Abstract
OBJECTIVE
Diabetes is an important risk factor for severe coronavirus disease 2019 (COVID-19), but little is known about the marginal effect of additional risk factors for severe COVID-19 among individuals with diabetes. We tested the hypothesis that sociodemographic, access to health care, and presentation to care characteristics among individuals with diabetes in Mexico confer an additional risk of hospitalization with COVID-19.
RESEARCH DESIGN AND METHODS
We conducted a cross-sectional study using public data from the General Directorate of Epidemiology of the Mexican Ministry of Health. We included individuals with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 between 1 March and 31 July 2020. The primary outcome was the predicted probability of hospitalization, inclusive of 8.5% of patients who required intensive care unit admission.
RESULTS
Among 373,963 adults with COVID-19, 16.1% (95% CI 16.0–16.3) self-reported diabetes. The predicted probability of hospitalization was 38.4% (37.6–39.2) for patients with diabetes only and 42.9% (42.2–43.7) for patients with diabetes and one or more comorbidities (obesity, hypertension, cardiovascular disease, and chronic kidney disease). High municipality-level of social deprivation and low state-level health care resources were associated with a 9.5% (6.3–12.7) and 17.5% (14.5–20.4) increased probability of hospitalization among patients with diabetes, respectively. In age-, sex-, and comorbidity-adjusted models, living in a context of high social vulnerability and low health care resources was associated with the highest predicted probability of hospitalization.
CONCLUSIONS
Social vulnerability contributes considerably to the probability of hospitalization among individuals with COVID-19 and diabetes with associated comorbidities. These findings can inform mitigation strategies for populations at the highest risk of severe COVID-19.
Introduction
Since the initially reported case of novel coronavirus disease 2019 (COVID-19) in December 2019 (1), 37.7 million cases and more than 1 million deaths have been reported worldwide as of October 2020 (2). Heightened COVID-19 severity has been widely reported among people with diabetes (3–5), which affects nearly a half-billion people globally, 79% of whom live in low- and middle-income countries (LMICs) (6). One of the LMICs with the highest case numbers of COVID-19 is Mexico (817,503 cases and 83,781 deaths as of 12 October 2020) (2), where diabetes affects 15.2% of the population (12.8 million adults) (6) and is the leading cause of disability and mortality (7,8). Before the COVID-19 outbreak, type 2 diabetes, which is highly prevalent among individuals of low socioeconomic status in Mexico (9), represented one of the most significant challenges to Mexican health care (10,11), a fragmented health system, in a major process of transition, with well-documented disparities in health care delivery (12–15). The convergence of the COVID-19 and diabetes epidemics has widened these preexisting health disparities in Mexico (16), thus raising the urgent need to better understand factors associated with poor outcomes among individuals with diabetes and COVID-19.
Observational studies conducted in Mexico, as elsewhere, have documented a strong association between diabetes and poor COVID-19 outcomes (3,17). Preliminary data have also shown that high municipality poverty among individuals living in Mexico is strongly associated with severe COVID-19 (16). However, there is scant evidence about how sociodemographic characteristics, health care resources, as well as presentation to care characteristics might relate to COVID-19 severity and, ultimately, to poor clinical outcomes among individuals with diabetes, particularly in LMICs. Given that one of the most pressing challenges of the COVID-19 epidemic is resource allocation for individuals at high risk of severe COVID-19 (18), particularly in lower-income contexts (19), identifying additional characteristics associated with increased risk of severe COVID-19 among individuals with diabetes and common comorbidities could contribute to improve resource allocation and guide efforts to mitigate risk of infection in this patient population.
In this study, we 1) describe sociodemographic, access to health care, and presentation to care characteristics of individuals with COVID-19 and diabetes according to comorbidity status in Mexico; 2) evaluate the interaction between these characteristics and the likelihood of hospitalization with COVID-19 among individuals with diabetes and associated comorbidities; and 3) present case-based scenarios of the risk attributed to these characteristics to severe COVID-19 among individuals with diabetes and associated comorbidities. We hypothesize that the likelihood of hospitalization will be higher for patients with diabetes who have comorbidities than for those without and that this likelihood will be even higher for patients living in contexts of high social vulnerability.
Research Design and Methods
Data Source and Study Population
We performed an observational, cross-sectional study using public data from the General Directorate of Epidemiology of the Mexican Ministry of Health, an open-source registry that provides data on individuals tested for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Mexico between 1 March and 31 July 2020 (20). The data analyzed in this study were downloaded on 31 July 2020 from the public website https://www.gob.mx/salud/documentos/datos-abiertos-152127?idiom=es. COVID-19 cases were confirmed with a positive test for the SARS-CoV-2 virus, which were certified by the National Institute for Diagnosis and Epidemiological Referral.
Epidemiological surveillance of SARS-CoV-2 is performed using sentinel surveillance, which consists of 475 units currently active in the Viral Respiratory Disease Epidemiological Surveillance System. Sampling by the Respiratory Disease Monitoring Units includes only 10% of suspected cases with mild (outpatient) symptoms and 100% of suspected cases with severe symptoms (respiratory distress). In addition, 100% of the cases that meet the definition of severe acute respiratory infection are sampled in all medical units in the country (20).
Our study population included the adult Mexican population diagnosed with COVID-19 and treated in a public health care unit (N = 396,560). After excluding 5.7% of observations with incomplete basic sociodemographic information, the analytical sample included 373,963 adults aged 18–85 years.
Variables and Definitions
Diabetes, Clinical Covariates, and Sociodemographic Characteristics
Diabetes status was available in the data set based on self-reported diagnosis. Obesity, hypertension, cardiovascular disease (CVD), and chronic kidney disease (CKD) were also available through self-report and were grouped as comorbidities given the high prevalence of these conditions among individuals with diabetes (21). Based on these diagnoses, we defined four mutually exclusive groups within the study sample: no comorbidities, one or more comorbidities (no diabetes), diabetes only, and diabetes with one or more comorbidities. Chronic obstructive lung disease (COPD) and asthma were also included in the analysis as adjustment variables. Age, sex, indigenous language spoken (yes/no), and health care provider type were also available at the individual level.
Health Care Provider Type Variable Context
There are two main public providers of health services in Mexico: 1) social security institutions, which offer health services to individuals working in the formal sector of the economy (nearly 50% of the population) and their families, and 2) the Ministry of Health, which provides primary and secondary health services to the nonsalaried population, which includes those working in the informal sector of the economy, the self-employed, and the unemployed (22). Although before the COVID-19 pandemic individuals had access only to their assigned health care provider institution, these restrictions were lifted for individuals with COVID-19, hence facilitating care for these individuals across both types of public health care providers.
Health System and Presentation to Care Variables
We included a municipality-level social deprivation index as a proxy of individual-level socioeconomic status. This index is based on access to basic public services, housing conditions, and wage earnings in 2015 (23). We also included a state-level index of human resource and hospital equipment availability with data available before the COVID-19 outbreak. This index was constructed using factor analysis (24) and followed the official guidelines for the organization and execution of the Hospital Conversion COVID-19 for the Mexican National Health System Institutions (25). Details on index construction are provided in Supplementary Appendix 5. Lastly, we included two variables pertaining to the timing of presentation to care: 1) month of presentation to care (March-April, May, June, or July) and 2) timing of presentation to care from initial symptom onset, defined as follows: 0–3 days, 4–7 days, ≥8 days.
Statistical Analysis
The primary outcome in this study was severity of COVID-19, which was defined according to need for hospitalization versus ambulatory care. Hospitalized care included the subset of patients who required admission to the intensive care unit (ICU), which corresponded to 8.5% of the hospitalized population. First, we described the sociodemographic, clinical, health care resource, and presentation to care characteristics (column percentages and 95% CI) according to the level of health care received (ambulatory versus hospitalized care). Differences between them were evaluated using χ2 tests. Next, we conducted multiple logistic regression analysis of hospitalization according to comorbidity status and presented the model estimates in terms of predicted probabilities (and 95% CI) and separately for the combination of diabetes with each associated comorbidity. Regression models were adjusted for all covariates of interest as detailed above. We then estimated the incremental predicted probability of hospitalization according to these covariates among patients with COVID-19 with diabetes and with diabetes and one or more comorbidities. Incremental probabilities were calculated with respect to the overall adjusted probability of being hospitalized among individuals with diabetes. Lastly, we estimated the predicted probability (and 95% CI) of hospitalization according social vulnerability, health care resource, and presentation to care characteristics, stratified by sex and comorbidity group. Analyses were performed using Stata MP 15.1 statistical software.
Results
The baseline characteristics of the study population, according to ambulatory versus hospitalized care, are presented in Table 1. This analysis included 373,963 patients with laboratory-confirmed SARS-CoV-2, among whom 276,185 (73.8%) required ambulatory care and 97,778 (26.2%) required hospitalization. Diabetes was self-reported among 10.5% of patients who presented to ambulatory care and among 32.2% of patients who were hospitalized. The same proportion of men and women presented to ambulatory care, whereas most patients who were hospitalized were men (61.2%). A higher proportion of patients who were hospitalized spoke an indigenous language (1.4% vs. 0.9%), were in the highest municipality social deprivation level (21.0% vs. 19.6%), and had a comorbidity, compared with patients in ambulatory care. A higher proportion of patients hospitalized lived in a state with the lowest health care resource index compared with patients in ambulatory care, who had the highest proportion of patients living in a state with the highest health care resource index (Table 1).
Table 1.
Characteristics of patients with COVID-19 according to ambulatory/hospitalized setting, Mexico 2020
| Total | Ambulatory | Hospitalized | P value | |
|---|---|---|---|---|
| N (%) | 373,963 (100) | 276,185 (73.8) | 97,778 (26.2) | |
| Sex | ||||
| Male | 53.0 (52.8–53.1) | 50.0 (49.8–50.2) | 61.2 (60.9–61.5) | <0.001 |
| Female | 47.0 (46.9–47.2) | 50.0 (49.8–50.2) | 38.8 (38.5–39.1) | |
| Age (years) | ||||
| 18–35 | 29.8 (29.6–29.9) | 36.8 (36.7–37.0) | 9.8 (9.6–9.9) | <0.001 |
| 36–50 | 34.7 (34.5–34.8) | 37.8 (37.6–38.0) | 25.9 (25.6–26.1) | |
| 51–65 | 24.1 (24.0–24.3) | 19.6 (19.4–19.7) | 37.0 (36.7–37.3) | |
| ≥65 | 11.4 (11.3–11.5) | 5.8 (5.7–5.9) | 27.4 (27.1–27.6) | |
| Indigenous language | 1.0 (1.0–1.0) | 0.9 (0.8–0.9) | 1.4 (1.3–1.5) | <0.001 |
| Municipality social deprivation level | ||||
| Lowest (wealthiest) | 21.0 (20.8–21.1) | 21.7 (21.5–21.8) | 19.0 (18.7–19.2) | <0.001 |
| Low | 20.9 (20.7–21.0) | 20.1 (20.0–20.3) | 22.9 (22.7–23.2) | |
| Middle | 18.5 (18.4–18.6) | 19.2 (19.1–19.4) | 16.5 (16.2–16.7) | |
| High | 19.7 (19.6–19.8) | 19.4 (19.2–19.5) | 20.7 (20.4–20.9) | |
| Highest (poorest) | 20.0 (19.8–20.1) | 19.6 (19.5–19.8) | 21.0 (20.7–21.2) | |
| Diabetes | 16.1 (16.0–16.3) | 10.5 (10.3–10.6) | 32.2 (31.9–32.5) | <0.001 |
| Hypertension | 19.9 (19.8–20.0) | 14.3 (14.2–14.4) | 35.7 (35.4–36.0) | <0.001 |
| Obesity | 19.4 (19.2–19.5) | 17.7 (17.6–17.9) | 24.0 (23.7–24.3) | <0.001 |
| CVD | 2.0 (2.0–2.1) | 1.4 (1.3–1.4) | 3.9 (3.8–4.1) | <0.001 |
| CKD | 2.0 (1.9–2.0) | 0.9 (0.9–1.0) | 4.9 (4.8–5.1) | <0.001 |
| COPD | 1.5 (1.4–1.5) | 0.8 (0.8–0.9) | 3.3 (3.2–3.4) | <0.001 |
| Asthma | 2.7 (2.6–2.7) | 2.8 (2.8–2.9) | 2.3 (2.2–2.4) | <0.001 |
| Health care provider type | ||||
| Ministry of Health | 57.8 (57.7–58.0) | 65.3 (65.1–65.5) | 36.8 (36.5–37.1) | <0.001 |
| Social security | 42.2 (42.0–42.3) | 34.7 (34.5–34.9) | 63.2 (62.9–63.5) | |
| Human resources and hospital equipment* | ||||
| Lowest | 22.9 (22.7–23.0) | 21.0 (20.8–21.1) | 28.1 (27.9–28.4) | <0.001 |
| Low | 18.0 (17.9–18.1) | 17.1 (17.0–17.3) | 20.4 (20.2–20.7) | |
| Middle | 20.1 (20.0–20.3) | 21.2 (21.1–21.4) | 17.1 (16.8–17.3) | |
| High | 11.8 (11.7–11.9) | 11.8 (11.7–11.9) | 11.7 (11.5–11.9) | |
| Highest | 27.2 (27.1–27.4) | 28.9 (28.7–29.0) | 22.6 (22.3–22.9) | |
| Month of presentation to care | ||||
| March-April | 8.1 (8.0–8.2) | 6.5 (6.4–6.6) | 12.5 (12.3–12.7) | <0.001 |
| May | 23.1 (23.0–23.2) | 21.7 (21.6–21.9) | 27.0 (26.7–27.3) | |
| June | 37.1 (37.0–37.3) | 37.9 (37.8–38.1) | 34.9 (34.6–35.2) | |
| July | 31.7 (31.6–31.9) | 33.9 (33.7–34.0) | 25.6 (25.4–25.9) | |
| Symptom onset to presentation to care (days) | ||||
| 0–3 | 46.4 (46.2–46.5) | 47.5 (47.3–47.7) | 43.2 (42.9–43.6) | <0.001 |
| 4–7 | 39.5 (39.3–39.6) | 39.3 (39.1–39.5) | 40.0 (39.7–40.3) | |
| ≥8 | 14.1 (14.0–14.3) | 13.2 (13.1–13.3) | 16.8 (16.6–17.0) | |
| ICU admission | — | 3.6 (3.5–3.7) | ||
| ICU admission and mechanical ventilation | — | 4.0 (3.9–4.1) |
Data are % (95% CI) unless otherwise indicated. P value represents the difference in proportions by each characteristic in ambulatory vs. hospitalized care (χ2 test).
Human resources and hospital equipment index represents state-level health system resources available in 2018. Data presented encompass publicly available data on individuals with confirmed SARS-CoV-2 in Mexico between 1 March and 31 July 2020.
Predicted Probability of Hospitalization According to Comorbidity Status
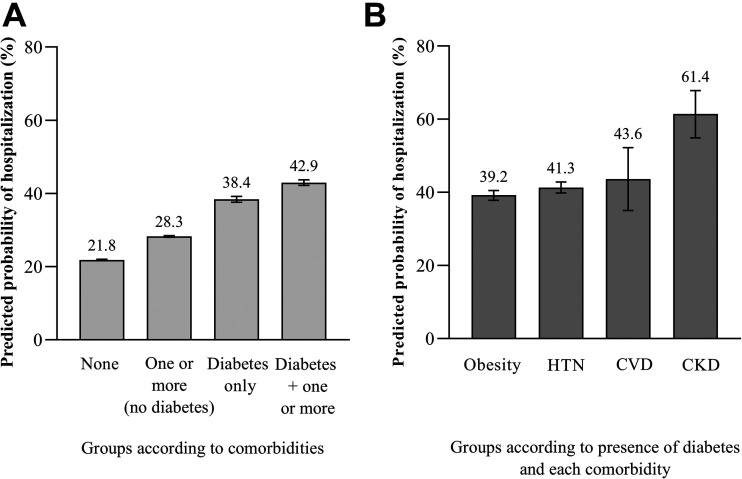
Figure 1 illustrates the adjusted predicted probability of hospitalization compared with ambulatory care according to comorbidity status and according to the combination of diabetes and each associated comorbidity. The predicted probability of hospitalization according to comorbidity status (highest to lowest) was as follows: diabetes with one or more comorbidities (42.9% [95% CI 42.2–43.7]), diabetes only (38.4% [95% CI 37.6–39.2]), one or more comorbidities but no diabetes (28.3% [95% CI 28.0–28.5]), and no self-reported comorbidities (21.8% [95% CI 21.7–22.0]). When stratified according to presence of diabetes and each comorbidity, the group with diabetes and CKD had the highest predicted probability of hospitalization (61.4% [95% CI 54.9–67.8]). The age- and sex-adjusted percentage of patients in ambulatory and hospitalized care according to comorbidity group is presented in Supplementary Appendix 1.
Figure 1.
Predicted probability of hospitalization according to comorbidities of obesity, hypertension (HTN), CVD, and CKD among 373,963 patients with COVID-19 in Mexico. Four groups according to comorbidity status (A) and according to diabetes and each comorbidity (B) are shown on the x axis, and the predicted probability of hospitalization is presented on the y axis. The model is adjusted for age, sex, indigenous language spoken, municipality social deprivation level, health care provider type, state-level health care resource index, month of presentation to care, days from symptom onset to presentation to care, COPD, and asthma. Among patients that required hospitalization, 8.5% of patients required ICU-level care.
Incremental Probability of Hospitalization According to Sociodemographic, Health Care Resource, and Presentation to Care Characteristics
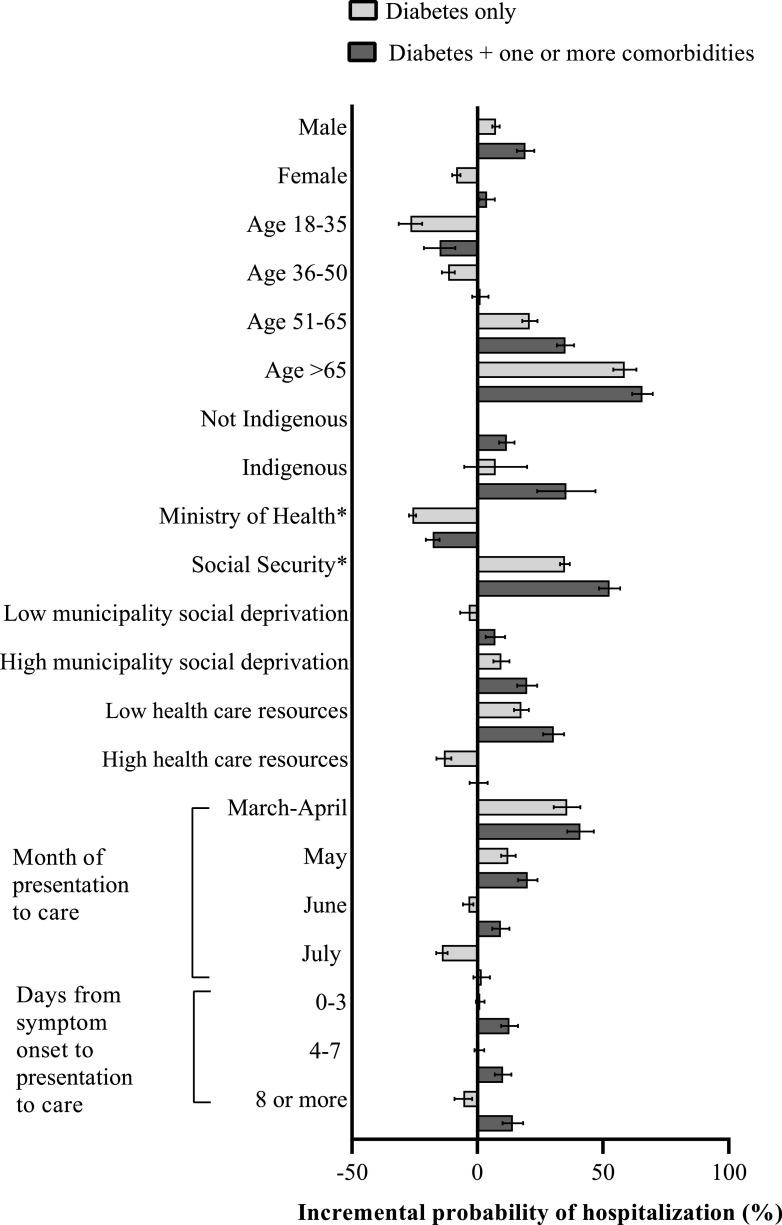
Figure 2 presents the incremental probability of hospitalization for each covariate of interest among individuals with diabetes only and diabetes with one or more comorbidities. Among individuals with diabetes and no comorbidities, the characteristics that conferred an additional risk of hospitalization (listed from highest to lowest) were age >65 (58.7% [95% CI 54.1–63.2]); being part of the initial cohort of reported COVID-19 cases in the country (i.e., patients who presented to care in March or April) (35.7% [95% CI 30.4–41.0]); social security provider type (34.8% [95% CI 32.9–36.8]); age 51–65 years (20.8% [95% CI 17.8–23.9]); low state-level health care resources (17.5% [95% CI 14.5–20.4]); being part of the later cohort of reported COVID-19 cases (i.e., presentation to care in May) (12.3% [95% CI: 9.4–15.2]); high municipality deprivation level (9.5% [95% CI 6.3–12.7]), and male sex (7.4% [95% CI 5.9–8.9]). Among individuals with diabetes with one or more comorbidities, all of the characteristics listed above also conferred an increased risk of hospitalization, with an effect size of higher magnitude than those with only self-reported diabetes (all estimated predicted probabilities are provided in Supplementary Appendix 2). Additional characteristics that conferred the highest risk of hospitalization among individuals with diabetes with one or more comorbidities were indigenous language (35.4% [95% CI 23.8–47.0] vs. 11.7% [95% CI 8.6–14.8] for those who did not speak an indigenous language) and presenting to care ≥8 days after symptom onset (14.1% [95% CI 10.0–18.2]). The predicted probability of hospitalization according to all covariates of interest in the overall study sample is provided in Supplementary Appendix 3.
Figure 2.
Incremental probability of hospitalization according to sociodemographic characteristics, health care resources, and presentation to care characteristics among 373,963 patients with COVID-19 and diabetes in Mexico, with and without one or more comorbidities. The incremental probability is calculated with respect to the overall adjusted probability of being hospitalized among individuals with diabetes as shown in Fig. 1. Model adjusted for all characteristics listed. Comorbidities included are as follows: obesity, hypertension, CVD, and CKD. Model additionally adjusted for COPD and asthma. Among patients that required hospitalization, 8.5% of patients required ICU-level care. *Ministry of Health and Social Security refer to health care provider type.
Case-Based Scenarios of the Cumulative Predicted Probability of Hospitalization
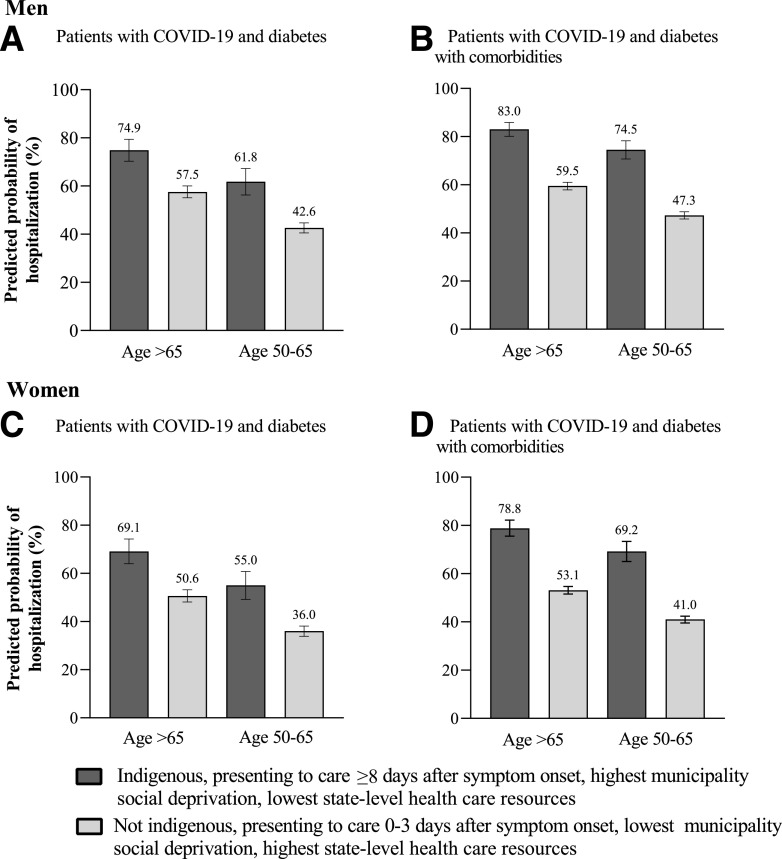
Figure 3 illustrates case scenarios of the overall contribution that socioeconomic, health care resource, and presentation to care characteristics have on the probability of hospitalization among individuals with COVID-19. As seen in Fig. 3A, men with COVID-19 and diabetes only, who were >65 years, spoke an indigenous language, presented to care ≥8 days after symptom onset, and who came from a context of high social vulnerability had a 74.9% (95% CI 70.3–79.4) predicted probability of hospitalization, whereas men with the same age and comorbidity status but who presented to care earlier and who came from a context of low social vulnerability had a predicted probability of hospitalization of 57.5% (17.4% difference). A similar gradient was observed for individuals aged 50–65 years (Fig. 3A). Figure 3B presents these same case scenarios in the population with COVID-19 and diabetes with one or more comorbidities. A similar gradient was observed as that seen in Fig. 3A, although the probability of hospitalization was overall higher for all the case scenarios, and the difference between scenarios with the same age and comorbidity status but different social context was more pronounced, as follows: 83.0% (95% CI 80.1–85.9) vs. 59.5% (95% CI 57.9–61.4) for age >65 years, a difference of 23.5%, and 74.5% (95% CI 70.7–78.3) vs. 47.3% (95% CI 45.8–48.8) for age 50–65 years, a difference of 27.2%.
Figure 3.
Case scenarios of the predicted probability of hospitalization according to social vulnerability, health care resources, and presentation to care characteristics for men (top panels) and for women (bottom panels). A and C: Case scenarios for patients with COVID-19 and diabetes. B and D: Case scenarios for patients with COVID-19 and diabetes with comorbidities. Model adjusted for all characteristics listed in addition to health care provider type and month of presentation to care. Comorbidities included are as follows: obesity, hypertension, CVD, and CKD. Model additionally adjusted for COPD and asthma. Among patients that required hospitalization, 8.5% of patients required ICU-level care. A sensitivity analysis of case scenarios that excluded timing of presentation to care did not appreciably change these estimates (Supplementary Appendix 4).
Figure 3C and 3D illustrate the same case scenarios described above but for women. Women with COVID-19 and diabetes only, age >65 years, who spoke an indigenous language, presented to care ≥8 days after symptom onset, and who came from a context of high social vulnerability had a 69.1% (95% CI 64.0–74.3) predicted probability of hospitalization, whereas women of the same age and comorbidity status but who presented to care earlier and who came from a context of low social vulnerability had a predicted probability of hospitalization of 50.6% (95% CI 48.1–53.2), an 18.5% difference. Women in the 50–65 years group had a difference of 19.5% (55.0% [95% CI 49.2–60.8] vs. 36.0% [95% CI 33.9–38.1]). Similar to what was observed among men with diabetes and one or more comorbidities, there were more pronounced differences between scenarios with the same age and comorbidity status but different social contexts (Fig. 3D). Women in the >65 years group had a difference of 25.7% in the predicted probability of hospitalization when comparing contexts of high versus low social vulnerability (78.8% [95% CI 75.5–82.2] vs. 53.1% [95% CI 51.5–54.7]) and 28.3% in the 50–65 years group (69.2% [95% CI 65.0–73.4] vs. 41.0% [95% CI 39.5–42.4]). Given that the timing of presentation to care may reflect the natural course of illness instead of serving solely as a proxy of access to care, we conducted a sensitivity analysis of case scenarios that excluded this variable, which did not appreciably change the reported predicted probabilities (Supplementary Appendix 4).
Conclusions
In this study of 373,963 individuals with laboratory-confirmed SARS-CoV-2 in Mexico, we identified key socioeconomic, health care resource, and presentation to care characteristics that confer an additional risk of severe COVID-19 among individuals with diabetes and associated comorbidities. In addition to older age and male sex, social security provider type, presenting to care in the early months of the COVID-19 epidemic, living in a municipality with high social deprivation, and living in a state with low health care resources were all associated with an increase in the probability of hospitalization among individuals with COVID-19 and diabetes. Additionally, speaking an indigenous language was associated with an incremental probability of severe COVID-19 among individuals with diabetes and one or more comorbidities. These findings confirm what has been previously documented on the role of diabetes and associated comorbidities on severe COVID-19 (3,26,27) and add to the existing literature (28) on social disparities uncovered by this epidemic by highlighting social vulnerability characteristics that amplify the risk of severe COVID-19 in this high-risk population.
We also found that in age-, sex-, and comorbidity-adjusted case scenarios of the risk of hospitalization, there was a strong social gradient in the predicted probability of hospitalization among individuals with COVID-19 and diabetes. The social gradient was most pronounced for women than men and for the group with diabetes and one or more comorbidities. Importantly, the social gradient was also observed across all groups for individuals 50–65 years of age. These findings suggest that in addition to the well-documented risk associated with advanced age and diabetes status on the likelihood of developing severe COVID-19 (3), social vulnerability as well as limited access to health care resources can potentiate the risk of hospitalization among individuals with COVID-19 and diabetes with and without associated comorbidities, particularly among women.
Two other important findings emerged from our analysis. First, we found considerable disparities in the incremental risk of hospitalization with COVID-19 by age, particularly among the 18–35 vs. >65 age-groups. While social distancing strategies have been emphasized for the ≥65 age-group globally (29), this approach has important limitations in low resource settings. In Mexico, where groups of low socioeconomic status have been subject to a disproportionate burden of COVID-19 (16), crowding of households shared by extended family members is thought to be an important contributor to the higher rates of infection observed in this population (28). Complying with social distancing requirements may be particularly challenging in these settings given that family members who rely on work outside the home may increase the infection risk of elderly family members. While there have been recommendations on use of masks and regular hand hygiene, other key containment strategies include rapid allocation of housing to those in need of isolation or quarantine, early and targeted testing, and proactive contact tracing (30). These measures have been partially implemented in highly contagious districts of Mexico City (31), but they have not yet been implemented extensively in all the states of Mexico, particularly in neighborhoods with the highest rates of disease transmission.
Second, we found substantial differences in the incremental risk of hospitalization among those who received care in social security facilities compared with Ministry of Health facilities. Given that the installed capacity of social security hospitals is greater than that of Ministry of Health hospitals (32), the incremental risk of hospitalization observed in our analysis may be merely reflective of a larger admitting capacity. It is also possible that patients who were more symptomatic presented to social security facilities under the perception that these would be best equipped to deliver adequate care. Although the COVID-19 pandemic has prompted the strengthening of hospital services provided by the Ministry of Health through the recruitment of health personnel and the expansion of ICU beds, there is still a critical lack of health personnel trained in the specialized treatment of severe cases (33).
The COVID-19 epidemic has revealed preexisting health disparities in Mexico and other contexts. Despite efforts aimed at increasing health care access for socioeconomically vulnerable groups (32,34), the unprecedented stress that this pandemic has brought to the fragmented Mexican health system has highlighted important shortcomings in previous attempts at reducing health inequalities in Mexico (22,32). It is noteworthy that shortly before the COVID-19 outbreak, the new government administration abolished or dismantled important social and health programs (15,35). While strategies to reduce metabolic disease in the country and improve regular access to comprehensive and high-quality health care should be prioritized, reallocation of health care resources to vulnerable groups during epidemics such as COVID-19 is also of paramount importance. Resources should be used to finance focused interventions aimed at reducing the risk of infection in vulnerable populations, such as financial and social support to help them stay at home during the acute phase of an epidemic, and to improve timely access to health care in case of infection. While the Mexican government has implemented some measures to improve access to all public health care providers, preexisting disparities in access to care remain an important barrier to mitigating the risk of severe COVID-19 among individuals with diabetes and associated comorbidities who live in contexts of high social vulnerability, most notably patients with diabetes in indigenous communities.
Our study has several important limitations. First, our study sample includes only individuals who presented to care, which may be reflective of a population that has better baseline access to health care, and could have underestimated the association between social vulnerability and severe COVID-19.
Second, diabetes diagnosis was derived from self-report, which could have led to an underestimation of the prevalence of diabetes, particularly undiagnosed diabetes, as well as of associated comorbidities. However, self-reported diabetes has been validated in numerous studies and is considered a reliable measure of diagnosed diabetes (36,37). Additionally, we did not have data on the history of diabetes, such as the duration of illness and glycemic control, which may be associated with severe COVID-19 (38).
Third, the data set was developed to monitor the COVID-19 epidemic, and as such, we only have information on the health status at the time patients presented to care, and there is no information on patient follow-up.
Fourth, we lacked socioeconomic information at the individual level (i.e., schooling, employment, health insurance, etc.). However, the municipality social deprivation index used in this study is considered a reliable measure of an individual’s socioeconomic context in Mexico (23).
Finally, we also lacked information on the health resources (capital and labor) currently available to combat the epidemic, which in our model could be underestimating the role of health care resources on COVID-19 severity.
The intersection of the COVID-19 and diabetes epidemics has brought forth important implications for individuals living with diabetes and associated comorbidities and for health systems struggling to meet the increased demand for hospitalized and ICU care. In this study, we provide further evidence for the association between diabetes and severe COVID-19, and we document that social vulnerability potentiates this risk considerably among individuals with diabetes and associated comorbidities. Given the high prevalence of diabetes in Mexico and globally, coupled with the urgent need to reduce the burden of COVID-19 on individuals and health systems, our findings can help guide resource allocation aimed at reducing gaps in access to health care, in general, and to implement specific measures to mitigate the risk of SARS-CoV-2 infection among socially vulnerable populations with the highest risk of severe COVID-19. These findings also point to the need to design specific strategies to protect highly vulnerable populations in future epidemics.
Article Information
Acknowledgments. The authors thank the Dirección General de Epidemiología for their extraordinary efforts in collecting individual-level data on COVID-19 cases throughout Mexico and for providing open access to this invaluable resource.
Funding. J.A.S. is supported by National Institute of Diabetes and Digestive and Kidney Diseases grant number T32DK007028. J.M.-G. is supported by National Institute of Allergy and Infectious Diseases grant number T32AI007433.
Duality of Interest. D.J.W. reports serving on a data monitoring committee for Novo Nordisk. J.B.M. is an Academic Associate for Quest Diagnostics. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. S.G.S.-R. and J.A.S. conceived the idea of the manuscript. S.G.S.-R., J.A.S., and C.C. wrote the first draft of the manuscript. S.G.S.-R., J.A.S., and E.S.-M. performed analysis. E.S.-M. led the formal analysis. E.S.-M. performed the data curation. J.M.-G., J.B.M., D.J.W., V.J.W., and O.G.-D. provided critical inputs on multiple iterations. All authors approved the final version. E.S.-M. is the guarantor of the work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
S.G.S.-R. and J.A.S. contributed equally to this work and share first authorship.
This article contains supplementary material online at https://doi.org/10.2337/figshare.13148636.
This article is part of a special article collection available at https://care.diabetesjournals.org/collection/diabetes-and-COVID19.
References
- 1.World Health Organization Pneumonia of unknown cause – China, 5 January 2020. Accessed 30 July 2020. Available from https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/
- 2.Johns Hopkins Coronavirus Resource Center COVID-19 Map. Accessed 30 July 2020. Available from https://coronavirus.jhu.edu/map.html
- 3.Bello-Chavolla OY, Bahena-López JP, Antonio-Villa NE, et al. . Predicting mortality due to SARS-CoV-2: a mechanistic score relating obesity and diabetes to COVID-19 outcomes in Mexico. J Clin Endocrinol Metab 2020;105:dgaa346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddle MC, Buse JB, Franks PW, et al. . COVID-19 in people with diabetes: urgently needed lessons from early reports. Diabetes Care 2020;43:1378–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Q, Zhang X, Jiang F, et al. . Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care 2020;43:1382–1391 [DOI] [PubMed] [Google Scholar]
- 6.International Diabetes Federation International Diabetes Federation Atlas, 9th edition, 2019. Accessed 18 December 2019. Available from https://www.diabetesatlas.org/en/resources/
- 7.Alegre-Díaz J, Herrington W, López-Cervantes M, et al. . Diabetes and cause-specific mortality in Mexico City. N Engl J Med 2016;375:1961–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escobedo J, Rodríguez-Abrego G, Aranda J, Zurita B, Ramirez T, Herrera J. Disability-adjusted life-years (DALYs) for diabetes in Mexico in 2005: a cross-sectional burden of disease analysis. Lancet 2013;381:S46 [Google Scholar]
- 9.Basto-Abreu A, Barrientos-Gutiérrez T, Rojas-Martínez R, et al. . Prevalence of diabetes and poor glycemic control in Mexico: results from Ensanut 2016. Salud Publica Mex 2020;62:50–59 [in Spanish] [DOI] [PubMed] [Google Scholar]
- 10.Gutierrez JP, Garcia-Saiso S, Aracena BM. Mexico’s household health expenditure on diabetes and hypertension: what is the additional financial burden? PLoS One 2018;13:e0201333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barquera S, Campos-Nonato I, Aguilar-Salinas C, Lopez-Ridaura R, Arredondo A, Rivera-Dommarco J. Diabetes in Mexico: cost and management of diabetes and its complications and challenges for health policy. Global Health 2013;9:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juan López M, Martínez Valle A, Aguilera N. Reforming the Mexican Health System to achieve effective health care coverage. Health Syst Reform 2015;1:181–188 [DOI] [PubMed] [Google Scholar]
- 13.Doubova SV, García-Saisó S, Pérez-Cuevas R, et al. . Barriers and opportunities to improve the foundations for high-quality healthcare in the Mexican Health System. Health Policy Plan 2018;33:1073–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puig A, Pagán JA, Wong R. Assessing quality across healthcare subsystems in Mexico. J Ambul Care Manage 2009;32:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reich MR Restructuring health reform, Mexican style. Health Syst Reform 2020;6:1–11 [DOI] [PubMed] [Google Scholar]
- 16.Gutierrez JP, Bertozzi SM. Non-communicable diseases and inequalities increase risk of death among COVID-19 patients in Mexico. PLos One 2020;15:e0240394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiglie J, Platt J, Cromer SJ, et al. . Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19. Diabetes Care 2020;43:2938–2944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Ethics and COVID-19: resource allocation and priority-setting, 2020. Accessed 5 August 2020. Available from https://www.who.int/ethics/publications/ethics-and-covid-19-resource-allocation-and-priority-setting/en/
- 19.Walker PGT, Whittaker C, Watson OJ, et al. . The impact of COVID-19 and strategies for mitigation and suppression in low- and middle-income countries. Science 2020;369:413–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secretaría de Salud Datos Abiertos - Dirección General de Epidemiología, 11 May 2020. Accessed 30 July 2020. Available from https://www.gob.mx/salud/documentos/datos-abiertos-152127?idiom=es
- 21.Iglay K, Hannachi H, Joseph Howie P, et al. . Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin 2016;32:1243–1252 [DOI] [PubMed] [Google Scholar]
- 22.Frenk J, González-Pier E, Gómez-Dantés O, Lezana MÁ, Knaul FM. Reforma integral para mejorar el desempeño del sistema de salud en México. Salud Publica Mex 2007;49(Suppl. 1):S23–S36 [DOI] [PubMed] [Google Scholar]
- 23.Consejo Nacional de Población CONAPO. Datos Abiertos del Índice de Marginación, 18 March 2016. Accessed 30 July 2020. Available from http://www.conapo.gob.mx/es/CONAPO/Datos_Abiertos_del_Indice_de_Marginacion
- 24.Tinsley HEA, Brown HD. Handbook of Applied Multivariate Statistics and Mathematical Modeling, 1st ed., Cambridge MA, Academic Press, 2000. Accessed 30 July 2020. Available from https://www.elsevier.com/books/handbook-of-applied-multivariate-statistics-and-mathematical-modeling/tinsley/978-0-12-691360-6 [Google Scholar]
- 25.Lineamiento de Reconversión Hospitalaria, 5 April 2020. Accessed 5 August 2020. Available from https://coronavirus.gob.mx/wp-content/uploads/2020/04/Documentos-Lineamientos-Reconversion-Hospitalaria.pdf
- 26.Petrilli CM, Jones SA, Yang J, et al. . Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ 2020;369:m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hernández-Galdamez DR, González-Block MÁ, Romo-Dueñas DK, et al. . Increased risk of hospitalization and death in patients with COVID-19 and pre-existing noncommunicable diseases and modifiable risk factors in Mexico. Arch Med Res 2020;5:683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodríguez-Izquierdo E, Pérez-Jiménez S, Merino-Pérez L, Mazari-Hiriart M. Spatial analysis of COVID-19 and inequalities in Mexico City. Accessed 13 October 2020. Available from https://www.un.org/development/desa/dpad/wp-content/uploads/sites/45/COVID-19-Mexico-City.pdf
- 29.World Health Organization COVID-19 : Resources for Care for Older Persons. Accessed 13 October 2020. Available from https://www.who.int/maternal_child_adolescent/links/covid-19-mncah-resources-care-for-older-persons/en/
- 30. Maru D, Maru S, Bass E, Masci J. Health Affairs Blog: To Stem The Spread of COVID-19, Address The Challenges Of Crowded Housing, 26 May 2020. Accessed 11 October 2020. Available from https://www.healthaffairs.org/do/10.1377/hblog20200521.144527/full/
- 31.COVID-19. Listado de colonias, pueblos y barrios de atención prioritaria por covid-19, 9 August 2020. Accessed 13 October 2020. Available from https://covid19.cdmx.gob.mx/comunicacion/nota/listado-de-colonias-pueblos-y-barrios-de-atencion-prioritaria-por-covid-19
- 32.Organisation for Economic Cooperation and Development (OECD) OECD Reviews of Health Systems: Mexico, 2016. Accessed 13 October 2020. Available from https://www.oecd-ilibrary.org/social-issues-migration-health/oecd-reviews-of-health-systems-mexico-2016_9789264230491-en
- 33.Pan American Health Organization Rehabilitation considerations during the COVID-19 outbreak, 28 April 2020. Accessed 13 October 2020. Available from https://www.paho.org/en/documents/rehabilitation-considerations-during-covid-19-outbreak
- 34.Frenk J, Gómez-Dantés O, Knaul FM. The democratization of health in Mexico: financial innovations for universal coverage. Bull World Health Organ 2009;87:542–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Frenk J, Gómez-Dantés O, Knaul FM. A dark day for universal health coverage. Lancet 2019;393:301–303 [DOI] [PubMed] [Google Scholar]
- 36.Pastorino S, Richards M, Hardy R, et al.; National Survey of Health and Development Scientific and Data Collection Teams . Validation of self-reported diagnosis of diabetes in the 1946 British birth cohort. Prim Care Diabetes 2015;9:397–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jackson JM, DeFor TA, Crain AL, et al. . Validity of diabetes self-reports in the Women’s Health Initiative. Menopause 2014;21:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu L, She Z-G, Cheng X, et al. . Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab 2020;31:1068. [DOI] [PMC free article] [PubMed] [Google Scholar]