Abstract
Background
The purpose of this study was to evaluate both the biodistribution and safety of 64Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)-Trastuzumab, a novel 64Cu-labeled positron emission tomography (PET) tracer for human epidermal growth factor receptor 2 (HER2) in patients with breast cancer.
Methods
PET images at 1, 24, and 48 h after 296 MBq of 64Cu-NOTA-Trastuzumab injection were obtained from seven patients with breast cancer. Both the primary tumors’ and metastatic lesions’ maximum standardized uptake value (SUVmax) was evaluated. The mean SUVmax (SUVmean) was evaluated in the other organs, including the blood pool, liver, kidney, muscle, spleen, bladder, and the lungs, as well as the bones. Moreover, the internal radiation dosimetry was calculated using the OLINDA/EXM software. Safety was assessed based on feedback regarding adverse reactions and safety-related issues within 1 month after 64Cu-NOTA-Trastuzumab administration.
Results
64Cu-NOTA-Trastuzumab PET images showed that the overall SUVmean values in each organ negatively correlated with time. The liver’s average SUVmean values were measured at 5.3 ± 0.7, 4.8 ± 0.6, and 4.4 ± 0.5 on 1 h, 24 h, and 48 h after injection, respectively. The average SUVmean blood values were measured at 13.1 ± 0.9, 9.1 ± 1.2, and 7.1 ± 1.9 on 1 h, 24 h, and 48 h after injection, respectively. The SUVmax of HER2-positive tumors was relatively higher than HER2-negative tumors (8.6 ± 5.1 and 5.2 ± 2.8 on 48 h after injection, respectively). Tumor-to-background ratios were higher in the HER2-positive tumors than in the HER2-negative tumors. No adverse events related to 64Cu-NOTA-Trastuzumab were reported. The calculated effective dose with a 296 MBq injection of 64Cu-NOTA-Trastuzumab was 2.96 mSv. The highest absorbed dose was observed in the liver (0.076 mGy/MBq), followed by the spleen (0.063 mGy/MBq), kidney (0.044 mGy/MBq), and heart wall (0.044 mGy/MBq).
Conclusions
64Cu-NOTA-Trastuzumab showed a specific uptake at the HER2-expressing tumors, thus making it a feasible and safe monitoring tool of HER2 tumor status in patients with breast cancer.
Trial registration
CRIS, KCT0002790. Registered 02 February 2018, https://cris.nih.go.kr
Keywords: HER-2, 64Cu-NOTA-Trastuzumab, Breast cancer, Positron emission tomography, Computed tomography
Background
The specific receptors that are expressed in cancer cells have been considered as targets for the treatment of tumors, resulting in an improved therapeutic performance [1]. Among them, the human epidermal growth factor receptor (HER)—which is involved in the growth of cancer cells—is a target of a representative molecular therapeutic agent [1, 2]. The overexpression of HER—an intrinsic protein tyrosine kinase—is closely related to rapid-progress tumors [3]. A member of the HER receptor family, HER2/neu (HER2), is overexpressed in breast, ovarian, bladder, prostate, and non-small cell lung cancer [3]. Recently, several therapeutic agents targeting HER2 have been developed to improve treatment outcomes, which include Trastuzumab, Lapatinib, and Pertuzumab [4].
The expression of HER2 is evaluated by tumor tissue acquisition, which entails an inevitably invasive procedure [2, 5, 6]. The discordance rate of HER2 expression between primary tumors and distant metastatic lesions is 4.9–17.7% [7]; therefore, it is necessary to re-evaluate HER2 expression in metastatic tumors. Moreover, HER2 expression may change over time after cancer develops [8], thus necessitating continuous HER2 evaluation. However, repeated biopsies are discomforting for the patient. Overcoming this limitation requires a noninvasive evaluation of HER2 expression of using radioisotopes [2, 5, 6, 9].
Various attempts have been made to noninvasively evaluate the expression of HER2 using radioisotopes, including the evaluation of the HER2 expression using single-photon emission computerized tomography (SPECT) with 111In-Trastuzumab [10, 11]. We investigated the possibility of evaluating HER2 expression; however, it presented both low sensitivity and limited spatial resolution [10]. To overcome these limitations, a diagnostic method using positron emission tomography (PET) has been studied. Clinical trials of antibodies such as Trastuzumab labeled with radioisotopes, including 124I and 89Zr, have been conducted [9, 11, 12], demonstrating HER2 expression capability of lesions in patients with HER2 expressing tumors [9, 11, 12]. Moreover, the use of HER2-targeted PET imaging, using 64Cu-tetra-azacyclododecanetetra-acetic acid (DOTA)-Trastuzumab, has been attempted [5, 6, 11, 13, 14]; related clinical trials in the USA and Japan showed effective identification of HER2 expression in breast cancer patients [5, 6, 13, 14].
We have previously developed the 64Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA)-Trastuzumab, targeting HER2-expressing tumors and conducted both in vitro and in vivo experiments, showing that 64Cu-NOTA-Trastuzumab can be used as a PET-diagnostic application for HER2-positive breast cancer [2]. Here, we evaluated the safety and pharmacokinetics of 64Cu-NOTA-Trastuzumab in breast cancer patients.
Methods
Participants
We recruited a total of seven patients with breast cancer between September 2017 and September 2019 with the following selection criteria: (1) aged 40–80 years, (2) at least one measurable lesion, (3) a histopathologically diagnosed breast cancer with a HER2 expression, and (4) an Eastern Cooperative Oncology Group score of 2 or lower.
This study was approved by the Korean Ministry of Food and Drug Safety (MFDS), and the Institutional Review Board of KIRAMS (IRB No.: KIRAMS 2017-09-006-020). All procedures were performed following the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. Informed consent was obtained from all participants. This preliminary clinical trial is registered with the Clinical Research Information Service (https://cris.nih.go.kr), registration number KCT0002790.
Preparation of 64Cu-NOTA-Trastuzumab
64Cu-NOTA-Trastuzumab was produced from the immunoconjugate NOTA-Trastuzumab, radiolabeled with 64Cu from 50-meV cyclotron irradiation [2]. Briefly, Trastuzumab (Herceptin®; F. Hoffmann-La Roche, Basel, Switzerland) was dissolved in 0.1 M 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 8.5) at a concentration of 10 mg/ml and mixed with a 20-fold molar excess of p–SCN-Bn-NOTA in 100% ethanol. The immunoconjugate (NOTA-Trastuzumab) was purified after an overnight incubation at 4 °C and was concentrated to 5 mg/mL with 0.1 M ammonium acetate buffer (pH 6). For radiolabeling, 370 MBq of 64CuCl2 was added to 5 mg of NOTA-Trastuzumab. The reaction mixtures were incubated at room temperature for 1 h, with a radiolabeling efficiency of > 95%. The reaction mixtures formulated with saline were sterilized by filtration through a 0.22 μm Millex GV filter (Merck Millipore, Billerica, MA, USA).
PET protocol
The 64Cu-NOTA-Trastuzumab PET images were acquired using a GE Discovery 710 PET/ computed tomography (CT) (GE Healthcare, Milwaukee, WI, USA). After a 45 mg Trastuzumab intravenous injection for at least 15 min, participants were intravenously injected with 64Cu-NOTA-Trastuzumab (296 MBq). The mean administered activity was 278.4 ± 13.0 MBq (range 259.0–297.0 MBq). No adverse or clinically detectable pharmacologic effects were found in any of the seven participants; moreover, no significant changes either in vital signs or the results of laboratory studies were reported. PET images were obtained at 60 min after intravenous injection of 64Cu-NOTA-Trastuzumab. Delayed PET images were obtained between 20 and 25 h, and 46 and 49 h after 64Cu-NOTA-Trastuzumab injection. All participants were scanned from the mid-thigh to the vertex of the skull.
18F-Fluorodeoxyglucose (FDG) PET/CT was performed 1 day before the 64Cu-NOTA-Trastuzumab PET. After 6 h of fasting, 370 MBq of 18F-FDG was intravenously injected. The blood glucose level before 18F-FDG injection did not exceed 7.2 mmol/L. One h after injection, PET images were acquired using GE Discovery 710 PET/CT (GE Healthcare, Milwaukee, WI, USA).
PET images were reconstructed using a conventional iterative algorithm and ordered-subsets expectation–maximization with parameters of four iterations and eight subsets. For attenuation correction, CT scans were obtained (130 kVp, 30 mA, 0.6 s/CT rotation, and 6 pitch), after voiding the bladder.
Radiation dosimetry
The internal dosimetry of 64Cu-NOTA-Trastuzumab was evaluated using accumulated radioactivity in PET images. The organ time-activity curve of radioactivity in the target region (ID) divided by target mass (g) was acquired from each organ to calculate residence time. The time-activity curve was expressed by three time points at 1, 24, and 48 h. The residence times were calculated by accumulated radioactivity divided by subject administered activity. The S value of the source-to-target region energy, deposited per unit mass, was calculated using OLINDA/EXM version 1.1 software, using an adult female as the model. The organ-absorbed doses were calculated as the self-dose and cross-dose from each organ region.
Biodistribution
The biodistribution of 64Cu-NOTA-Trastuzumab was evaluated using the maximum standardized uptake value (SUVmax) and the mean standardized uptake value (SUVmean) from the three sequential PET images using GE AW software (GE Healthcare, Milwaukee, WI, USA). Regarding normal-organ distribution, the blood, liver, kidney, muscle, spleen, bladder, lung, and bone were analyzed. Regarding tumors, the primary tumor, metastatic lymph nodes (LNs), and metastatic bone lesions were also evaluated. A 2–3 cm ellipsoidal volume of interest was drawn inside the organ on the PET images to calculate the SUV.
The lesion-to-background ratios were calculated to test the degree of 64Cu-NOTA-Trastuzumab uptake at the lesion sites. The SUVmean either of the liver or blood was used as the background. The SUVmax of the breast tumors, metastatic LNs, and metastatic bone lesions was used for lesion assessment.
Safety
Safety was assessed both before and after 64Cu-NOTA-Trastuzumab administration, acquiring feedback—including adverse reactions and other safety-related issues—1 month later. Adverse events, vital signs, physical examination data, and laboratory test results were all considered in the safety evaluation.
Results
Participant characteristics
Seven patients with breast cancer were recruited. One screened participant was excluded, because of a failure to produce radioisotopes, thus evaluating a total of six participants.
During the initial diagnosis, the immunohistochemistry (IHC) results from core needle biopsy found three patients with HER2-positive tumors and another three with HER2-negative tumors; however, after neo-adjuvant chemotherapy, the final IHC results from tumor excision showed two patients with HER2-positive tumors and four with HER2-negative tumors. One patient with an IHC score of 3+ from core needle biopsy had the result changed into IHC score 1+ from excision after neoadjuvant chemotherapy, thus classifying this patient as HER2-negative.
Patient cancer staging was checked from IIA to IV. The period between histology evaluation and 64Cu-NOTA-Trastuzumab imaging was 1–3 months. All participants—except for participant 1—underwent neoadjuvant chemotherapy with adriamycin and cyclophosphamide before the 64Cu-NOTA-Trastuzumab PET/CT scan. The tumor size at the time of 64Cu-NOTA-Trastuzumab PET/CT scan was measured at 1.7–4.0 cm.
Detailed participant characteristics are described in Table 1.
Table 1.
Participant characteristics
| Subject no | Age (years) | Histology | Stage | IHC score* (CNB) | SISH | IHC score† (excision) | Neoadjuvant chemotherapy | History of Trastuzumab treatment | Interval from CNB to 64Cu-NOTA-Trastuzumab (months) | Tumor size (cm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45 | IDC | IV | 3+ | N/A | N/A | – | – | 1 | 7.3 |
| 2 | 42 | IDC | IIIB | 3+ | N/A | 3+ | AC #3 | – | 2 | 2.4 |
| 3 | 46 | IDC | IIIA | 3+ | N/A | 1+ | AC #4 | – | 3 | 1.7 |
| 4 | 45 | IDC | IIA | – | N/A | – | AC #4 | – | 3 | 2.2 |
| 5 | 56 | IDC | IIIB | 2+ | – | 1+ | AC #4 | – | 3 | 14.0 |
| 6 | 54 | IDC | IIB | – | N/A | – | AC #3 | – | 2 | 4.6 |
IHC immunohistochemistry, SISH silver-enhanced in situ hybridization, CNB core needle biopsy, IDC invasive ductal carcinoma, N/A not applicable, AC anthracycline, and cyclophosphamide
*IHC score with core needle biopsy at the initial diagnosis
†IHC score with tumor excision after neoadjuvant chemotherapy
Safety
No adverse events related to the use of 64Cu-NOTA-Trastuzumab were observed.
Radiation dosimetry
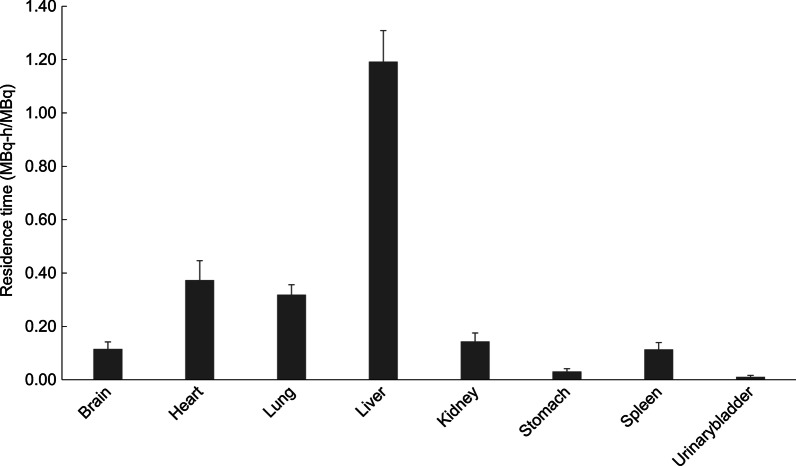
The estimated radiation-absorbed dose for each organ is described in Table 2. The organ with the highest absorbed doses was the liver, with 0.076 ± 0.007 mGy/MBq. The effective dose was calculated as 0.010 ± 0.001 mSv/MBq. Therefore, when injected with 296 MBq of 64Cu-NOTA-Trastuzumab, the effective dose was 2.96 mSv. Figure 1 shows the residence time for each organ.
Table 2.
Dosimetry of 64Cu-NOTA-Trastuzumab (OLINDA)
| Organ | Absorbed dose (mGv/MBq) | ||
|---|---|---|---|
| 64Cu-NOTA-Trastuzumab | 64Cu-DOTA-Trastuzumab [6] | [89Zr]Trastuzumab [9] | |
| Adrenals | 0.005 ± 0.001 | 0.031 ± 0.004 | 0.80 |
| Brain | 0.009 ± 0.002 | 0.015 ± 0.003 | 0.39 |
| Breasts | 0.002 ± 0.000 | 0.020 ± 0.001 | 0.42 |
| Gallbladder wall | 0.006 ± 0.001 | 0.035 ± 0.008 | 0.86 |
| LLI wall | 0.000 ± 0.000 | 0.018 ± 0.002 | 0.58 |
| Small intestine | 0.001 ± 0.000 | 0.019 ± 0.001 | 0.57 |
| Stomach wall | 0.008 ± 0.002 | 0.024 ± 0.002 | 0.63 |
| ULI wall | 0.002 ± 0.000 | 0.022 ± 0.002 | 0.65 |
| Heart wall | 0.042 ± 0.008 | 0.340 ± 0.046 | 1.11 |
| Kidneys | 0.044 ± 0.009 | 0.103 ± 0.034 | 1.23 |
| Liver | 0.076 ± 0.007 | 0.237 ± 0.117 | 1.63 |
| Lungs | 0.034 ± 0.004 | 0.057 ± 0.070 | 0.59 |
| Muscle | 0.001 ± 0.000 | 0.023 ± 0.006 | 0.49 |
| Ovaries | 0.001 ± 0.000 | 0.018 ± 0.002 | 0.59 |
| Pancreas | 0.005 ± 0.001 | 0.032 ± 0.003 | 0.78 |
| Red marrow | 0.001 ± 0.000 | 0.017 ± 0.001 | 0.69 |
| Osteogenic cells | 0.001 ± 0.000 | 0.035 ± 0.001 | 0.79 |
| Skin | 0.001 ± 0.000 | 0.015 ± 0.001 | 0.34 |
| Spleen | 0.063 ± 0.010 | 0.142 ± 0.040 | 0.86 |
| Thymus | 0.003 ± 0.000 | 0.030 ± 0.002 | 0.57 |
| Thyroid | 0.000 ± 0.000 | 0.016 ± 0.001 | 0.43 |
| Urinary | 0.003 ± 0.001 | 0.023 ± 0.006 | 0.42 |
| Uterus | 0.001 ± 0.000 | 0.018 ± 0.002 | 0.58 |
| Total body | 0.004 ± 0.000 | 0.029 ± 0.004 | 0.55 |
| Effective dose (mSv/MBq) | 0.010 ± 0.001 | 0.036 ± 0.009 | 0.61 |
Fig. 1.
Residence time derived from serial positron emission tomography images (PET). Mean organ residence times (± standard deviation) for 64Cu-NOTA-Trastuzumab
Normal-organ biodistribution and tumor uptake
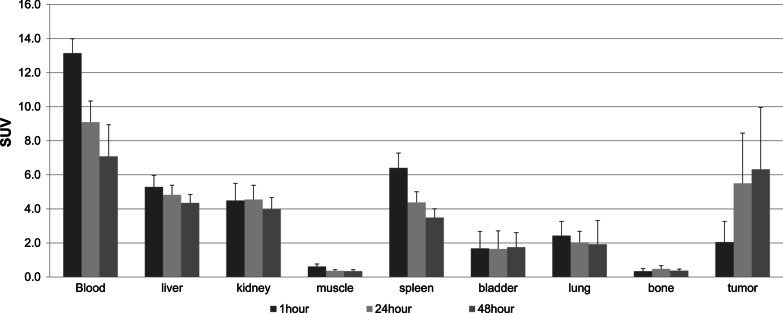
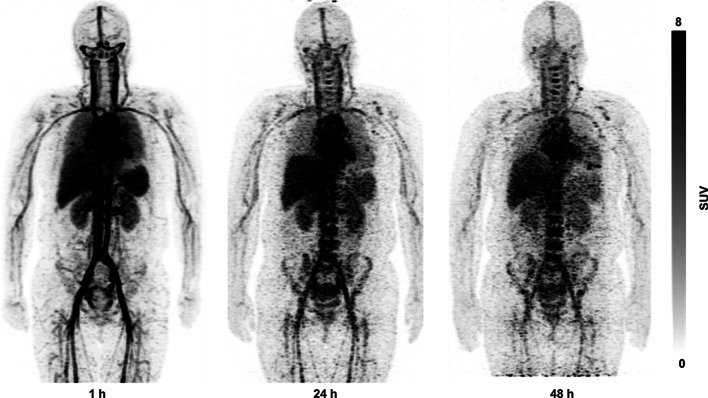
The uptakes of 64Cu-NOTA-Trastuzumab in normal organs, including blood pool, liver, kidneys, muscles, spleen, bladder, lungs, and bones, are presented in Fig. 2 as SUVmean. Maximum intensity projection (MIP) images of participant 1 show the whole-body distribution of 64Cu-NOTA-Trastuzumab in Fig. 3.
Fig. 2.
Mean standardized uptake value (SUVmean) with a standard error of 64Cu-NOTA-Trastuzumab in normal organs and maximum standardized uptake value (SUVmax) with a standard error of 64Cu-NOTA-Trastuzumab in tumors
Fig. 3.
Maximum intensity projection images of 64Cu-NOTA-Trastuzumab PET at 1, 24, and 48 h after injection
The uptake of 64Cu-NOTA-Trastuzumab in the blood showed a high value at 1 h after injection and a decreasing pattern over time. The SUVmean of the liver also showed a high value at 1 h after injection and a gradual decrease over time. The bladder’s SUVmean was maintained at a low value at 1–48 h after injection. Overall, the uptakes of 64Cu-NOTA-Trastuzumab in the blood, liver, kidneys, and spleen were relatively high.
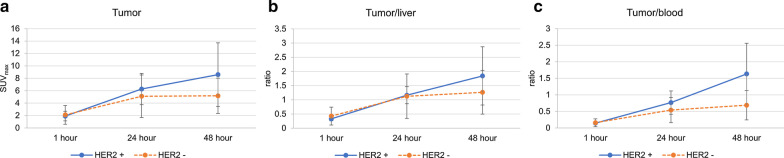
The average values of SUVmax for HER2 positive tumors were: 1.9 ± 0.8 at 1 h; 6.3 ± 2.5 at 24 h; and 8.6 ± 5.1 at 48 h after 64Cu-NOTA-Trastuzumab injection. In the case of HER2 negative tumors, the average values of SUVmax were: 2.1 ± 1.5 at 1 h; 5.1 ± 3.4 at 24 h; and 5.2 ± 2.8 at 48 h after 64Cu-NOTA-Trastuzumab injection. The lesion-to-liver ratios at 48 h after injection were 1.8 ± 1.0 and 1.3 ± 0.8 for HER2 positive and negative tumors, respectively. The lesion-to-blood ratios at 48 h were 1.6 ± 0.9 and 0.7 ± 0.4 for HER2 positive and negative tumors, respectively. Figure 4 shows changes in both the SUVmax of the tumors and tumor-to-background ratios depending on time. Overall, the values of both SUVmax and tumor-to-background ratios were higher in HER2-positive tumors than HER2-negative tumors. The values of HER2-positive tumors increased over time up to 48 h after injection; however, HER2-negative tumors did not show the same increase in uptake values observed for the HER2-positive tumors.
Fig. 4.
The changes of SUVmax (a), tumor-to-liver ratio (b), and tumor-to-blood pool ratio (c) of HER2-positive and HER2-negative tumors over time
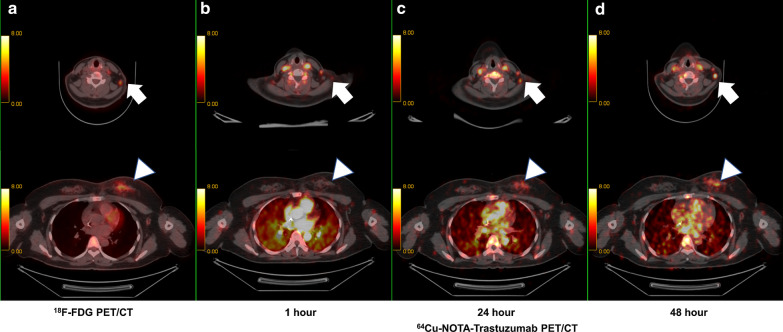
Participant 1’s images of 64Cu-NOTA-Trastuzumab PET/CT are shown in Fig. 5; Fig. 5a (first column) is the 18F-FDG PET/CT image and Fig. 5b–d (second to fourth columns) shows 64Cu-NOTA-Trastuzumab PET/CT images regarding time (on 1, 24, and 48 h, respectively). Participant 1 was a 45-year-old, left breast cancer patient with multiple metastatic lymph nodes and bones. The tumor showed a HER2-positive expression (HER2 3 +). The upper-row arrows show the metastatic lymph node on the left side of the neck. High 18F-FDG uptake was shown in the 18F-FDG PET/CT (Fig. 5a upper row), and the uptakes of 64Cu-NOTA-Trastuzumab increased over time in the same lesion with the FDG PET/CT images (Fig. 5b–d upper row). The SUVmax of the metastatic lymph node was: 1.2 at 1 h; 6.5 at 24 h; and 11.6 at 48 h after injection. The lower row of Fig. 5 shows the primary tumor in the left breast. 18F-FDG uptake appears in the left breast cancer (Fig. 5a, lower row, arrowhead). The uptakes of 64Cu-NOTA-Trastuzumab were also found in the same lesion with increases over time (Fig. 5b–d, lower row, arrowhead). The SUVmax of the primary tumor was: 2.2 at 1 h; 5.8 at 24 h; 9.7 at 48 h after injection.
Fig. 5.
64Cu-NOTA-Trastuzumab PET images of HER2-positive breast cancer (arrowheads, lower row) and metastatic lymph node (arrows, upper row). The primary tumor and metastatic lymph nodes were clearly observed by 18F-FDG PET/CT (a) and 64Cu-NOTA-Trastuzumab PET/CT (b–d). In HER2-expressing lesions, the uptakes of NOTA increased over time to 48 h after injection
Discussion
This study demonstrated that a novel HER2-targeted PET ligand, 64Cu-NOTA-Trastuzumab, was safe, had no adverse effects, and provided a relatively low exposure to radiation (2.96 mSv from a 296-MBq injection). Moreover, the uptakes of 64Cu-NOTA-Trastuzumab were observed in the HER2-expressing tumors including primary breast cancer, metastatic lymph nodes, and metastatic bones.
Due to the tumor heterogeneity, HER2 expression could differ between the primary and the metastatic lesions and may vary depending on disease progression [7, 8]. Therefore, evaluating the HER2 expression before HER2-targeted therapy to enhance the patients’ treatment efficacy is vital.
64Cu-DOTA-Trastuzumab PET—an evaluation method of HER2 expression in a noninvasive procedure—was a feasible modality based on clinical trials [5, 6, 13, 14]. Compared to other PET agents—such as 89Zr and 124I for evaluating HER2 expression—64Cu has the benefit of reducing radiation exposure with a relatively short half-life [2]. Moreover, PET/CT can be performed in an outpatient setting, thus making it advantageous. Previous studies have reported that 64Cu-DOTA-Trastuzumab PET has difficulty in distinguishing either metastatic lesions or tumors, in the liver and around the blood vessels due to the high physiologic uptakes of 64Cu-DOTA-Trastuzumab [6], which are found in both the liver and blood [15]. However, in a chelator comparison study for 64Cu labeled compound administration in vivo, a reduction in non-specific uptake was observed for the 64Cu-NOTA compound compared to the 64Cu-DOTA compound [16]. Further, the 64Cu-NOTA compound showed considerably lower accumulation in the liver than the 64Cu-DOTA compound [17]. Therefore, we developed 64Cu-NOTA-Trastuzumab to achieve improved PET imaging for HER2 expression, compared to 64Cu-DOTA-Trastuzumab [2].
Previous studies with 64Cu-DOTA-Trastuzumab [6, 18] evaluated breast cancer patients with only HER2-positive tumors; however, despite the limitation of the small sample number, our study included both patients with HER2-positive and negative tumors. In our study, HER2-positive tumors showed a high 64Cu-NOTA-Trastuzumab uptake, whereas HER2-negative tumors did not. Adding to the previous preclinical results [2], this study suggested that 64Cu-NOTA-Trastuzumab can effectively differentiate HER2-positive from HER2-negative tumors in patients with breast cancer.
The biodistribution of 64Cu-NOTA-Trastuzumab in normal organs showed high uptakes in both the blood and liver, in line with the in vivo study [2]. Nevertheless, 64Cu-NOTA-Trastuzumab showed that it is advantageous regarding the relatively low uptake in the liver compared to 64Cu-DOTA-Trastuzumab. Conversely, blood uptake is relatively higher than 64Cu-DOTA-Trastuzumab. The relatively low uptake in the liver could be due to the stable copper-binding ability of NOTA, potentially reducing free copper accumulation; however, a direct comparison of the results is limited, as the liver uptake between 64Cu-NOTA-Trastuzumab and 64Cu-DOTA-Trastuzumab was compared in different tumor models [19]. 64Cu-NOTA-Trastuzumab showed a relatively low effective dose (0.014 mSv/MBq) compared to the latest radiolabeled Trastuzumab studies (64Cu-DOTA-Trastuzumab, 0.036 mSv/MBq; 89Zr-Trastuzumab, 0.61 mSv/MBq), potentially reducing patient radiation exposure [6, 9].
The uptake of 64Cu-NOTA-Trastuzumab in HER2-expressing tumors was not observed 1 h after injection. Nevertheless, the specific uptake increased 24 h after injection, and a further increase of specific uptake of 64Cu- NOTA-Trastuzumab in HER2-expressing tumors could be observed after 48 h following the injection, showing a distinctive feature from the background. It is suggested that 64Cu- NOTA-Trastuzumab PET at 48 h after injection can evaluate the HER2 expression in the clinical setting. Mild diffuse uptakes were also observed in the tumors with a negative expression of HER2, as HER2 expression is not all-or-none as determined by the immunohistochemistry methods [5, 20]. Namely, even if the tumor’s HER2 expression is negative, its SUVmax positively correlates to the tumor IHC score [5, 20]. Therefore, it is important to carefully set the cut-off values of SUVmax or SUV ratio between the tumor and the background to determine HER2 expression using a 64Cu-NOTA-trastuzumab PET image.
This study has some limitations. First, the number of enrolled participants was relatively small. Second, since all the patients—except for participant 1—underwent neoadjuvant chemotherapy, the SUV of tumors might have reflected tumor cell suppression. Therefore, the SUV might be lower than expected. Namely, regarding participants 2 and 3, with HER2 positive tumors as an initial diagnosis, the 18FDG PET/CT—performed before and after neoadjuvant chemotherapy—showed both decreased tumor size and metabolic activity after chemotherapy (data not shown). Therefore, the SUV of HER2 positive tumors on 64Cu-NOTA-Trastuzumab PET could have been underestimated. Consequently, a further study with a larger sample size and 64Cu-NOTA-Trastuzumab PET before neoadjuvant chemotherapy is needed to evaluate the exact efficacy of 64Cu-NOTA-Trastuzumab PET imaging.
Conclusion
This preliminary clinical trial showed that 64Cu-NOTA-Trastuzumab PET is both safe and feasible. 64Cu-NOTA-Trastuzumab showed a specific uptake at the HER2-expressing tumors with a relatively low liver uptake. 64Cu-NOTA-Trastuzumab can be used to evaluate radiation dosimetry and prediction of treatment-response in targeted therapy for HER2-positive breast cancer with HER2-targeted therapy.
Acknowledgements
Not applicable.
Abbreviations
- 64Cu-DOTA-Trastuzumab
64Cu-tetra-azacyclododecanetetra-acetic acid-Trastuzumab
- 64Cu-NOTA-Trastuzumab
64Cu-1,4,7-triazacyclononane-1,4,7-triacetic acid-Trastuzumab PET: Positron emission tomography
- CT
Computed tomography
- FDG
18F-Fluorodeoxyglucose
- HER2
Human epidermal growth factor receptor 2
- SPECT
Single-photon emission computerized tomography
- SUVmax
Maximum standardized uptake value
- SUVmean
Mean SUVmax
Authors’ contributions
All authors contributed to the study conception and design of this study. IL, BHB, BIK, CWC, MS, HK, WCN, SML, and ILim designed and performed the clinical trial, analyzed data, and wrote the manuscript; SW, KIK, KCL, and JHK made contributions to conception by analyzing and interpreting data; All authors gave final approval of the final content of the manuscript.
Funding
This study was supported by a grant from the Korea Institute of Radiological and Medical Sciences (KIRAMS), funded by the Ministry of Science and ICT, Republic of Korea (No. 50547-2020) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (Ministry of Science and ICT) (Nos. 2020R1A2C2102492, 2019M2D2A1A02057204, and 2017M2A2A7A02070985).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Korean Ministry of Food and Drug Safety (MFDS), and the Institutional Review Board of KIRAMS (IRB No.: KIRAMS 2017-09-006-020). All procedures were performed following the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This clinical trial has been registered with the Clinical Research Information Service (https://cris.nih.go.kr), registration number KCT0002790. Informed consent was obtained from all individual participants included in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Woo Chul Noh and Ilhan Lim should be considered joint corresponding authors.
Contributor Information
Ilhan Lim, Email: ilhan@kcch.re.kr.
Woo Chul Noh, Email: nohwoo@kirams.re.kr.
References
- 1.Falzone L, Salomone S, Libra M. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol. 2018;9:1300. doi: 10.3389/fphar.2018.01300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woo SK, Jang SJ, Seo MJ, Park JH, Kim BS, Kim EJ, et al. Development of 64Cu-NOTA-Trastuzumab for HER2 targeting: a radiopharmaceutical with improved pharmacokinetics for human studies. J Nucl Med. 2019;60(1):26–33. doi: 10.2967/jnumed.118.210294. [DOI] [PubMed] [Google Scholar]
- 3.Yan M, Schwaederle M, Arguello D, Millis SZ, Gatalica Z, Kurzrock R. HER2 expression status in diverse cancers: review of results from 37,992 patients. Cancer Metastasis Rev. 2015;34(1):157–164. doi: 10.1007/s10555-015-9552-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreutzfeldt J, Rozeboom B, Dey N, De P. The trastuzumab era: current and upcoming targeted HER2+ breast cancer therapies. Am J Cancer Res. 2020;10(4):1045–1067. [PMC free article] [PubMed] [Google Scholar]
- 5.Sasada S, Kurihara H, Kinoshita T, Yoshida M, Honda N, Shimoi T, et al. 64Cu-DOTA-trastuzumab PET imaging for HER2-specific primary lesions of breast cancer. Ann Oncol. 2017;28(8):2028–2029. doi: 10.1093/annonc/mdx227. [DOI] [PubMed] [Google Scholar]
- 6.Tamura K, Kurihara H, Yonemori K, Tsuda H, Suzuki J, Kono Y, et al. 64Cu-DOTA-trastuzumab PET imaging in patients with HER2-positive breast cancer. J Nucl Med. 2013;54(11):1869–1875. doi: 10.2967/jnumed.112.118612. [DOI] [PubMed] [Google Scholar]
- 7.Houssami N, Macaskill P, Balleine RL, Bilous M, Pegram MD. HER2 discordance between primary breast cancer and its paired metastasis: tumor biology or test artefact? Insights through meta-analysis. Breast Cancer Res Treat. 2011;129(3):659. doi: 10.1007/s10549-011-1632-x. [DOI] [PubMed] [Google Scholar]
- 8.Branco FP, Machado D, Silva FF, André S, Catarino A, Madureira R, et al. Loss of HER2 and disease prognosis after neoadjuvant treatment of HER2+ breast cancer. Am J Transl Res. 2019;11(9):6110–6116. [PMC free article] [PubMed] [Google Scholar]
- 9.Laforest R, Lapi SE, Oyama R, Bose R, Tabchy A, Marquez-Nostra BV, et al. [89Zr]Trastuzumab: evaluation of radiation dosimetry, safety, and optimal imaging parameters in women with HER2-positive breast cancer. Mol Imaging Biol. 2016;18(6):952–959. doi: 10.1007/s11307-016-0951-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaykema SB, de Jong JR, Perik PJ, Brouwers AH, Schroder CP, Oude Munnink TH, et al. 111In-trastuzumab scintigraphy in HER2-positive metastatic breast cancer patients remains feasible during trastuzumab treatment. Mol Imaging. 2014 doi: 10.2310/7290.2014.00011. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Li X, Zhu L, Liu J, Xu W, Wang P. Preclinical and clinical applications of specific molecular imaging for HER2-positive breast cancer. Cancer Biol Med. 2017;14(3):271–280. doi: 10.20892/j.issn.2095-3941.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo X, Zhou N, Chen Z, Liu T, Xu X, Lei X, et al. Construction of 124I-trastuzumab for noninvasive PET imaging of HER2 expression: from patient-derived xenograft models to gastric cancer patients. Gastric Cancer. 2020;23(4):614–626. doi: 10.1007/s10120-019-01035-6. [DOI] [PubMed] [Google Scholar]
- 13.Mortimer JE, Bading JR, Park JM, Frankel PH, Carroll MI, Tran TT, et al. Tumor uptake of 64Cu-DOTA-Trastuzumab in patients with metastatic breast cancer. J Nucl Med. 2018;59(1):38–43. doi: 10.2967/jnumed.117.193888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kurihara H, Hamada A, Yoshida M, Shimma S, Hashimoto J, Yonemori K, et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015;5:8. doi: 10.1186/s13550-015-0082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Owen CA, Jr, Hazelrig JB. Metabolism of Cu-64-labeled copper by the isolated rat liver. Am J Physiol. 1966;210(5):1059–1064. doi: 10.1152/ajplegacy.1966.210.5.1059. [DOI] [PubMed] [Google Scholar]
- 16.Dearling JL, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, et al. Imaging cancer using PET—the effect of the bifunctional chelator on the biodistribution of a 64Cu-labeled antibody. Nucl Med Biol. 2011;38(1):29–38. doi: 10.1016/j.nucmedbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Silva RA, Jain S, Lears KA, Chong HS, Kang CS, Sun X, et al. Copper-64 radiolabeling and biological evaluation of bifunctional chelators for radiopharmaceutical development. Nucl Med Biol. 2012;39(8):1099–1104. doi: 10.1016/j.nucmedbio.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mortimer JE, Bading JR, Colcher DM, Conti PS, Frankel PH, Carroll MI, et al. Functional imaging of human epidermal growth factor receptor 2-positive metastatic breast cancer using 64Cu-DOTA-trastuzumab PET. J Nucl Med. 2014;55(1):23–29. doi: 10.2967/jnumed.113.122630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortimer JE, Shively JE. Functional imaging of human epidermal growth factor receptor 2-positive breast cancers and a note about NOTA. J Nucl Med. 2019;60(1):23–25. doi: 10.2967/jnumed.118.220905. [DOI] [PubMed] [Google Scholar]
- 20.Ulaner GA, Hyman DM, Lyashchenko SK, Lewis JS, Carrasquillo JA. 89Zr-Trastuzumab PET/CT for detection of human epidermal growth factor receptor 2-positive metastases in patients with human epidermal growth factor receptor 2-negative primary breast cancer. Clin Nucl Med. 2017;42(12):912–917. doi: 10.1097/RLU.0000000000001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.