Abstract
Mechanical stress determines bone mass and structure. It is not known whether mechanical loading affects expression of bone regulatory genes in a combined deficiency of estrogen and vitamin D. We studied the effect of mechanical loading on the messenger RNA (mRNA) expression of bone regulatory genes during vitamin D and/or estrogen deficiency. We performed a single bout in vivo axial loading with 14 N peak load, 2 Hz frequency and 360 cycles in right ulnae of nineteen weeks old female control Wistar rats with or without ovariectomy (OVX), vitamin D deficiency and the combination of OVX and vitamin D deficiency (N = 10/group). Total bone RNA was isolated 6 hours after loading, and mRNA expression was detected of Mepe, Fgf23, Dmp1, Phex, Sost, Col1a1, Cyp27b1, Vdr, and Esr1. Serum levels of 25(OH)D, 1,25(OH)2D and estradiol were also measured at this time point. The effect of loading, vitamin D and estrogen deficiency and their interaction on bone gene expression was tested using a mixed effect model analysis. Mechanical loading significantly increased the mRNA expression of Mepe, and Sost, whereas it decreased the mRNA expression of Fgf23 and Esr1. Mechanical loading showed a significant interaction with vitamin D deficiency with regard to mRNA expression of Vdr and Esr1. Mechanical loading affected gene expression of Mepe, Fgf23, Sost, and Esr1 independently of vitamin D or estrogen, indicating that mechanical loading may affect bone turnover even during vitamin D deficiency and after menopause.
Keywords: estrogen deficiency, gene expression, mechanical loading, phosphate, Vitamin D deficiency
1. INTRODUCTION
Mechanoresponse is a cascade of events initiated by load‐induced deformation–strain–of bone. 1 Bone formation occurs when bone is exposed to peak mechanical loading above a certain threshold. In response to mechanical loading, osteocytes produce signaling molecules which regulate osteoblast and osteoclast function to maintain adequate bone mass and architecture. 2 , 3
Estrogens are important for bone maintenance. Estrogen deficiency, which occurs after menopause causes severe bone loss. 4 Several studies have confirmed that the estrogen receptor (ER), particularly ER‐α responds to loading in both in vivo and in vitro models. 5 , 6 , 7 In post‐menopausal women, weight bearing exercise and hormone replacement therapy may have synergistic effects on whole body bone mineral density (BMD). 8 In agreement with the human condition rats develop osteopenia following ovariectomy, 5 , 9 which results in decreased bone strength and microarchitecture. Mechanical loading or estrogen replacement also increased BMD in ovariectomy (OVX) rats. 9
Vitamin D is another factor important for bone turnover and mineralization. The effect of the active metabolite 1,25(OH)2D could be indirect via an adequate supply of calcium and phosphate at the mineralization site by stimulating calcium absorption from the gut. 10 Vitamin D deficiency may drop 1,25(OH)2D, and less calcium will be available for bone mineralization. Vitamin D deficiency stimulates PTH, thereby increasing bone turnover. 10 An important factor in phosphate homeostasis is fibroblast growth factor 23 (FGF23), which is a phosphaturic hormone produced by osteocytes and osteoblasts. FGF23 is stimulated by dietary phosphate and 1,25(OH)2D signaling through VDR, whereas FGF23 inhibits 1,25(OH)2D and PTH. 11 FGF23 is also locally influenced by matrix extracellular phosphoglycoprotein (MEPE) and dentin matrix protein (DMP1), and phosphate regulating gene with homologies to endopeptidase (PHEX) that are produced by osteoblasts and osteocytes. 11
25‐hydroxyvitamin D (25(OH)D) is converted by the enzyme 1‐α‐hydroxylase (Cyp27B1) into the active metabolite 1,25(OH)2D in the kidney, 10 but also in osteoblasts, which locally converts 25(OH)D to 1,25(OH)2D. 12 This local conversion seems to be differently regulated from the kidney, since local CYP27B1 expression in bone is not affected by renal regulators, such as parathyroid hormone (PTH), 13 but is likely affected by mechanical loading, as demonstrated by induced messenger RNA (mRNA) expression of CYP27B1 in primary human osteoblasts, suggesting a possible role for mechanical loading in local vitamin D activation in bone. 13 The biological explanation for this may be that the presence of sufficient 1,25(OH)2D is crucial for mechanoresponse. Mechanotransduction has a well documented anabolic effect on bone tissue mineralization. 3 , 4 Anabolic effect on bone tissue mineralization is shown to be regulated by Mepe. 14 A microarray experiment revealed that Mepe mRNA expression was up‐regulated after mechanical loading of the rat tibia. 14 This was confirmed by other investigators, who showed that mechanical loading of bone increased the expression of Mepe 15 , 16 and Dmp1 16 and decreased the expression of Fgf23, 17 which indicates that the phosphate‐related genes may be regulated by mechanical loading. Sufficient availability of local vitamin D may be essential for an optimal mechanoresponse.
While both vitamin D deficiency and estrogen deficiency may impair mechanoresponse in bone and the combination of both deficiencies is common in elderly persons, it is not known whether mechanical loading‐dependent regulation of bone genes is affected in conditions of combined vitamin D and estrogen deficiency. Yet, no study has examined the effect of in vivo mechanical loading on local conversion of 25(OH)D to 1,25(OH)2D in osteocytes and osteoblasts. Although, the effect of in vivo axial loading was studied in rodent models of vitamin D deficiency 18 , 19 or estrogen deficiency 4 , 9 but the effect of mechanical loading in a combined estrogen and vitamin D deficiency model has not been investigated.
This study hypothesizes that mechanical loading induced regulation of phosphate‐related genes is impaired during a combination of both vitamin D deficiency and estrogen deficiency. To test this hypothesis we measured gene expression levels of phosphate‐related genes: Mepe, Fgf23, Phex, and Dmp1 in bone after in vivo mechanical loading in rats with vitamin D deficiency, estrogen deficiency or combined vitamin D and estrogen deficiency. In addition, the effect of in vivo mechanical loading on Cyp27b1 mRNA expression was investigated.
2. METHODS
2.1. Experimental animals
Eighty, 13 weeks old female Wistar rats (220‐240 g) (Harlan Laboratories, Netherlands), were included in this study. Rats were group housed (5/cage) and fed with free access to regular rodent diet (Teklad Global 16% Protein Rodent Diet, Madison, WI) and water. At the age of 15 weeks, rats were grouped into four groups: sham (N = 20), OVX (N = 20), vitamin D deficiency (sham‐D, N = 20) and, OVX with vitamin D deficiency (OVX‐D, N = 20). Eight separate rats were used to calibrate stress strain relation, and two separate rats were labeled with calcein for detection of bone formation by histomorphometry. The animal experiments were approved by the Institutional Animal Care and Use Committee, VU University Amsterdam, The Netherlands (Endo 13‐01).
2.2. Vitamin D deficiency
Vitamin D deficiency was induced by feeding a daily portion of 20 g vitamin D deficient diet (TD 120503 Vit. D Def diet: 20% lactose, 1% Ca, 0.65% P; Harlan Laboratories, Madison, WI) from the 15th to 19th week age. In addition, three intraperitoneal injections with 32 ng paricalcitol (19‐nor‐1,25‐dihydroxyvitamin D2; Zemplar: AbbVie, Netherlands), a synthetic and biologically active analogue of calcitriol were given in the first two weeks, week 15 to 17. 20 Paricalcitol acts by augmenting renal 24‐hydroxylase activity, which degrades endogenous 25(OH)D and 1,25(OH)2D. Absence of vitamin D impairs absorption of Ca and P and increases PTH. This was counter‐balanced by supplementation of 1% Ca and 0.65% P and 20% lactose in the vitamin D deficient diet. The high lactose in this diet increases the passive vitamin D independent Ca absorption in the intestine. 18 , 19 , 20 This model of vitamin D deficiency enables to investigate the direct role of vitamin D deficiency in the mechanoresponse without calcium or phosphate deficiency, excluding confounding factors such as hyperparathyroidism and high bone turnover and bone loss which may lead to impairment of mechanical competence.
2.3. Estrogen deficiency
Estrogen deficiency was induced by ovariectomy at 15 weeks age. Rats were anesthetized with a combination of Temgesic (Buprenorphine 0.03mg/100g; Reckitt Benckiser, Slough, England) and isoflurane (±2%) (Tevapharm, Haarlem,Netherlands). OVX was performed after dorsal midline incision. The two ovaries were dissected and the muscle and skin were sutured after the procedure. Rimadyl (Caprofen 5mg/kg; Zoetis, Florham park, NJ) was given post‐surgery. For sham surgery a single dorsal midline incision was made, and both muscle and the skin were sutured. The rats were monitored after OVX or sham surgery for any post‐surgical complications. In the combined ovariectomy and vitamin D deficient group, vitamin D deficient diet was started the same day as the surgery.
2.4. Load‐strain relationship and histomorphometry
To establish the force required to elicit strain required for bone formation after mechanical loading, eight rats were loaded ex vivo to detect the deformation in the loaded ulna. For each condition two rats were included: (a) sham, (b) OVX, (c) sham‐D, and (d) OVX‐D. The right ulnae of these rats were harvested after sacrifice and a strain gage (EA‐06‐015DJ‐120/LE; Micro‐measurements, Raleigh, NC) was attached to the anterior mid‐shaft of the ulnar diaphysis with a cyanoacrylate adhesive. Mechanical loading was performed on the ulnae equipped with the strain gage for 360 cycles at a frequency of 2 Hz using the Servo hydraulic Instron testing device (Instron, Norwood, MA) and with a stepwise increasing force of 4, 8, 10, 12, and 14 N. The strain signals were recorded with HBM MGCplus (HBM Inc, Marlboro, MA) connected to the Instron device.
To confirm axial load induced bone adaptation, two rats were loaded in vivo with a single bout of 14 N peak load, for 360 cycles at 2 Hz frequency and injected with calcein (10 mg/kg; Sigma‐Aldrich, Netherlands) at two intervals, 1 day and 10 days after mechanical loading. Ulnae were excised, fixed and embedded undecalcified in methyl methacrylate. Five micrometres sections were cut, using a sledge microtome (Leica SM2500S; Leica Microsystems BV, Germany). Endosteal and periosteal single labeled surface (sLS/BS) and double labeled surface (dLS/BS) was measured using a fluorescence microscope, (Nikon eclipse E800, Japan) connected to NIS Elements software version 3 (Nikon Instruments Inc, Japan). MARs (µm/day) were calculated.
2.5. In vivo axial mechanical loading
Ten rats from each group (sham, OVX, sham‐D, and OVX‐D) were assigned to a load group, and 10 were assigned to a control group. In the load groups, the right ulna of each rat was loaded in vivo at 19 weeks age and the left ulna was kept as contra‐lateral control. Mechanical loading was applied by a servo hydraulic Instron testing device. The rats were anesthetized with ketamine (75 mg/kg) (Alfasam, Netherlands) and dexdomitor (1 mg/kg) (ORION Pharma, Germany). A single bout of 14 N load was applied at 2 Hz frequency for 360 cycles. Thirty minutes after the mechanical loading experiment, an Atipam (5mg/mL; Eurofet, Netherlands) injection was given to reverse sedation. The rats were allowed to have normal cage activity, for 6 hours after which they were euthanized by CO2 asphyxiation. A pilot experiment in our lab showed that 6 hours after a single bout of loading the gene expression of phosphate related genes: Fgf23, Mepe, and Dmp1 was stimulated (Figure S1). The non‐loaded rats were sacrificed on the same day. Post‐mortem, blood was collected by cardiac puncture and serum was stored at −80°C. Ulnae were harvested, transported in liquid nitrogen and stored at −80°C. Tibiae were harvested and prepared for histology. Hematoxylin and eosin stains were performed on 5 µm sections of decalcified, paraffin embedded bone tissue.
2.6. Serum analysis
Vitamin D metabolites 25(OH)D and 1,25(OH)2D were measured with two‐dimensional isotope dilution ultra‐pressure liquid chromatography tandem mass spectrometry (2‐D ID‐UPLC‐MS/MS; Waters, Milford, MA). Sample preparation was based on a method described by Strathmann et al, 21 and immunoextraction was performed as described previously. 22 Intra‐assay and inter‐assay coefficients of variation (CV) for 1,25(OH)2D were 3.5% (3.1 to >200 pmol/L) and 5.5% (167 pmol/L). 22 , 23 For 25(OH)D, the intra‐assay CV was <6% and the inter‐assay CV was <8% for three concentrations between 25 and 180 nmol/L. 23 Estradiol was measured using an in‐house developed 2D UPLC‐MS/MS method (Waters Acquity UPLC system coupled to a Waters Xevo TQ‐S Tandem‐MS). 13C labeled estradiol was used as internal standard. Lower limit of quantitation was 20 pmol/L. Intra‐assay variation was 6% at a level of 23 pmol/L and 5% at a level of 300 pmol/L. Serum creatinine, was measured by enzymatic assay and Ca, and P were measured by colorimetric assays (Roche Modular Analytics P800, Germany).
2.7. RNA isolation
The ulna diaphyses were cleaned, bone marrow was flushed from the diaphysis with RNAse‐free water, pulverized and extracted with Trizol (Invitrogen, Carlsbad, CA) using the Freezer mill 6750 (Spex Certiprep, Metuchen, NY) followed by a phenol extraction, washed with chloroform/isoamylalcohol extraction and again extracted with Trizol according to the manufacturer's instructions. The RNA pellet was dissolved in RNase‐free water and stored at −80°C prior to use. The quality of the RNA samples was determined with the RNA 6000 Nano Assay Kit (Agilent Technologies). The yield of RNA was measured by Nanodrop spectrophotometer (A260) (NanoDrop ND‐1000 Spectrophotometer). 14
2.8. Complementary DNA preparation and quantitative polymerase chain reaction
One hundred nanogram of total RNA was reverse‐transcribed using 10 ng/μL random primers (Roche, Basel, Switzerland) and 5 U/μL M‐MLV Reverse Transcriptase (Promega) in a mixture containing 5 mM MgCl, 1X RT‐buffer, 1 mM dNTPs each, 1 M betaine and 0.40 U/μL RNAsin for 10 minutes at 25°C, 1 hour at 37°C and 5 minutes at 95°C in a total volume of 20 μL. For real‐time quantitative polymerase chain reaction (qPCR) reaction the complementary DNA (cDNA) was diluted five folds with RNase free water. For a 10 μL qPCR reaction, 2 μL of diluted cDNA was mixed with a 5 µL SYBR Green qPCR mastermix (Roche Diagnostics, Germany) and 2 µL H2O. One microliter mixture of 0.5 µL reverse and 0.5 µL forward primers (each 10 pmol/L) was added in the qPCR reaction. The PCR reaction consisted of an initial denaturation step of 95°C for 10 minutes, followed by 45 cycles of a three‐step amplification as follows: 95°C for 10 seconds, 60°C for 5 seconds, 72°C for 10 seconds. Primers were designed for the rat genes: Fgf23, Phex, Mepe, Dmp1, Cyp27b1, Vdr, Sost, Esr1, and Col1a1 (Table 1). The Light Cycler 480 release 1.5.0 SP4 software (Roche, Germany) was used to analyze gene expression data. The gene expression was normalized with three different housekeeping genes Hprt, Pbgd, and Sdha followed by ΔC p calculation using formula: . ΔC p was calculated as a difference of the target genes with the geometric mean of the three housekeeping genes. 24
Table 1.
Primer sequences for genes used in qPCR analyses
| Primer | Sequence | Length (bp) |
|---|---|---|
| Col1a1 F | 5′‐CATGTTCAGCTTTGTGGACCT‐3′ | 94 |
| Col1a1 R | 5′‐GCAGCTGACTTCAGGGATGT‐3′ | |
| Fgf23 F | 5′‐GCGGCAACATTTTTGGAT‐3′ | 61 |
| Fgf23 R | 5′‐CCTTTATAGACTGGATTATTCAAAATAT‐3′ | |
| Mepe F | 5′‐CCAAGCTTCCTTGAAGGTGA‐3′ | 60 |
| Mepe R | 5′‐CAGACACAGCCTGCATCG‐3′ | |
| Phex F | 5′‐TACTGCCTGAAGCCAGAATG‐3′ | 67 |
| Phex R | 5′‐CCACAGAAAGATTTACTTTGCTCA‐3′ | |
| Cyp27b1 F | 5′‐TCGAGTCCAACTGCCTTCTCA‐3′ | 101 |
| Cyp27b1 R | 5′‐AGAAGAGTTCAGCCAGGAAGC‐3′ | |
| Dmp1 F | 5′‐ATCTGAAAGCTCCGAAGAGAGG‐3′ | 100 |
| Dmp1 R | 5′‐CTCCTCTCCAGACTCACTGC‐3′ | |
| Esr1 F | 5′‐ACTACCTGGAGAACGAGCCC‐3′ | 175 |
| Esr1 R | 5′‐CACACAGCACAGTAGCGAGT‐3′ | |
| Sost F | 5′‐ACATGCAGCCTTCGTTGCT‐3′ | 225 |
| Sost R | 5′‐ACGAAGCGGGTGTAGTGCAG‐3′ | |
| Vdr F | 5′‐GCATCCAAAAGGTCATCGGC‐3′ | 105 |
| Vdr R | 5′‐TGATCACCTCAATGGCGCTT‐3′ | |
| Hprt F | 5′‐GTGTCATCAGCGAAAGTGGA‐3′ | 98 |
| Hprt R | 5′‐TACTGGCCACATCAACAGGA‐3′ | |
| Pbgd F | 5′‐ATGTCCGGTAACGGCGGC‐3′ | 135 |
| Pbgd R | 5′‐CAAGGTTTTCAGCATCGCTACCA‐3′ | |
| Sdha F | 5′‐GACGGGCCACTCACTCTTAC‐3′ | 195 |
| Sdha R | 5′‐CCATAGCCCCCAGTAGCAAT‐3′ |
Abbreviations: bp, base pair, qPCR, quantitative polymerase chain reaction.
2.9. Statistical analysis
Statistical Package for Social Sciences (SPSS, IBM Corp. Armonk, NY) version 22 was used to analyze serum parameters. For serum parameters E2, 1,25(OH)2D and 25(OH)D, all the values below detection limits were valued as detection limit divided by 2. Normality of the serum data was checked by visual inspection of histogram and Q‐Q plots, and Kruskal Wallis analysis of variance (ANOVA) with Dunn Bonferroni correction was applied for multiple comparisons across groups for 25(OH)D, 1,25(OH)2D, and E2. Two way ANOVA was applied to test the interaction effects of vitamin D (vitamin D sufficient versus deficient) and estrogen (estrogen sufficient versus deficient) on body weights, Ca, P, and Creatinine. The gene expression data were log transformed to attain the assumption of normality. Mixed effect model analysis was performed with R version 3.3.1 on gene expression data with intervention: load (non‐loaded versus loaded), vitamin D (vitamin D sufficient versus deficient), estrogen (estrogen sufficient versus deficient) as fixed factors, the interaction effect between loading*vitamin D, loading*estrogen, and vitamin D*estrogen was also established as fixed factors. The individual rat was included as a random effect. β‐estimate values were calculated in the model as the change in log gene expression values between non‐loaded versus loaded (load), vitamin D sufficient versus deficient (vitamin D) and, estrogen sufficient versus deficient (estrogen), and interaction effect of mechanical loading and vitamin D (load*vitamin D). P < .05 was considered statistically significant.
3. RESULTS
Seven rats were excluded from the study due to surgical complications or non‐effective OVX, resulting in 73 rats for the final analysis. No fractures or microcracks were observed in any of the rats. Additionally two samples were lost during RNA isolation procedure. The average body weight of the rats at week 19 is presented in Table 2. Body weights were significantly higher in week 19 in estrogen sufficient rats (sham and sham‐D) as compared with estrogen deficient rats (OVX and OVX‐D) (P < .01). To confirm success of the OVX and vitamin D deficiency in the rats, we measured concentrations of E2, 25(OH)D and 1,25(OH)2D in serum. Serum concentrations of E2 were very low or undetectable in OVX rats (25th and 75th percentiles): (OVX 10(10,20) pmol/L and OVX‐D 10(10,28) pmol/L). Serum concentrations of both 25(OH)D and 1,25(OH)2D were very low or undetectable in the sham‐D group, median (25th and 75th percentiles): (6(3,8) nmol/L and 5(5,10) pmol/L). In the OVX‐D group median (25th and 75th percentiles) serum concentrations of 25(OH)D and 1,25(OH)2D were 12(5,16) nmol/L and 5(5,57) pmol/L, which was still very low. Serum Creatinine, Ca and P levels were not different in vitamin D deficient rats (sham‐D and OVX‐D) as compared with vitamin D sufficient rats (sham and OVX) (P = .3) (Table 2).
Table 2.
Body weights of rats and serum biochemistry in sham, OVX, sham‐D, and OVX‐D rats
| Parameters | sham (N = 19) | OVX (N = 19) | sham‐D (N = 18) | OVX‐D (N = 17) |
|---|---|---|---|---|
| Body weight, g1,a | 261.9 ± 16.6 | 307.8 ± 33.3 | 257.4 ± 23.9 | 291.6 ± 36.7 |
| E2, pmol/L2,b | 26 (12,123) | 10 (10,20) | 77 (10,239) | 10 (10,28) |
| 25(OH)D, nmol/L3,4b | 83 (56,110) | 95(61,120) | 6 (3,8) | 12 (5,16) |
| 1,25(OH)2D, pmol/L3,4 | 54 (26,66) | 115 (74,179) | 5 (5,10) | 5 (5,57) |
| Serum creatinine, μmol/L1,a | 29.2 ± 4.1 | 31.6 ± 4.4 | 27.5 ± 3.9 | 30.5 ± 8.5 |
| Serum total calcium, mmol/La | 3.0 ± 0.1 | 3.1 ± 0.1 | 3.1 ± 0.1 | 3.0 ± 0.1 |
| Serum phophorus, mmol/L1,a | 2.8 ± 0.4 | 3.3 ± 0.4 | 3.1 ± 0.9 | 3.2 ± 0.4 |
Note: Mean ± SDa, two way ANOVA (body weight), P < .01: (estrogen sufficient vs estrogen deficient)1; median (25th & 75th percentiles)b; Kruskal Wallis ANOVA with post hoc Bonferroni Correction (E2, 25(OH)D, 1,25(OH)2D); P < .05: (OVX vs sham)2, (sham‐D vs sham)3, (OVX‐D vs OVX)4; two way ANOVA (serum creatinine, Ca and P), P < .05, (estrogen sufficient vs estrogen deficient)1
Abbreviations: ANOVA, analysis of variance; OVX, ovariectomy.
3.1. A single bout of 14 N induced sufficient strain for mechanoresponse
A single bout loading of 14 N at a frequency of 2 Hz and 360 cycles on the right ulna resulted in an average strain of 2265 µε, which varied from 1180 to 4000 µε. A single bout loading of 14 N at 2 Hz and 360 cycles on the right ulna increased sLS/BS (+27.1%) and average dLS/BS (+4.4%) (Figure 1). Average mineral apposition rate (MAR) in the right loaded ulna was 0.83 µm/day, whereas in the left contralateral controls average MAR was 0.53 µm/day.
Figure 1.
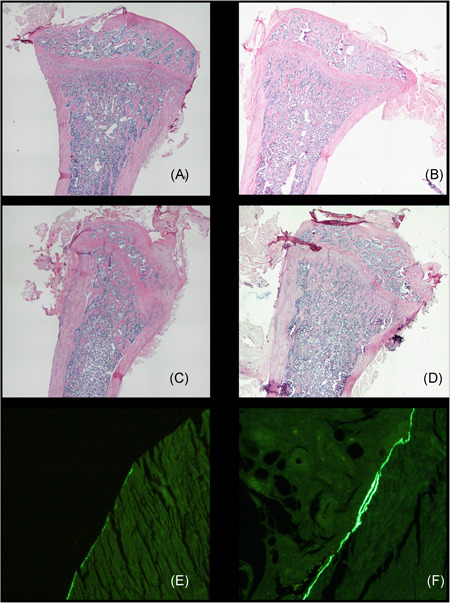
Typical examples of HE stained longitudinal overviews of the proximal tibia from (A) sham, (B) sham‐D, (C) ovariectomy (OVX), and (D) OVX‐D rats, showing both cortical and trabecular bone structure is not dramatically changed. Magnification (4×10). Detail of calcein double labeled transversal sections from the diaphysis of (E) non‐loaded and (F) loaded ulna, demonstrating loading induced endo‐cortical double labeled surface. Magnification (20×10). HE, hematoxylin and eosin
3.2. Mechanical loading affected bone gene expression of Fgf23, Mepe, Sost and Esr1 but did not affect Cyp27b1 (1α‐hydroxylase)
The effect of mechanical loading in loaded rats versus contra‐lateral controls was tested on phosphate related genes: Fgf23, Mepe, Dmp1, and Phex. Mechanical loading decreased mRNA expression of Fgf23 (P = .02), but increased the mRNA expression of Mepe (P = .01) in the loaded rats as compared to controls (Figures 2A,C). However, mechanical loading did not affect the expression of Phex (P = .73) and Dmp1 (P = .8) (Figures 2B,D) in the loaded rats as compared with controls. Mechanical loading decreased the expression of Esr1 (P = .01) and increased mRNA expression of Sost (P = .02, data not shown) (Figure 3B). Mechanical loading did not alter gene expression of Cyp27b1 in the loaded rats as compared with controls (P = .1) (Figure 4A).
Figure 2.
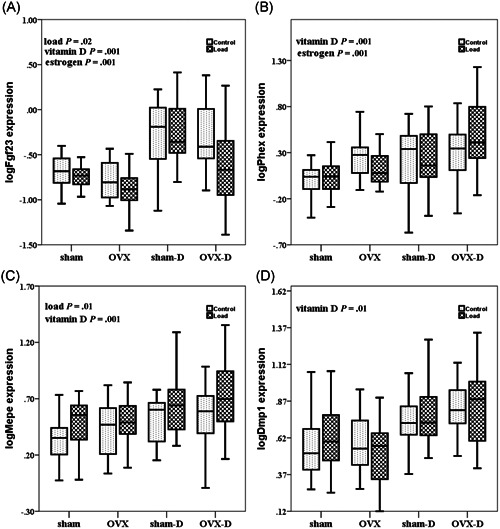
Box whisker plots of log transformed relative bone gene expression of Fgf23, Phex, Mepe, Dmp1 during estrogen deficiency (OVX) and/or vitamin D deficiency and after mechanical loading. The box represents median, 25th and 75th percentiles, and the whiskers represent highest and lowest gene expression values. Each group comprises 10 rats. Open boxes are non‐loaded and filled boxed are loaded ulnae, sham is sham surgery, D is vitamin D deficient. P values are calculated as follows: load, non‐loaded versus loaded; vitamin D, vitamin D sufficient versus deficient; estrogen, estrogen sufficient versus deficient; load*vitamin D, interaction between mechanical loading and vitamin D. OVX, ovariectomy
Figure 3.
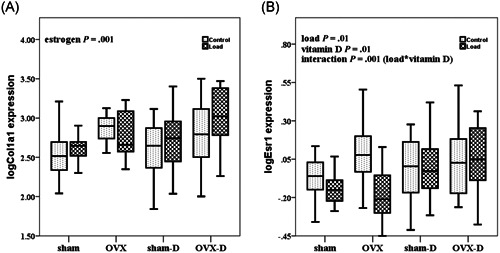
Box whisker plots of relative log transformebone gene expression of Col1a1 and Esr1 during estrogen deficiency (OVX) and/or vitamin D deficiency and after mechanical loading. The box represents median, 25th and 75th percentiles, and the whiskers represent highest and lowest gene expression values. Each group comprises 10 rats. Open boxes are non‐loaded and filled boxes are loaded ulnae, sham is sham surgery, D is vitamin D deficient. P values are calculated as follows: load, non‐loaded versus loaded; vitamin D, vitamin D sufficient versus deficient; estrogen, estrogen sufficient versus deficient; load*vitamin D, interaction between mechanical loading and vitamin D. OVX, ovariectomy
Figure 4.
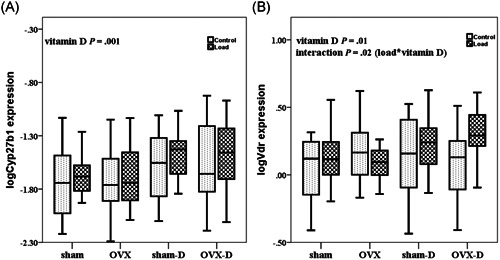
Box whisker plots of log transformed relative bone gene expression of Cyp27b1 and Vdr during estrogen deficiency (OVX) and/or vitamin D deficiency and after mechanical loading. The box represents median, 25th and 75th percentiles, and the whiskers represent highest and lowest gene expression values. Each group comprises 10 rats. Open boxes are non‐loaded and filled boxes are loaded ulnae, sham is sham surgery, and D is vitamin D deficient. P values are calculated as follows: load, non‐loaded versus loaded; vitamin D, vitamin D sufficient versus deficient; estrogen, estrogen sufficient versus deficient; load*vitamin D, interaction between mechanical loading and vitamin D. OVX, ovariectomy
3.3. Vitamin D deficiency affected gene expression with respect to both vitamin D metabolism and bone metabolism
Vitamin D deficiency increased expression of phosphate related genes Fgf23 (P = .001), Phex (P = .001), Mepe (P = .001), and Dmp1 (P = .01) (Figure 2A‐D). Vitamin D deficiency also increased gene expression of Esr1 (P = .01) (Figure 3B), and Sost (P = .01), Cyp27b1 (P = .001) and Vdr (P = .01) (Figure 4A,B).
3.4. Estrogen deficiency affected bone gene expression
Fgf23 mRNA expression (P = .001) was decreased, whereas mRNA expression of Phex (P = .001) and Col1a1 (P = .001) was increased in OVX rats as compared with control rats (Figures 2A,B and 3A). Esr1 gene expression was not significantly different in OVX rats as compared to estrogen sufficient rats (P = .06) (Figure 3B).
3.5. Mechanical loading interacted with vitamin D and estrogen on vitamin D receptor and estrogen receptor but not on bone gene expression
We found significant interaction between the effects of mechanical loading and vitamin D deficiency (load*vitamin D) on mRNA expression of Esr1 (P = .001) and Vdr (P = .02) (Figures 3B and 4B; Table 3). However, no significant interaction was observed between effects of mechanical loading and vitamin D deficiency (load*vitamin D), mechanical loading and OVX (load*estrogen), and effects of vitamin D deficiency and OVX (vitamin D*estrogen) regarding bone gene expression of Fgf23, Phex, Mepe, Dmp1, Col1a1, Sost, and Cyp27b1 (Figures 2A‐D,3A, and 4A).
Table 3.
Significant gene expression values and their β‐estimates in the mixed effect model analysis
| Factors | Significant gene expression | β‐Estimates | P values |
|---|---|---|---|
| Load | Fgf23 | −0.3 | .02 |
| Esr1 | −0.2 | .01 | |
| Mepe | +0.29 | .01 | |
| Vitamin D | Cyp27b1 | +0.45 | .001 |
| Dmp1 | +0.5 | .01 | |
| Esr1 | +0.2 | .01 | |
| Fgf23 | +0.91 | .001 | |
| Mepe | +0.37 | .001 | |
| Phex | +0.5 | .001 | |
| Vdr | +0.26 | .01 | |
| Estrogen | Fgf23 | −0.41 | .001 |
| Col1a1 | +0.6 | .001 | |
| Phex | +0.42 | .001 | |
| Load*vitamin D | Esr1 | −0.25 | .001 |
| Vdr | −0.26 | .02 |
Note: β‐Estimates were calculated as the differences in log gene expression values in the factors: estrogen, estrogen sufficient versus deficient; load, non‐loaded versus loaded; load*vitamin D, interaction between mechanical loading and vitamin D; vitamin D, vitamin D sufficient versus deficient.
4. DISCUSSION
This study showed that mechanical loading altered gene expression of Mepe, Fgf23, Sost, and Esr1 independently of vitamin D deficiency, estrogen deficiency or the combination of both, which led to the rejection of our hypothesis that stated mechanical loading‐induced changes in bone gene expression is impaired during all three conditions: vitamin D deficiency, estrogen deficiency or the combination of them. However, interactions of mechanical loading with vitamin D deficiency were detected in Vdr and Esr1 mRNA expression. Moreover, we hypothesized that mechanical loading would induce Cyp27b1 gene expression, which was not shown. It should be noted that the vitamin D deficiency model used in this study was designed to investigate the direct role of vitamin D in mechanoresponsiveness, excluding confounding factors of a clinical vitamin D deficiency, such as changes in calcium phosphate, and hyperparathyroidism, high turnover or bone loss.
This study confirmed that several mechanically responsive bone genes were differentially expressed after mechanical loading. In this study, mechanical loading down‐regulated Esr1 mRNA expression, confirming a role for ER‐α in mechanoresponse. 6 , 7 , 25 In our study, mechanical loading did not increase Col1a1 mRNA expression, as was reported previously after 4 to 12 days of in vivo mechanical loading. 17 This was likely due to the short interventional period of six hours after mechanical loading in the present study. Mechanical loading increased Mepe expression, similarly to previous studies. 14 , 15 , 16 Mechanical loading reduced Fgf23 gene expression, in line with a microarray study reporting down‐regulation of Fgf23, during matrix formation phase after in vivo mechanical loading. 17 Sost mRNA expression was also increased 6 hours after mechanical loading. This was an unexpected finding since mechanical loading is thought to inhibit sclerostin expression. 26 However, an upregulation of Sost gene expression was reported previously in a microarray study at 3 hours after a single‐bout of loading. 4 This increase was followed by a decline 12 hours after loading suggesting a biphasic response. The chosen timepoint of 6 hours after loading could therefore have caused this surprising result. In summary though, a single bout of axial ulna loading was sufficient to induce osteogenic response.
Both vitamin D as well as estrogen deficiency affected gene expression but these changes did not seem to influence the mechanoresponsiveness. Vitamin D deficiency increased expression of Fgf23, Phex, Dmp1, Mepe, Esr1, and Sost, and OVX increased Col1a1 and Phex mRNA expression but decreased Fgf23 mRNA expression. In our experimental animal model of diet‐induced vitamin D deficiency without hypocalcemia and hypophosphatemia, bone turnover was not affected, which was reflected in the gene expression profile, assuming we studied the direct role of absence of vitamin D. Estrogen deficiency is temporarily associated with high bone turnover, which may explain the increase in Col1a1 expression. Taken together these results suggest that vitamin D deficiency or estrogen deficiency do not have a profound effect on mechanoresponsiveness.
A surprising result of this study was that mechanical loading did not affect Cyp27b1 mRNA expression, which may indicate that mechanical loading does not contribute to the local conversion of 25(OH)D to 1,25(OH)2D, increasing the local availability of 1,25(OH)2D. This is in contrast to a previous in vitro study that showed mechanical loading, applied as pulsatile fluid flow, increased CYP27B1 expression. 13
In this study the vitamin D deficiency was induced while calcium and phosphate were maintained constant to investigate a direct role of 1,25(OH)2D on the expression of mechanically sensitive genes and phosphate‐related genes. In this model, both Cyp27b1 and Vdr mRNA expressions were increased with vitamin D deficiency. This was unsuspected because the gene promotor of CYP27B1 contains negative regulatory VDRE elements which downregulates the transcription of 25(OH)D‐1‐α‐hydroxylase (CYP27B1) enzyme. 27 , 28 Though, a high calcium diet in vitamin D deficient rats was shown to increase Vdr expression previously, 29 it is unlikely that PTH contributed to the increased Cyp27b1 mRNA expression during vitamin D deficiency in our study, since a diet of Ca, P and lactose is known to lower both PTH and renal Cyp27b1 expression 30 also in the same model of initiation and induction of vitamin D deficient rats as used in this study. 20 Therefore, only a direct effect of 1,25(OH)2D can be expected in our vitamin D deficiency model.
In the present study, mechanical loading significantly interacted with vitamin D deficiency in relation to the gene expression of Esr1 and Vdr. Although mechanical loading increased Mepe and decreased Fgf23 expression, no significant interactions of mechanical loading, estrogen deficiency and vitamin D deficiency were observed. These results imply that although vitamin D deficiency and estrogen deficiency alone influence the expression of mechanically responsive genes and phosphate related genes, the effect of mechanical loading on these genes is independent of vitamin D deficiency and/or estrogen deficiency. The clinical consequence of this finding is that mechanoresponsiveness remains intact, even if estrogen or vitamin D are not sufficient. However, extrapolation to the clinical situation is complex since in the clinical situation other consequences of long‐term vitamin D deficiency on bone turnover and bone structure modify the mechanical properties of bone and consequently alter the mechanoresponsiveness. Nevertheless, we suggest that in conditions of elderly post‐menopausal osteoporosis, where both vitamin D and estrogen deficiency may prevail, regular exercise can deliver benefits in improving bone health. 10 , 31
A limitation of this study is that gene expression could not directly be associated to bone structure and turnover, given that the time intervals of gene expression response and bone structure response did not match. We used a 14 N of load across all the groups because an average strain of approximately 2000 to 3000 με, 32 , 33 which is optimal for bone formation, was obtained. Given the dynamic nature of bone tissue, strain may vary among bone samples even within identical regions. Variations of strain among bones have been reported by three different methods: load‐strain calibration, digital image correlation and finite element analysis. 34 Nevertheless, we confirmed the bone's adaptive response to loading in two rats by dynamic histomorphometry
Another limitation of this study is the induction of vitamin D deficiency by a vitamin D deficient diet in combination with paricalcitol injections, which resulted in low or undetectable serum levels of both 25(OH)D and 1,25(OH)2D, except for the OVX‐D group, in which the 1,25(OH)2D was not below the detection limit. This might be due to the fact that 1,25(OH)2D, being a fat‐soluble vitamin, may adhere to the fat in the adipocytes accumulated in these rats due to ovariectomy. Several studies have shown that adipocytes can convert 25(OH)D to 1,25(OH)2D by inducing CYP27B1, 35 , 36 , 37 which might have contributed to impaired reduction of 1,25(OH)2D levels in our OVX‐D model.
5. CONCLUSION
In conclusion, the present study confirmed that mechanical loading modulated the expression of mechanically responsive genes in rat bone and that this response was independent of vitamin D and estrogen. Cyp27b1 expression was not induced by in vivo mechanical loading in rat bone, which suggests that a single bout of mechanical loading in vivo does not increase the local availability of 1,25(OH)2D in rat bone.
AUTHOR CONTRIBUTIONS
AN, HE, and AV performed the animal experiments. AN, HE, and FC performed the qPCR analysis. WW performed the statistical analysis. AN, HE, AV, WW, AS, FC, GP, DM, DV, PL, and NB have contributed to the acquisition, analysis and interpretation of the data as well as to the drafting and critical revision of the paper. All authors have read and approved the final submitted manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was funded by the European Commission through MOVE‐AGE, an Erasmus Mundus Joint Doctorate program (2011‐2015). The work of Ferdy Kurniawan Cayami was supported by the High Reputation of International Publication (RPIBT) Grant of Diponegoro University, Republic of Indonesia (No.329‐113/UN7.P4.3/PP/2019). We would like to thank Endocrinology Laboratory, Amsterdam UMC, Vrije Universiteit Amsterdam for performing the serum vitamin D metabolites and estrogen assays. We are thankful to Dilani Sellathurai and Maria Renes in the Department of Clinical Genetics, Amsterdam UMC, Vrije Universiteit Amsterdam for optimizing and performing qPCR analyses.
Nepal AK, van Essen HW, van der Veen AJ, et al. Mechanical stress regulates bone regulatory gene expression independent of estrogen and vitamin D deficiency in rats. J Orthop Res. 2021;39:42–52. 10.1002/jor.24775
REFERENCES
- 1. Rubin J, Rubin C, Jacobs CR. Molecular pathways mediating mechanical signaling in bone. Gene. 2006;367:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klein‐Nulend J, Bakker AD, Bacabac RG, et al. Mechanosensation and transduction in osteocytes. Bone. 2013;54(2):182‐190. [DOI] [PubMed] [Google Scholar]
- 3. Frost HM. Bone's mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275(2):1081‐1101. [DOI] [PubMed] [Google Scholar]
- 4. Zaman G, Saxon LK, Sunters A, et al. Loading‐related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone. 2010;46(3):628‐642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zaman G, Jessop HL, Muzylak M, et al. Osteocytes use estrogen receptor alpha to respond to strain but their ERalpha content is regulated by estrogen. J Bone Miner Res. 2006;21(8):1297‐1306. [DOI] [PubMed] [Google Scholar]
- 6. Lee K, Jessop H, Suswillo R, et al. Endocrinology: bone adaptation requires oestrogen receptor‐alpha. Nature. 2003;424(6947):389. [DOI] [PubMed] [Google Scholar]
- 7. Lee KC, Lanyon LE. Mechanical loading influences bone mass through estrogen receptor alpha. Exerc Sport Sci Rev. 2004;32(2):64‐68. [DOI] [PubMed] [Google Scholar]
- 8. Kohrt WM, Snead DB, Slatopolsky E, et al. Additive effects of weight‐bearing exercise and estrogen on bone mineral density in older women. J Bone Miner Res. 1995;10(9):1303‐1311. [DOI] [PubMed] [Google Scholar]
- 9. Tromp AM, Bravenboer N, Tanck E, et al. Additional weight bearing during exercise and estrogen in the rat: the effect on bone mass, turnover, and structure. Calcif Tissue Int. 2006;79(6):404‐415. [DOI] [PubMed] [Google Scholar]
- 10. Lips P, van Schoor NM. The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab. 2011;25(4):585‐591. [DOI] [PubMed] [Google Scholar]
- 11. Bergwitz C, Juppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Driel M, Koedam M, Buurman CJ, et al. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha‐hydroxylase expression and activity in human bone cells. FASEB J. 2006;20(13):2417‐2419. [DOI] [PubMed] [Google Scholar]
- 13. van der Meijden K, Bakker AD, van Essen HW, et al. Mechanical loading and the synthesis of 1,25(OH)2D in primary human osteoblasts. J Steroid Biochem Mol Biol. 2016;156:32‐39. [DOI] [PubMed] [Google Scholar]
- 14. Reijnders CM, van Essen HW, van Rens BT, et al. Increased expression of matrix extracellular phosphoglycoprotein (MEPE) in cortical bone of the rat tibia after mechanical loading: identification by oligonucleotide microarray. PLoS One. 2013;8(11):e79672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gluhak‐Heinrich J, Pavlin D, Yang W, et al. MEPE expression in osteocytes during orthodontic tooth movement. Arch Oral Biol. 2007;52(7):684‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris SE, Gluhak‐Heinrich J, Harris MA, et al. DMP1 and MEPE expression are elevated in osteocytes after mechanical loading in vivo: theoretical role in controlling mineral quality in the perilacunar matrix. J Musculoskelet Neuronal Interact. 2007;7(4):313‐315. [PMC free article] [PubMed] [Google Scholar]
- 17. Mantila Roosa SM, Liu Y, Turner CH. Gene expression patterns in bone following mechanical loading. J Bone Miner Res. 2011;26(1):100‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bouillon R. Vitamin D: from photosynthesis, metabolism, and action to clinical applications In: Groot LJD, Kretser DMd, Giudice LC, et al., eds. Endocrinology: Adult and Pediatric. 7th ed. Philadelphia: W.B. Saunders; 2016:1018‐1037. [Google Scholar]
- 19. Bouillon R, Carmeliet G, Verlinden L, et al. Vitamin D and human health: lessons from vitamin D receptor null mice. Endocr Rev. 2008;29(6):726‐776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stavenuiter AW, Arcidiacono MV, Ferrantelli E, et al. A novel rat model of vitamin D deficiency: safe and rapid induction of vitamin D and calcitriol deficiency without hyperparathyroidism. BioMed Res Int. 2015;2015:604275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Strathmann FG, Laha TJ, Hoofnagle AN. Quantification of 1alpha,25‐dihydroxy vitamin D by immunoextraction and liquid chromatography‐tandem mass spectrometry. Clin Chem. 2011;57(9):1279‐1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dirks NF, Martens F, Vanderschueren D, et al. Determination of human reference values for serum total 1,25‐dihydroxyvitamin D using an extensively validated 2D ID‐UPLC‐MS/MS method. J Steroid Biochem Mol Biol. 2016;164:127‐133. [DOI] [PubMed] [Google Scholar]
- 23. Heijboer AC, Blankenstein MA, Kema IP, et al. Accuracy of 6 routine 25‐hydroxyvitamin D assays: influence of vitamin D binding protein concentration. Clin Chem. 2012;58(3):543‐548. [DOI] [PubMed] [Google Scholar]
- 24. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Windahl SH, Saxon L, Borjesson AE, et al. Estrogen receptor‐alpha is required for the osteogenic response to mechanical loading in a ligand‐independent manner involving its activation function 1 but not 2. J Bone Miner Res. 2012;28(2):291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galea GL, Lanyon LE, Price JS. Sclerostin's role in bone's adaptive response to mechanical loading. Bone. 2017;96:38‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29(12):664‐673. [DOI] [PubMed] [Google Scholar]
- 28. Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1,25‐dihydroxyvitamin D(3). Endocrinol Metab Clin North Am. 2010;39(2):255‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brown AJ, Zhong M, Finch J, et al. The roles of calcium and 1,25‐dihydroxyvitamin D3 in the regulation of vitamin D receptor expression by rat parathyroid glands. Endocrinology. 1995;136(4):1419‐1425. [DOI] [PubMed] [Google Scholar]
- 30. Kaufmann M, Lee SM, Pike JW, et al. A high‐calcium and phosphate rescue diet and VDR‐expressing transgenes normalize serum vitamin D metabolite profiles and renal Cyp27b1 and Cyp24a1 expression in VDR null mice. Endocrinology. 2015;156(12):4388‐4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bischoff‐Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med. 2012;367(1):40‐49. [DOI] [PubMed] [Google Scholar]
- 32. Torrance AG, Mosley JR, Suswillo L, et al. Noninvasive loading of rat ulna in vivo induces a strain‐related modelling response uncomplicated by trauma or periosteal pressure. Calcif Tissue Int. 1994;54:241. [DOI] [PubMed] [Google Scholar]
- 33. Lee KC, Maxwell A, Lanyon LE. Validation of a technique for studying functional adaptation of the mouse ulna in response to mechanical loading. Bone. 2002;31(3):407‐412. [DOI] [PubMed] [Google Scholar]
- 34. Begonia M, Dallas M, Johnson LM, et al. Comparison of strain measurement in the mouse forearm using subject specific finite element models, strain gaging and digital image correlation. Biomech Model Mechanobiol. 2017;16(4):1243‐1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li J, Byrne ME, Chang E, et al. 1alpha,25‐Dihydroxyvitamin D hydroxylase in adipocytes. J Steroid Biochem Mol Biol. 2008;112(1‐3):122‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nimitphong H, Holick MF, Fried SK, et al. 25‐hydroxyvitamin D(3) and 1,25‐dihydroxyvitamin D(3) promote the differentiation of human subcutaneous preadipocytes. PLoS One. 2012;7(12):e52171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ching S, Kashinkunti S, Niehaus MD, et al. Mammary adipocytes bioactivate 25‐hydroxyvitamin D(3) and signal via vitamin D(3) receptor, modulating mammary epithelial cell growth. J Cell Biochem. 2011;112(11):3393‐3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


