Abstract
OBJECTIVE
Sodium–glucose cotransporter 2 (SGLT2) inhibitors reduce the risk for heart failure hospitalization potentially by inducing sodium excretion, osmotic diuresis, and plasma volume contraction. Few studies have investigated this hypothesis, but none have assessed cumulative sodium excretion with SGLT2 inhibition during standardized sodium intake in patients with type 2 diabetes.
RESEARCH DESIGN AND METHODS
The DAPASALT trial was a mechanistic, nonrandomized, open-label study in patients with type 2 diabetes with preserved kidney function on a controlled standardized sodium diet (150 mmol/day). It evaluated the effects of dapagliflozin on sodium excretion, 24-h blood pressure, and extracellular, intracellular, and plasma volumes at the start of treatment (ST) (days 2–4), end of treatment (ET) (days 12–14), and follow-up (FU) (days 15–18).
RESULTS
Fourteen patients were included in the efficacy analysis. Mean (SD) baseline sodium excretion (150 [32] mmol/24-h) did not significantly change during treatment (change at ST: −7.0 mmol/24-h [95% CI −22.4, 8.4]; change at ET: 2.1 mmol/24-h [−28.8, 33.0]). Mean baseline 24-h systolic blood pressure was 128 (10) mmHg and significantly reduced at ST (−6.1 mmHg [−9.1, −3.1]; P < 0.001) and ET (−7.2 mmHg [−10.0, −4.3]; P < 0.001). Dapagliflozin did not significantly alter plasma volume or intracellular volume, while extracellular volume changed at ST (−0.7 L [−1.3, −0.1]; P = 0.02). As expected, 24-h urinary glucose excretion significantly increased during dapagliflozin treatment and reversed during FU.
CONCLUSIONS
During standardized sodium intake, dapagliflozin reduced blood pressure without clear changes in urinary sodium excretion, suggesting that factors other than natriuresis and volume changes may contribute to the blood pressure–lowering effects.
Introduction
Data from recent trials have shown that sodium–glucose cotransporter 2 (SGLT2) inhibitors, such as dapagliflozin, improve cardiovascular (CV) and kidney outcomes, including heart failure hospitalizations and progression of kidney disease, in patients with and without type 2 diabetes and at different stages of chronic kidney disease (1–5). The underlying mechanisms for these CV benefits have been studied extensively but remain incompletely understood. A main hypothesis is that SGLT2 inhibitors confer CV protection through hemodynamic actions, including volume contraction secondary to glycosuria and natriuresis, leading to reduced cardiac pre- and afterload (6,7).
Previous studies of varying designs in healthy volunteers or patients with type 2 diabetes have indeed suggested that SGLT2 inhibitors cause transient increases in 24-h sodium excretion (8–11) along with reductions in plasma and extracellular volume (12,13). Studies in patients with heart failure showed either no change (14) or a modest increase in sodium excretion in parallel with a reduction in plasma volume (15). The prior studies were limited by the fact that sodium excretion was estimated from spot urine samples instead of 24-h urine collections, or dietary intake of sodium was not recorded or standardized. To date, no studies have formally assessed cumulative sodium excretion of SGLT2 inhibitors together with changes in plasma volume and extracellular volume in patients with type 2 diabetes during controlled sodium intake.
Therefore, the aim of this open-label, mechanistic study was to assess the effect of dapagliflozin on natriuresis, plasma volume, extracellular and intracellular volume, and 24-h blood pressure regulation in patients with type 2 diabetes and preserved kidney function during strictly controlled sodium intake. We hypothesized that SGLT2 inhibition increases urinary sodium excretion and osmotic diuresis and thereby affects both volume status and blood pressure.
Research Design and Methods
This phase IV, multicenter, open-label, mechanistic interventional study was conducted between July 2017 and March 2020. Originally, the study had three strata consisting of patients with type 2 diabetes and impaired kidney function (stratum 1), type 2 diabetes and preserved kidney function (stratum 2), and no diabetes but impaired kidney function (stratum 3), with a total sample size of 51 patients (17 patients per stratum). Here, we present data on the completed stratum of patients with type 2 diabetes and preserved kidney function (stratum 2) who were recruited at the Amsterdam University Medical Center (AUMC), location VU University Medical Center (VUMC), and at Ziekenhuisgroep Twente, Almelo, the Netherlands.
Study Population
Patients were recruited from study databases and by advertisements in local newspapers. Eligible patients were Caucasian, Asian, or Middle Eastern males and females of no child-bearing potential and aged 18 to ≤80 years. Eligible patients had a diagnosis of type 2 diabetes with glycated hemoglobin (HbA1c) ranging from 6.5% (48 mmol/mol) to <10% (<86 mmol/mol) and were treated with a stable dose of metformin, sulfonylurea (SU), or a combination of metformin and SU as standard of care for at least 3 months before enrollment. Patients had to have a preserved kidney function defined as an estimated glomerular filtration rate between >90 and ≤130 mL/min/1.73 m2 for patients aged ≤59 years, between >85 and ≤130 mL/min/1.73 m2 for patients aged 60–69 years, and between >75 and ≤130 mL/min/1.73 m2 for patients aged ≥70 years. In addition, a stable dose of an angiotensin receptor blocker (ARB) for at least 6 weeks was required to create a homogeneous cohort using a similar class of agents to inhibit the renin-angiotensin-aldosterone system. For inclusion, patients had to have a stable 24-h urinary sodium excretion on 2 successive days (<20% difference between days −3 and −2). Exclusion criteria were a history of unstable or rapidly progressing kidney disease, albumin-to-creatinine ratio >1,000 mg/g, symptoms of urinary retention, use of a pacemaker or other implanted electronic devices, type 1 diabetes, blood pressure ≥180/110 mmHg, and CV/vascular disease within 3 months before screening. Use of any glucose-lowering drugs (except metformin and SU), ACE inhibitors, or nonsteroidal anti-inflammatory drugs was not allowed during the study; treatment with diuretics during the study or within 2 weeks before the study also was not allowed. Written informed consent was obtained from all patients before any trial-related activities. The study protocol, protocol amendments, and all other protocol-specific documents were reviewed and approved by local authorities and the medical ethical review boards of the participating centers. The study complied with the Declaration of Helsinki and Good Clinical Practice guidelines.
Intervention
Eligible patients received dapagliflozin 10-mg tablets once daily for 14 ± 1 days. Patients were instructed to take their study medication in the morning during the treatment period.
Outcome Measures
The overarching objective was to assess the effect of dapagliflozin on change in 24-h sodium excretion. The primary end point of change in 24-h sodium excretion was defined as the change in average sodium excretion at baseline (average of days −3 to −1) relative to the average sodium excretion at start of treatment (ST) (average of days 2–4) on the basis of earlier clinical studies (10). Secondary objectives included the effect of dapagliflozin on changes in 24-h sodium excretion from baseline to end of treatment (ET) (days 12–14) and from ET to follow-up (FU) (days 15–17). Additional secondary end points of 24-h glucose excretion, systolic blood pressure (SBP), plasma volume, and extracellular volume were measured at baseline, ST, ET, and FU. Change in intracellular volume, body weight, 24-h urinary aldosterone, and N-terminal pro-B-type natriuretic peptide (NT-proBNP) were exploratory end points measured at the same time points. Change from baseline in fractional excretion of endogenous lithium, a surrogate for sodium reabsorption in the proximal tubule, was a post hoc exploratory end point.
Procedures and FU Visits
Run-in Period (Day −6 to Day −1)
Patients received food boxes (delivered by Sodexo, Rotterdam, the Netherlands) with a daily sodium content of 150 mmol, starting on day −6, and were required to adhere to the dietary requirements throughout the period of the study up to and including day 18 (Supplementary Fig. 1). Food questionnaires were required to be completed by the participants starting from day −6 to record any deviation in intake from the provided food boxes and liquid intake. Patients were allowed to consume nonstudy food products, but these were restricted to products that did not contain sodium. These food products were also recorded in the food questionnaires. Participants were given the following oral and written instructions about how to collect 24-h urine samples: refrain from strenuous exercise during the collection periods, collect 24-h urine in dedicated containers, and store collections in a refrigerator until delivery to the clinic. Twenty-four-hour urine samples were collected on day −3 to day −1 (baseline). Patients in whom 24-h sodium excretion did not differ by >20% between day −3 to day −2 were considered to be adherent to the dietary requirements and could proceed to the active treatment period. On day −1, 24-h ambulatory blood pressure monitoring (ABPM) (iCardiac Technologies, Pittsford, NY) was initiated.
Treatment Period (Day 1 to Day 14)
In-patient study visits were scheduled at days 1, 4, 5, 13, and 14. In total, four consecutive 24-h urine samples were collected at days 1–4 followed by three consecutive 24-h urine collections at days 12–14. On days 4 and 13, 24-h ABPM measurements were initiated. Blood samples were obtained in the fasting condition on the morning of days 1, 4, and 14. Plasma volume assessment and bioimpedance spectroscopy (BIS) (ImpediMed Limited, Pinkenba, Queensland, Australia) for assessment of extracellular volume and intracellular volume were performed on days 1, 4, and 14. Plasma volume was measured using the indocyanine green (ICG) (Verdye; Diagnostic Green, Ascheim-Dornach, Germany) indicator dilution method. ICG is a water-soluble dye that binds to plasma proteins (mainly albumin) and is a marker of the plasma volume distribution space. After an intravenous bolus injection of ICG 0.25 mg/kg over a period of 1 min, blood samples were obtained every 30 s from 2 to 5 min as described previously (16,17).
FU Period (Day 15 to Day 19)
Three consecutive 24-h urine samples were collected at days 15–17 (FU). A final in-patient study visit was scheduled at day 18 during which plasma volume and BIS assessments were performed. At the final in-patient study visit, blood samples were obtained in the fasting condition, and 24-h ABPM was initiated.
Laboratory Measurements
All samples were measured by standard in-house assays at COVANCE (Geneva, Switzerland), MLM Medical Laboratories (Mönchengladbach, Germany) (ICG), University Medical Center Groningen (urine and serum lithium by a validated inductively coupled plasma mass spectrometry assay), and AUMC and Ziekenhuisgroep Twente (HbA1c, creatinine, urinary sodium, and glucose).
Statistical Analysis
Sample size calculations were performed for each of the three strata individually. With a sample size of 15 patients and assuming an SD of 25 mmol/24 h in change from average baseline in 24-h sodium excretion, the study had 80% power to detect an increase in 24-h sodium excretion of at least 20 mmol/24 h from baseline with dapagliflozin at a two-sided α-level of 0.05. Under these conventions, the minimum detectable difference in change from average baseline in 24-h sodium excretion is ∼13.6 mmol/24 h. To account for early discontinuation because of the complex protocol and high demand on study participants, we enrolled 17 patients. Baseline characteristics were summarized using mean and SD or proportion. A longitudinal repeated-measures analysis was used for the change versus the baseline value. The model included a fixed effect of time point, interaction term between time point and baseline, and continuous baseline value as covariates. An unstructured covariance matrix structure was used to model correlations among the repeated measurements. Point estimates and corresponding 95% CIs for the least squares means at each time point were derived. P < 0.05 was considered to indicate statistical significance. Analyses were performed using SAS 9.4 statistical software.
Results
Participant Characteristics
Thirty-one patients were enrolled in the study, of whom 17 started treatment with dapagliflozin. Before database lock, one patient was excluded from analysis because of a >20% difference in urinary sodium excretion between days −3 and −2 and another because of missing urine volume for 24-h urine collection at day −2. After database lock, one additional patient was excluded because of nonadherence to dapagliflozin. Efficacy analyses were therefore performed in 14 patients, and their baseline characteristics are reported in Table 1. Efficacy data for the 15 patients included before database lock are reported in Supplementary Table 1. Safety was assessed in the 17 patients who started treatment.
Table 1.
Baseline characteristics
| Characteristic | Study participants (n = 14) |
|---|---|
| Age (years) | 63.9 (7.9) |
| Male sex, n (%) | 9 (64.3) |
| Race, n (%) | |
| White | 13 (92.9) |
| Asian | 1 (7.1) |
| Diabetes duration (years) | 10.2 (5.2) |
| Body weight (kg) | 98.7 (15.9) |
| BMI (kg/m2) | 31.9 (4.2) |
| Fasting plasma glucose (mmol/L) | 8.1 (1.4) |
| HbA1c (%) | 7.2 (0.6) |
| HbA1c (mmol/mol) | 55 (6.6) |
| SBP (mmHg) | 128.6 (13.6) |
| DBP (mmHg) | 74.7 (7.5) |
| eGFR (CKD-EPI) (mL/min/1.73 m2) | 94.3 (10.9) |
| UACR (mg/mmol), median (25th–75th percentile) | 0.8 (0.5–2.8) |
| Hemoglobin (g/L) | 137.9 (13.0) |
| Hematocrit (L/L) | 0.4 (0.0) |
| Metformin, n (%) | 14 (100) |
| SU derivative, n (%) | 5 (35.7) |
Data are mean (SD) unless otherwise indicated. Blood pressure recorded in supine position. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; UACR, urinary albumin-to-creatinine ratio.
Effect of Dapagliflozin on Urinary Excretion of Sodium, Glucose, Volume, and Lithium
Mean (SD) 24-h sodium excretion at baseline was 150 (32) mmol/24-h, confirming successful design and adherence to the diet. Dapagliflozin treatment did not significantly change 24-h sodium excretion (urinary 24-h sodium change at ST: −7.0 mmol/24-h [95% CI −22.4, 8.4; P = 0.34]; change at ET: 2.1 mmol/24-h [−28.8, 33.0; P = 0.89]) (Fig. 1A). Compared with ET, 24-h sodium excretion did not change during FU (change at days 15–17: −13.8 mmol/24-h [−30.9, 3.2]; P = 0.10) (Fig. 1A). Mean 24-h urinary sodium excretion from day −1 to day 1 tended toward an increase (27 mmol/24-h [−10, 63]) (Supplementary Table 2).
Figure 1.
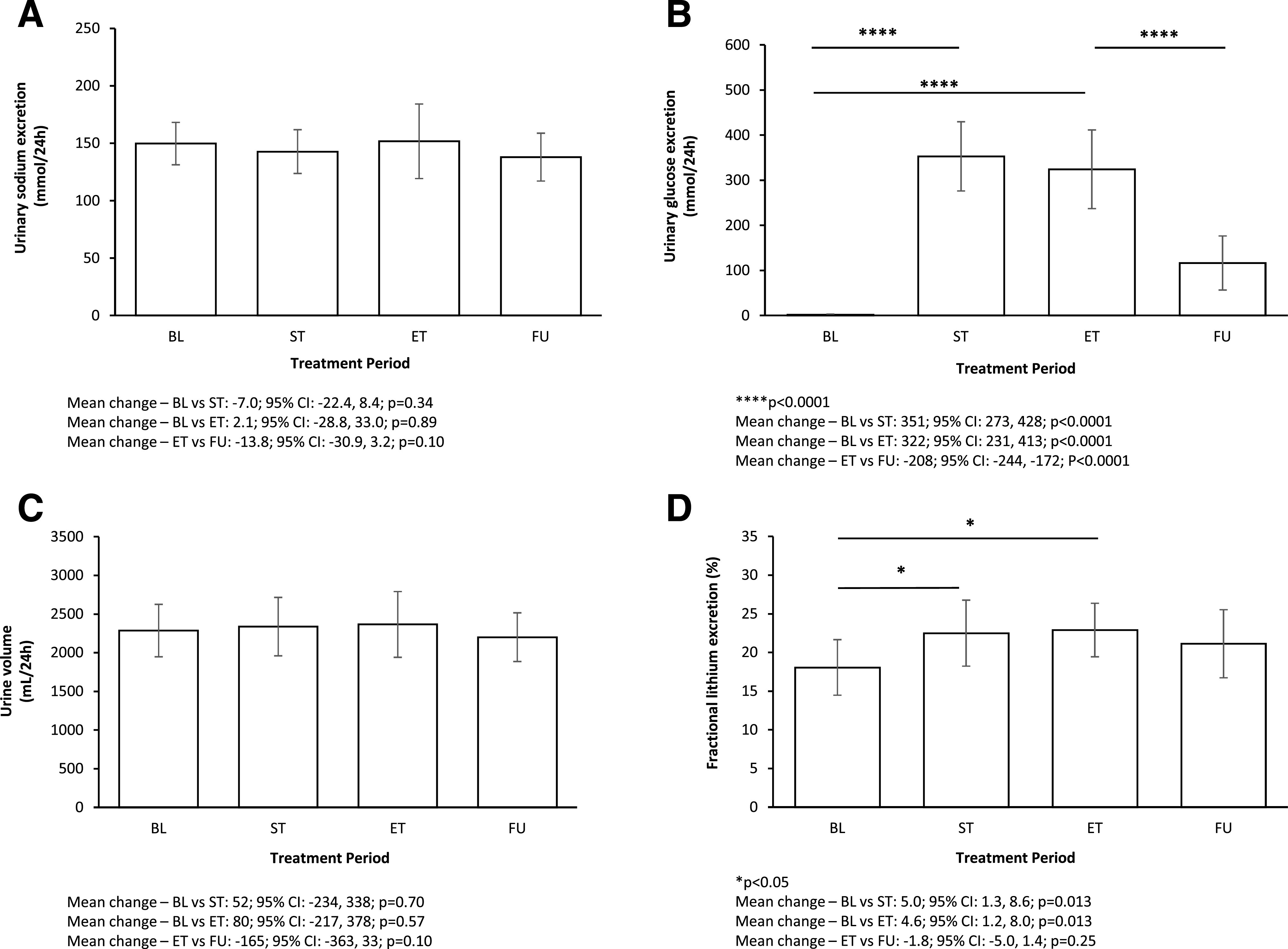
Urinary sodium excretion (A), urinary glucose excretion (B), urine volume (C), and fractional lithium excretion (D) at baseline (BL), ST, ET, and FU.
Mean (SD) baseline urinary glucose excretion was 1.9 (2.2) mmol/24-h, which, as expected, significantly increased during dapagliflozin treatment (urinary glucose change at ST: 351 mmol/24-h [95% CI 273, 428; P < 0.0001]; change at ET: 322 mmol/24-h [231, 413; P < 0.0001]) (Fig. 1B). Mean 24-h glucose excretion significantly decreased at FU compared with ET (change at days 15–17: −208 mmol/24-h [−244, −172]; P < 0.0001) (Fig. 1B).
At baseline, mean (SD) 24-h urine volume was 2,285 (587) mL/24-h, which did not statistically significantly change during treatment with dapagliflozin (urinary volume change at ST: 52 mL/24-h [95% CI −234, 338; P = 0.70]; change at ET: 80 mL/24-h [−217, 378; P = 0.57]) (Fig. 1C). During FU, urine volume also did not change compared with ET (change at days 15–17: −165 mL/24-h [−363, 33]; P = 0.10) (Fig. 1C).
Mean (SD) baseline fractional lithium excretion was 18.1% (5.3%), which significantly increased during dapagliflozin treatment (urinary fractional lithium change at ST: 5.0% [95% CI 1.3, 8.6; P = 0.013]; change at ET: 4.6% [1.2, 8.0; P = 0.013]) (Fig. 1D). During FU, fractional lithium excretion returned in the direction of baseline (change at FU: −1.8% [−5.0, 1.4]; P = 0.25) (Fig. 1D).
Plasma Volume, Extracellular Volume, and Intracellular Volume
Mean (SD) plasma volume at baseline was 3.75 (0.8) L, remained stable at ST (mean change −0.19 L [95% CI −0.9, 0.5]; P = 0.58) (Fig. 2A) and tended to be lower at ET (mean change −0.45 L [−1.1, 0.2]; P = 0.16). During FU, plasma volume significantly increased compared with ET, and the magnitude of the effect was identical to the decrease observed from baseline to ET (change at day 18: 0.43 L [0.0, 0.8]; P = 0.049) (Fig. 2A).
Figure 2.
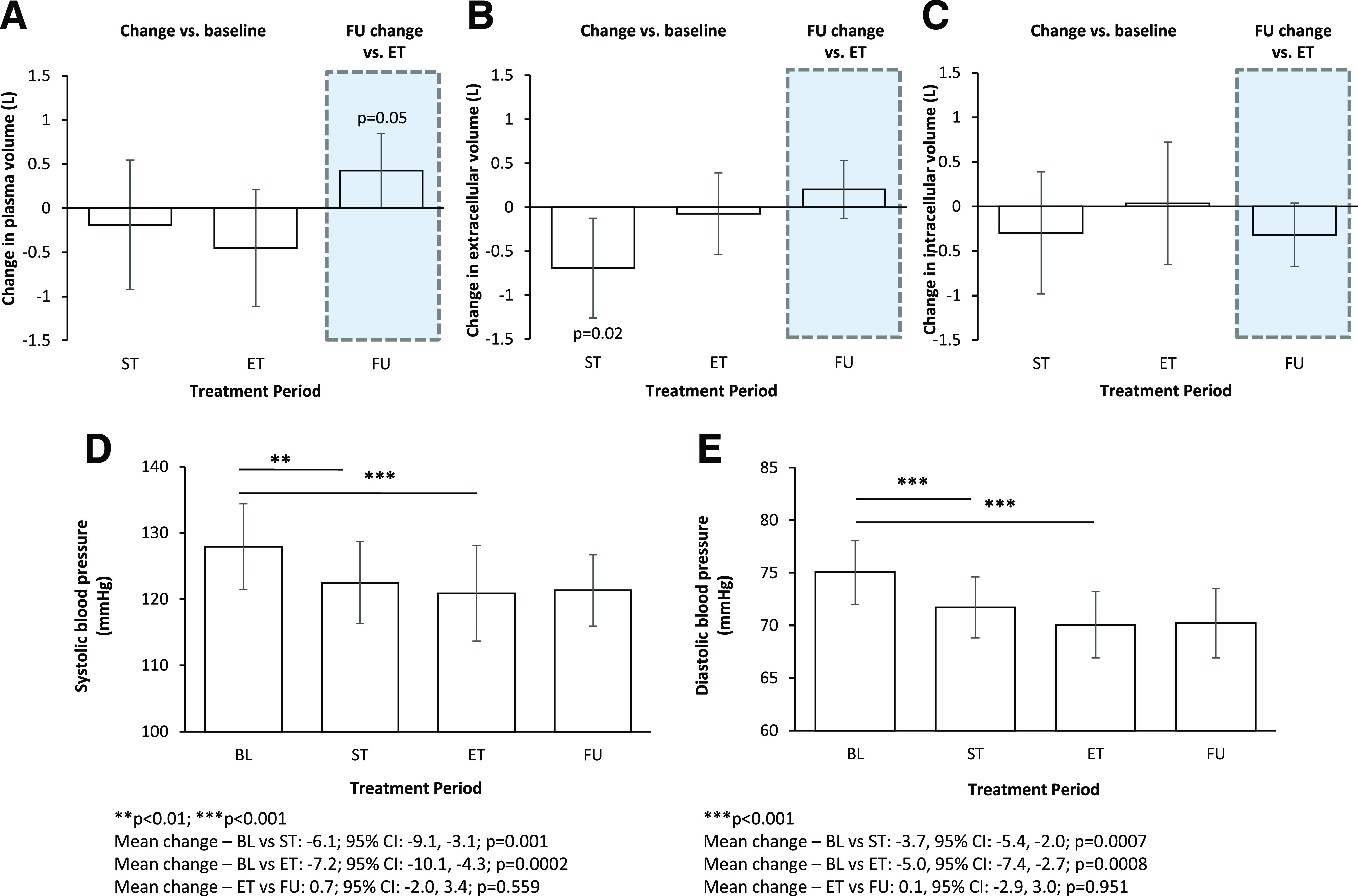
Changes in plasma volume (A), extracellular volume (B), intracellular volume (C), SBP (D), and DBP (E) from baseline (BL) to ST, from BL to ET, and from ET to FU.
At baseline, mean (SD) extracellular volume was 20.9 (4.5) L, which was significantly reduced after ST but not at ET (change at day 4: −0.7 L [95% CI −1.3, − 0.1; P = 0.02]; change at day 14: −0.1 L [−0.5, 0.4; P = 0.73]) (Fig. 2B). Mean extracellular volume during FU did not change compared with ET (change at day 18: 0.2 L [−0.1, 0.5]; P = 0.21) (Fig. 2B).
Mean (SD) intracellular volume at baseline was 26.6 (5.7) L, which did not significantly change during treatment with dapagliflozin (mean change at ST: −0.3 L [95% CI −1.0, 0.4; P = 0.36]; mean change at ET: 0.04 L [−0.7, 0.7; P = 0.91]) (Fig. 2C). Change in intracellular volume at FU compared with ET was −0.3 L (−0.7, −0.0; P = 0.08) (Fig. 2C).
Effect of Dapagliflozin on 24-h Blood Pressure Profiles
Mean (SD) 24-h SBP at baseline was 128 (10) mmHg, which significantly changed during dapagliflozin treatment (mean change at ST: −6.1 mmHg [95% CI −9.1, −3.1; P = 0.001]; mean change at ET: −7.2 mmHg [−10.1, −4.3; P < 0.001]) and remained reduced during FU compared with ET (0.7 mmHg [−2.0, 3.4]; P = 0.56) (Fig. 2D). Treatment with dapagliflozin also reduced 24-h diastolic blood pressure (DBP) (Fig. 2E).
Anthropometrics and Hormones
Mean (SD) body weight at baseline was 98.7 (15.9) kg, which was significantly reduced during dapagliflozin treatment (mean change at ST: −0.8 kg [95% CI −1.2, −0.5; P < 0.001]; mean change at ET: −1.8 kg [−2.5, −1.1; P < 0.001]). During FU, body weight returned in the direction of baseline (change from ET to end of FU: 0.4 kg [0.0, 0.9]; P = 0.07) (Table 2). Mean 24-h urinary aldosterone excretion was 14.0 (8.4) μg/24-h at baseline, which was significantly increased at ST (mean change at day 4: 3.6 μg/24-h [2.2, 5.1]; P < 0.001), while no differences were observed at ET (mean change at day 14: 0.8 μg/24-h [−2.4, 3.9]; P = 0.60) (Table 2). Mean NT-proBNP at baseline was 3.5 (2.6) pmol/L and did not change during dapagliflozin treatment (change at ST: −0.4 pmol/L [−2.0, 1.2; P = 0.62]; change at ET: 1.0 pmol/L [−1.2, 3.3; P = 0.32]) (Table 2). BNP and hematocrit also did not change during dapagliflozin treatment (Table 2).
Table 2.
Changes in anthropometrics, hormones, and hematocrit
| Baseline value | Change at ST* | Change at ET* | Change at FU† | ||||
|---|---|---|---|---|---|---|---|
| Parameter | Mean (SD) | Mean (95% CI) | P value | Mean (95% CI) | P value | Mean (95% CI) | P value |
| Body weight (kg) | 98.7 (15.9) | −0.8 (−1.2, 0.5) | <0.01 | −1.8 (−2.5, −1.1) | <0.01 | 0.4 (0.0, 0.9) | 0.068 |
| Urinary aldosterone (μg/24-h) | 14.0 (11.4) | 3.6 (2.2, 5.1) | <0.01 | 0.8 (−2.4, 3.9) | 0.60 | −0.5 (−2.8, −1.8) | 0.64 |
| NT-proBNP (pmol/L) | 3.5 (2.6) | −0.4 (−2.0, 1.2) | 0.62 | 1.0 (−1.2, 3.3) | 0.32 | 2.3 (−0.5, 5.1) | 0.096 |
| BNP (pg/mL) | 20.0 (10.7) | −2.1 (−8.0, 3.8) | 0.46 | 3.6 (−6.2, 13.4) | 0.44 | 12.4 (3.6, 21.2) | 0.010 |
| Hematocrit (L/L) | 0.4 (0.0) | −0.001 (−0.009, 0.006) | 0.67 | −0.009 (−0.02, 0.001) | 0.08 | −0.011 (−0.019, −0.003) | 0.013 |
Compared with baseline.
Compared with ET.
Adverse Events
Dapagliflozin was generally well tolerated. There were no serious adverse events, and no participants discontinued dapagliflozin after treatment commenced (Supplementary Table 3).
Conclusions
The findings from the DAPASALT (An Open Label, Phase IV, Mechanistic, Three-Arm Study to Evaluate the Natriuretic Effect of 2-Week Dapagliflozin treatment in Type 2 Diabetes Mellitus Patients with Either Preserved or Impaired Renal Function and Non-Diabetics with Impaired Renal Function) trial show that during standardized conditions, including a strictly standardized sodium intake with overall good compliance with the diet and stable use of ARBs, 24-h sodium excretion did not change in diuretic-naive patients with type 2 diabetes and preserved kidney function. Despite similar sodium balance, SBP acutely decreased, which persisted during the 2-week treatment period. Extracellular volume was reduced after 4 days of treatment, but this initial reduction dissipated after 14 days of treatment. Taken together, these data suggest that the blood pressure–lowering effect during treatment with SGLT2 inhibitors may be sodium excretion independent.
SGLT2 inhibitors exert their effect on the proximal tubule of the kidney by blocking glucose and sodium reabsorption (18), resulting in glucosuria and an increase in sodium and chloride delivery at the juxtaglomerular apparatus. Indeed, we observed an increase in fractional lithium excretion, a proxy for reduced sodium reabsorption at the proximal tubule, during dapagliflozin treatment. Enhanced natriuresis could be expected as a consequence of inhibition of proximal sodium reabsorption. The lack of increased natriuresis in our trial suggests that compensatory mechanisms in the distal tubule may be involved to compensate for the proximal decrease in sodium reabsorption. Increased activity of the renin-angiotensin-aldosterone system and, consequently, aldosterone-regulated increased sodium reabsorption through epithelial sodium channels might contribute to this, as reflected by the increase in urinary aldosterone excretion at ST despite all patients using a stable dose of ARBs. After drug discontinuation, we observed a trend toward sodium and volume retention. This might reflect that activation of compensatory mechanisms in the distal tubule may take >4 days to resolve.
In contrast to our findings, clinical studies in patients with type 2 diabetes reported that SGLT2 inhibitors induce transient increases in urine volume (8,9,19,20) and natriuresis (8–11). Indeed, a model-based prediction of the DAPASALT study also suggested that a transient increase in natriuresis can be expected at ST (21). However, few experimental and clinical studies were performed during standardized sodium intake, which is essential to properly assess natriuretic effects. A study in healthy volunteers receiving a standardized sodium diet and sodium tablets aimed at daily sodium intake of 110 mmol reported modest natriuresis in the first 6 h following dapagliflozin administration. However, no baseline assessment was performed, and therefore, actual 6-h sodium excretion was determined but no changes from baseline could be determined. In addition, poor tolerance to the sodium tablets was observed. Both limitations hamper the interpretation of the results (10). Another study in patients with type 2 diabetes and heart failure reported a similar acute increase in urinary sodium excretion over 6 h, but sodium intake was not standardized, fluid administration was high, and 24-h urine samples were not collected (15). The current study investigated the effect of dapagliflozin on cumulative urinary sodium excretion in well-controlled patients with type 2 diabetes and preserved kidney function following a strictly controlled sodium diet. The primary end point was chosen on the basis of earlier studies that suggested that changes in natriuresis would occur 2–4 days following dapagliflozin administration (10,15). Acute effects during the first hours after dapagliflozin administration were not assessed, and it is possible that a modest transient increase in sodium excretion was present but not detected. We observed a modest increase in 24-h urinary sodium excretion at day 1. Whether this increase is real or can be attributed to biological variation in sodium excretion, which has been reported previously in a well-controlled salt balance study, is difficult to ascertain (22). Nevertheless, even when the acute and transient increase in natriuresis reflects a genuine effect, it is questionable whether the magnitude is sufficient for the substantial and persistent reduction in blood pressure.
Under healthy circumstances, up to 180 g/day of glucose is filtered by the kidney glomerulus, and virtually all of it is subsequently reabsorbed in the proximal convoluted tubule predominantly by SGLT2 (23). Treatment with SGLT2 inhibitors increases glucose concentration in the distal nephron, resulting in a decrease in the osmotic gradient between the tubular fluid and interstitium. This reduces passive water reabsorption, resulting in osmotic diuresis (13,14). In the current study, glycosuria was consistently increased, although we did not observe a clear effect on urine volume, which contrasts the findings from in silico models (21). A previous study showed that dapagliflozin caused a larger increase in urine compared with plasma osmolality, making it less likely that osmotic diuresis explains the observed hemodynamic effects in our study (24). Given that glycosuria is persistent during treatment with dapagliflozin while the actual fluid balance of the participants was negative, the kidney likely uses other mechanisms to concentrate the urine and enhance water reabsorption. Antidiuretic hormone–regulated renal water conservation could possibly be involved. To conserve water, antidiuretic hormone indirectly increases renal urea absorption, thereby increasing urea-driven urine concentration, because urea is the most efficient organic osmolyte to concentrate urine and prevent dehydration (25–27). Preclinical studies have shown that dapagliflozin increases levels of the urea transporter UT-A1 in rats, supporting this hypothesis (28).
If the observed reductions in systemic blood pressure in this study are not (osmotic) diuretic related, other effects that could contribute include reductions in arterial stiffness, inhibition of the sympathetic nervous system (29–31), or restoration of endothelial function. Indeed, in a previous study, dapagliflozin reduced pulse wave velocity 2 days after initiation, a time course of effect similar to our study (32,33). Another compartment that could influence extracellular volume and blood pressure is the endothelial surface layer or glycocalyx (34,35). Damage to the endothelial glycocalyx has been observed in patients with type 2 diabetes, and SGLT2 inhibition has been shown to restore the structural integrity of the glycocalyx in vitro and in vivo (35–37).
In the current study, plasma volume did not statistically significantly change during treatment with dapagliflozin in patients who had no heart failure or volume overload at baseline, although the magnitude of effect in our small study was consistent with the reduction in plasma volume observed in a prior study with dapagliflozin (12). In addition, during FU, plasma volume significantly increased compared with ET, which could reflect a real effect of dapagliflozin on plasma volume. Extracellular volume was acutely reduced after 4 days, while we did not observe a clear effect on intracellular volume. Because extracellular volume is the sum of plasma volume and interstitial volume, this may suggest that although not directly measured, a proportionally larger decrease in interstitial volume occurred, which is consistent with the results suggested by Hallow et al. (13) in a modeling study of SGLT2 inhibitor effects. Importantly, heart failure is characterized by interstitial fluid accumulation and sodium retention, leading to peripheral and pulmonary edema, which may lead to differential effects of dapagliflozin on natriuresis and plasma volume in this population. The ability to selectively reduce interstitial fluid may be a unique feature of SGLT2 inhibitors compared with diuretics and has been suggested in a modeling study comparing dapagliflozin with the loop diuretic bumetanide. Although both drugs were associated with a reduction in interstitial fluid, dapagliflozin induced little or no change in blood volume, whereas bumetanide was associated with greater reductions in intravascular volume (6,13). However, so far, no clinical data have confirmed this notion. A differential effect in regulating interstitial fluid may be particularly important in patients with heart failure in whom, in many instances, arterial intravascular contraction is present and often provoked by diuresis. However, the fact that the effect of extracellular fluid contraction dissipated during prolonged treatment in our study would suggest a physiological adaptive response of the kidney to maintain body fluid volume, at least in patients with type 2 diabetes and preserved kidney function. Whether our findings can be extrapolated to patients with heart failure requires further study.
We acknowledge some limitations. In contrast to other studies with SGLT2 inhibitors, we did not observe an increase in hematocrit. The lack of effect on hematocrit may be explained by the multiple blood samplings performed during the relatively short study. To study the effect of dapagliflozin on natriuresis and systemic hemodynamics, we deliberately performed this study in a homogeneous cohort of patients with type 2 diabetes and preserved kidney function who were all using an ARB without concomitant diuretic treatment, thereby avoiding disease heterogeneity and potential confounding by preexisting kidney damage. The carefully selected cohort may limit the generalizability of our findings. In addition, extracellular and intracellular volumes were assessed by BIS, which is not the gold standard method to assess body composition. Nevertheless extracellular and intracellular volume assessed by BIS correlates well with the reference bromide method (38). In addition, although the study was sufficiently powered for the primary outcome, the size of our study cohort was small, limiting the precision of the effect estimates for some of the secondary end points. Finally, the open-label design does not allow definitive conclusions. Our results should be considered hypothesis generating.
In conclusion, we have demonstrated that treatment with dapagliflozin lowers blood pressure without clear effects on sodium excretion in patients with type 2 diabetes and preserved kidney function. Together with the absence of prolonged changes in plasma volume, extracellular volume, and intracellular volume, this suggests that other nondiuretic-related effects cannot be ruled out in the CV protective effects conferred by SGLT2 inhibitors.
Article Information
Acknowledgments. The authors are extremely grateful to the participants who volunteered in the study. The authors acknowledge the help of dietician Esther Pekel for developing the food menus and the following study nurses and assistants who were indispensable in the process of data collection: Jeannette Boerop, Ingrid Knufman, and Renée de Meijer (Diabetes Center, Department of Internal Medicine, AUMC, location VUMC). The authors also thank the study team members at AstraZeneca. Finally, the authors thank Parita Sheth (inScience Communications, London, U.K.) for assistance with figure preparation and editing; this support was funded by AstraZeneca.
Funding and Duality of Interest. The DAPASALT study was funded by AstraZeneca. M.H.A.M. has acted as a speaker/consultant for AstraZeneca, Eli Lilly, Novo Nordisk, and Sanofi; all honoraria are paid to his employer (AUMC, location VUMC). P.J.G., C.K., A.H., and N.A. are employees and shareholders at AstraZeneca. D.H.v.R. has acted as a consultant and received honoraria from Boehringer Ingelheim, Eli Lilly, Merck, Novo Nordisk, Sanofi, and AstraZeneca and has received research operating funds from Boehringer Ingelheim-Lilly Diabetes Alliance, AstraZeneca, and Novo Nordisk; all honoraria are paid to his employer (AUMC, location VUMC). H.J.L.H. is consultant for AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Gilead Sciences, Janssen, Merck, Mundipharma, Mitsubishi Tanabe, Novo Nordisk, and Retrophin. He received research support from AbbVie, AstraZeneca, Boehringer Ingelheim, and Janssen. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. R.A.S., M.H.A.M., M.J.B.v.B., A.H., D.H.v.R., and H.J.L.H. were involved in data collection. R.A.S., D.H.v.R., and H.J.L.H. wrote the drafts of the manuscript. P.J.G., C.K., D.H.v.R., and H.J.L.H. designed the study. All authors were involved in the data analysis and interpretation and participated in critical review of the manuscript drafts and approved the final version for submission. R.A.S., D.H.v.R., and H.J.L.H. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented at the European Society of Cardiology Congress, 2020–The Digital Experience, 29 August–1 September, 2020.
Footnotes
Clinical trial reg. no. NCT03152084, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13232276.
D.H.v.R. and H.J.L.H. contributed equally to this work.
References
- 1.Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 2.Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644–657 [DOI] [PubMed] [Google Scholar]
- 3.Wiviott SD, Raz I, Bonaca MP, et al. DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 4.Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet 2019;393:31–39 [DOI] [PubMed] [Google Scholar]
- 5.Heerspink HJL, Stefánsson BV, Correa-Rotter Ret al. Dapagliflozin in patients with chronic kidney disease. N Engl J Med 2020;383:1436–1446 [DOI] [PubMed] [Google Scholar]
- 6.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia 2018;61:2108–2117 [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE, Zinman B, Fitchett D, et al. How does empagliflozin reduce cardiovascular mortality? Insights from a mediation analysis of the EMPA-REG OUTCOME trial. Diabetes Care 2018;41:356–363 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Takano K, Iijima H, et al. Factors affecting canagliflozin-induced transient urine volume increase in patients with type 2 diabetes mellitus. Adv Ther 2017;34:436–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iijima H, Kifuji T, Maruyama N, Inagaki N. Pharmacokinetics, pharmacodynamics, and safety of canagliflozin in Japanese patients with type 2 diabetes mellitus. Adv Ther 2015;32:768–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilcox CS, Shen W, Boulton DW, Leslie BR, Griffen SC. Interaction between the sodium-glucose-linked transporter 2 inhibitor dapagliflozin and the loop diuretic bumetanide in normal human subjects. J Am Heart Assoc 2018;7:e007046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blau JE, Bauman V, Conway EM, et al. Canagliflozin triggers the FGF23/1,25-dihydroxyvitamin D/PTH axis in healthy volunteers in a randomized crossover study. JCI Insight 2018;3:e99123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lambers Heerspink HJ, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a glucose-regulating drug with diuretic properties in subjects with type 2 diabetes. Diabetes Obes Metab 2013;15:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hallow KM, Helmlinger G, Greasley PJ, McMurray JJV, Boulton DW. Why do SGLT2 inhibitors reduce heart failure hospitalization? A differential volume regulation hypothesis. Diabetes Obes Metab 2018;20:479–487 [DOI] [PubMed] [Google Scholar]
- 14.Mordi NA, Mordi IR, Singh JS, McCrimmon RJ, Struthers AD, Lang CC. Renal and cardiovascular effects of SGLT2 inhibition in combination with loop diuretics in patients with type 2 diabetes and chronic heart failure: the RECEDE-CHF trial. Circulation 2020;142:1713–1724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Griffin M, Rao VS, Ivey-Miranda J, et al. Empagliflozin in heart failure: diuretic and cardiorenal effects. Circulation 2020;142:1028–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacob M, Chappell D, Conzen P, et al. Impact of the time window on plasma volume measurement with indocyanine green. Physiol Meas 2008;29:761–770 [DOI] [PubMed] [Google Scholar]
- 17.Jacob M, Conzen P, Finsterer U, Krafft A, Becker BF, Rehm M. Technical and physiological background of plasma volume measurement with indocyanine green: a clarification of misunderstandings. J Appl Physiol (1985) 2007;102:1235–1242 [DOI] [PubMed] [Google Scholar]
- 18.van Bommel EJ, Muskiet MH, Tonneijck L, Kramer MH, Nieuwdorp M, van Raalte DH. SGLT2 inhibition in the diabetic kidney-from mechanisms to clinical outcome. Clin J Am Soc Nephrol 2017;12:700–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sha S, Devineni D, Ghosh A, et al. Pharmacodynamic effects of canagliflozin, a sodium glucose co-transporter 2 inhibitor, from a randomized study in patients with type 2 diabetes. PLoS One 2014;9:e105638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heise T, Seewaldt-Becker E, Macha S, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013;15:613–621 [DOI] [PubMed] [Google Scholar]
- 21.Hallow KM, Boulton DW, Penland RC, et al. Renal effects of dapagliflozin in people with and without diabetes with moderate or severe renal dysfunction: prospective modeling of an ongoing clinical trial. J Pharmacol Exp Ther 2020;375:76–91 [DOI] [PubMed] [Google Scholar]
- 22.Lerchl K, Rakova N, Dahlmann A, et al. Agreement between 24-hour salt ingestion and sodium excretion in a controlled environment. Hypertension 2015;66:850–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerich JE Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med 2010;27:136–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eickhoff MK, Dekkers CCJ, Kramers BJ, et al. Effects of dapagliflozin on volume status when added to renin-angiotensin system inhibitors. J Clin Med 2019;8:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marton A, Kaneko T, Kovalik JP, et al. Organ protection by SGLT2 inhibitors: role of metabolic energy and water conservation. Nat Rev Nephrol. 1 October 2020 [Epub ahead of print]. DOI: 10.1038/s41581-020-00350-x [DOI] [PubMed] [Google Scholar]
- 26.Kitada K, Daub S, Zhang Y, et al. High salt intake reprioritizes osmolyte and energy metabolism for body fluid conservation. J Clin Invest 2017;127:1944–1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakova N, Kitada K, Lerchl K, et al. Increased salt consumption induces body water conservation and decreases fluid intake. J Clin Invest 2017;127:1932–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, LaRocque LM, Efe O, Wang J, Sands JM, Klein JD. Effect of dapagliflozin treatment on fluid and electrolyte balance in diabetic rats. Am J Med Sci 2016;352:517–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chilton R, Tikkanen I, Cannon CP, et al. Effects of empagliflozin on blood pressure and markers of arterial stiffness and vascular resistance in patients with type 2 diabetes. Diabetes Obes Metab 2015;17:1180–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Striepe K, Jumar A, Ott C, et al. Effects of the selective sodium-glucose cotransporter 2 inhibitor empagliflozin on vascular function and central hemodynamics in patients with type 2 diabetes mellitus. Circulation 2017;136:1167–1169 [DOI] [PubMed] [Google Scholar]
- 31.Cherney DZ, Perkins BA, Soleymanlou N, et al. The effect of empagliflozin on arterial stiffness and heart rate variability in subjects with uncomplicated type 1 diabetes mellitus. Cardiovasc Diabetol 2014;13:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Solini A, Giannini L, Seghieri M, et al. Dapagliflozin acutely improves endothelial dysfunction, reduces aortic stiffness and renal resistive index in type 2 diabetic patients: a pilot study. Cardiovasc Diabetol 2017;16:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Bommel EJM, Smits MM, Ruiter D, et al. Effects of dapagliflozin and gliclazide on the cardiorenal axis in people with type 2 diabetes. J Hypertens 2020;38:1811–1819 [DOI] [PubMed] [Google Scholar]
- 34.Oberleithner H Two barriers for sodium in vascular endothelium? Ann Med 2012;44(Suppl. 1):S143–S148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper S, Teoh H, Campeau MA, Verma S, Leask RL. Empagliflozin restores the integrity of the endothelial glycocalyx in vitro. Mol Cell Biochem 2019;459:121–130 [DOI] [PubMed] [Google Scholar]
- 36.Ikonomidis I, Pavlidis G, Thymis J, et al. Effects of glucagon-like peptide-1 receptor agonists, sodium-glucose cotransporter-2 inhibitors, and their combination on endothelial glycocalyx, arterial function, and myocardial work index in patients with type 2 diabetes mellitus after 12-month treatment. J Am Heart Assoc 2020;9:e015716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herat LY, Magno AL, Rudnicka C, et al. SGLT2 inhibitor-induced sympathoinhibition: a novel mechanism for cardiorenal protection. JACC Basic Transl Sci 2020;5:169–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbosa EJ, Glad CA, Nilsson AG, et al. Extracellular water and blood pressure in adults with growth hormone (GH) deficiency: a genotype-phenotype association study. PLoS One 2014;9:e105754. [DOI] [PMC free article] [PubMed] [Google Scholar]


