 1371–1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and PASI 100 (b) responses at week 16 by baseline patient demographics, disease characteristics and prior biologic therapy.
Figure S2. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and PASI 100 (b) responses at week 52 by baseline patient demographics, disease characteristics and prior biologic therapy.
Figure S3. Mean PASI improvement from baseline to week 52 by weight deciles in patients treated with risankizumab (LOCF)*.
Figure S4. Representative images from 3 patients (a–c) treated with risankizumab at baseline, week 4, week 16, and week 52.
Figure S5. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and PASI 100 (b) responses at week 16 by prior treatment experience.
Figure S6. Proportion of patients (non‐responder imputation) achieving sPGA 0/1 (a) and sPGA 0 (b) responses at week 52 by prior treatment experience.
Table S1. Inclusion and exclusion criteria.
Table S2. Independent variables and their interactions analyzed for their effects on risankizumab compared with ustekinumab using logistic regression.
Table S3. Logistic regression models tested comparing proportion of patients achieving efficacy thresholds on risankizumab compared with ustekinumab at weeks 16 and 52.
Video S1. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment.
Video S2. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients with PASI 18 or above.
Video S3. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients with body mass index of 30 or more.
Video S4. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients with weight of 100 kg and above.
Video S5. Time‐lapse of individual patients’ percent improvement in PASI through 52 weeks of risankizumab treatment: subgroup of patients who failed 1 or more biologics.


