Abstract
OBJECTIVE
Diabetes and hyperglycemia are important risk factors for poor outcomes in hospitalized patients with coronavirus disease 2019 (COVID-19). We hypothesized that achieving glycemic control soon after admission, in both intensive care unit (ICU) and non-ICU settings, could affect outcomes in patients with COVID-19.
RESEARCH DESIGN AND METHODS
We analyzed pooled data from the Glytec national database including 1,544 patients with COVID-19 from 91 hospitals in 12 states. Patients were stratified according to achieved mean glucose category in mg/dL (≤7.77, 7.83–10, 10.1–13.88, and >13.88 mmol/L; ≤140, 141–180, 181–250, and >250 mg/dL) during days 2–3 in non-ICU patients or on day 2 in ICU patients. We conducted a survival analysis to determine the association between glucose category and hospital mortality.
RESULTS
Overall, 18.1% (279/1,544) of patients died in the hospital. In non-ICU patients, severe hyperglycemia (blood glucose [BG] >13.88 mmol/L [250 mg/dL]) on days 2–3 was independently associated with high mortality (adjusted hazard ratio [HR] 7.17; 95% CI 2.62–19.62) compared with patients with BG <7.77 mmol/L (140 mg/dL). This relationship was not significant for admission glucose (HR 1.465; 95% CI 0.683–3.143). In patients admitted directly to the ICU, severe hyperglycemia on admission was associated with increased mortality (adjusted HR 3.14; 95% CI 1.44–6.88). This relationship was not significant on day 2 (HR 1.40; 95% CI 0.53–3.69). Hypoglycemia (BG <70 mg/dL) was also associated with increased mortality (odds ratio 2.2; 95% CI 1.35–3.60).
CONCLUSIONS
Both hyperglycemia and hypoglycemia were associated with poor outcomes in patients with COVID-19. Admission glucose was a strong predictor of death among patients directly admitted to the ICU. Severe hyperglycemia after admission was a strong predictor of death among non-ICU patients.
Introduction
Diabetes and hyperglycemia have emerged as important risk factors for hospitalization, disease severity, acute kidney injury, acute respiratory distress syndrome, intensive care unit (ICU) admissions, and death in patients with coronavirus disease 2019 (COVID-19) (1–6). The American Diabetes Association and the American Association of Clinical Endocrinologists recommend a target blood glucose (BG) range of 7.83–10.0 mmol/L (140–180 mg/dL) for a majority of hospitalized patients (7,8). Most ICU studies using intravenous insulin have consistently shown that for some patients, glycemic target levels can be achieved within as little as 6 h (9–12). For non-ICU patients, randomized controlled trials using standard subcutaneous basal bolus insulin regimens have demonstrated that glycemic levels of 140–180 mg/dL can be achieved within 2–3 days (13–15) (although these studies were not conducted in a setting of COVID-19). Achieving glycemic targets after admission within these general timeframes, which we consider to be soon after admission, is associated with better outcomes in patients with diabetes or stress hyperglycemia in general medicine, surgery, and critical care (10,11,13,16–20).
Prior studies have shown hyperglycemia on admission to the hospital is a predictor of death and other severe outcomes of COVID-19, but whether intervention to improve glycemia can improve outcomes has not been addressed by careful examination of postadmission glycemia. This retrospective study asks whether this hypothesis can be supported by determining whether glycemia in the first 2–3 days will better predict outcomes than admission glycemia. To test this hypothesis in patients with COVID-19 in both ICU and non-ICU settings, we extracted individual- and event-level data from the Glytec database to determine the impact of glycemic control on hospital outcomes in patients admitted with COVID-19. To account for the effect of severity of illness and temporality of glycemic control on outcomes, we stratified patients according to admission setting (non-ICU vs. ICU) and achieved glucose level soon after admission.
Research Design and Methods
Data Source
We analyzed the Glytec database for patients with a COVID-19–positive laboratory test from 1 March 2020 to 8 May 2020. Glytec is an insulin software titration company that contracts with hospitals and health systems across the U.S. As part of its integrative software, Glytec maintains a database of laboratories and patient demographics for its sites, as well as admission and discharge dates, locations, and death notifications. The data set for this study included patients from 91 hospitals located in 12 different states in the U.S. All sites have an agreed contract to allow deidentified data in aggregate form to be used for research purposes. The raw data set was validated, and a fully deidentified data set was analyzed.
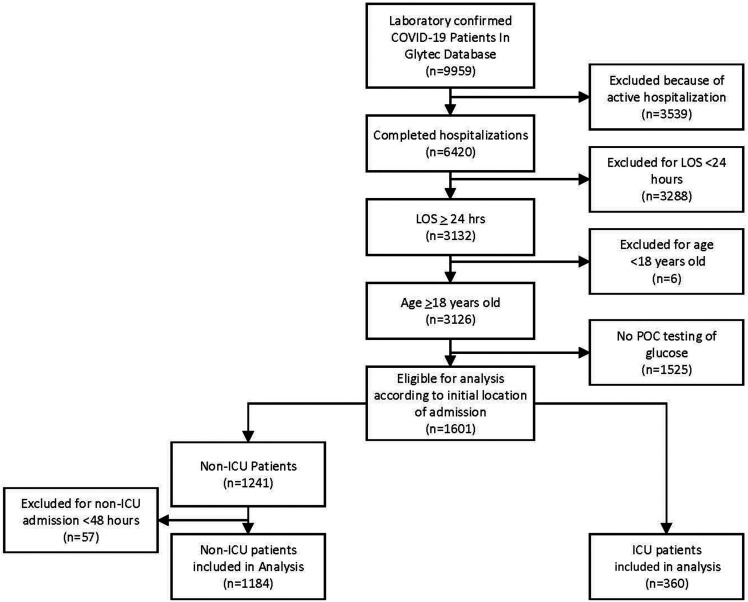
To obtain estimates related to glycemic control, for this analysis, we included patients requiring glucose monitoring. Of the 9,959 patients testing COVID-19 positive, we excluded 3,539 who were active admissions (had neither died nor been discharged from the hospital), 3,288 who had been hospitalized for <24 h, and 6 age <18 years. To examine the impact of glycemic control in the hospital, we also excluded 1,525 patients who had only laboratory serum glucose but no point-of-care (POC) glucose testing. The remaining 1,601 patients were deidentified and transferred from the Glytec database for analysis. In the non-ICU group, 57 patients were excluded with a length of stay (LOS) <48 h, because these patients were not in the hospital long enough to experience the opportunity to receive treatment that would allow for glycemic goals to be achieved. We also excluded an additional 314 patients from the non-ICU group because they did not have POC BG data to calculate the day 2–3 mean BG and 76 patients from the ICU group because they did not have sufficient POC BG data to calculate the day 2 mean BG. This set of 1,544 patients, 1,184 non-ICU and 360 ICU patients, had the requisite admission characteristics. Patients were classified as non-ICU or ICU according to their initial hospital destination. Only 12% of the study population was using commercial glucose management software. Our analysis did not include details. No data were available about different treatment strategies, which may have been different between ICUs and across hospital settings and health systems. See Fig. 1 for a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flowchart of the study population.
Figure 1.
PRISMA.
Hemoglobin A1c data within 90 days of admission were reviewed if available from the hospital records or transferred into the hospital records from an outside laboratory. All POC glucose data were abstracted. No continuous glucose monitor data were analyzed.
Variable Definitions
Diabetes was defined in patients with an ICD-10 code for a diagnosis of diabetes in the electronic health record and in patients who had an elevated hemoglobin A1c concentration (≥48 mmol/mol; 6.5%) within 3 months of admission. Hypoglycemia was defined as at least one BG concentration <3.9 mmol/L (70 mg/dL). Hyperglycemia in hospitalized patients was defined as per the American Diabetes Association as BG level >7.77 mmol/L (140 mg/dL) (21). We chose <7.77 mmol/L (140 mg/dL) as our reference group (but not as a target range) and 7.83–10 mmol/L (141–180 mg/dL) as an alternate reference. Hyperglycemia was defined as a mean glucose concentration >10 mmol/L (180 mg/dL) and severe hyperglycemia as a mean glucose concentration >13.88 mmol/L (250 mg/dL). Transfer to ICU was defined as a patient initially categorized as non-ICU moving to a subsequent ICU location. Acute kidney injury was defined as an increase in serum creatinine from the admission value by 0.5 mg/dL or a doubling of the admission value. Patients were grouped by their admission to a non-ICU or ICU location and then separated into one of four categories of mean achieved BG. We established the glucose ranges as ≤7.77 mmol/L (140 mg/dL) (reference group), 7.83–10 mmol/L (141–180 mg/dL), 10.1–13.88 mmol/L (181–250 mg/dL), and >13.88 mmol/L (250 mg/dL). The level of achieved glycemia was defined for non-ICU patients as the mean BG on days 2–3 and for ICU patients as the mean BG on day 2.
Outcomes of Interest
The main outcome of interest was time to mortality, assessed by survival and hazard functions. Secondary outcomes included comparative hospital mortality rate, development of acute kidney injury, LOS, and transfer to the ICU according to achieved BG group.
Exposures
The primary exposure was glycemic control during days 2–3 in a non-ICU setting or on day 2 of ICU admission for critically ill patients directly admitted to the ICU. We also examined the impact of hypoglycemia (<3.9 mmol/L; 70 mg/dL) on mortality and morbidity.
Potential Confounders
Men, older patients, and those with higher BMI, history of diabetes, or higher admission glucose seem to have worse outcomes during the COVID-19 pandemic (21–23). In addition, patients with hyperglycemia but without diabetes tend to be sicker and have worse outcomes in the hospital compared with patients with diabetes (21,24). To account for these potential confounders of the association of glycemic control with hospital outcomes in patients with COVID-19, sex, age, BMI, history of diabetes, and admission glucose were included in the regression models.
Statistical Analysis
Our retrospective analysis of the collected observational data set was stratified by whether patients were admitted to the non-ICU or ICU setting. We provided descriptive statistics by summarizing continuous variables by mean ± SD or median with interquartile range and categorical variables by count and proportion. For the survival outcome of interest (i.e., time to death), we provided the estimated 25th percentile in addition to Kaplan-Meier curves. We evaluated marginal differences in baseline characteristics and clinical outcomes among groups defined by achieved mean BG on days 2–3 for patients with non-ICU admission or achieved mean BG on day 2 for patients with direct ICU admission. We used the nonparametric Kruskal-Wallis test to compare continuous variables and χ2 test (or Fisher’s exact test) to compare categorical variables. A survival analysis was performed to assess the effect on survival outcome (i.e., time to death) of the achieved mean BG group (≤7.77, 7.83–10, 10.1–13.88, and >13.88 mmol/L; ≤140, 141–180, 181–250, and >250 mg/dL) on days 2–3 for the non-ICU group or mean BG on day 2 for the ICU group. The Kaplan-Meier method was used to estimate the survival function of time to death. Unweighted and weighted log-rank tests were used to compare the survival functions among the achieved mean BG categories. Multivariate Cox regression assuming a constant hazard ratio (HR) over time was performed to estimate the HRs associated with different BG categories relative to the reference group with achieved mean BG ≤7.77 mmol/L (140 mg/dL), while adjusting for sex, age, BMI, history of diabetes, and admission BG. We also conducted multivariate logistic regression to investigate the association between hypoglycemia and binary outcomes such as inpatient mortality, acute kidney injury, and ICU transfer, adjusting for sex, age, BMI, history of diabetes, and hemoglobin A1c. A P value <0.05 was considered statistically significant.
Results
Patients hospitalized with COVID-19 were admitted to a non-ICU setting (n = 1,184) or were directly admitted to the ICU (n = 360). Demographics and outcomes for the entire non-ICU and ICU groups are presented in Table 1. Of the non-ICU patients, 40% had diabetes. The admission BG on average for the non-ICU cohort was 8.9 ± 4.4 mmol/L (159.5 ± 78.6 mg/dL). In this non-ICU group, the incidence of adverse outcomes included transfer to the ICU (34%), hypoglycemia (20%), acute kidney injury (21%), and mortality (16%). The median LOS was 7.87 days before discharge or death, and the median time from admission to ICU transfer was 2.24 days for the 34% who transferred to the ICU.
Table 1.
Demographics and laboratory features by location of treatment on admission (non-ICU and ICU)
| Non-ICU (n = 1,184) | ICU (n = 360) | |
|---|---|---|
| Sex | ||
| Female | 546 (46) | 166 (46) |
| Male | 638 (54) | 194 (54) |
| Age, years | 64.3 ± 16.0 | 64.5 ± 15.4 |
| BMI, kg/m2 | 30.5 ± 8.3 | 30.3 ± 8.9 |
| Weight, kg | 89.1 ± 26.0 | 88.7 ± 27.4 |
| Diabetes diagnosis or hemoglobin A1c ≥6.5 | 479 (40) | 144 (40) |
| Admission creatinine, mg/dL | 1.6 ± 2.0 | 1.6 ± 1.6 |
| Admission albumin, g/dL | 3.5 ± 0.5 | 3.3 ± 0.6 |
| Admission anion gap, mEq/L | 13.1 ± 4.0 | 15.1 ± 5.6 |
| Admission lactic acid, mg/dL | 1.7 ± 1.0 | 2.2 ± 2.0 |
| Admission potassium, mEq/L | 4.0 ± 0.6 | 4.2 ± 0.7 |
| Admission BG, mg/dL | 159.5 ± 78.6 | 181.3 ± 105.6 |
| At least one BG <70, mg/dL | 226 (20) | 72 (20) |
| Acute kidney injury | 244 (21) | 98 (27) |
| In-hospital death | 175 (16) | 104 (31) |
| LOS, days | 7.9 (4.7, 14.0) | 9.1 (5.2, 17.0) |
| Transfer to ICU from non-ICU | 398 (34) | |
| ICU transfer time (n = 398), days | 2.24 (0.92, 4.05) |
Data are presented as n (%), mean ± SD, or median (interquartile range).
Of the ICU patients, 40% had diabetes. The admission BG on average for the ICU cohort was 10.1 ± 5.9 mmol/L (181.3 ± 105.6 mg/dL). In this ICU group, the incidence of adverse outcomes included hypoglycemia (20%), acute kidney injury (27%), and mortality (31%). The median LOS was 9.06 days before discharge or death.
The outcomes stratified according to four defined achieved glycemia categories for both non-ICU and ICU patients are presented in Table 2. In the non-ICU population, the mean BG was >13.88 mmol/L (250 mg/dL) for 41 patients (4.7%), between 10 and 13.88 mmol/L (181–250 mg/dL) for 161 (18.5%), between 7.83 and 10 mmol/L (141–180 mg/dL) for 236 (27.1%), and ≤7.77 mmol/L (140 mg/dL) for 432 (49.7%). In the non-ICU population, all four groups stratified by glycemic range soon after admission had a similar rate of transfer to the ICU (Table 2). The admission BG was positively associated with the achieved BG on days 2–3 (P < 0.001). Mortality was highest in the >13.88 mmol/L (250 mg/dL) group at 21%. Mortality rates in the other groups were 17%, 14%, and 15%, but there were no significant differences in these rates among the four groups (P = 0.73).
Table 2.
Demographics and laboratory features of non-ICU and ICU patients by achieved mean BG
| Variable | Non-ICU day 2–3 mean BG, mg/dL | ICU day 2 mean BG, mg/dL | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ≤140 (n = 432) | 141–180 (n = 236) | 181–250 (n = 161) | >250 (n = 41) | P | ≤140 (n = 125) | 141–180 (n = 75) | 181–250 (n = 65) | >250 (n = 22) | P | |
| Sex | 0.40 | 0.41 | ||||||||
| Female | 209 (48) | 100 (42) | 71 (44) | 21 (51) | 56 (45) | 39 (52) | 30 (46) | 7 (32) | ||
| Male | 223 (52) | 136 (58) | 90 (56) | 20 (49) | 69 (55) | 36 (48) | 35 (54) | 15 (68) | ||
| Age, years | 65.7 ± 15.4 | 63.4 ± 15.3 | 61.1 ± 14.5 | 57.1 ± 14.4 | <0.001 | 63.4 ± 15.2 | 64.7 ± 15.0 | 65.9 ± 12.8 | 65.3 ± 13.4 | 0.84 |
| BMI, kg/m2 | 30.9 ± 8.5 | 30.4 ± 7.4 | 31.9 ± 7.9 | 33.8 ± 11.7 | 0.32 | 31.1 ± 9.6 | 31.4 ± 9.3 | 29.0 ± 6.5 | 35.4 ± 11.6 | 0.09 |
| Weight, kg | 89.5 ± 25.1 | 88.9 ± 24.1 | 95.5 ± 27.2 | 95.4 ± 35.9 | 0.07 | 90.4 ± 27.1 | 91.9 ± 29.5 | 84.0 ± 19.0 | 107.9 ± 42.3 | 0.07 |
| Diabetes diagnosis or hemoglobin A1c ≥6.5 | 143 (33) | 158 (67) | 118 (73) | 35 (85) | <0.001 | 27 (22) | 51 (68) | 42 (65) | 18 (82) | <0.001 |
| Admission serum creatinine, mg/dL | 1.7 ± 2.1 | 1.7 ± 2.2 | 1.6 ± 2.1 | 1.2 ± 1.3 | 0.005 | 1.6 ± 1.5 | 1.6 ± 1.6 | 1.6 ± 1.4 | 2.7 ± 3.0 | 0.17 |
| Admission albumin, g/dL | 3.5 ± 0.5 | 3.5 ± 0.6 | 3.5 ± 0.4 | 3.6 ± 0.5 | 0.65 | 3.2 ± 0.5 | 3.3 ± 0.6 | 3.3 ± 0.5 | 3.2 ± 0.6 | 0.51 |
| Admission anion gap, mEq/L | 13.1 ± 3.9 | 13.2 ± 4.1 | 14.4 ± 4.0 | 13.3 ± 3.5 | 0.16 | 14.5 ± 3.1 | 17.0 ± 7.3 | 16.0 ± 7.0 | 16.8 ± 4.6 | 0.37 |
| Admission lactic acid, mg/dL | 1.6 ± 1.0 | 1.9 ± 1.4 | 1.9 ± 1.0 | 1.8 ± 0.8 | 0.001 | 2.1 ± 2.1 | 2.7 ± 2.7 | 2.4 ± 1.8 | 1.8 ± 0.7 | 0.62 |
| Admission potassium, mEq/L | 4.0 ± 0.6 | 4.1 ± 0.7 | 4.1 ± 0.6 | 4.2 ± 0.5 | 0.10 | 4.1 ± 0.7 | 4.4 ± 0.7 | 4.2 ± 0.8 | 4.7 ± 0.8 | <0.001 |
| Admission BG, mg/dL | 133.8 ± 57.8 | 173.5 ± 76.5 | 217.5 ± 87.0 | 252.3 ± 101.4 | <0.001 | 130.8 ± 63.8 | 211.9 ± 108.1 | 250.9 ± 118.0 | 251.6 ± 117.0 | <0.001 |
| At least one BG <70, mg/dL | 103 (24) | 59 (25) | 32 (20) | 7 (17) | 0.49 | 30 (24) | 21 (28) | 15 (23) | 4 (18) | 0.82 |
| Acute kidney injury | 105 (24) | 46 (19) | 31 (19) | 4 (10) | 0.10 | 35 (28) | 20 (27) | 19 (29) | 10 (45) | 0.39 |
| In-hospital death | 66 (17) | 32 (14) | 23 (15) | 7 (21) | 0.73 | 34 (29) | 24 (33) | 20 (33) | 9 (45) | 0.52 |
| LOS, days | 7.9 (4.7, 13.9) | 7.9 (5.0, 14.9) | 6.8 (4.0, 11.0) | 4.9 (3.3, 8.2) | <0.001 | 10.7 (6.2, 19.0) | 9.2 (5.1, 19.1) | 9.3 (5.2, 17.0) | 6.2 (4.3, 9.8) | 0.10 |
| Transfer to ICU from non-ICU | 142 (33) | 76 (32) | 48 (30) | 11 (27) | 0.80 | |||||
| ICU transfer time, days | 1.94 (0.91, 3.08) | 1.92 (1.03, 4.41) | 1.54 (0.89, 2.87) | 1.28 (0.55, 5.95) | 0.70 | |||||
Data are presented as n (%), mean ± SD, or median (interquartile range).
In the ICU population, the mean BG was >13.88 mmol/L (250 mg/dL) for 22 patients (7.7%), between 10.1 and 13.88 mmol/L (181–250 mg/dL) for 65 (22.6%), between 7.83 and 10 mmol/L (141–180 mg/dL) for 75 (26.1%), and ≤7.77 mmol/L (140 mg/dL) for 125 (43.6%). The ≤7.77 mmol/L (140 mg/dL) group compared with the >13.88 mmol/L (250 mg/dL) group had a smaller proportion of patients with diabetes (22% vs. 82%). The ≤7.77 mmol/L (140 mg/dL) group tended to be younger and have a lower BMI. The admission BG was positively associated with the achieved BG on day 2 (P < 0.001). Mortality was highest in the >13.88 mmol/L (250 mg/dL) group (45%) and lowest in the ≤7.77 mmol/L (140 mg/dL) group (29%). The differences in ICU mortality rates were not statistically significant (P = 0.52). We did not find a significant effect of BMI on the distribution of time to death or the hazard of death, either marginally or jointly with other potential predictors.
In those patients stratified into the achieved BG >13.88 mmol/L (250 mg/dL) group, the overall mortality was 25% (16/63): 21% (7/41) in the non-ICU setting and 45% (9/22) in the ICU setting.
Survival Analysis
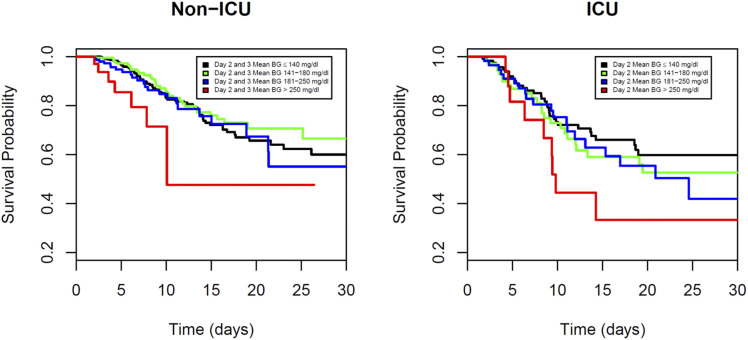
A survival analysis was performed separately for the non-ICU and ICU patients to assess the survival differences among the four achieved glucose groups. In Fig. 2, we plotted the Kaplan-Meier curves stratified by the achieved glucose group separately for the non-ICU and ICU patients. In the non-ICU patients, the >13.88 mmol/L (250 mg/dL) group had a substantially lower survival compared with the other achieved glycemia groups (log-rank test P = 0.027). The Kaplan-Meier curves for the group achieving glucose of >250 mg compared with the group achieving ≤7.77 mmol/L (140 mg/dL) did not generate 50th percentile comparisons of survival, because more than half of the patients were still alive at the end of their hospital stay. Therefore, the median (or 50th percentile) survival time could not be estimated. Instead, we estimated and compared the 25th percentile of survival time for the >13.88 mmol/L (250 mg/dL) and ≤7.77 mmol/L (140 mg/dL) groups. For the non-ICU patients, the estimated 25th percentile survival times for these two achieved glycemia groups were 7.83 and 14.08 days, respectively, and for the ICU patients, these times were 6.33 and 9.63 days, respectively. For the ICU patients, from Fig. 2, the group achieving glucose of >13.88 mmol/L (250 mg/dL) had the lowest survival compared with the other groups. However, the P values from the log-rank and the Wilcoxon tests were not statistically significant, possibly because of a lack of power related to the smaller sample sizes.
Figure 2.
Survival probability by achieved BG in non-ICU and ICU settings. Kaplan-Meier survival curves showed significantly lower survival probability among patients with mean BG >250 mg/dL in the non-ICU setting (log-rank test P = 0.027). Multivariate Cox regression analysis showed the highest mortality for patients with mean BG >250 mg/dL (HR 7.17; 95% CI 2.62–19.62) compared with BG <140 mg/dL (reference group). Survival probability curves were not significantly different for patients directly admitted to the ICU (log-rank test P = 0.21). In the ICU group, multivariate Cox regression analysis showed mortality was higher but not statistically significant for patients with mean BG >250 mg/dL (HR 1.40; 95% CI 0.53–3.69) compared with the reference group (<140 mg/dL).
A multivariate Cox regression analysis was conducted separately for the non-ICU and the ICU patients to estimate the effects of the stratified groups according to mean glycemia soon after admission, with the reference group chosen as those achieving a mean BG <7.77 mmol/L (140 mg/dL), while adjusting for sex, age, baseline BMI, history of diabetes, and hemoglobin A1c. Non-ICU patients with a mean BG >13.88 mmol/L (250 mg/dL) 2–3 days after admission had the highest mortality risk compared with patients in the reference group (<7.77 mmol/L; 140 mg/dL), with an estimated HR of 7.60 (95% CI 1.95–29.60). This association was not significantly modified by adding admission glucose to the model (HR 7.17; 95% CI 2.62–19.62). This relationship was not significant on admission (adjusted HR 1.47; 95% CI 0.68–3.14). In ICU patients, severe hyperglycemia on admission (BG >250 mg/dL) was associated with increased mortality (HR 3.14; 95% CI 1.44–6.88); however, such an association with achieved glucose level on day 2 was modestly high but not significant, with an estimated HR of 1.40 (95% CI 0.53–3.70) (Fig. 2). Regarding ICU patients, the mean BG >13.88 mmol/L (250 mg/dL) group compared with the reference group had an estimated HR of 1.40 (95% CI 0.53–34.70) (Fig. 2).
In a logistic regression analysis, hypoglycemia was associated with increased mortality in the non-ICU group (odds ratio [OR] 1.69; 95% CI 1.16–2.45), although not in the ICU group (OR 0.98; 95% CI 0.55–1.74). In a multivariate logistic regression analysis, patients with hypoglycemia in the non-ICU setting were at increased odds for mortality (OR 2.2; 95% CI 1.35–3.60) after adjusting for sex, age, BMI, history of diabetes, and hemoglobin A1c.
In patients with a mean BG ≤7.77 mmol/L (140 mg/dL), in both ICU and non-ICU settings, 24% had at least one reading of <3.9 mmol/L (70 mg/dL). Among those in the non-ICU setting, hypoglycemia was associated with an increased risk of acute kidney injury (OR 2.15; 95% CI 1.33–3.49) and mortality (OR 2.20; 95% CI 1.15–3.56).
We performed a secondary analysis using a mean BG of 141–180 mg/dL as the reference group, because this is a common target range in the hospital. The survival analysis results were similar to those of the original reference group (mean BG ≤7.77 mmol/L; 140 mg/dL). In the multivariate Cox regression analysis, the >13.88 mmol/L (250 mg/dL) group had an estimated HR of 6.78 (95% CI 1.74–26.46) for non-ICU patients and 1.20 (95% CI 0.45–3.2) for ICU patients compared with the group of patients with BG between 7.83 and 10 mmol/L (141–180 mg/dL).
Conclusions
This study sought to investigate whether poorly controlled glycemia, within a timeframe where patients can meet glucose targets with standard hospital therapy, represents a predictor of worse outcomes in patients with COVID-19. Our results indicate that 2–3 days glycemia predicted outcomes better than baseline values for non-ICU admissions but not direct ICU admissions. Reaching a glucose value of ≤7.77 mmol/L (140 mg/dL) or 7.83–10 mmol/L (141–180 mg/dL) within 2 days after ICU admission or between 2 and 3 days in the non-ICU setting, respectively, is associated with reduced mortality, which was statistically significant in the non-ICU setting and trending toward significance in the ICU population. The greater survival of the individuals with better control after admission compared with patients with uncontrolled glucose levels (>13.88 mmol/L; 250 mg/dL) is clinically meaningful and indicates the need to start treatment of hyperglycemia on admission. To account for severity of illness on presentation in patients with COVID-19, we stratified this cohort according to admission setting. The mortality for ICU patients (31%) was almost twice that in the non-ICU population (16%). Admission glucose was significantly associated with mortality in ICU patients. The high ICU transfer rate in this population suggests delayed recognition of severity, and it is not known whether improving glucose control could significantly change this course.
The mean BG on days 2–3 serves as a surrogate metric for the achievement of successful treatment in a non-ICU setting (13,15,25). The mean BG on day 2 serves as a surrogate metric for the impact in an ICU setting, because this target is commonly achieved within the first 24 h of hospitalization (9–12,26).
More than half of the patients with hyperglycemia admitted to either the non-ICU (53%) or ICU (56%) setting continued to have a mean BG concentration exceeding 10 mmol/L (180 mg/dL) by days 2–3 in the non-ICU group and by day 2 in the ICU group. Among patients in the non-ICU setting, those averaging severe hyperglycemia on days 2–3 had a significantly increased risk of mortality, with a sevenfold higher mortality risk compared with reference glycemia patients, who had a mean BG ≤7.77 mmol/L (140 mg/dL), indicating that early severe hyperglycemia is a strong independent marker for mortality. The high percentages of patients with COVID-19 presenting with hyperglycemia who did not reach a mean BG <10 mmol/L (180 mg/dL) represent a potentially missed opportunity to treat hyperglycemia and improve clinical outcomes. Our study suggests that more than half of the patients with a BG >10.1 mmol/L (180 mg/dL) could have benefitted from earlier and more aggressive treatment of hyperglycemia during their hospitalization. In non-ICU patients, the admission BG category compared with achieved glycemia did not differentiate Kaplan-Meier curves or hazard of mortality (Cox regression). We found that in ICU patients, however, the admission BG compared with the achieved BG category reflected the outcome to a greater extent.
Among patients who achieved a mean glycemia of ≤7.77 mmol/L (140 mg/dL), 24% experienced hypoglycemia. There was a twofold increase in the odds of death in patients who experienced hypoglycemia. This was independent of a history of diabetes, and those without diabetes had higher mortality if hypoglycemia occurred (OR 1.66; 95% CI 1.046–2.63). Additional analyses are needed to determine the exact cause of hypoglycemia (e.g., insulin therapy or multiorgan failure).
The mean BG ≤7.77 mmol/L (140 mg/dL) group was chosen as a reference group before analysis, because a value greater than this is considered abnormal for hospitalized patients in several societies’ guidelines (7,25,27). Because 7.83–10 mmol/L (141–180 mg/dL) is the recommended treatment target, we performed a sensitivity analysis with 7.83–10 mmol/L (141–180 mg/dL) serving as the alternate reference group. This analysis produced similar results to an analysis using ≤7.77 mmol/L (140 mg/dL) as the reference group, when compared with a mean achieved BG >13.88 mmol/L (250 mg/dL) (HR 6.78; 95% CI 1.74–26.46).
Inpatient hyperglycemia in those with or without a prior diagnosis of diabetes is associated with an increased risk of complications and mortality (28). It seems prudent to prevent severe hyperglycemia (7,29), and selecting a specific target not exceeding 10 mmol/L (180 mg/dL) in critically ill patients is supported by common sense (30–32). This maximum target glucose concentration is the opinion of the authors and is not backed by hard evidence. In patients with COVID-19, it has not been clear whether hyperglycemia is simply a marker of disease severity or whether intensive treatment of the hyperglycemia can reduce mortality or other adverse outcomes. However, it is difficult to infer the impact of glycemic control on outcomes from studies of adverse outcomes linked to admission hyperglycemia. On the basis of prior randomized controlled trials in the non-ICU and ICU settings (10,11,16–18), we know the first few days of hospitalization serve as a window of opportunity to treat to inpatient goals and achieve improved outcomes. We believe this is the first study reporting on the impact of achieved glycemia early in hospitalization in the ICU and non-ICU settings.
Hyperglycemia is a well-known marker of disease severity, and its association with poor outcomes in patients with COVID-19 has been reproduced multiple times. However, most analyses have not accounted for disease severity on admission or temporality of glucose control, nor have they adjusted for relevant factors now known to be independent predictors of poor outcomes during COVID-19 (i.e., sex, age, and BMI) that are also associated with diabetes (33). Our analytic approach accounted for temporality (dysglycemia before outcomes), confounders, severity of disease on admission, and performance (achieving target within a window in which target can be met) and provides a rational approach to interpret glucose control interventions.
We note the following limitations. Given the retrospective nature of the analysis, selection bias and misclassification were possible. We used laboratory data only for the diagnosis of COVID-19, and we were unable to gauge the severity of the disease based on clinical characteristics or other factors, such as a need for invasive ventilation. Nonavailability of ICU beds may have classified sick patients as non-ICU on presentation. We could not determine from this database 1) whether there was a delay in therapy or 2) which treatment strategies were used (including whether patients were receiving drugs such as chloroquine or dexamethasone, known to modify glucose levels). Further research with individual-level data on treatment type and timing may help clarify these questions. Although there could have been selection bias in being directed to a non-ICU or ICU destination on admission, it is noteworthy that in both cohorts, the HR for poor control soon after admission was higher than it was on admission, indicating that in this study, the results were consistent with our hypothesis that achieving glycemic control soon after admission in both ICU and non-ICU settings could affect outcomes in patients with COVID-19. The reason for transfer from the non-ICU to ICU setting could have been delayed recognition of severity, inadequate care, or worsening of disease. For ICU patients with achieved glucose concentrations of >250 mg/dL on day 2, the HR suggested a 40% increase in death; however, this estimate was imprecise, with a wide CI, and nonsignificant. On the basis of all the evidence, this association is likely and may be confirmed with a larger sample size from observational cohorts. For ethical reasons, testing higher glucose targets is not recommended.
All patients in this study had BG levels measured solely from POC testing, which puts them in a group potentially biased for glycemic excursions or other factors warranting frequent BG testing (e.g., corticosteroid use, enteral or parenteral feedings, or hypoglycemia risk factors). After we stratified our 1,601 eligible patients into non-ICU versus ICU categories and then further stratified them by admission BG, we were left with a small number of ICU patients (n = 22) with BG >250 mg/dL on day 2. Because of the limitations of our database, we did not have individual treatment data. Future analytics should include all treatments to better gauge who was receiving insulin intravenously or via basal bolus subcutaneously as well as documentation of other treatments in the hospital (e.g., hydroxychloroquine, high doses of steroids, or vasopressors) that could have affected glycemic control to determine the optimal type of insulin management.
We recognize that acceptance of achieved glycemia as a surrogate marker of glycemic control will require validation with prospective cohort studies and randomized controlled trials (34). This is because we currently do not know whether patients had better COVID-19 outcomes because the BG came down or whether the BG came down because patients had better COVID-19 outcomes (35).
This retrospective study adds to prior information based mainly on admission glycemia, but conclusions are limited by lack of information on important covariates, missing data, and potential for residual bias and confounding, and prospective studies are needed to fully test the underlying hypothesis. We found that severe hyperglycemia early in the course of hospitalization in patients with COVID-19 admitted to a non-ICU setting was associated with a sevenfold increase in mortality risk. In addition, admission glucose was a strong predictor of death among patients directly admitted to the ICU. We also observed higher odds of dying among patients with hypoglycemia. Our results suggest patients with COVID-19 should promptly receive treatment to improve glycemic control.
Article Information
Acknowledgments. The authors thank Annamarie Sucher-Jones (Diabetes Technology Society) for her expert editorial assistance.
Funding and Duality of Interest. D.C.K. is a consultant to Dexcom, Eoflow, Fractyl, Lifecare, Novo, Roche, and Thirdwayv. G.E.U. is partly supported by research grants from the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under award UL1TR002378 from the Clinical and Translational Science Award program and NIH grant U30 P30DK11102 and has received research grant support to Emory University for investigator-initiated studies from Novo Nordisk, Dexcom, and AstraZeneca. J.C.M., R.B., V.G., J.C., and R.M. all worked at Glytec at the time of the writing of this article. F.J.P. is supported in part by NIH awards 1K23GM128221-01A1, P30DK111024, and P30DK111024-05S1 and has received unrestricted research support from Merck and Dexcom and consulting fees from Boehringer Ingelheim. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. D.C.K., J.C.M., G.E.U., R.B., J.C., V.G., R.M., and F.J.P. designed the study. D.C.K. and J.C.M. drafted the first version of the manuscript. D.C.K., J.C.M., and G.E.U. edited multiple versions of the manuscript. L.P. performed the data analysis, created survival figures, and reviewed/edited the manuscript. R.B. pulled the data and takes responsibility for the integrity of the data. J.C. contributed to the discussion and finalized the PRISMA and tables. F.J.P. designed the analysis plan and critically reviewed successive versions of the manuscript. All authors contributed to the interpretation of the results and review of the manuscript. J.C.M. and R.B. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
References
- 1.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G, Zangrillo A, Zanella A, et al.; COVID-19 Lombardy ICU Network . Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020;323:1574–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo W, Li M, Dong Y, et al. . Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 31 March 2020 [Epub ahead of print]. DOI:10.1002/dmrr.3319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X, Yu Y, Xu J, et al. . Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study [published correction appears in Lancet Respir Med 2020;8:e26]. Lancet Respir Med 2020;8:475–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu C, Chen X, Cai Y, et al. . Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med 2020;180:934–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association 15. Diabetes care in the hospital: Standards of Medical Care in Diabetes—2020. Diabetes Care 2020;43(Suppl. 1):S193–S202 [DOI] [PubMed] [Google Scholar]
- 8.Ceriello A, Standl E, Catrinoiu D, et al.; “Diabetes and Cardiovascular Disease (D&CVD)” Study Group of the European Association for the Study of Diabetes (EASD) . Issues for the management of people with diabetes and COVID-19 in ICU. Cardiovasc Diabetol 2020;19:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Juneja R, Roudebush CP, Nasraway SA, et al. . Computerized intensive insulin dosing can mitigate hypoglycemia and achieve tight glycemic control when glucose measurement is performed frequently and on time. Crit Care 2009;13:R163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowery NT, Gunter OL, Dossett LA, et al. . Failure to achieve euglycemia despite aggressive insulin control signals abnormal physiologic response to trauma. J Crit Care 2011;26:295–302 [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Park KN, Choi SP, et al. . Time to reach target glucose level and outcome after cardiac arrest patients treated with therapeutic hypothermia. J Crit Care 2015;30:1204–1209 [DOI] [PubMed] [Google Scholar]
- 12.Newton CA, Smiley D, Bode BW, et al. . A comparison study of continuous insulin infusion protocols in the medical intensive care unit: computer-guided vs. standard column-based algorithms. J Hosp Med 2010;5:432–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Smiley D, Zisman A, et al. . Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007;30:2181–2186 [DOI] [PubMed] [Google Scholar]
- 14.Umpierrez GE, Hor T, Smiley D, et al. . Comparison of inpatient insulin regimens with detemir plus aspart versus neutral protamine hagedorn plus regular in medical patients with type 2 diabetes. J Clin Endocrinol Metab 2009;94:564–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquel FJ, Gianchandani R, Rubin DJ, et al. . Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita-Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial [published corrections appear in Lancet Diabetes Endocrinol 2017;5:e1; Lancet Diabetes Endocrinol 2017;5:e3]. Lancet Diabetes Endocrinol 2017;5:125–133 [DOI] [PubMed] [Google Scholar]
- 16.Borgquist O, Wise MP, Nielsen N, et al.; TTM-Trial Investigators . Dysglycemia, glycemic variability, and outcome after cardiac arrest and temperature management at 33°C and 36°C. Crit Care Med 2017;45:1337–1343 [DOI] [PubMed] [Google Scholar]
- 17.Woo JH, Lim YS, Yang HJ, et al. . The relationship between the decreased rate of initial blood glucose and neurologic outcomes in survivors of out-of-hospital cardiac arrest receiving therapeutic hypothermia. Neurocrit Care 2017;26:402–410 [DOI] [PubMed] [Google Scholar]
- 18.Kobata H, Sugie A, Suehiro E, et al. . Association between blood glucose levels the day after targeted temperature initiation and outcome in traumatic brain injury: a post-hoc analysis of the B-HYPO study. J Neurotrauma 2017;34:987–995 [DOI] [PubMed] [Google Scholar]
- 19.Ceriello A Hyperglycemia and the worse prognosis of COVID-19. Why a fast blood glucose control should be mandatory. Diabetes Res Clin Pract 2020;163:108186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceriello A, De Nigris V, Prattichizz F. Why is hyperglycaemia worsening COVID-19 and its prognosis? Diabetes Obes Metab. 28 May 2020 [Epub ahead of print]. DOI: 10.1111/dom.14098 [DOI] [PMC free article] [PubMed]
- 21.Wang S, Ma P, Zhang S, et al. . Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia 2020;63:2102–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Xu S, Yu M, et al. . Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol 2020;146:110–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du R-H, Liang L-R, Yang C-Q, et al. . Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study [Eur Respir J 2020;56(3):2050524]. Eur Respir J 2020;55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bode B, Garrett V, Messler J, et al. . Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States [published correction appears in J Diabetes Sci Technol. 10 June 2020 (Epub ahead of print) DOI:10.1177/1932296820932678]. J Diabetes Sci Technol 2020;14:813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Umpierrez GE, Hellman R, Korytkowski MT, et al.; Endocrine Society . Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2012;97:16–38 [DOI] [PubMed] [Google Scholar]
- 26.Umpierrez G, Cardona S, Pasquel F, et al. . Randomized controlled trial of intensive versus conservative glucose control in patients undergoing coronary artery bypass graft surgery: GLUCO-CABG trial. Diabetes Care 2015;38:1665–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moghissi ES, Korytkowski MT, DiNardo M, et al.; American Association of Clinical Endocrinologists; American Diabetes Association . American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Diabetes Care 2009;32:1119–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corsino L, Dhatariya K, Umpierrez G. Management of diabetes and hyperglycemia in hospitalized patients. In Endotext. Feingold KR, Anawalt B, Boyce A, et al., Eds. South Dartmouth, MA, MDText.com, Inc.; 2000–2017. pp. 1–33 [Google Scholar]
- 29.Gunst J, De Bruyn A, Van den Berghe G. Glucose control in the ICU. Curr Opin Anaesthesiol 2019;32:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finfer S, Chittock DR, Su SY, et al.; NICE-SUGAR Study Investigators . Intensive versus conventional glucose control in critically ill patients. N Engl J Med 2009;360:1283–1297 [DOI] [PubMed] [Google Scholar]
- 31.Gunst J, Van den Berghe G. Blood glucose control in the ICU: how tight? Ann Transl Med 2017;5:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yatabe T, Inoue S, Sakaguchi M, Egi M. The optimal target for acute glycemic control in critically ill patients: a network meta-analysis. Intensive Care Med 2017;43:16–28 [DOI] [PubMed] [Google Scholar]
- 33.Selvin E, Juraschek SP. Diabetes epidemiology in the COVID-19 pandemic. Diabetes Care 2020;43:1690–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Libby P, King K. Biomarkers: a challenging conundrum in cardiovascular disease. Arterioscler Thromb Vasc Biol 2015;35:2491–2495 [DOI] [PubMed] [Google Scholar]
- 35.Fleming TR, DeMets DL. Surrogate end points in clinical trials: are we being misled? Ann Intern Med 1996;125:605–613 [DOI] [PubMed] [Google Scholar]