Abstract
OBJECTIVE
To further evaluate the safety and efficacy of the Control-IQ closed-loop control (CLC) system in children with type 1 diabetes.
RESEARCH DESIGN AND METHODS
After a 16-week randomized clinical trial (RCT) comparing CLC with sensor-augmented pump (SAP) therapy in 101 children 6–13 years old with type 1 diabetes, 22 participants in the SAP group initiated use of the CLC system (referred to as SAP-CLC cohort), and 78 participants in the CLC group continued use of CLC (CLC-CLC cohort) for 12 weeks.
RESULTS
In the SAP-CLC cohort, mean percentage of time in range 70–180 mg/dL (TIR) increased from 55 ± 13% using SAP during the RCT to 65 ± 10% using CLC (P < 0.001), with 36% of the cohort achieving TIR >70% plus time <54 mg/dL <1% compared with 14% when using SAP (P = 0.03). Substantial improvement in TIR was seen after the 1st day of CLC. Time <70 mg/dL decreased from 1.80% to 1.34% (P < 0.001). In the CLC-CLC cohort, mean TIR increased from 53 ± 17% prerandomization to 67 ± 10% during the RCT and remained reasonably stable at 66 ± 10% through the 12 weeks post-RCT. No episodes of diabetic ketoacidosis or severe hypoglycemia occurred in either cohort.
CONCLUSIONS
This further evaluation of the Control-IQ CLC system supports the findings of the preceding RCT that use of a closed-loop system can safely improve glycemic control in children 6–13 years old with type 1 diabetes from the 1st day of use and demonstrates that these improvements can be sustained through 28 weeks of use.
Introduction
The Control-IQ closed-loop control (CLC) system, consisting of an insulin pump (t:slim ×2 insulin pump with Control-IQ Technology; Tandem Diabetes Care, San Diego, CA) and a continuous glucose monitor (CGM) (Dexcom G6; Dexcom, San Diego, CA), has been approved by the U.S. Food and Drug Administration for clinical use in patients with type 1 diabetes ≥14 years old. Approval was based on the results of a 26-week randomized clinical trial (RCT) that demonstrated efficacy and safety, with an improvement of 11% in time in time in range 70–180 mg/dL (TIR) with CLC compared with sensor-augmented pump (SAP) therapy, and significant reductions in CGM-measured hyperglycemia, hypoglycemia, and glycemic variability, and in HbA1c (1).
We conducted a second RCT in 101 6- to 13-year-olds and similarly found this CLC system to be safe and effective, with an improvement in TIR of 11% over 16 weeks with CLC compared with SAP (2). No episodes of diabetic ketoacidosis or severe hypoglycemia occurred in either group. To further assess the safety and efficacy of the system in this age range, the study was designed with a 12-week extension phase in which the SAP group in the RCT initiated the CLC system and the CLC group continued to use the system with reduced study contact, the results of which are reported herein.
Research Design and Methods
This study was conducted at four pediatric diabetes centers in the U.S. The protocol was approved by a central Institutional Review Board (Tampa, FL), written informed consent was obtained from the parent or guardian of each participant, and assent was obtained from each participant when applicable. An Investigational Device Exemption was approved by the U.S. Food and Drug Administration. An independent Data and Safety Monitoring Board provided study oversight. The protocol is available at nejm.org, and details are provided on clinicaltrials.gov (NCT03844789). Key aspects of the protocol are described herein.
The randomized trial included 101 participants age 6–13 years old with type 1 diabetes for at least 1 year. Before entering the RCT, 20% used multiple daily injections and 80% a pump for insulin delivery, and 92% were using a CGM. HbA1c ranged from 5.7 to 10.1% (mean 7.7 ± 1.0%; 40% with HbA1c ≥8.0%). Participants were randomly assigned 3:1 to use the CLC system (consisting of a Tandem t:slim ×2 insulin pump with Control-IQ Technology and a Dexcom G6 CGM) or SAP for 16 weeks. The RCT was completed by 100 of the 101 participants.
After the 16-week randomized trial, the 100 participants completing the RCT entered an extension phase and were monitored for an additional 12 weeks for a total of 28 weeks of follow-up. In this phase, 22 participants in the RCT SAP group initiated use of the CLC system (referred to as SAP-CLC cohort), and 78 participants in the RCT CLC group continued use of CLC (CLC-CLC cohort).
During the extension phase, the SAP-CLC cohort had a study contact schedule similar to the CLC-CLC cohort in the RCT phase with phone contacts after 1, 3, 5, and 9 weeks and visits after 7 and 12 weeks. For the CLC-CLC cohort, study contacts were reduced in the extension phase to phone contacts after 4 and 8 weeks and a single visit at 12 weeks (28 weeks from randomization). Pump, CGM, blood glucose meter, and ketone meter data were downloaded and reviewed at each visit. HbA1c was measured at the beginning and end of the RCT (which served as the extension phase baseline) and at the end of the extension phase by a central laboratory at the University of Minnesota Advanced Research and Diagnostic Laboratory. Patient-reported outcomes questionnaires were completed at the beginning and end of the RCT and at the end of the extension phase and will be reported separately. The 10-item System Usability Scale was completed at the end of the extension phase by parents and by participants 10 years and older.
Reportable adverse events included serious adverse events, adverse events occurring in association with a study device or procedure, severe hypoglycemia (defined as hypoglycemia requiring assistance due to altered consciousness), diabetic ketoacidosis as defined by the Diabetes Control and Complications Trial (3), or hyperglycemia with ketonemia for which a health care provider was contacted.
Statistical Analysis
Outcomes included CGM metrics and HbA1c. CGM-measured outcomes were calculated by pooling data at RCT baseline (14 days before randomization), weeks 1–16 (RCT), and weeks 17–28 (extension phase).
A paired t test, signed rank test, or the McNemar test, as appropriate, was used to evaluate the change in each outcome between the RCT and the extension phase separately in each cohort. P values are two-sided and have been adjusted for multiple comparisons to control the false discovery rate using the adaptive Benjamini-Hochberg procedure (4). All analyses were conducted on available cases only. Analyses were conducted with SAS 9.4 software (SAS Institute, Cary, NC).
Results
The age range of the 100 participants at the start of the extension phase was 6–14 years; characteristics of the two cohorts are shown in Supplementary Table 1. The extension phase was completed by all 22 of the SAP-CLC cohort (100%; used SAP during the RCT and initiated CLC in the extension phase) and by 76 of 78 of the CLC-CLC cohort (97%; used CLC in both the RCT and extension phase) (Supplementary Fig. 1). None of the SAP-CLC cohort and 2 of the CLC-CLC cohort had at least one unscheduled visit, and 7 (32%) and 33 (42%), respectively, had at least one unscheduled phone contact (Supplementary Table 2).
SAP-CLC Cohort
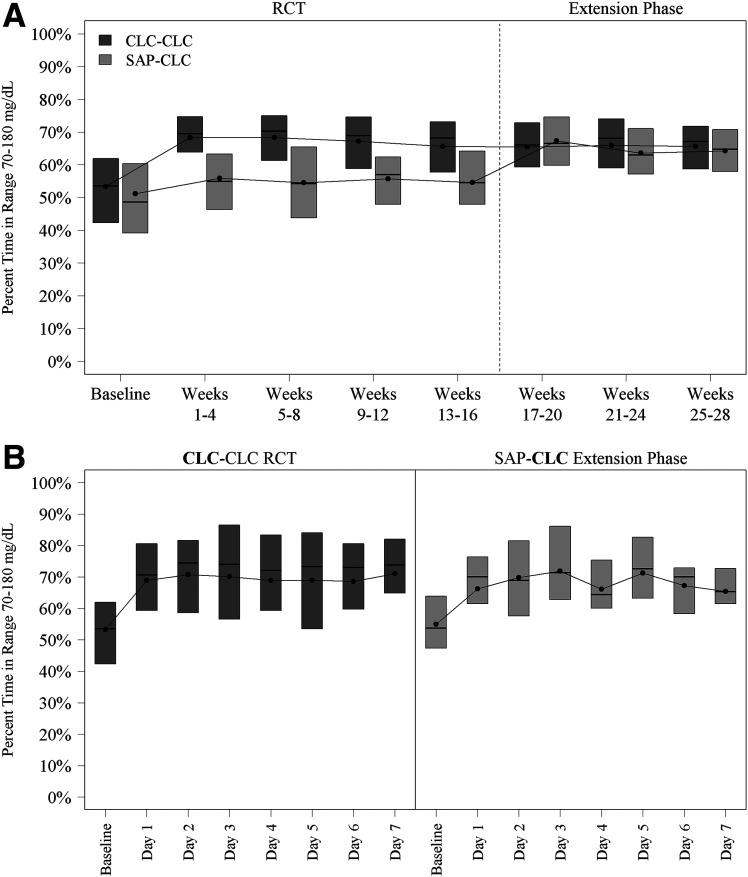
Among the 22 participants in the SAP-CLC cohort, mean TIR increased from 55 ± 13% during the 16-week RCT to 65 ± 10% during the extension phase (mean change from baseline 9.7% ± 6.2%, P < 0.001) (Table 1). The treatment effect was evident on the 1st day of CLC use and remained consistent over the 12 weeks of follow-up (Fig. 1). Daytime (6 a.m.–12 midnight) mean TIR was 56% ± 14% during the RCT vs. 61 ± 11% during the extension phase, and nighttime (12 midnight–6 a.m.) was 54% ± 16 vs. 75% ± 13%, respectively (Supplementary Table 3). Time <70 mg/dL was low at baseline (median 1.80%) but nevertheless significantly decreased to 1.34% during follow-up (median change from baseline −0.62%, P < 0.001). Significant reductions from baseline were seen in all hyperglycemia metrics and most hypoglycemia metrics (Table 1). Over the 12-week extension, 8 participants (36%) had both TIR >70% plus time <54 mg/dL <1% compared with 3 (14%) during the 16-week RCT (P = 0.03).
Table 1.
Overall CGM-measured outcomes in the SAP-CLC cohort
| RCT baseline* (n = 23) | RCT follow-up (n = 22) | Extension phase follow-up (n = 22) | Change from RCT follow-up to extension follow-up (n = 22) | P value† extension phase follow-up vs. RCT follow-up | |
|---|---|---|---|---|---|
| Hours of CGM data | 311 ± 23 | 2,609 ± 128 | 1,981 ± 86 | — | — |
| Glucose control | |||||
| Percent TIR 70–180 mg/dL | 51 ± 16 | 55 ± 13 | 65 ± 10 | 9.7 ± 6.2 | <0.001 |
| Percent TIR 70–140 mg/dL | 31 ± 14 | 35 ± 11 | 42 ± 8 | 7.5 ± 5.6 | <0.001 |
| Mean glucose (mg/dL) | 189 ± 34 | 179 ± 26 | 167 ± 18 | −12.1 ± 11.6 | <0.001 |
| Glucose coefficient of variation (%) | 38 ± 4 | 39 ± 4 | 38 ± 4 | −1.1 ± 3.2 | 0.12 |
| Glucose SD (mg/dL) | 71 ± 16 | 70 ± 13 | 64 ± 12 | −6.5 ± 6.0 | <0.001 |
| Hyperglycemia | |||||
| Percent time >180 mg/dL | 47 ± 17 | 43 ± 14 | 34 ± 10 | −9.0 ± 6.6 | <0.001 |
| Percent time >250 mg/dL | 20.7 (12.4–32.6) | 18.4 (9.4–24.6) | 10.1 (6.3–15.8) | −5.2 (−10.3 to −2.3) | <0.001 |
| Percent time >300 mg/dL | 8.0 (2.5–15.0) | 6.8 (2.9–11.2) | 3.5 (1.8–6.6) | −3.0 (−4.6 to −0.9) | <0.001 |
| High blood glucose index | 12.5 ± 6.1 | 10.8 ± 4.5 | 8.3 ± 3.2 | −2.5 ± 2.0 | <0.001 |
| Rate of hyperglycemia events per week (>300 mg/dL)‡ | 7.1 (2.1–10.5) | 5.6 (3.4–8.1) | 3.0 (2.1–5.1) | −1.6 (−3.2 to −1.2) | <0.001 |
| Hypoglycemia | |||||
| Percent time <70 mg/dL | 1.03 (0.25–2.14) | 1.80 (1.13–3.04) | 1.34 (0.92–1.95) | −0.62 (−1.20 to −0.05) | <0.001 |
| Percent time <54 mg/dL | 0.08 (0.00–0.30) | 0.29 (0.12–0.62) | 0.25 (0.12–0.41) | −0.05 (−0.21–0.02) | 0.04 |
| Low blood glucose index | 0.35 (0.18–0.68) | 0.55 (0.41–0.92) | 0.48 (0.34–0.58) | −0.17 (−0.29 to −0.01) | <0.001 |
| Rate of hypoglycemia events per week (<70 mg/dL)§ | 2.1 (0.6–4.6) | 4.0 (2.9–6.4) | 2.9 (2.8–4.1) | −1.0 (−2.4 to −0.1) | <0.001 |
| Rate of more severe hypoglycemia events per week (<54 mg/dL)‖ | 0.0 (0.0–0.6) | 0.6 (0.1–1.0) | 0.4 (0.2–0.8) | −0.1 (−0.4 to 0.1) | 0.06 |
| Meeting target, n (%) | |||||
| TIR 70–180 mg/dL >70% | 3 (13) | 3 (14) | 8 (36) | NA | 0.03 |
| TIR 70–180 mg/dL >70% plus time <70 mg/dL <4% | 2 (9) | 3 (14) | 8 (36) | NA | 0.03 |
| TIR 70–180 mg/dL >70% plus time <54 mg/dL <1% | 3 (13) | 3 (14) | 8 (36) | NA | 0.03 |
Data are presented as mean ± SD, median (IQR), or as indicated otherwise. NA, not applicable.
RCT phase baseline is defined as the 14 days before randomization.
P values from a paired t test, Wilcoxon signed rank test, or McNemar test, as appropriate. P values were adjusted to control the false discovery rate.
At least 15 consecutive minutes >300 mg/dL.
At least 15 consecutive minutes <70 mg/dL.
At least 15 consecutive minutes <54 mg/dL.
Figure 1.
A: Box plots of TIR 70–180 mg/dL by 4-week period. B: Box plots of TIR 70–180 mg/dL by day in the week after initiation of the closed-loop system.
Mean HbA1c was 7.6 ± 0.9% at the start of the extension phase (i.e., the end of the RCT) and 7.3 ± 0.7% at the end of the 12-week extension phase. The percentage of participants with an HbA1c level <7.5% was 45% at the start of the extension phase and 62% at the end of the extension phase (Supplementary Table 4).
CLC-CLC Cohort
Among the 78 participants in the CLC-CLC cohort, mean TIR increased from 53 ± 17% at RCT baseline (prerandomization) to 67 ± 10% during the 16-week RCT and remained reasonably stable at 66 ± 10% through the 12-week extension phase (Table 2 and Fig. 1), although statistically this represented a small decrease (change in mean TIR from the RCT to the extension phase of −1.7% ± 3.8%, P < 0.001). Daytime (6 a.m.–12 midnight) mean TIR was 53% ± 17% at RCT baseline, 63% ± 11% during the RCT, and 61% ± 11% during the extension phase, and nighttime (12 midnight–6 a.m.) was 54% ± 20%, 80% ± 9%, and 79% ± 11%, respectively (Supplementary Table 5). Median time <70 mg/dL was 1.2% at RCT baseline, 1.57% during the RCT, and 1.50% during the extension phase.
Table 2.
Overall CGM-measured outcomes in the CLC-CLC cohort
| RCT baseline* (n = 77) | RCT follow-up (n = 78) | Extension phase follow-up (n = 78) | Change from RCT follow-up to extension follow-up (n = 78) | P value† extension phase follow-up vs. RCT follow-up | |
|---|---|---|---|---|---|
| Hours of CGM data | 306 ± 33 | 2,637 ± 134 | 1,980 ± 145 | — | — |
| Glucose control | |||||
| Percent TIR 70–180 mg/dL | 53 ± 17 | 67 ± 10 | 66 ± 10 | −1.7 ± 3.8 | <0.001 |
| Percent TIR 70–140 mg/dL | 33 ± 15 | 44 ± 10 | 42 ± 11 | −1.7 ± 4.1 | <0.001 |
| Mean glucose (mg/dL) | 183 ± 34 | 162 ± 18 | 165 ± 19 | 3.4 ± 7.1 | <0.001 |
| Glucose coefficient of variation (%) | 38 ± 5 | 38 ± 4 | 38 ± 4 | 0.0 ± 1.8 | 0.83 |
| Glucose SD (mg/dL) | 69 ± 14 | 61 ± 11 | 62 ± 11 | 1.2 ± 4.5 | 0.02 |
| Hyperglycemia | |||||
| Percent time >180 mg/dL | 45 ± 18 | 31 ± 10 | 33 ± 11 | 1.9 ± 3.8 | <0.001 |
| Percent time >250 mg/dL | 17.2 (8.6–27.6) | 7.8 (5.1–14.3) | 9.0 (6.7–15.4) | 1.2 (−0.9–2.6) | <0.001 |
| Percent time >300 mg/dL | 6.3 (1.9–14.2) | 2.6 (1.5–5.5) | 2.9 (1.8–5.9) | 0.4 (−0.4–1.3) | 0.003 |
| High blood glucose index | 11.5 ± 5.9 | 7.5 ± 3.1 | 8.0 ± 3.3 | 0.5 ± 1.2 | <0.001 |
| Rate of hyperglycemia events per week (>300 mg/dL)‡ | 5.6 (2.2–8.7) | 3.0 (1.7–5.2) | 3.1 (2.2–5.5) | 0.3 (−0.3–1.1) | 0.01 |
| Hypoglycemia | |||||
| Percent time <70 mg/dL | 1.20 (0.50–2.36) | 1.57 (0.79–2.44) | 1.50 (0.64–2.10) | −0.12 (−0.58–0.09) | 0.006 |
| Percent time <54 mg/dL | 0.14 (0.03–0.36) | 0.23 (0.10–0.45) | 0.18 (0.06–0.39) | −0.03 (−0.13–0.02) | 0.002 |
| Low blood glucose index | 0.47 (0.23–0.71) | 0.57 (0.30–0.72) | 0.48 (0.26–0.63) | −0.04 (−0.16–0.02) | <0.001 |
| Rate of hypoglycemia events per week (<70 mg/dL)§ | 3.0 (1.1–5.1) | 3.7 (1.7–5.8) | 3.4 (1.4–5.2) | −0.2 (−1.3–0.3) | 0.02 |
| Rate of more severe hypoglycemia events per week (<54 mg/dL)‖ | 0.0 (0.0–0.6) | 0.5 (0.1–0.8) | 0.3 (0.1–0.7) | 0.0 (−0.3–0.1) | 0.003 |
| Meeting target n (%) | |||||
| TIR 70–180 mg/dL >70% | 12 (16) | 37 (47) | 28 (36) | NA | 0.03 |
| TIR 70–180 mg/dL >70% plus time <70 mg/dL <4% | 11 (14) | 33 (42) | 25 (32) | NA | 0.06 |
| TIR 70–180 mg/dL >70% plus time <54 mg/dL <1% | 12 (16) | 34 (44) | 26 (33) | NA | 0.06 |
Data are presented as the mean ± SD, median (IQR), or as indicated otherwise. NA, not applicable.
RCT phase baseline is defined as the 14 days before randomization.
P values from a paired t test, Wilcoxon signed rank test, or McNemar test, as appropriate. P values were adjusted to control the false discovery rate.
At least 15 consecutive minutes >300 mg/dL.
At least 15 consecutive minutes <70 mg/dL.
At least 15 consecutive minutes <54 mg/dL.
The percentage of participants with both TIR >70% plus time <54 mg/dL <1% was 16% at RCT baseline, 44% during the RCT, and 33% during the extension phase (Table 2). The percentage of participants with TIR >70% was 16% at RCT baseline, 47% during the RCT phase, and 36% during the extension phase. There were 13 participants who met the target TIR of >70% in the RCT but not in the extension phase. Among these 13 participants, the changes in TIR from the RCT to the extension phase were small. The median change was −3.6%, ranging from −6.7% to −1.1%, with 11 of 13 having a decrease <5% and the lowest TIR being 65.1%. Of 21 participants with TIR >70% during daytime in the RCT, 6 had TIR ≤70% in the extension as did 3 of 65 overnight.
Mean HbA1c was 7.6 ± 1.0% at randomization, 7.0 ± 0.8% at the end of the 16-week RCT, and 7.2 ± 0.9% at the end of the 12-week extension phase. The percentages of participants with HbA1c <7.5% were 45%, 74%, and 63%, respectively (Supplementary Table 6). Eleven participants with HbA1c <7.0% at the end of the RCT had an HbA1c level ≥7.0% at the end of the extension phase. Among these 11 participants, median HbA1c at the end of the extension phase was 7.1%, with 7 of the 11 having an HbA1c ≤7.1% and the highest HbA1c being 7.8%. All of these participants whose baseline (pre-RCT) HbA1c was ≥7.0% had a reduction in HbA1c from the RCT baseline to the end of the extension phase.
Glucose Monitoring and Closed-Loop System Use
In both cohorts, all participants were still using the closed-loop system at the end of the 12 weeks (which equated to 28 total weeks for those in the CLC-CLC cohort). Median percentage of CGM use over the 12 weeks of the extension phase was 97% (interquartile range [IQR] 95–98%) in both cohorts (Supplementary Table 7). Median percentage of time the system was in closed-loop mode over the 12 weeks of the extension phase was 94% (IQR 92–95%) in the SAP-CLC cohort and 94% (IQR 92–96%) in the CLC-CLC cohort (Supplementary Table 8). The System Usability Scale survey showed a high degree of satisfaction with the closed-loop system by both parents and the children 10 years and older: 90% of parents and 87% of children 10 years and older across both cohorts agreed or strongly agreed that they would like to use the CLC system frequently (Supplementary Tables 9 and 10 and Supplementary Fig. 2).
Participants performed a median of 0.17 (IQR 0.06–0.50) finger stick blood glucose measurements per day in the SAP-CLC cohort and 0.20 (IQR 0.05–0.48) in the CLC-CLC cohort during the 12-week extension phase.
Safety and Other Outcomes
There were no diabetic ketoacidosis or severe hypoglycemia events in either cohort during the extension phase. Of the 100 participants, 8 reported a total of 10 adverse events. All reported events were related to hyperglycemia with or without ketosis, with eight due to a presumed pump infusion set problem, one due to loss of CGM connectivity and insufficient insulin in the pump, and one due to illness (Supplementary Table 11). Evaluation of uploaded study ketone meters showed that a blood ketone level ≥1.0 mmol/L occurred on 16 (0.08%) of 20,150 days, not all of which were reported as an adverse event.
Insulin and BMI data are provided in Supplementary Tables 12 and 13.
Conclusions
The results of this further evaluation of the Control-IQ CLC system support the findings of the preceding RCT that the system is safe and effective for improving glycemic control in children age 6–13 years old with type 1 diabetes. The extension phase included two cohorts. In the cohort that used SAP in the preceding RCT (SAP-CLC cohort), mean TIR increased by 9.7% with CLC compared with SAP. In the cohort that used CLC in the RCT (CLC-CLC cohort), improvements in TIR observed in the RCT were maintained through the 12 weeks of the extension phase, under reduced monitoring.
The improvement in TIR observed in the SAP-CLC cohort of 9.7% is consistent with the treatment group difference observed in the preceding RCT of 10.7% for CLC compared with SAP (2). It is noteworthy that the improvement in TIR was observed after the 1st day of CLC use and then was sustained for the 12 weeks, suggesting that the system can be highly effective from the time of initiation. Although the amount of hypoglycemia was already low at baseline, likely because most were using an insulin pump with a predictive low glucose suspend feature, there nevertheless was a reduction observed in time <70 mg/dL.
In the CLC-CLC cohort, from a clinical perspective, TIR was reasonably stable across the entire 28 weeks of follow-up. Mean TIR decreased by 1.7% from the RCT to the extension phase. However, this change, although statistically significant, is far below the threshold of 5% to be considered clinically meaningful (5).
No severe hypoglycemia or diabetic ketoacidosis events occurred in either cohort in the extension phase. Combined with the RCT, this represents no events during 579 person-months of use of the Control-IQ system.
Use of the CGM and closed-loop mode was consistently high across follow-up. There was no drop-off in the CLC-CLC cohort between the randomized trial and extension phase. This is reflective of the high satisfaction with the system reported by parents and participants on the System Usability Scale.
There are no other published studies of this size and length evaluating a CLC system in this age-group. In a 3-month study of the Medtronic MiniMed 670G system in 105 children age 7–13 years old, Forlenza et al. (6) reported an increase in TIR 70–180 mg/dL from 56% at baseline to 65% during follow-up, with median time in auto mode being 81%. Real-world data on use of the 670G system in youth have indicated a substantially lower time in auto mode and a discontinuation rate of 30% within 6 months (7,8). In a 12-week crossover trial, Thabit et al. (9) reported higher overnight TIR using CLC of similar magnitude to that found in our study in 25 children 6–18 years old with type 1 diabetes.
The strengths of this study include its multicenter design, high rate of retention, with 97% of randomized participants completing the extension study, and high participant adherence to the assigned interventions.
However, there are some limitations. Participants came from a more advantaged socioeconomic background, had more experience with diabetes technology, and had better glycemic control at baseline than would be expected in the general population of children in this age-group with type 1 diabetes. Further studies are needed to evaluate the system in those with less advantaged socioeconomic status and those with poor glycemic control. Additionally, participants were monitored for a maximum of 28 weeks on the closed-loop system. It is still unknown whether the improvements in glycemic control can be sustained over a longer term in a real-world setting.
In conclusion, this extension study has further demonstrated that the ControlIQ CLC system can safely improve glycemic control in 6–13-year-olds with type 1 diabetes from the 1st day of use and that these improvements can be sustained through 28 weeks of use. Real-world use data will be valuable to assess whether similar results are achieved and are maintained for a longer time period.
Article Information
Funding. This study was funded by Tandem Diabetes, Inc. and the National Institute of Diabetes and Digestive and Kidney Diseases (UC4 108483). Tandem Diabetes Care provided the experimental closed-loop systems used in the trial, systemrelated supplies, including the Dexcom CGM and Roche glucometer, and technical expertise.
Duality of Interest. R.P.W. reports receiving grant support, consulting fees, and supplies, provided to his institution, from Dexcom, advisory fees from Medtronic, and grant support, provided to his institution, from Tandem Diabetes Care and Bigfoot Biomedical, grant support, paid to his institution, advisory board fees, and supplies, provided to his institution, from Eli Lilly, and grant support, paid to his institution, and supplies, provided to his institution, from MannKind and Novo Nordisk. G.P.F. reports receiving grants support and lecture fees from Medtronic, MiniMed, Insulet, and Tandem, grant support from Abbott, and grant support and consulting fees from Eli Lilly. S.A.W. reports speaker and consultancy fees from Medtronic, Insulet, and Sanofi outside the submitted work. B.A.B. reports receiving grant support and advisory board fees from Medtronic Diabetes and ConvaTec, grant support and presentation fees from Insulet, advisory board fees from Novo Nordisk and Profusa, grant support from Eli Lilly, grant support and equipment from Dexcom, and holding patent 61197230 on a hypoglycemia prediction algorithm. R.W.B. reports receiving consulting fees, paid to his institution, from Insulet, Bigfoot Biomedical, and Eli Lilly, grant support and supplies, provided to his institution, from Tandem and Dexcom, and supplies from Ascenia and Roche. No other potential conflicts of interest relevant to this article were reported.Author Contributions. L.G.K. wrote and edited the manuscript and performed statistical analyses. R.P.W., M.D.B., K.J.R., L.E., G.P.F., E.C., M.J.S., E.J., L.C., E.E., L.J.H., S.A.W., M.D.D., B.A.B., M.O., C.K., B.B.D., D.C., and R.W.B. researched data, contributed to discussion, and reviewed and edited the manuscript. R.W.B. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Portions of the results were presented at the 13th International Conference on Advanced Technologies & Treatments for Diabetes, Madrid, Spain, 19–22 February 2020.
Footnotes
A listing of the study group members can be found in the supplementary material online.
Clinical trial reg. no. NCT03844789, clinicaltrials.gov
This article contains supplementary material online at https://doi.org/10.2337/figshare.13260212.
References
- 1.Brown SA, Kovatchev BP, Raghinaru D, et al.; iDCL Trial Research Group . Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Breton MD, Kanapka LG, Beck RW, et al.; iDCL Trial Research Group . A randomized trial of closed-loop control in children with type 1 diabetes. N Engl J Med 2020;383:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nathan DM, Genuth S, Lachin J, et al.; Diabetes Control and Complications Trial Research Group . The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993;329:977–986 [DOI] [PubMed] [Google Scholar]
- 4.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B 1995;57:289–300 [Google Scholar]
- 5.Battelino T, Danne T, Bergenstal RM, et al. . Clinical targets for continuous glucose monitoring data interpretation: recommendations from the International Consensus on Time in Range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Forlenza GP, Pinhas-Hamiel O, Liljenquist DR, et al. . Safety evaluation of the MiniMed 670G system in children 7-13 years of age with type 1 diabetes. Diabetes Technol Ther 2019;21:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berget C, Messer LH, Vigers T, et al. . Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system. Pediatr Diabetes 2020;21:310–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Messer LH, Berget C, Vigers T, et al. . Real world hybrid closed-loop discontinuation: predictors and perceptions of youth discontinuing the 670G system in the first 6 months. Pediatr Diabetes 2020;21:319–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thabit H, Tauschmann M, Allen JM, et al. . Home use of an artificial beta cell in type 1 diabetes. N Engl J Med 2015;373:2129–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]