Abstract
Background
Investigation of preschool asthma is important since not all children outgrow their illness during this age. Data are scarce on the role of rhinovirus (RV) infections in this patient group.
Objectives
To investigate the role of RV infections in preschool asthma: (i) susceptibility factors, (ii) clinical course, and (iii) medium‐term outcome.
Methods
A total of 130 asthmatic children aged 4‐6 years from the multinational PreDicta cohort were prospectively followed for a 12‐month period. Allergy tests and a standard health questionnaire were carried out at study entry. Respiratory virus presence in nasopharyngeal washes was studied at illness visits and at 3 scheduled visits.
Results
At study entry, mean age of the children was 5.3 years. Of 571 visits, 54% were positive for any virus and 39% for RV. Patient characteristics were only assessed with RV infection due to low number of other viruses. The use of supplementary vitamin D was inversely associated with RV infection (P < .05). RV infection was associated with more severe course of acute illness in terms of more severe nighttime coughing, more sleep disturbances, and more days with runny nose (all P < .05). RV infection was also associated with more severe disease course during the 12‐month follow‐up in terms of more nights with awakenings and more days of exercise‐related symptoms (both P < .05).
Conclusions
Vitamin D supplementation may have an anti‐rhinovirus effect. Both short‐ and medium‐term outcomes suggest RV infection to be an important clinical marker of instable preschool asthma.
Keywords: asthma, child, preschool, rhinovirus, vitamin D
This study investigated the role of rhinovirus (RV) infections in preschool asthma in 130 asthmatic children from PreDicta cohort over 1‐year follow‐up. During all visits, RV was the most commonly detected virus. Vitamin D supplementation was associated with decreased number of rhinovirus infections in preschool children with asthma. Detection of RV was associated with more severe course of acute illness (more severe nighttime coughing, more sleep disturbances, and more days with runny nose) and more compromised medium‐term prognosis (more nights with awakenings and more days of exercise‐related symptoms).

Abbreviations
- ICAM‐1
intercellular adhesion molecule 1
- IL
interleukin
- IκBα
inhibitor of NF‐κB
- NF‐κB
nuclear factor kappa‐light‐chain‐enhancer of activated B cells
- PCR
polymerase chain reaction
- RV
rhinovirus
1. INTRODUCTION
Asthma is the most common chronic disease among children in industrialized countries. In Europe, the prevalence of childhood asthma has increased during last decades; in 2017, the prevalence at the age of 4 years varied from 2% to 13% depending on country. 1 Thus, asthma creates a major burden on public health, with the mean cost per patient per year estimated to be 1700 € in Europe considering all asthmatics. 2
Respiratory viral infections are the almost exclusive trigger of wheezing episodes in early life, a condition affecting approximately 30% of children. 3 Rhinovirus (RV) is the most commonly identified culprit, and interestingly, RV‐induced wheezing has been shown to be a strong predictor of recurrent wheezing and a major risk factor for school‐age asthma (odds ratios up to 20‐45 depending on concomitant asthma risk allele and/or aeroallergy). 4 , 5 , 6 Other main risk factors of asthma include atopic dermatitis, family history of asthma, aeroallergen sensitization, exposure to smoke, and male gender. 7 Among children with asthma, RV infections are also the main trigger of acute exacerbations. 7 , 8 However, data are scarce on the role of RV infections in preschool age children with asthma. 9 , 10 The investigation of preschool asthma is important since many children outgrow their illness during this age while in others the disease persists.
RV is a potentially valuable clinical marker of asthma persistence/morbidity in preschool age children with recurrent wheezing/asthma. Therefore, our goal was to examine factors associated with susceptibility to virus infections as well as clinical aspects of such infections in asthmatic preschoolers, with a special focus on RV. We had three specific aims: first, to identify characteristics of children with preschool asthma associated with (susceptibility to) symptomatic or asymptomatic RV infections; second, to assess whether RV infections are associated with more severe clinical course of acute respiratory illness in comparison to other viruses or where no virus was identified, and third, to evaluate whether susceptibility to RV infections is associated with a more compromised 12‐month asthma course. We hypothesized that there are likely to be factors that increase susceptibility for RV infections and, further, that RV infections may be associated with more severe short‐ and medium‐term clinical course of preschool asthma.
2. METHODS
2.1. Subjects
This study was part of the PreDicta cohort (postinfectious immune reprogramming and its association with persistence and chronicity of respiratory allergic diseases). Recruitment was carried out in five study centers in Europe: in Athens, Greece, Turku, Finland, Lodz, Poland, Ghent, Belgium, Erlangen, and Germany. Characteristics of the subjects, inclusion and exclusion criteria, and study design have been described in detail before. 11 In brief, main inclusion criteria consisted of a written informed consent from the child's parents/guardians, age 4‐6 years, gestational age of 36 weeks or above and a doctor‐diagnosed asthma within the last 2 years, mild to moderate persistence's severity according to GINA. 12 Main exclusion criteria were severe or brittle asthma, children receiving immunotherapy, other chronic nonatopic disease. The study was commenced only after obtaining written informed consent from the parents or a guardian. The study protocol was approved by the Ethics Committee of all participating hospitals.
2.2. Study protocol
At study entry, a guardian filled out a standardized questionnaire on symptoms of asthma, rhinitis, eczema, infections, immunizations, environmental factors, and family history in the preceding 12‐month period. In order to be included at baseline, children had to be asymptomatic for at least 4‐6 weeks. Baseline blood tests and nasopharyngeal swab samples were obtained (see below). Follow‐up visits were arranged at 6‐month intervals for 2 years, where guardians were asked to fill out a questionnaire on symptoms of asthma/rhinitis activity (Figure S1). Respiratory symptoms and medications were also recorded in a daily symptom diary.
In addition to the scheduled visits, guardians were instructed to contact the study center if the child developed signs of a respiratory tract infection, exacerbation of asthma, a symptom score according to the diary cards of 4 or over, or a decrease in forced expiratory volume in one second >15% or in peak expiratory flow >30%. An additional visit to the clinic was then arranged, for pathogen identification.
At each visit, nasopharyngeal sample was collected with the use of a nasal applicator swab, which has a tip with flocked soft nylon fiber (ESwab 482CE; Copan Italia). Immediately after collection, the specimen was transported to the laboratory and stored at −80°C for later analysis.
2.3. Definitions
Childhood asthma refers to repeated attacks of airway obstruction and intermittent symptoms of increased airway responsiveness to triggering factors, such as exercise, allergen exposure, and viral infection. 12 , 13 “Wheezing” refers to expiratory breathing difficulty as a result of airway obstruction with a high‐pitched bilateral sound during expiration. The presence of allergic sensitization was defined by having one or more positive values for allergen‐specific IgE (>0.35 kU/L, Phadiatop Combi; Phadia, Uppsala, Sweden). Skin prick tests were performed optionally for additional atopy determination using 7 common allergens: normal saline, histamine (10 mg/mL), dermatophagoides mix, cockroach, cat, alternaria, and grass mix in all centers. In addition, birch and Ambrosia were used in Ghent, Lodz, Erlangen and Turku and Parietaria and olive in Athens. Virus infection refers to a positive virus detection using PCR, irrespective of respiratory symptoms.
2.4. Outcomes
Aim 1: The first study question investigated whether certain host characteristics at baseline were associated with the presence of RV vs. non‐RV. RV infection was defined as a positive RT‐PCR test of nasopharyngeal swab sample whether symptomatic or not during the 24‐month follow‐up period. RV group may include other viruses. Non‐RV group includes other virus and virus‐negative findings.
Aim 2: The second study question investigated whether the detection of RV vs. non‐RV in an acute respiratory illness was associated with more severe disease course. The symptom data were based on the diary records.
Aim 3: The third study question investigated whether susceptibility RV vs. non‐RV infection as defined in aim 1 was associated with more severe medium‐term (12‐month) prognosis, based on all clinical visits and diary records.
2.5. Laboratory methods
Virus transport mediums (ESwab 482CE; Copan Diagnostics, Murrieta, CA, USA) were transported from study centers on dry ice and analyzed for respiratory viruses at the Department of Virology, University of Turku. Entero‐, rhino‐ (species A, B, and C), and respiratory syncytial viruses were detected using an in‐house RT‐PCR test. 14 The first one hundred Finnish samples were also tested for adenovirus, human metapneumovirus, coronavirus 229E/NL63, parainfluenza viruses 1, 2, or 3, influenza viruses A or B, respiratory syncytial virus A or B, rhinovirus A/B, and coronavirus OC43/HKU1 by a commercial multiplex PCR test (Seeplex RV12 ACE Detection kit). Thereafter, the test was replaced by a new test version of the same manufacturer and all remaining samples were assessed by Anyplex RV16 Detection kit (Seegene, Soul), which identifies adenovirus, influenza A and B, parainfluenza viruses types 1‐4, rhinovirus A, B, and C, respiratory syncytial virus A and B, human bocavirus 1‐4, metapneumovirus, coronavirus 229E, NL63 and OC43, and enterovirus.
2.6. Statistics
The association between categorical baseline characteristics and susceptibility to rhinovirus infections was analyzed using chi‐squared test or Fisher's exact test and continuous baseline characteristics using Mann‐Whitney U test. Analyses were extended to group baseline characteristics as number of sensitizations, allergic diseases ever (atopic eczema, allergic rhinitis diagnosis, adverse reaction to food, bee, wasp or latex, and/or skin prick test positive for aeroallergens at study entry); parental allergic illnesses ever (eczema, allergic rhinitis, asthma and/or adverse reaction to food); moderate‐to‐severe asthma (asthma severity at least moderate persistent, asthma treatment during last 3 months, peak expiratory flow <80%), exposure to any air impurity factor (animal exposure on a weekly basis, parental smoking, trucks through street at least frequently though the day, mold spots, and/or window condensation at home), and exposures to animal and other air impurity factors separately. The associations between RV infection and symptoms of asthma were examined with Mann‐Whitney U test. The associations between continuous parameters describing activity of asthma and RV were analyzed using Mann‐Whitney U test, because the continuous variables were not normally distributed. A two‐sided P < .05 was considered statistically significant, and no adjustment was made for multiplicity. We used SAS/STAT(r) version 9.4 of the SAS System for Windows (SAS Institute Inc, Cary, NC, USA).
3. RESULTS
3.1. Study cohort
The enrollment of the study subjects and data sets for each study aim are shown in Figure 1. Originally, 167 children were recruited into the case group (diagnosed asthma) and 75 children into the control group in 5 study centers. Over 1‐year period, one patient dropped out after the baseline visit, 70 patients were not sampled for viruses from follow‐up visits, while 41 patients had data from only 1 follow‐up visit. A total of 131 patients, one of which in control group, had virus data from at least 2 follow‐up visits. Finally, we have analyzed 130 patients for whom virus data from at least 2 follow‐up visits were available (Turku = 42, Lodz n = 39, Athens n = 26, Ghent n = 12, Erlangen n = 11).
Figure 1.
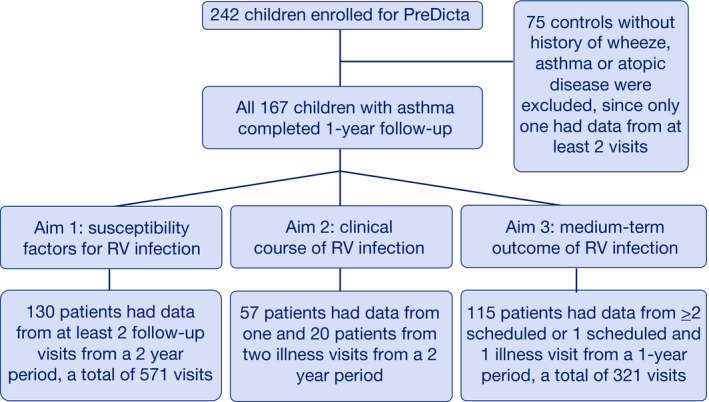
Study flowchart
3.2. Patient characteristics
Baseline characteristics of 130 study children who had virus data available from at least two scheduled visits are shown in Table 1, and Table S1. At study entry, 62% of them were boys, 55% had aeroallergen sensitization, 38% had active atopic eczema, and 38% had vitamin D supplementation. The severity of asthma was mainly mild persistent (59%) or intermittent (25%), and 83% of subjects had peak expiratory flow in normal range. Inhaled corticosteroid was the most common asthma control medication (83%), 34% of parents smoked and 42% had indoor pets. Of all 571 visits (scheduled and illness visits), viruses were detected in 307 (54%). RV was the most common finding (39%), whereas each other virus was found in <5% of visits. RV was detected significantly more often at illness compared to scheduled visits (58% vs 34%, P < .0001). Virus coinfections were found in 6.7% (38/571) of all visits (Table S2). No difference in their prevalence was found between RV‐positive (16/222, 7.2%) vs. RV‐negative groups (22/349, 6.3%) (P = .67).
Table 1.
Baseline characteristics
| Characteristics | N = 130 |
|---|---|
| Age, mean (SD) | 5.3 (0.7%) |
| Male | 80 (62%) |
| Allergic diseases ever | |
| Atopic eczema | 74 (57%) |
| Symptoms within past 12 months | 50 (38%) |
| Allergic rhinitis diagnosis | 91 (70%) |
| Symptoms within past 12 months | 109 (84%) |
| Adverse reaction to food | 48 (37%) |
| Adverse reaction to bee or wasp | 20 (15%) |
| Adverse reaction to latex | 1 (1%) |
| Asthma severity | |
| Intermittent | 32 (25%) |
| Mild persistent | 77 (59%) |
| Moderate persistent | 21 (16%) |
| Severe persistent | 0 (0%) |
| Asthma treatment during last 3 months | |
| Inhaled corticosteroid | 108 (83%) |
| Leukotriene receptor antagonist | 55 (42%) |
| Long‐acting beta2‐agonist | 20 (51%) |
| Status at study entry | |
| Skin prick test positive for aeroallergens | 72 (55%) |
| Peak expiratory flow | |
| >80% | 101/121 (83%) |
| 60%‐80% | 16/121 (13%) |
| <60% | 4/121 (3%) |
| Parental smoking | 44 (34%) |
| Parental allergic illnesses ever | |
| Eczema | 47 (36%) |
| Allergic rhinitis | 81 (62%) |
| Asthma | 51 (39%) |
| Adverse reaction to food | 34 (26%) |
| Animal exposure on a weekly basis | |
| Indoor pets | 54 (42%) |
| Cat | 24/77 (31%) |
| Dog | 30/76 (39%) |
| Farm animal | 4/76 (5%) |
| Other | 17/76 (22%) |
| Breastfed | 110 (85%) |
| Vitamin D supplementation | 45/119 (38%) |
| In milk | 62/112 (55%) |
| In cereal | 40/103 (39%) |
| In yogurt | 48/112 (43%) |
Data are shown as number of patients (%) unless otherwise expressed.
See more patient characteristics in Table S1.
3.3. Susceptibility to rhinovirus infections
Patient characteristics at baseline were only compared with RV (n = 109) vs. non‐RV (n = 21) infection due to low number of other virus findings. The use of vitamin D supplementation was inversely associated with RV infection at any visit (scheduled and illness visits) (proportion of children with supplementation, RV+ vs RV‐ group, 76% vs 89%, P = .049, Table S3). The association persisted after adjusting to study center, P = .0496). Use of vitamin D‐fortified food items (milk, cereal, or yoghurt) was not associated with RV detection (P > .1, Table S2). We further analyzed the association between vitamin D supplementation at study entry and symptomatic rhinovirus illnesses but no association was found: positive RV findings in children without vit‐D vs with vit‐D, 69.6% (32/46) vs 70.0% (14/20), P > .9. No other associations were found (Table S3) even if baseline characteristics were grouped as number of sensitizations, atopic disease ever, parental allergic illnesses ever moderate‐to‐severe asthma, or any pollutant exposure (all P > .1, data not shown).
3.4. Clinical characteristics of virus infections
Clinical characteristics of acute events were studied by investigating the connection between the virus findings during the course of an acute respiratory illness and symptom severity. Of 130 asthmatic children, 57 children had virus information available from illness visit 1 and 20 children from illness visit 2 (Table S4). RV infection was associated with increased cough during the night (P = .029) and sleep disturbances (P = .015), but less ophthalmic symptoms (P = .041) at illness visit 1 and with increased runny nose (P = .008) at illness visit 2 (Figure 2, Online Table E3).
Figure 2.
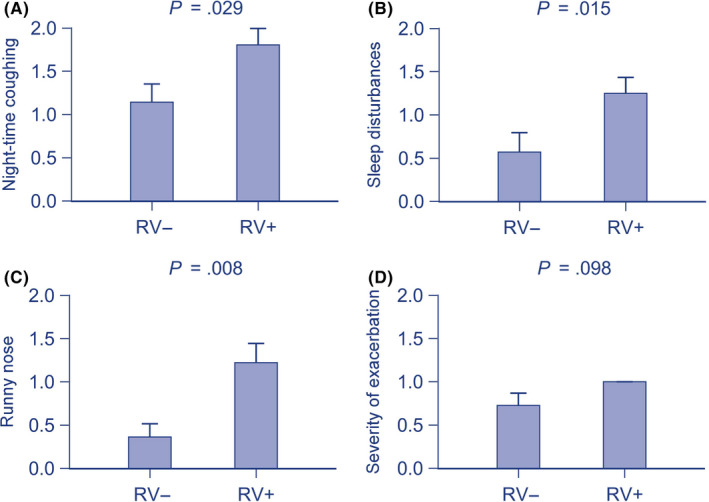
The association between rhinovirus detection and respiratory symptoms at illness visits. Significant and positive associations were found with (A) nighttime coughing, (B) sleep disturbances, (C) runny nose, and a tendency with (D) the severity of exacerbation. Data are shown as mean (standard error of mean) for better visualization and are analyzed using Mann‐Whitney U test
3.5. Virus findings and asthma activity
The connection between RV infections at any time‐point (scheduled or illness visits) and asthma activity was analyzed within 1‐year follow‐up (Table S5). It was found that RV infection was associated with increased activity of asthma in terms of more nights with awakenings (P = .026) and more days with exercise‐related symptoms (P = .041, Figure 3). Moreover, RV infection at baseline was associated with increased days of rhinitis within the 12‐month follow‐up (50% vs 25% had rhinitis >4 days per week within 6 months, P = .024).
Figure 3.
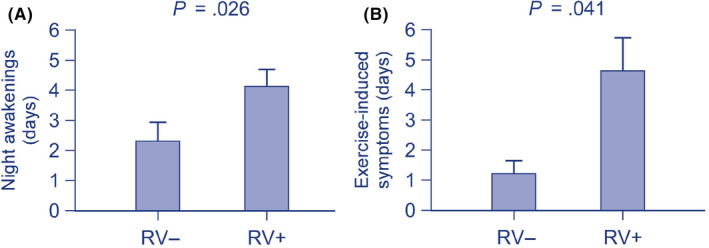
The association between rhinovirus detection and activity of asthma within 12 months. Significant and positive associations were found with (A) night awakenings and (B) exercise‐induced symptoms. Data are shown as mean (standard error of mean) for better visualization and are analyzed using Mann‐Whitney U test
4. DISCUSSION
This study had three important findings in children with preschool asthma: First, of many personal (age, sex, atopic characteristics, asthma severity, physical activity, breast feeding, and vitamin D supplementation), family (atopic characteristics and education level), and environmental (pet ownership, farm animal exposure, and air pollutants) factors, only vitamin D supplementation was associated with decreased susceptibility to RV infections. Interestingly, atopic sensitization was not. Second, RV triggered acute respiratory illness was associated with more severe course of the illness; and third, susceptibility to RV infection was associated with increased asthma activity during the following 12‐month period.
Our findings of potential protective role of vitamin D against respiratory infections are supported by many clinical studies in children and in vitro studies, 15 , 16 , 17 , 18 although contradictory reports also exist in adults. 19 , 20 Low levels of vitamin D or lack of supplementation have been associated with increased risk of respiratory infections as well as coinfections in children. 15 , 16 Specific association to RV infections, however, has received less attention, and results have been inconclusive. 21 , 22 High vitamin D levels may have partly explained the lack of meaningful correlations in some cases. 21 Our findings support the protective role of vitamin D against RV infections which is particularly important in asthmatic children since RV is the major trigger of exacerbations 23 and vitamin D levels were inversely related to measures of asthma severity. 24 Collectively, available data strongly support antiviral effects of vitamin D3 supplementation (recommended 7.5‐25 µg/day in many European countries but not necessarily during summer times) and its usage in young asthmatic patients. In addition, many food items are fortified with vitamin D. Our data support the view that vitamin D supplementation, and not vitamin D fortified food items, is the best source.
Asthma‐prone children appear to be more susceptible to RV infections than healthy children. 7 Our findings in preschool children support these findings by showing that RV detection during acute illness was positively associated with measures of illness severity. However, the exact mechanisms are not precisely known but most plausible explanation is that asthmatic children have weaker antiviral defense than healthy children. This is likely to be due to predisposition (genetically low interferon α/β/γ/λ levels and low interleukin [IL]‐10 levels thereby weakened antiviral defense and anti‐inflammatory control), T helper2 type risk factors of asthma (allergen exposure and high‐affinity IgE receptor cross‐linking have been shown to impair type I and III interferon production and increased production of T helper2 cytokines IL‐4, IL‐5, and IL‐13 in airway secretions counteract T helper1 responses), and interactions between the underlying inflammatory pathways (RV infections and allergens enhance, for example, the airway epithelial cell production of IL‐25 and IL‐33, which promote T helper2 airway inflammation; T helper2 polarized immune responses may increase the expression of ICAM‐1, and disrupted airway epithelium favors RV replication). 7 Delayed maturation of antiviral responses may also contribute at this age range. 25 Thus, genetically weak antiviral mechanisms may be further attenuated by airway inflammation and, in our case, also by low vitamin D status. Moreover, antiviral mechanisms are likely to be, at least partly, RV‐specific since genetic variants at the 17q21 locus and in CDHR3 allele may markedly increase the risk of severe RV infections although not investigated in our study. 5 , 6
Negative findings in many investigated personal, family, and environmental factors further support the compromised virus defense as the key element in the susceptibility to RV infections. More severe RV infections are known to be closely associated with atopic asthma, 4 , 26 but surprisingly atopic characteristics (investigated separately and as a group) were not associated with susceptibility to RV infection even though a half of our children were sensitized to aeroallergens. Also, air pollutants, especially exposure to allergens, but also traffic pollutants such as NO2, increase risk of virus‐induced exacerbation of asthma. 27 , 28 However, we did not find association between exposure to these pollutants (separately or as a group) and susceptibility to RV infections. The probable explanation for these null findings is that our children were using regular asthma controller medication. Parental smoking does not appear to have strong influence on the susceptibility to RV infections. 26 , 29 As expected, breast feeding during infancy was not associated with virus susceptibility at preschool age.
Our findings also indicate that susceptibility to RV infections, whether symptomatic or asymptomatic, serves as an important clinical marker for more compromised prognosis of asthma. It is known previously that RV‐induced wheezing is an important risk factor for subsequent wheezing and later asthma among young wheezing children. 7 Already at the time of first severe wheezing episode, its presence can predict school‐age atopic asthma (odds ratio 5.0 in normal population). 26 This risk value of RV detection in wheezing children has been extended to the first three years of life. 4 It has been speculated that this risk may be limited to early wheezing only. Our results contrast these speculations and extend the prognostic value of RV infection in preschool to school‐age asthma. RV detection among wheezing children appears to be particularly important since it also effectively predicts steroid‐responders. 30 , 31 It should be noted that increased RV expression in asthmatics may suppress secondary virus infections, thus further promoting the role of RV in asthmatic subjects. 32
The PreDicta cohort has many strengths including careful characterization of study subjects, serial virus detection as well as prospective, multi‐center, and multinational design. In‐house RT‐PCR effectively covered all RV species. 33 There are also weaknesses. Sample size only allowed RV‐specific analyses since the number of other virus detections was low. However, RV was our main focus. A longer than 12‐month follow‐up time may have revealed more risk factors. Days with exercise symptoms were determined subjectively by parents. Also, sample size for some risk factors was likely to be low, but analyses were repeated by uniting them into larger groups, for example, by uniting any atopic characteristics under one variable. Correction for multiple comparisons was not done due to explorative nature of the analysis. Due to large study setting and multidisciplinary approach, several predefined subanalyses to assess causality and interrelations have not yet been completed.
In conclusion, vitamin D supplementation may have beneficial role in antiviral defense in preschool asthmatic children when used with recommended doses. However, an intervention study is warranted. Our findings also highlight the significance of RV infection as a meaningful clinical marker for short‐ and medium‐term outcomes in preschool asthma. This information adds to the potential of direct RV detection, as well as RV antibody detection, 34 to be used as an approach for personalized medication. 35
CONFLICTS OF INTEREST
The authors declare no conflict of interest in connection with this paper.
Supporting information
Supplementary Material
Jartti T, Liimatainen U, Xepapadaki P, et al. Clinical correlates of rhinovirus infection in preschool asthma. Allergy.2021;76:247–254. 10.1111/all.14479
Funding information
Supported by European Commission's Seventh Framework program (260895 PREDICTA) and Dr Jartti's group was supported by Pediatric Research Foundation, Paulo Foundation and Sigrid Juselius Foundation, all in Helsinki, Finland.
REFERENCES
- 1. Uphoff EP, Bird PK, Antó JM, et al. Variations in the prevalence of childhood asthma and wheeze in MeDALL cohorts in Europe. ERJ Open Res. 2017;3(3):00150‐2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nunes C, Pereira AM, Morais‐Almeida M. Asthma costs and social impact. Asthma Res Pract. 2017;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jartti T, Smits HH, Bønnelykke K, et al.; EAACI Task Force on Clinical Practice Recommendations on Preschool Wheeze . Bronchiolitis needs a revisit: distinguishing between virus entities and their treatments. Allergy 2019;74(1):40‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rubner FJ, Jackson DJ, Evans MD, et al. Early life rhinovirus wheezing, allergic sensitization, and asthma risk at adolescence. J Allergy Clin Immunol. 2017;139(2):501‐507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Çalışkan M, Bochkov YA, Kreiner‐Møller E, et al. Rhinovirus wheezing illness and genetic risk of childhood‐onset asthma. N Engl J Med. 2013;368(15):1398‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bønnelykke K, Coleman AT, Evans MD, et al. Cadherin‐related family member 3 genetics and rhinovirus C respiratory illnesses. Am J Respir Crit Care Med. 2018;197(5):589‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jartti T, Gern JE. Role of viral infections in the development and exacerbation of asthma in children. J Allergy Clin Immunol. 2017;140(4):895‐906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guibas GV, Tsolia M, Christodoulou I, Stripeli F, Sakkou Z, Papadopoulos NG. Distinction between rhinovirus‐induced acute asthma and asthma‐augmented influenza infection. Clin Exp Allergy. 2018;48(5):536‐543. [DOI] [PubMed] [Google Scholar]
- 9. Konstantinou GN, Papadopoulos NG, Manousakis E, Xepapadaki P. Virus‐induced asthma/wheeze in preschool children: longitudinal assessment of airflow limitation using impulse oscillometry. J Clin Med. 2019;8(9):1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konstantinou GN, Xepapadaki P, Manousakis E, et al. Assessment of airflow limitation, airway inflammation, and symptoms during virus‐induced wheezing episodes in 4‐ to 6‐year‐old children. J Allergy Clin Immunol. 2013;131(1):87‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xepapadaki P, Bachert C, Finotto S, et al. Contribution of repeated infections in asthma persistence from preschool to school age: design and characteristics of the PreDicta cohort. Pediatr Allergy Immunol 2018;29(4):383‐393. [DOI] [PubMed] [Google Scholar]
- 12. GINA 2019 report: Global Strategy for Asthma Management and Prevention. https://ginasthma.org/gina‐reports/
- 13. Papadopoulos NG, Arakawa H, Carlsen K‐H, et al. International consensus on (ICON) pediatric asthma. Allergy 2012;67(8):976‐997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Peltola V, Waris M, Österback R, Susi P, Ruuskanen O, Hyypiä T. Rhinovirus transmission within families with children: incidence of symptomatic and asymptomatic infections. J Infect Dis. 2008;197(3):382‐389. [DOI] [PubMed] [Google Scholar]
- 15. Jartti T, Ruuskanen O, Mansbach JM, Vuorinen T, Camargo CA Jr. Low serum 25‐hydroxyvitamin D levels are associated with increased risk of viral coinfections in wheezing children. J Allergy Clin Immunol. 2010;126(5):1074‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Camargo CA, Ganmaa D, Frazier AL, et al. Randomized trial of vitamin D supplementation and risk of acute respiratory infection in Mongolia. Pediatrics 2012;130(3):e561‐e567. [DOI] [PubMed] [Google Scholar]
- 17. Telcian AG, Zdrenghea MT, Edwards MR, et al. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93‐101. [DOI] [PubMed] [Google Scholar]
- 18. Greiller CL, Suri R, Jolliffe DA, et al. Vitamin D attenuates rhinovirus‐induced expression of intercellular adhesion molecule‐1 (ICAM‐1) and platelet‐activating factor receptor (PAFR) in respiratory epithelial cells. J Steroid Biochem Mol Biol. 2019;187:152‐159. [DOI] [PubMed] [Google Scholar]
- 19. Castro M, King TS, Kunselman SJ, et al. Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 2014;311(20):2083‐2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denlinger LC, King TS, Cardet JC, et al.; AsthmaNet Investigators . Vitamin D supplementation and the risk of colds in patients with asthma. Am J Respir Crit Care Med. 2016;193(6):634‐641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koistinen A, Turunen R, Vuorinen T, et al. Vitamin D, virus etiology, and atopy in first‐time wheezing children in Finland. Pediatr Allergy Immunol. 2014;25(8):834‐837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tovey ER, Stelzer‐Braid S, Toelle BG, et al. Rhinoviruses significantly affect day‐to‐day respiratory symptoms of children with asthma. J Allergy Clin Immunol. 2015;135(3):663‐669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jolliffe DA, Greenberg L, Hooper RL, et al. Vitamin D supplementation to prevent asthma exacerbations: a systematic review and meta‐analysis of individual participant data. Lancet Respir Med. 2017;5(11):881‐890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brehm JM, Celedón JC, Soto‐Quiros ME, et al. Serum vitamin D levels and markers of severity of childhood asthma in Costa Rica. Am J Respir Crit Care Med. 2009;179(9):765‐771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Georgountzou A, Papadopoulos NG. Postnatal innate immune development: from birth to adulthood. Front Immunol. 2017;8:957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lukkarinen M, Koistinen A, Turunen R, Lehtinen P, Vuorinen T, Jartti T. Rhinovirus‐induced first wheezing episode predicts atopic but not nonatopic asthma at school age. J Allergy Clin Immunol. 2017;140(4):988‐995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chauhan AJ, Inskip HM, Linaker CH, et al. Personal exposure to nitrogen dioxide (NO2) and the severity of virus‐induced asthma in children. Lancet 2003;361(9373):1939‐1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Murray CS, Poletti G, Kebadze T, et al. Study of modifiable risk factors for asthma exacerbations: virus infection and allergen exposure increase the risk of asthma hospital admissions in children. Thorax 2006;61(5):376‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Turunen R, Jartti T, Bochkov YA, Gern JE, Vuorinen T. Rhinovirus species and clinical characteristics in the first wheezing episode in children. J Med Virol. 2016;88(12):2059‐2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lehtinen P, Ruohola A, Vanto T, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after a first wheezing episode associated with rhinovirus infection or eczema. J Allergy Clin Immunol. 2007;119(3):570‐575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jartti T, Nieminen R, Vuorinen T, et al. Short‐ and long‐term efficacy of prednisolone for first acute rhinovirus‐induced wheezing episode. J Allergy Clin Immunol. 2015;135(3):691‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pinky L, Dobrovolny HM. Coinfections of the respiratory tract: viral competition for resources. PLoS One 2016;11(5):e0155589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jartti T, Palomares O, Waris M, et al. Distinct regulation of tonsillar immune response in virus infection. Allergy 2014;69(5):658‐667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Megremis S, Niespodziana K, Cabauatan C, et al. Rhinovirus species‐specific antibodies differentially reflect clinical outcomes in health and asthma. Am J Respir Crit Care Med. 2018;198(12):1490‐1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Papadopoulos NG, Čustović A, Cabana MD, et al. Pediatric asthma: an unmet need for more effective, focused treatments. Pediatr Allergy Immunol. 2019;30(1):7‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


