Abstract
Understanding the links between sleep and brain development is important, as rapid eye movement (REM) sleep and non‐REM (NREM) sleep seem to contribute to different aspects of brain maturation. If children have sleep problems, REM sleep and NREM sleep are likely to have different consequences for their developing brain, depending on their age. We highlight important discoveries from human and animal research on the role sleep plays in brain development. A hypothetical model is presented to explain the dynamic relationship of REM sleep and NREM sleep with different processes of brain maturation, with implications for current neonatal care and future research.
Keywords: brain development, child maturation, neonatal care, rapid eye movement, sleep ontogeny
Abbreviations
- EEG
electroencephalogram
- GA
gestational age
- NREM
non–rapid eye movement
- REM
rapid eye movement
Key notes.
Sleep is important for brain development, and rapid eye movement (REM) sleep and non‐REM (NREM) sleep have distinct functions in this process.
This review highlights important discoveries from human and animal research on the role sleep plays in brain development.
A hypothetical model is presented to explain the dynamic relationship of REM sleep and NREM sleep with different processes of brain maturation, with implications for current neonatal care and future research.
1. INTRODUCTION
Sleep plays an essential role in health and cognitive performance. Known functions of sleep include recuperating from oxidative stress or toxins accumulated during wakefulness, 1 replenishing energy stores 2 and synthesis of macromolecules, such as growth hormones. 3 They also include executive functioning, 4 memory consolidation, 5 immuno‐protection 6 and repair of neural damage. 7 New functions of sleep are still being discovered, such as its role in driving brain development. 8 The amount of sleep peaks during foetal and neonatal life, and this is also a time of rapid brain development. 9 Research has also shown that sleep quantity and brain development both gradually decrease across a person's lifespan. 9 Several studies have shown that sleep deprivation during early development has a detrimental impact on brain maturation, 10 , 11 and this suggests that sleep is important for brain development. However, there is still a considerable lack of clarity about the nature of the relationship between sleep and brain development and how this relates to the different states of sleep.
This review looks at the current understanding of the role of sleep in brain development across the different development stages and focuses on the distinct functions of rapid eye movement (REM) sleep and non‐REM (NREM) sleep. There is a scarcity of experimental studies on the relationships between sleep deprivation and brain development in human newborns or young children for ethical reasons. Consequently, most studies have focused on animals or older children and adults and it is important to take care when translating these results to the developing human brain. This narrative review provides a concise overview of the human brain and sleep development, respectively. It also explores the role of sleep in brain development.
2. HUMAN BRAIN DEVELOPMENT
Human brain development starts early after conception. By the end of the embryonic period, 8 weeks after conception, the rudimentary structures of the brain have been established. There are three major processes during the prenatal period: neuron proliferation, migration and differentiation. 12 Newly formed neurons establish connections after migration and early neural networks form. Apoptosis contributes to the formation of neuronal networks by removing extra neurons and synapses, thereby enabling efficient and synchronised synaptic transmission. 13 By the end of the foetal period, all major fibre pathways have been formed and the process of myelination starts. 14 , 15 After birth, brain development continues for an extended period, up to early adult age. In the first postnatal years, the level of connectivity in the developing brain exceeds that of the adult brain. 16 During early development, many connections are gradually pruned back under the influence of the child's early experiences, which means that child play is an important driver of development. 17 From 3 to 4 years of age, the human brain increases 4‐fold in size and changes continue through childhood and adolescence. 18
This means that brain development can be roughly divided into two major periods and processes. The first period covers the prenatal and early postnatal years, where brain development focusses on the accumulation of neuronal connections. The second are the years from late infancy to adolescence, where brain development centres more around pruning of neuronal connections. Deviation from the carefully orchestrated process of brain development, for example because of a brain injury, can have a long‐lasting impact on motor, cognitive and behavioural functioning. 19 The major long‐term socio‐economic impact of abnormal brain development fuels the search for drivers of normal brain development in order to identify potential protective elements. It has been suggested that sleep plays a vital role in brain development.
3. HUMAN SLEEP ONTOGENY
Pioneering work by Roffwarg et al 9 more than 50 years ago showed that sleep duration and sleep states change as children develop. Today, much more is known about the maturation of sleep characteristics from the foetus to the adult. An overview of the wake and sleep state distribution across child development is shown in Figure 1. An overview of the sleep characteristics for each developmental stage is provided in Table 1.
FIGURE 1.
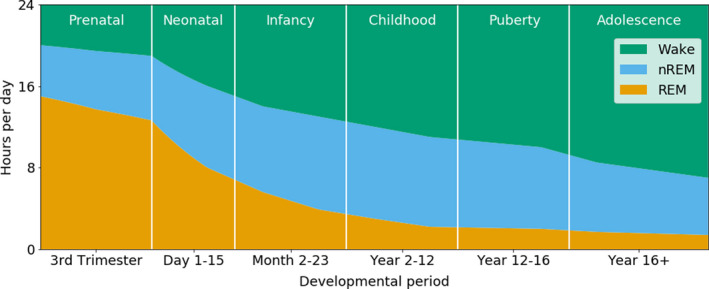
Distribution of human sleep states across development. The number of hours spent in wake (green), NREM sleep (blue) and REM sleep (orange) is shown for each developmental stage. REM sleep dominates in the prenatal and neonatal periods. From infancy onwards, NREM sleep becomes increasingly more present. NREM, non‐REM; REM, rapid eye movement. Based on Roffwarg et al 9 and Kaplan et al 20
TABLE 1.
Sleep characteristics during each stage of child development
| Developmental stage | Sleep state | Characteristic parameters |
|---|---|---|
| Overview (birth to 18 y of age) |
Parameters listed for each stage are characteristic of general sleep or specifically active sleep/REM or quiet sleep/NREM. The most important sleep parameters in preterm and neonatal stages are behaviour, physiology and brain activity. From infancy onwards, brain activity is the most important parameter for sleep classification. |
|
| Preterm and neonatal stage (birth to 1 mo) | General sleep |
Movement:
Note: A large percentage of preterm sleep is classified as indeterminate or transitional sleep, which is mixture of two or more sleep and wake states |
| Active sleep |
REMs:
EEG: Continuous (30‐31 wk of GA)
Continuous mixture of different frequencies (37 wk of GA) From 46 wk of GA:
|
|
| Quiet sleep |
Eyes: No eye movements (37 wk of GA) Chin electromyography:
Heart rate/respiratory rate: Regular (37 wk of GA) EEG:
|
|
| Infancy (2‐23 mo) | General sleep |
EEG: K‐complexes (5‐6 mo) |
| REM sleep |
EEG: Like adults, but slower and higher voltage Dominant frequency increases:
|
|
| NREM sleep |
Chin electromyography: Preserved EEG:
|
|
| Childhood (2‐12 y) | General sleep |
EEG:
|
| REM sleep |
EEG: Like adults, but slower and higher voltage
|
|
| NREM sleep |
Chin electromyography: Preserved EEG:
|
|
| Teenage years (13‐18 y) | General sleep |
EEG: K‐complexes repeat every 1‐3 s |
| REM sleep |
EEG: Adult‐like/mixed frequency activity with bursts of sawtooth waves |
|
| NREM sleep |
Chin electromyography: Preserved EEG:
|
Adapted from Grigg‐Damberger. 21
Abbreviations: EEG, electroencephalography; GA, gestational age; NREM, non‐REM; REM, rapid eye movement.
3.1. Sleep in foetuses and preterm infants
A foetus spends most of its time in the womb asleep. Prenatal sleep does not match typical adult patterns, as it is highly disorganised. Prenatal ultrasound studies have studied sleep behaviour in foetuses from as early as 21 weeks of gestation. 22 In contrast to the typical REM and NREM sleep states in older children and adults, a more subtle distinction between sleep states can be made for foetuses and infants born preterm, before 37 weeks of gestation, based on behavioural and physiological parameters (Table 1). Active sleep, which is a foetal and preterm neonatal version of REM sleep, is characterised by extensive facial and body movements and variations in heart rate and respiration. 23 Muscle atonia has not developed at this stage of development. Quiet sleep, which is the foetal and preterm neonatal equivalent of NREM sleep, shows a more regular heart rate and breathing pattern and hardly any body movements. 23 Sleep studies in infants born preterm show that active sleep occurs twice as much as quiet sleep during the preterm period, up to 40 weeks of gestation (Figure 1). 23 , 24 With increasing gestational age, the ratio between active sleep and quiet sleep drops to about 50/50, 9 , 25 and at the age of 6 months, infants spend about twice as much time in what is by then called NREM sleep, than REM sleep (Figure 1). 9 , 25 During the first year of life, infants rapidly increase both the amount of time they spend awake during the day and the length of the longest period of sustained sleep during the night. 24
Over time, patterns of active sleep and quiet sleep slowly mature and are replaced by REM sleep and NREM sleep, respectively, at around 3‐5 months of age. 26 Around that time, a cortical electroencephalogram (EEG) will start showing the first signs of adult REM and NREM sleep features (Table 1). By 5‐8 months, EEGs of NREM stages show clear signs of slow waves, with delta bands of 0.5‐4.0 Hz and sleep spindles of 7‐14 Hz. 26
3.2. Sleep from infancy to adolescence
Using polysomnography, Scholle et al 27 explored how sleep patterns developed from infancy onwards in 209 participants ranging from 1 to 18 years of age. The 2007 sleep state classification criteria of the American Academy of Sleep Medicine were applied. 28 The study included eight age groups: two toddler groups, aged 12‐23 months and 2.1‐3.6 years, respectively, a group of preschool‐age children, aged 4.1‐5.9 years, and five groups of school‐age children, collectively aged 6‐18 years and grouped according to the Tanner scale of pubertal development. 27 The authors showed that sleep patterns changed across childhood and adolescence (Figure 1). For example, the percentage of wakefulness after sleep onset and the percentage REM sleep decreased with maturation, while the percentage of NREM stage 2 sleep increased with age. Furthermore, sleep cycles in younger children were relatively short, while the sleep cycles in adolescents were longer, suggesting that the length of sleep cycles increased with maturation.
3.3. Sleep in adolescence
Adolescents have a shorter daily sleep time than younger children (Figure 1), with more variations in sleep duration and sleep‐wake rhythms, which are often influenced by external factors such as increased academic or social pressure. 29 By this age, sleep is very similar to adult sleep and adolescents go through REM sleep (consisting of 1 stage) and three stages of NREM sleep in a cyclic matter. The first few cycles of the sleep period are characterised by a high percentage of slow‐wave sleep, synchronised slow cortical oscillations of NREM stage 3 sleep. The later sleep period is characterised by a higher percentage of REM sleep (Figure 2). The adolescent period is characterised by sleep phase delays, in which adolescents go to bed late and get up early to go to school. 30 This has been reported to affect electrophysiological parameters. Research by Jenni et al 31 showed that, after sleep deprivation, the spectral power of the NREM sleep EEG was enhanced in the low‐frequency range and reduced in the sigma range. Furthermore, sleep deprivation resulted in a stronger increase in slow‐wave activity, but a slower build‐up of homeostatic sleep pressure in older adolescents, with a mean age of 14.2 years, than in younger adolescents with a mean age of 11.9 years. Insufficient sleep in adolescents has been related to worse performance at school, mood‐related disturbances, obesity and substance abuse. 29 In addition, sleep problems in childhood 32 and adolescence 33 might serve as a marker for a wide range of problems, such as anxiety, depression and attention problems.
FIGURE 2.
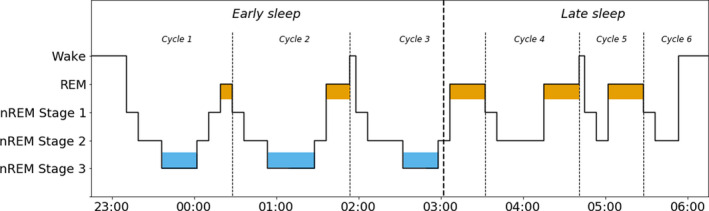
The adolescent sleep cycle. During a night's sleep, REM and NREM sleep states alternate in a cyclic fashion, occasionally interrupted by brief periods of near wakefulness or wakefulness. Early sleep is dominated by the three stages of NREM sleep, and in particular the slow‐wave sleep of NREM stage 3 (depicted in blue). Late sleep is characterised by a high incidence of REM sleep (orange). NREM, non‐REM; REM, rapid eye movement. Based on Diekelman and Born 5
4. THE ROLE OF SLEEP IN BRAIN DEVELOPMENT
Unlike the original statement by Roffwarg et al, 9 that REM sleep was the main sleep state that was associated with brain development, the current view is that both REM sleep and NREM sleep are important for optimal brain development, but have different functional contributions. 11 , 34
4.1. REM sleep and endogenous stimulation of early neuronal structure and circuitry
Within the field of sleep research, there is strong support for the view that REM sleep is associated with brain development. Studies in rats have shown that deprivation of REM sleep during early development delayed maturation of the visual cortex 35 and impaired maturation of the motor system. 10 These studies, and others, have contributed to the general understanding that REM sleep in early maturation, including both REM in early infancy and active sleep in the preterm and neonatal periods, provides the neural stimulation needed to develop and prepare the neural circuitry for later higher cognitive processing. Li et al 36 used a model of motor learning in 3‐week‐old mice and showed that the formation and maintenance of synapses were stimulated during REM sleep. REM sleep facilitated the formation of new spines in the mouse motor cortex during development and after a motor learning task, as well as strengthening of the synapses that had already formed and were necessary for performance improvement after motor learning. The implication that REM sleep stimulates synapse formation has been further demonstrated by identifying various key molecular factors of synaptogenesis that are upregulated during REM sleep. These are as follows: brain‐derived neurotrophic factor, 37 immediate early gene activity–regulated cytoskeleton‐associated protein 38 and protein transcription factor cyclic adenosine monophosphate response element‐binding protein. 39
On an electrophysiological level, REM sleep in early maturation is characterised by synchronised delta activity 21 and theta oscillations (Table 1). 40 In particular, theta oscillations have been associated with increased synapse density and functional maturation of neural circuits. 41 A major feature of this association with neural maturation are the twitches and jerks that characterise early REM sleep. These movements were initially discarded as random by‐products, but work in rats has shown that they are highly structured, as early as the neonatal period. 10 Twitching is produced by the red nucleus of the brainstem, and it provides sensory feedback that activates the cells in the sensorimotor network. 10 This activation during early REM sleep takes the form of synchronised neural oscillations that stimulate the sensorimotor cortices and distant structures, such as the brainstem and hippocampus. 42 The importance of twitches for neurodevelopment is emphasised by behavioural studies that have shown a correlation between decreased startles and twitches in human neonates and poor behavioural and neurological outcomes at a later age. 43 This suggests that twitches not only influence motor development, they also tweak the neural circuitry involved in sensory processing. Indeed, in addition to motor development, REM sleep also seemed to be involved in the maturation of the visual circuit in kittens. de Lima et al 44 found that during REM sleep, retinal ganglion cells and then cortical neurons of the lateral geniculate nucleus were activated, much like the response to visual input during wakefulness. Shaffery et al 45 suggested that REM sleep deprivation has a similar detrimental effect on the development of the visual sensory system to monocular or binocular deprivation, namely deficits in both visual acuity and high‐level visual perception.
To summarise, during early maturation REM sleep seems to provide the stimulation needed for preliminary development and the survival of sensorimotor neuronal networks. It does this by driving the generation of endogenous, intense and generalised neural activity across sensorimotor systems.
4.2. NREM sleep and how slow waves stimulate synaptic and cortical maturation
The link between NREM sleep and brain development has not been studied as extensively as the link with REM sleep. However, the research that has been carried out has suggested a functional association, especially with NREM stage 3 sleep and its hallmark characteristic: synchronous cortical oscillations, known as slow waves, with delta bands of 0.5‐2.0 Hz. 25 This slow‐wave activity affects virtually every cortical neuron, moving it into alternating states of prolonged firing and neuronal silencing. 46 Initial interest in this phenomenon can be attributed to the observation that during human brain development, none of the EEG frequency bands change as strongly as the slow‐wave activity band. 46 The amplitude of slow‐wave activity shows a U‐shaped distribution across development: initially increasing during childhood, peaking just before puberty and then decreasing in puberty and adolescence. A cross‐sectional study by Kurth et al 47 assessed the development of slow‐wave activity topography in relation to cortical maturation in participants from 2 to 20 years of age. They found that the development of the two processes paralleled each other, which suggests a functional association. Early childhood is marked by prominent slow‐wave activity in the posterior regions of the brain. This prominence shifts via the central derivations to the frontal cortex in adolescence, thereby matching the posterior‐to‐anterior time course seen in cortical maturation. 47 The synaptic homeostasis hypothesis proposes that synaptic downscaling occurs during NREM stage 3 sleep, which is needed to process new experiences learned during the day. 48 This return to baseline levels of neuronal activity, as a result of slow‐wave activity, has been linked to synaptic pruning. One of the strongest pieces of evidence for this functional association with synaptic pruning comes from human studies, in which slow‐wave activity in the cortical region increased during sleep after this region had been exposed to a learning task while the subject was awake. 49
Another characteristic of NREM sleep that appears to be linked to human brain development involves sleep spindles, which are sinusoidal waves that are paced from the thalamus at 11‐16 Hz. 50 This EEG feature is most prominent in NREM stage 2 sleep. It is also thought that it appears in NREM stage 3, but is masked by slow‐wave activity. 50 Characteristics of spindles have been linked to several brain development processes. Sleep spindle duration and amplitude have been related to thalamocortical projections, and spindle density has been associated with the activity of the reticular nucleus of the thalamus. 51 The relationship between spindles and regional brain development appears evident. However, it still seems unclear whether NREM spindles have a causal functional link with developing thalamocortical brain connectivity or whether the spindle characteristics are merely a reflection of thalamocortical development. 50 A final characteristic of NREM sleep is K‐complexes, which are transient waves with a sharp amplitude of 12‐14 Hz. These are mostly present in NREM stage 2 sleep. 52 It is thought that deepening of K‐complexes facilitates the transition from NREM stage 2 sleep to slow‐wave activity in NREM stage 3 sleep, suggesting a potential association between these complexes and slow‐wave activity. 52 However, they are two distinct functional features of NREM sleep. K‐complexes have been linked to memory processing in adults, 53 but a link with brain development has not been discovered.
To summarise, NREM sleep is hypothesised to contribute to brain development by optimising neuronal networks via mechanisms of synaptic downscaling and pruning.
4.3. Influence of the developmental period
There is an interesting pattern in the link between the stages of brain development and REM sleep and NREM sleep. REM sleep seems important for forming synapses, especially early in development. This is supported by human pharmacological studies in adults, for example one in which REM sleep deprivation, which was induced with the use of monoamine oxidase inhibitors, did not cause changes in mental or cognitive functioning. 8 Moreover, an experimental study showed no effect of REM sleep deprivation on synaptic plasticity in adolescent rodents—typically aged between the postnatal period of weaning, postnatal day 21, to adulthood, postnatal day 60. 54 These studies seem to suggest that REM sleep relates less to brain development in the later stages of maturation. To a similar extent, correlations between NREM sleep and brain functioning vary considerably in strength and even direction across development. 55 NREM sleep has been positively associated with brain maturation at later developmental stages, from childhood onwards. However, research on preterm infants has shown the opposite effect, which is that a higher percentage of quiet sleep, and therefore less active sleep, was related to worse motor and cognitive development at 6 months 56 and 18 months. 57 Taken together, these findings suggest that the respective roles of REM sleep and NREM sleep in brain development are influenced by a child's developmental period.
4.4. A combined theory of sleep states and brain development
An emerging concept regarding the role of sleep in brain development is that the two sleep states, REM and NREM, could be functionally linked to different processes of maturation in the central nervous system, with different roles at different ages. A neonate or infant only spends 10%‐30% of the time awake, 58 and they have little opportunity for high neuronal stimulation. The high percentage of sleep at this age is greatly devoted to REM sleep. 58 Roffwarg et al's initial theory of sleep ontogeny 9 suggests that REM sleep is the equivalent of wakefulness and that the need for REM sleep decreases once wakefulness is more established. Although the percentage of REM sleep does not decrease in parallel with the overall decrease in the percentage of sleep, Roffwarg et al's theory 9 does fit the principles of stimulus‐induced neuron development. The neuronal substrates of REM sleep facilitate stimulus‐induced neuronal activity, but in early development these stimuli are mostly generated endogenously and not mainly provided by external factors during the wake state. As a child matures, it spends more time awake, thus enabling high neuronal activity via interaction with the environment. Considering the high demands of environmental interaction on neuronal activity, and the fact that the brain must uphold energy homeostasis, being awake longer would provide a lot of stress to the brain. 46 The survival and function of the neurons are then increasingly more reliant on downgrading these activity levels. It has been suggested that this could take place during slow‐wave activity of NREM stage 3 sleep, when neurons return to a synchronised level of low electrical activity. 46
Combining Roffwarg et al's theory of sleep ontogeny 9 with the NREM theory of synaptic homeostasis could suggest that early REM sleep provides the endogenous neural stimulation that the child is not yet able to achieve exogenously during the very early stages of life. This functionally links REM sleep to the rudimentary processes of brain development, laying the groundwork for brain morphology and early neural circuitry. NREM sleep seems more functionally important for later brain maturation, by regulating synaptic homeostasis. It provides synaptic adjustment to the sensory inputs acquired from increased interaction with the outside world. Although more research is needed, it could explain why human neonates seem to compensate for sleep deprivation by increasing their time in NREM sleep and not in REM sleep. 59 This is because a prolonged period awake would require a counterbalance of downgraded synaptic activity during subsequent sleep, rather than a continuation of high neural activity.
5. DISCUSSION
Over the few past decades, sleep has emerged as an important contributor to normal brain development. This review highlights the importance of both REM sleep and NREM sleep in brain development, but demonstrates that they make different functional contributions. These contributions are likely to be influenced by a child's developmental stage. We speculate that a combination of Roffwarg et al's theory of sleep ontogeny, 9 and the synaptic homeostasis theory of NREM sleep, could explain this dynamic concept. However, more work is needed to clarify this. There is extensive knowledge on changes in regulation during the transition from REM to NREM during sleep. 60 Despite this, the developmental change in regulation within a separate sleep state remains largely unknown and is currently being explored. An example of this change is that the percentage of REM sleep steeply decreases with development and the percentage of NREM sleep increases.
Although sleep scientists have come a long way since Roffwarg et al 9 published their groundbreaking paper in 1966, there is still a lot to discover in the field of sleep research. Sleep is free of cost and available to everyone and holds high potential therapeutic value. We need to further clarify the potential of sleep to optimise brain development. Animal studies on the developmental function of sleep have produced strong results. However, the physiological, metabolic and environmental differences between rodents and humans mean these results cannot be assumed to humans. The field will benefit from improving translational animal models. We also need safe methods to be developed that will enable detailed assessments of the relationships between the REM sleep and NREM sleep and brain development in humans at all developmental stages. For example, rather than focusing on sleep deprivation in newborn infants, the relationship with brain development could be assessed by linking sleep characteristics in the neonatal period to longitudinal brain outcome measures.
6. CONCLUSION
This narrative review will hopefully help future studies to further characterise the individual implication of REM sleep and NREM sleep in brain development and produce better therapeutic targets. Understanding how sleep is regulated will be a major step forward for both research and clinical practice. It is important to understand how sleep states relate to brain development during periods of increased risk of developmental deviation, such as in the preterm and the neonatal period. We conclude that it seems of utmost importance to safeguard the natural progression of sleep as children develop. This can be achieved by making sure they have sufficient sleep quality and duration. Hospitals should also limit paediatric patients' exposure to excessive light and noise and stress and plan elective care and elective medical treatment around the child's sleep cycle. Healthy sleep renders healthy brains, especially when children are most vulnerable.
CONFLICT OF INTEREST
The authors have no conflicts of interest to declare.
ACKNOWLEDGEMENTS
We thank anonymous peer reviewers for their feedback. Special mention to Mark Blumberg and Ko Hagoort for their valuable suggestions. We thank Tim Pijl for creating the figures.
Biographies
Marit S. Knoop

Eline R. de Groot

Jeroen Dudink

Knoop MS, de Groot ER, Dudink J. Current ideas about the roles of rapid eye movement and non–rapid eye movement sleep in brain development. Acta Paediatr. 2021;110:36–44. 10.1111/apa.15485
REFERENCES
- 1. Reimund E. The free radical flux theory of sleep. Med Hypotheses. 1994;43(4):231‐233. [DOI] [PubMed] [Google Scholar]
- 2. Benington JH, Heller HC. Restoration of brain energy metabolism as the function of sleep. Prog Neurobiol. 1995;45(4):347‐360. [DOI] [PubMed] [Google Scholar]
- 3. Mackiewicz M, Shockley KR, Romer MA, et al. Macromolecule biosynthesis: a key function of sleep. Physiol Genomics. 2007;31(3):441‐457. [DOI] [PubMed] [Google Scholar]
- 4. Slama H, Chylinski DO, Deliens G, Leproult R, Schmitz R, Peigneux P. Sleep deprivation triggers cognitive control impairments in task‐goal switching. Sleep. 2018;41(2):zsx200. [DOI] [PubMed] [Google Scholar]
- 5. Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11(2):114‐126. [DOI] [PubMed] [Google Scholar]
- 6. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zada D, Bronshtein I, Lerer‐Goldshtein T, Garini Y, Appelbaum L. Sleep increases chromosome dynamics to enable reduction of accumulating DNA damage in single neurons. Nat Commun. 2019;10(1):1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437(7063):1264‐1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Roffwarg HP, Muzio JN, Dement WC. Ontogenetic development of the human sleep‐dream cycle. Science. 1966;152:604‐619. [DOI] [PubMed] [Google Scholar]
- 10. Blumberg MS, Marques HG, Iida F. Twitching in sensorimotor development from sleeping rats to robots. Curr Biol. 2013;23(12):R532‐R537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shaffery JP. Sleep and brain development In: Dringenberg HC, ed. Handbook of Behavioral Neuroscience. Cambridge, MA: Elsevier; 2019:413–424. [Google Scholar]
- 12. Molnár Z, Clowry GJ, Šestan N, et al. New insights into the development of the human cerebral cortex. J Anat. 2019;235(3):432‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neurosci Lett. 1982;33(3):247‐252. [DOI] [PubMed] [Google Scholar]
- 14. Yakowlev PI, Lecours AR. The myelogenetic cycles of regional maturation of the brain in early life In: Minkowski A, ed. Regional Development of the Brain in Early Life. Oxford: Blackwell; 1967:3–70. [Google Scholar]
- 15. Saleem SN. Fetal MRI: an approach to practice: a review. J Adv Res. 2014;5(5):507‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387(2):167‐178. [DOI] [PubMed] [Google Scholar]
- 17. Piaget J. Play, Dreams and Imitation in Childhood. New York, NY: Norton Library; 1945. [Google Scholar]
- 18. Gerrard‐Morris A, Taylor HG, Yeates KO, et al. Cognitive development after traumatic brain injury in young children. J Int Neuropsychol Soc. 2010;16(1):157‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gilmore JH, Knickmeyer RC, Gao W. Imaging structural and functional brain development in early childhood. Nat Rev Neurosci. 2018;19(3):123‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kaplan S, Deniz OG, Önger ME, et al. Electromagnetic field and brain development. J Chem Neuroanat. 2016;75:52‐61. [DOI] [PubMed] [Google Scholar]
- 21. Grigg‐Damberger MM. Ontogeny of sleep and its functions in infancy, childhood, and adolescence In: Nevšímalová S, Bruni O, eds. Sleep Disorders in Children. Cham: Springer; 2017:3–29. [Google Scholar]
- 22. Robertson SS. Cyclic motor activity in the human fetus after midgestation. Dev Psychobiol. 1985;18(5):411‐419. [DOI] [PubMed] [Google Scholar]
- 23. Werth J, Atallah L, Andriessen P, Long X, Zwartkruis‐Pelgrim E, Aarts RM. Unobtrusive sleep state measurements in preterm infants – a review. Sleep Med Rev. 2017;32:109‐122. [DOI] [PubMed] [Google Scholar]
- 24. Hoppenbrouwers T, Hodgman JE, Sterman MB, Harper RM. Temporal distribution of sleep states, somatic and autonomic activity during the first half of life. Sleep. 1982;5:131‐144. [PubMed] [Google Scholar]
- 25. Graven S. Sleep and brain development. Clin Perinatol. 2006;33(3):693‐706. [DOI] [PubMed] [Google Scholar]
- 26. Mizrahi E. Atlas of Neonatal Electroencephalography. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 27. Scholle S, Beyer U, Bernhard M, et al. Normative values of polysomnographic parameters in childhood and adolescence: quantitative sleep parameters. Sleep Med. 2011;12(6):542‐549. [DOI] [PubMed] [Google Scholar]
- 28. Iber C, Ancoli‐Israel S, Chesson A, Quan SF. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 29. Colrain IM, Baker FC. Changes in sleep as a function of adolescent development. Neuropsychol Rev. 2011;21(1):5‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crowley SJ, Acebo C, Carskadon MA. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007;8(6):602‐612. [DOI] [PubMed] [Google Scholar]
- 31. Jenni OG, Achermann P, Carskadon MA. Homeostatic sleep regulation in adolescents. Sleep. 2005;28(11):1446‐1454. [DOI] [PubMed] [Google Scholar]
- 32. Wong MM, Brower KJ, Fitzgerald HE, Zucker RA. Sleep problems in early childhood and early onset of alcohol and other drug use in adolescence. Alcohol Clin Exp Res. 2004;28(4):578‐587. [DOI] [PubMed] [Google Scholar]
- 33. Gregory AM, Rijsdijk FV, Lau JY, Dahl RE, Eley TC. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32(2):189‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Peirano PD, Algarín CR. Sleep in brain development. Biol Res. 2007;40(4):471‐478. [PubMed] [Google Scholar]
- 35. Shaffery JP, Lopez J, Bissette G, Roffwarg HP. Rapid eye movement sleep deprivation in post‐critical period, adolescent rats alters the balance between inhibitory and excitatory mechanisms in visual cortex. Neurosci Lett. 2006;393(2‐3):131‐135. [DOI] [PubMed] [Google Scholar]
- 36. Li W, Ma L, Yang G, Gan WB. REM sleep selectively prunes and maintains new synapses in development and learning. Nat Neurosci. 2017;20(3):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krueger JM, Obal JF. Sleep function. Fron Biosci. 2003;8:d511‐d519. [DOI] [PubMed] [Google Scholar]
- 38. Grønli J, Soulé J, Bramham CR. Sleep and protein synthesis‐dependent synaptic plasticity: impacts of sleep loss and stress. Front Behav Neurosci. 2014;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole‐brain networks. Curr Biol. 2013;23(17):R774‐R788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Peever J, Fuller PM. The biology of REM sleep. Curr Biol. 2017;27(22):R1237‐R1248. [DOI] [PubMed] [Google Scholar]
- 41. Del Rio‐Bermudez C, Blumberg MS. Active sleep promotes functional connectivity in developing sensorimotor networks. BioEssays. 2018;40(4):1700234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Del Rio‐Bermudez C, Kim J, Sokoloff G, Blumberg MS. Theta oscillations during active sleep synchronize the developing rubro‐hippocampal sensorimotor network. Curr Biol. 2017;27(10):1413‐1424.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brown JL. States in newborn infants. Merrill Palmer Q Behav Dev. 1964;10(4):313‐327. [Google Scholar]
- 44. de Lima AD, Montero VM, Singer W. The cholinergic innervation of the visual thalamus: an EM immunocytochemical study. Exp Brain Res. 1985;59(1):206‐212. [DOI] [PubMed] [Google Scholar]
- 45. Shaffery JP, Sinton CM, Bisset G, Roffwarg HP, Marks GA. Rapid eye movement sleep deprivation modifies expression of long‐term potentiation in visual cortex of immature rats. Neuroscience. 2002;110(3):431‐443. [DOI] [PubMed] [Google Scholar]
- 46. Ringli M, Huber R. Developmental aspects of sleep slow waves: linking sleep, brain maturation and behavior. Prog Brain Res. 2011;193:63‐82. [DOI] [PubMed] [Google Scholar]
- 47. Kurth S, Ringli M, Geiger A, LeBourgeois M, Jenni OG, Huber R. Mapping of cortical activity in the first two decades of life: a high‐density sleep electroencephalogram study. J Neurosci. 2010;30(40):13211‐13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tononi G, Cirelli C. Sleep function and synaptic homeostasis. Sleep Med Rev. 2006;10(1):49‐62. [DOI] [PubMed] [Google Scholar]
- 49. Ghilardi M, Ghez C, Dhawan V, et al. Patterns of regional brain activation associated with different forms of motor learning. Brain Res. 2000;871(1):127‐145. [DOI] [PubMed] [Google Scholar]
- 50. McCain IJ, Lustenberger C, Achermann P, Lassonde JM, Kurth S, LeBourgeois MK. Developmental changes in sleep spindle characteristics and sigma power across early childhood. Neural Plast. 2016;2016: 1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bonjean M, Baker T, Lemieux M, Timofeev I, Sejnowski T, Bazhenov M. Corticothalamic feedback controls sleep spindle duration in vivo. J Neurosci. 2011;31(25):9124‐9134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Leger D, Debellemaniere E, Rabat A, Bayon V, Benchenane K, Chennaoui M. Slow‐wave sleep: from the cell to the clinic. Sleep Med Rev. 2018;41:113‐132. [DOI] [PubMed] [Google Scholar]
- 53. Cash SS, Halgren E, Dehghani N, et al. The human K‐Complex represents an isolated cortical down‐state. Science. 2009;324(5930):1084‐1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lopez J, Roffwarg HP, Dreher A, Bissette G, Karolewicz B, Shaffery JP. Rapid eye movement sleep deprivation decreases long‐term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153(1):44‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Clawson BC, Durkin J, Aton SJ. Form and function of sleep spindles across the lifespan. Neural Plast. 2016;2016:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Freudigman KA, Thoman EB. Infant sleep during the first postnatal day: an opportunity for assessment of vulnerability. Pediatrics. 1993;92(3):373‐379. [PubMed] [Google Scholar]
- 57. Shellhaas RA, Burns JW, Hassan F, Carlson MD, Barks JD, Chervin RD. Neonatal sleep–wake analyses predict 18‐month neurodevelopmental outcomes. Sleep. 2017;40(11):zsx144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Barbeau DY, Weiss MD. Sleep disturbances in newborns. Children (Basel). 2017;4(10):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Canet EM, Gaultier CL, D'Allest AM, Dehan MI. Effects of sleep deprivation on respiratory events during sleep in healthy infants. J Appl Physiol. 1989;66(3):1158‐1163. [DOI] [PubMed] [Google Scholar]
- 60. Lu J, Sherman D, Devor M, Saper CB. A putative flip‐flop switch for control of REM sleep. Nature. 2006;441:590‐594. [DOI] [PubMed] [Google Scholar]


