Abstract
Background
Secukinumab demonstrated superior efficacy over ustekinumab in the treatment of moderate to severe plaque psoriasis over 16 weeks in the CLARITY study and over 52 weeks in the CLEAR study.
Objective
To compare the efficacy and safety of secukinumab vs. ustekinumab over 52 weeks in CLARITY.
Methods
Analysis of 52‐week data from CLARITY (NCT02826603), a phase 3b study in which patients were randomized to receive secukinumab 300 mg (n = 550) or ustekinumab 45/90 mg (n = 552) per label.
Results
At week 52, secukinumab was superior to ustekinumab in the proportion of patients who achieved ≥ 90% improvement in Psoriasis Area and Severity Index (73.2% vs. 59.8%; odds ratio [OR], 1.84 [95% CI, 1.41–2.41]; P < 0.0001), Investigator’s Global Assessment modified 2011 responses of clear (0) or almost clear (1) skin (76.0% vs. 60.2%; OR, 2.12 [95% CI, 1.61–2.79]; P < 0.0001) and Dermatology Life Quality Index response of no effect (0/1) (69.9% vs. 61.2%; P = 0.0028). Proportions of patients with any adverse events were comparable between treatment arms.
Conclusions
This second head‐to‐head study confirmed the superior efficacy of secukinumab over ustekinumab in skin clearance and quality of life through 52 weeks, with safety comparable to that reported in previous trials. Clinicaltrials.gov identifier: NCT02826603.
Introduction
Several classes of biologics with different mechanisms of action and distinct efficacy and safety profiles are available for the treatment of psoriasis, and differentiation among these therapeutic options is important for sound decision‐making regarding treatment. The tumour necrosis factor alpha inhibitors (TNFis) adalimumab, etanercept and infliximab were the first biologic mainstays of treatment; however, the optimal response threshold for treatment success continues to improve with the advent of newer therapies, and ≥90% improvement in Psoriasis Area and Severity Index (PASI 90) 1 is frequently not achieved by patients treated with TNFis. 2 , 3 , 4 , 5 , 6 , 7 Ustekinumab, a fully human anti‐interleukin (IL) 12/23 monoclonal antibody targeting the p40 subunit shared by IL‐12 and IL‐23, is approved for treatment of moderate to severe plaque psoriasis, 8 , 9 psoriatic arthritis 10 , 11 and Crohn’s disease. 12 , 13 , 14 Compared with the TNFi etanercept, ustekinumab has demonstrated superior PASI responses in phase 3 clinical trials, 15 although ustekinumab similarly fails to consistently achieve PASI 90 responses in the majority of treated patients. 8 , 9 Biologics targeting IL‐17A have a distinct mechanism of action, improved efficacy in skin and joints and a more favourable safety profile compared with TNFis. 16 , 17 Secukinumab, a fully human anti‐IL 17A monoclonal antibody and the first approved biologic to selectively target and inhibit the biologic function of IL‐17A, is approved to treat psoriasis, 17 , 18 psoriatic arthritis 19 , 20 , 21 , 22 and ankylosing spondylitis. 23 , 24
Secukinumab has demonstrated robust efficacy, safety and durability over 5 years, 25 supporting its use in the long‐term management of psoriasis. Compared with ustekinumab, secukinumab has demonstrated superiority in the treatment of moderate to severe plaque psoriasis through 52 weeks in the phase 3b CLEAR study. 18 , 26 CLARITY is the second head‐to‐head trial comparing secukinumab with ustekinumab, targeting a larger patient population (N = 1102 vs. 676) with a greater proportion of US patients (64.2% vs. 12.6%) than CLEAR. In addition to geographic differences, patients enrolled in CLARITY were slightly heavier (approximately 92 vs. 87 kg) and more racially diverse (74.8% vs. 86.8% Caucasian) compared with those enrolled in CLEAR. 18 , 26 , 27 The larger and demographically distinct patient cohort of CLARITY vs. CLEAR provides an important test for the long‐term efficacy of biologics with different mechanisms of action in the general population. The superior efficacy of secukinumab vs. ustekinumab was previously shown in CLARITY through week 16. 27 Here, we report the efficacy and safety of secukinumab vs. ustekinumab over 52 weeks in CLARITY.
Methods
Study population
The study population included adult patients with moderate to severe chronic plaque psoriasis, as defined by PASI ≥ 12, Investigator’s Global Assessment modified 2011 (IGA mod 2011) score ≥ 3 and affected body surface area involvement ≥ 10% with disease inadequately controlled by topical treatments, phototherapy and/or previous systemic therapy. Key exclusion criteria included forms of psoriasis other than plaque psoriasis; drug‐induced psoriasis; ongoing use of prohibited treatments; previous exposure to secukinumab or any other biologic drug directly targeting IL‐17A or IL‐17RA, ustekinumab or any therapies targeting IL‐12 or IL‐23; pregnant or nursing (lactating) women; active, ongoing inflammatory diseases other than psoriasis that could confound the evaluation of the benefit of secukinumab therapy; active systemic infections during the 2 weeks prior to randomization; history of an ongoing, chronic, or recurrent infectious disease or evidence of tuberculosis infection; history of lymphoproliferative disease or any known malignancy or history of malignancy of any organ system within the past 5 years; and history of hypersensitivity to any of the study drug constituents. 27
Study design
In this multicenter, head‐to‐head, double‐blind, parallel‐group, phase 3b study (NCT02826603), patients were randomized 1:1 to receive secukinumab 300 mg (n = 550) or ustekinumab 45/90 mg (n = 552) per the label (Fig. 1). Patients assigned to the secukinumab group received secukinumab 300 mg at baseline; weeks 1, 2, 3 and 4 and then every 4 weeks thereafter until week 48. Patients assigned to the ustekinumab group received subcutaneous ustekinumab (45 mg for patients weighing ≤ 100 kg or 90 mg for patients weighing >100 kg) at baseline, week 4, and then every 12 weeks thereafter until week 40.
Figure 1.
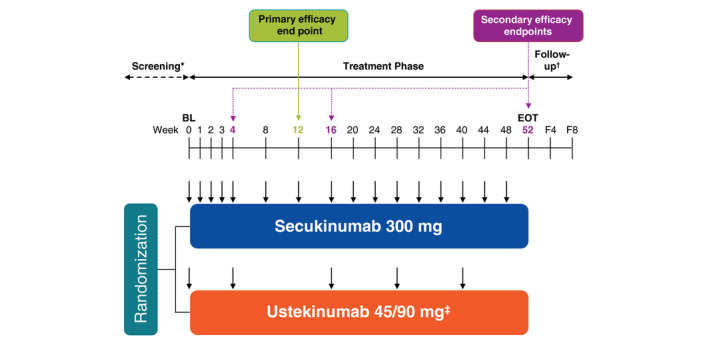
Design of the CLARITY study. BL, baseline; EOT, end of treatment phase; F4, follow‐up visit approximately 4 weeks after the EOT visit; F8, follow‐up visit approximately 8 weeks after the EOT visit. *The screening phase duration was at least 2 weeks and up to 4 weeks. †For patients with premature treatment discontinuation only. ‡Ustekinumab dose was based on bodyweight at baseline; 45 mg for patients ≤100 kg; 90 mg for patients >100 kg. ↓Active dose administration; patients received placebo at several time points to maintain blinding (not shown).
This study was conducted in accordance with the Declaration of Helsinki principles and was approved by institutional review boards or independent ethics committees. Written informed consent was obtained from all patients.
Study objectives
The co‐primary outcomes, as reported previously, 27 were to assess the superiority of secukinumab over ustekinumab in the achievement of IGA mod 2011 scores of 0 (clear) or 1 (almost clear) and PASI 90 at week 12. Key secondary and exploratory outcomes included achievement of PASI 75, PASI 90, PASI 100, IGA mod 2011 0/1, IGA mod 2011 0 and Dermatology Life Quality Index (DLQI) responses of no effect (0/1) through week 52. Other secondary objectives were to investigate the clinical safety of secukinumab compared with ustekinumab as assessed by adverse event (AE) monitoring, vital signs and clinical laboratory variables.
Statistical analysis
Differences between treatment arms were assessed using logistic regression analysis of efficacy response for PASI 75, PASI 90, PASI 100, and IGA mod 2011 0/1 and 0 responses or the Fisher exact test for DLQI 0/1 response. Missing values were handled by multiple imputation except for DLQI 0/1, for which missing values were handled by using the last observation carried forward. Safety was evaluated through week 52 per the incidence of treatment‐emergent AEs.
Results
Demographics and defining clinical characteristics
A total of 1102 patients, of whom 64.2% were from the United States, were randomized to either secukinumab 300 mg (n = 500) or ustekinumab 45/90 mg (n = 552). Demographic and baseline characteristics were similarly balanced across treatment arms, as previously published. 27 The rate of discontinuation was low and balanced between treatment arms, with 61 patients (11.1%) receiving secukinumab and 64 (11.6%) receiving ustekinumab discontinuing treatment. The most frequent reason for discontinuation of treatment in both arms was patient/guardian choice, including 20 patients (3.6%) receiving secukinumab and 19 (3.4%) receiving ustekinumab. Safety was the next most frequent reason for discontinuation, with 17 patients (3.1%) receiving secukinumab and 9 (1.6%) receiving ustekinumab discontinuing due to AEs.
Efficacy
Secukinumab 300 mg showed superiority over ustekinumab 45/90 mg in the achievement of PASI 75, PASI 90 and PASI 100 (Fig. 2), as well as IGA mod 2011 0/1 and IGA mod 0 responses (Fig. 3) at every time point from week 4 through 52. At week 52, a greater proportion of patients receiving secukinumab 300 mg than those receiving ustekinumab 45/90 mg achieved PASI 75 (89.0% vs. 82.1%; odds ratio [OR], 1.74 [95% CI, 1.21–2.50]; P = 0.0013), PASI 90 (73.2% vs. 59.8%; OR, 1.84 [95% CI, 1.41–2.41]; P < 0.0001) and PASI 100 (48.9% vs. 33.5%; OR, 1.92 [95% CI, 1.48–2.47]; P < 0.0001) responses. A greater proportion of patients receiving secukinumab 300 mg than those receiving ustekinumab 45/90 mg also achieved IGA mod 2011 0/1 (76.0% vs. 60.2%; OR, 2.12 [95% CI, 1.61–2.79]; P < 0.0001) and IGA mod 2011 0 (50.3% vs. 33.8%; OR, 2.00 [95% CI, 1.55–2.57]; P < 0.0001) responses. Twelve patients (2.2%) who received secukinumab and 28 (5.1%) who received ustekinumab experienced ≥ 1 relapse during the study period.
Figure 2.
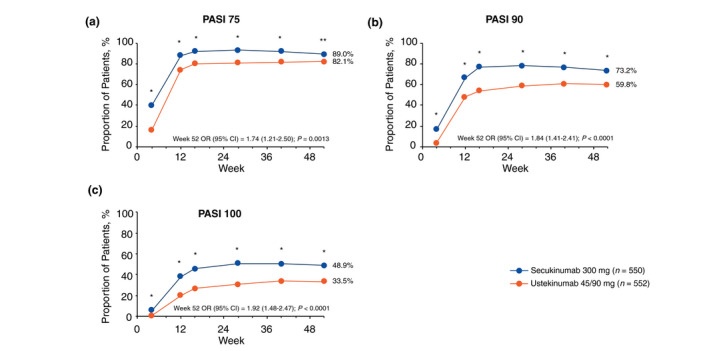
Improvement in skin symptoms as measured by (a) PASI 75, (b) PASI 90 and (c) PASI 100 responses through week 52 (multiple imputation). OR, odds ratio; PASI, Psoriasis Area and Severity Index. *P < 0.0001 vs. ustekinumab 45/90 mg. **P < 0.01 vs. ustekinumab 45/90 mg.
Figure 3.
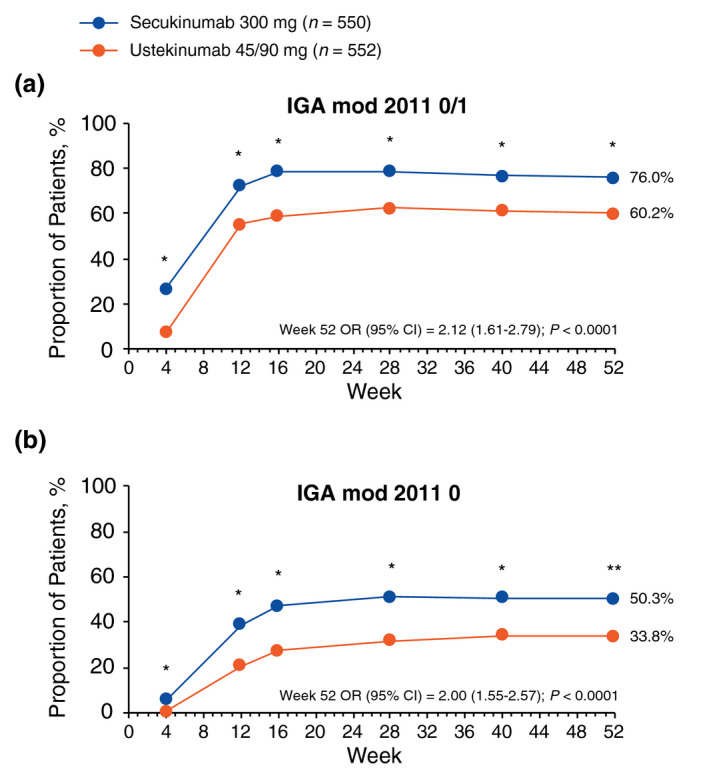
Improvement in skin symptoms as measured by (a) IGA mod 2011 0/1 and (b) IGA mod 2011 0 responses through week 52 (multiple imputation). IGA mod 2011, Investigator’s Global Assessment modified 2011; OR, odds ratio. *P < 0.0001 vs. ustekinumab 45/90 mg. **P < 0.01 vs. ustekinumab 45/90 mg.
Quality of life
Secukinumab led to rapid and sustained improvement in health‐related quality of life compared with ustekinumab as measured by DLQI (Fig. 4). At week 52, a higher proportion of patients treated with secukinumab than those treated with ustekinumab achieved DLQI 0/1 (69.9% vs. 61.2%; P = 0.0028) responses. Significant differences between secukinumab 300 mg and ustekinumab 45/90 mg in the achievement of DLQI 0/1 responses were consistent at every time point from week 4 through 52.
Figure 4.
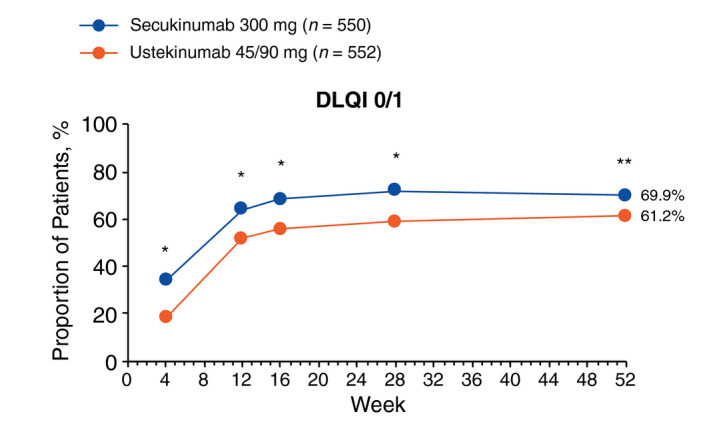
DLQI 0/1 response assessed by Fisher exact test through week 52 (last observation carried forward). DLQI, Dermatology Life Quality Index. *P < 0.0001 vs. ustekinumab 45/90 mg. **P < 0.01 vs. ustekinumab 45/90 mg.
Safety
Safety findings were based on 550 patients with 529.0 patient years of exposure to secukinumab and 552 patients with 529.6 patient years of exposure to ustekinumab. Treatment‐emergent AEs were similar in extent and severity to those observed previously in each treatment arm (Table 1). Proportions of patients with any AEs were comparable between the secukinumab and ustekinumab arms (68.5% vs. 70.7%, respectively); incidences of AEs considered by the investigator to be possibly related to the study drug were comparable between treatment groups (secukinumab, 20.0%; ustekinumab, 20.8%). The incidence of Candida infections was slightly higher in the secukinumab arm than in the ustekinumab arm (2.4% vs. 0.7%), with oral candidiasis accounting for most Candida infections in the secukinumab arm (1.5%); none of the Candida infections were considered serious or resulted in treatment discontinuation. No opportunistic infections or reactivations of latent tuberculosis were reported in either treatment arm. Reactivation of viral hepatitis was not observed in any patients in the secukinumab arm. One patient in the ustekinumab arm who was not tested for hepatitis at baseline had elevated levels of aspartate transaminase and alanine aminotransferase and was diagnosed with hepatitis C on Day 11. Inflammatory bowel disease was reported by two patients (0.4%) in the secukinumab arm and no patients in the ustekinumab arm; 1 patient with inflammatory bowel disease in the secukinumab arm had a history of this disease. Malignant or unspecified tumours, as defined by the Standardized Medical Dictionary for Regulatory Activities Query, occurred less frequently in patients receiving secukinumab (0.9%) than in those receiving ustekinumab (1.3%). Overall percentages of patients who discontinued the study treatment due to AEs were low and comparable between treatment groups (3.8% in the secukinumab group and 2.4% in the ustekinumab group). Analysis of laboratory parameters and vital signs showed no new safety signals.
Table 1.
Treatment‐emergent adverse events through week 52 (safety population)
| Secukinumab 300 mg (n = 550) | Ustekinumab 45/90 mg (n = 552) | |
|---|---|---|
| Duration of exposure, patient years | 529.0 | 529.6 |
| Any AEs, n (%) | 377 (68.5) | 390 (70.7) |
| Deaths* | 2 (0.4) | 0 |
| Non‐fatal SAEs | 27 (4.9) | 21 (3.9) |
| Discontinued study treatment due to any AEs | 21 (3.8) | 13 (2.4) |
| Most frequent AEs by preferred term, n (%) † | ||
| Nasopharyngitis | 55 (10.0) | 54 (9.8) |
| Upper respiratory tract infection | 49 (8.9) | 61 (11.1) |
| Diarrhoea | 26 (4.7) | 24 (4.3) |
| Headache | 26 (4.7) | 25 (4.5) |
| Sinusitis | 25 (4.5) | 18 (3.3) |
| Cough | 17 (3.1) | 16 (2.9) |
| Hypertension | 17 (3.1) | 22 (4.0) |
| Back pain | 14 (2.5) | 20 (3.6) |
| Oropharyngeal pain | 14 (2.5) | 17 (3.1) |
| Urinary tract infection | 13 (2.4) | 10 (1.8) |
| Conjunctivitis | 12 (2.2) | 6 (1.1) |
| Contact dermatitis | 12 (2.2) | 8 (1.4) |
| Pruritus | 12 (2.2) | 18 (3.3) |
| Arthralgia | 9 (1.6) | 14 (2.5) |
| Bronchitis | 9 (1.6) | 18 (3.3) |
| Nausea | 6 (1.1) | 13 (2.4) |
| AEs of special interest, n (%) | ||
| Infections and infestations (SOC) | 236 (42.9) | 219 (39.7) |
| Hypersensitivity (SMQ) (narrow) ‡ | 43 (7.8) | 21 (3.8) |
| Candida infections | 13 (2.4) | 4 (0.7) |
| Malignant or unspecified tumours (SMQ) | 5 (0.9) | 7 (1.3) |
| Neutropenia (NMQ) (narrow) | 3 (0.5) | 0 |
| MACE (MI, stroke, cardiovascular death) (NMQ) | 1 (0.2) | 2 (0.4) |
| Inflammatory bowel disease (NMQ) (narrow) | 2 (0.4)§ | 0 |
| Hepatitis viral reactions (HLT) | 0 | 1 (0.2) |
AE, adverse event; HLT, high level term; MACE, major adverse cardiac event; MedDRA, Medical Dictionary for Regulatory Activities; MI, myocardial infarction; NMQ, Novartis MedDRA Query; SAE, serious adverse event; SMQ, Standardized MedDRA Query; SOC, system organ class.
Two patients in the secukinumab group died. A 44‐year‐old man with an ongoing medical history of arteriosclerosis, obesity (baseline bodyweight, 188 kg), hypertension and peripheral swelling died due to sudden cardiac death. Additionally, a 50‐year‐old man with an ongoing medical history of hypertension, hyperlipidaemia, hypothyroidism and obesity (baseline bodyweight, 150 kg) died due to ‘acute intoxication by cocaine’ (toxicity to various agents [preferred term]). No causal relationship between these deaths and the study medication was suspected by the investigator.
Occurred at an incidence of ≥ 2% in either treatment arm. AEs are listed in decreasing order of frequency in the secukinumab arm.
The higher incidence of hypersensitivity (SMQ) in the secukinumab group compared with the ustekinumab group was mainly driven by the cases of contact dermatitis (12 patients [2.2%]), urticaria (5 patients [0.9%]), dermatitis and eczema (4 patients each [0.7%]), and dermatitis acneiform and rash (3 patients each [0.5%]). Only 1 patient (receiving secukinumab) had an anaphylactic reaction.
The 2 cases of inflammatory bowel disease were colitis erosive, and colitis ulcerative and hemorrhagic diarrhoea (preferred terms). In these two patients, 1 case was suspected to be related to the study drug. This patient had an active medical history of colitis ulcerative, which was exacerbated during the study. The study treatment was withdrawn, and the event was considered resolved after 45 days of its occurrence following treatment.
No deaths were reported in the ustekinumab group. Two patients in the secukinumab group died (Table 1). A 44‐year‐old man with an ongoing medical history of arteriosclerosis, obesity (baseline bodyweight, 188 kg), hypertension and peripheral swelling died due to sudden cardiac death. Additionally, a 50‐year‐old man with an ongoing history of hypertension, hyperlipidaemia, hypothyroidism and obesity (baseline bodyweight, 150 kg) died due to ‘acute intoxication by cocaine’ (toxicity to various agents [preferred term]). No causal relationships between these deaths and the study medication were suspected by the investigator.
Discussion
CLARITY is the second head‐to‐head, multicenter, double‐blind, parallel‐group, phase 3b trial that compared the efficacy of secukinumab 300 mg with that of ustekinumab 45/90 mg in 1102 patients with moderate to severe plaque‐type psoriasis in a mostly US population. Results from the complete 52‐week CLARITY study confirm the clinically relevant and statistically significant superiority of secukinumab compared with ustekinumab in skin clearance and quality‐of‐life improvement at all time points in patients with moderate to severe plaque psoriasis. The durability of these effects was maintained over 52 weeks with no loss of efficacy.
In general, maximum PASI responses were observed at week 16 and sustained through week 52, with higher response rates in the secukinumab group than in the ustekinumab group. Patients receiving secukinumab also experienced rapid and sustained improvements in quality of life compared with those receiving ustekinumab as evidenced by the exploratory endpoint DLQI. Frequencies of AEs were similar between treatment groups and consistent with previous studies. 18 , 26
Safety results observed through 52 weeks of CLARITY are consistent with the established long‐term safety profiles of both secukinumab and ustekinumab. 28 , 29 , 30 In the current study, more patients receiving secukinumab than ustekinumab experienced candidiasis (2.4% vs. 0.7%, respectively) and IBD (0.4% vs. 0%), although the incidence of these AEs was similar to that in previous reports of patients receiving secukinumab. 28 Additionally, the overall safety profile observed for secukinumab was consistent with that reported in other studies of biologics targeting IL‐17A. 21 , 22 , 31 , 32 , 33 No new safety signals, including malignancies, reactivation of tuberculosis and opportunistic infections, were identified.
Secukinumab acts rapidly, with significantly more patients receiving secukinumab achieving efficacy responses at week 4 vs. those receiving ustekinumab. This rapid onset appears consistent with recent findings from the ECLIPSE trial comparing secukinumab with the IL‐23 inhibitor guselkumab. 34 In ECLIPSE, a numerically greater proportion of patients receiving secukinumab achieved PASI 90 at weeks 4 through 16 compared with those receiving guselkumab, although no formal statistical analyses were performed at these early time points. 34
Studies comparing effectiveness and safety of available biologic therapies are important for informed shared decision‐making in the treatment in psoriasis. Ustekinumab, an IL‐12/23 inhibitor, effectively treats moderate to severe plaque psoriasis as demonstrated in the PHOENIX 1, PHOENIX 2 and ACCEPT studies 8 , 9 , 15 ; however, approximately 40% to 50% of patients in these trials did not achieve PASI 90, the updated standard for treatment success in patients with moderate to severe psoriasis. 1
Results presented here confirm the long‐term benefit of secukinumab in the treatment of moderate to severe psoriasis. CLEAR has previously shown superior efficacy of secukinumab over ustekinumab at clearing skin of patients with moderate to severe plaque psoriasis after 52 weeks of treatment, as measured by PASI and IGA mod 2011 scores. 18 , 26 CLARITY targets a larger (N = 1102 vs. 676) and more diverse patient population compared with CLEAR, with a greater proportion of patients from the United States (64.2% vs. 12.6%). 27 Possibly partially attributed to the greater US distribution, the mean bodyweight of patients receiving secukinumab was higher in CLARITY than in CLEAR (mean [SD], 91.0 [24.9] kg vs. 87.4 [20.0] kg), as was the bodyweight of patients receiving ustekinumab (93.0 [24.9] kg vs. 87.2 [22.1] kg). Higher body mass index or bodyweight correlates with poorer outcomes and response to treatment with ustekinumab. 9 Additionally, secukinumab has shown maintenance of therapeutic response over 5 years in the long‐term study SCULPTURE 25 and is being evaluated in additional long‐term extension studies ERASURE (NCT01365455) and FIXTURE (NCT01358578). Results from these studies support secukinumab as an effective option for long‐term treatment of moderate to severe psoriasis. Moreover, secukinumab has shown superior efficacy in the real world compared with ustekinumab as determined by PASI 90 after 1 year; this difference was significant regardless of previous exposure of patients to biologics. 35
One limitation of CLARITY was the lack of a predefined statistical threshold for superiority of exploratory end points through week 52. In line with the definition of superiority for primary and key secondary end points, significantly higher responses with secukinumab vs. ustekinumab through week 52, as determined by logistic regression analysis, were considered superior. Another limitation was the lack of patient‐reported outcomes beyond DLQI. However, secukinumab has previously been shown to improve patient‐reported outcomes to a greater extent than ustekinumab. In CLEAR, patients receiving secukinumab reported a greater improvement in participation measures including presenteeism, work productivity loss and overall daily impairment compared with those receiving ustekinumab (P < 0.01 for all measures).
Nonetheless, these findings provide dermatologists with important information on key efficacy and safety outcomes in support of secukinumab as a widely applicable biologic for the treatment for psoriasis.
Conclusion
The CLARITY study confirms the superiority of secukinumab over ustekinumab in achieving clear skin among patients with moderate to severe plaque psoriasis, with superior speed of onset and efficacy maintained up to 52 weeks. Secukinumab was also superior to ustekinumab in improving patients’ health‐related quality of life, as measured by DLQI. The observed safety profile of secukinumab over 52 weeks was comparable to that of ustekinumab and aligned with previous findings. Results presented here support the rapid onset of action and the long‐term efficacy of secukinumab, which have already been observed in CLEAR, in a larger study of demographically distinct patients. The robust response of different populations to secukinumab supports its wide application to the clinical treatment of patients with psoriasis.
Acknowledgements
The authors thank Richard Karpowicz, PhD, of Health Interactions, Inc, Hamilton, NJ, USA, for providing medical writing/editorial support, which was funded by Novartis Pharmaceuticals Corporation, East Hanover, NJ, in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Funding sources: This study is funded by Novartis Pharma AG, Basel, Switzerland.
Potential conflicts of interest: J. Bagel is an investigator and/or consultant and/or speaker for AbbVie, Amgen, Boehringer Ingelheim, Janssen, LEO Pharma, Novartis, Celgene, Eli Lilly, Sun Pharma and Valeant. J. Nia and P. Hashim have nothing to disclose. M. Patekar, A. de Vera, and B. Paguet are employees of Novartis Pharma AG, Basel, Switzerland. K. Ahmad is an employee of Novartis Healthcare Pvt Ltd, Hyderabad, India. S. Xia is an employee of Beijing Novartis Pharma Co, Ltd, Shanghai, China. E. Muscianisi is an employee and stockholder of Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA. A. Blauvelt has served as a scientific consultant and clinical study investigator for AbbVie, Aclaris, Almirall, Arena, Athenex, Boehringer Ingelheim, Bristol‐Myers Squibb, Dermavant, Dermira, Eli Lilly, FLX Bio, Forte, Galderma, Janssen, LEO Pharma, Novartis, Ortho, Pfizer, Regeneron, Sandoz, Sanofi Genzyme, Sun Pharma and UCB, and as a paid speaker for AbbVie. M. Lebwohl is an employee of Icahn School of Medicine at Mount Sinai, which receives research funds from AbbVie, Amgen, Arcutis, AstraZeneca, Boehringer Ingelheim, Celgene, Clinuvel, Eli Lilly, Incyte, Janssen, Kadmon, LEO Pharma, Medimmune, Novartis, Ortho Dermatologics, Pfizer, Sciderm, UCB and ViDac; he is also a consultant for Allergan, Almirall, Arcutis, Avotres Therapeutics, BirchBioMed, Boehringer Ingelheim, Bristol‐Myers Squibb, Cara Therapeutics, Castle Biosciences, Corrona, Dermavant Sciences, Foundation for Research and Education in Dermatology, Inozyme Pharma, LEO Pharma, Meiji Seika Pharma, Menlo, Mitsubishi, Neuroderm, Pfizer, Promius/Dr. Reddy’s Laboratories, Theravance and Verrica.
References
- 1. Ryan C, Korman NJ, Gelfand JM et al Research gaps in psoriasis: opportunities for future studies. J Am Acad Dermatol 2014; 70: 146–167. [DOI] [PubMed] [Google Scholar]
- 2. Leonardi CL, Powers JL, Matheson RT et al Etanercept as monotherapy in patients with psoriasis. N Engl J Med 2003; 349: 2014–2022. [DOI] [PubMed] [Google Scholar]
- 3. Menter A, Tyring SK, Gordon K et al Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol 2008; 58: 106–115. [DOI] [PubMed] [Google Scholar]
- 4. Reich K, Nestle FO, Papp K et al Infliximab induction and maintenance therapy for moderate‐to‐severe psoriasis: a phase III, multicentre, double‐blind trial. Lancet 2005; 366: 1367–1374. [DOI] [PubMed] [Google Scholar]
- 5. Saurat JH, Stingl G, Dubertret L et al Efficacy and safety results from the randomized controlled comparative study of adalimumab vs. methotrexate vs. placebo in patients with psoriasis (CHAMPION). Br J Dermatol 2008; 158: 558–566. [DOI] [PubMed] [Google Scholar]
- 6. Papp KA, Tyring S, Lahfa M et al A global phase III randomized controlled trial of etanercept in psoriasis: safety, efficacy, and effect of dose reduction. Br J Dermatol 2005; 152: 1304–1312. [DOI] [PubMed] [Google Scholar]
- 7. Menter A, Feldman SR, Weinstein GD et al A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate‐to‐severe plaque psoriasis. J Am Acad Dermatol 2007; 56: 31.e1–31.e15. [DOI] [PubMed] [Google Scholar]
- 8. Leonardi CL, Kimball AB, Papp KA et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 76‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 1). Lancet 2008; 371: 1665–1674. [DOI] [PubMed] [Google Scholar]
- 9. Papp KA, Langley RG, Lebwohl M et al Efficacy and safety of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with psoriasis: 52‐week results from a randomised, double‐blind, placebo‐controlled trial (PHOENIX 2). Lancet 2008; 371: 1675–1684. [DOI] [PubMed] [Google Scholar]
- 10. McInnes IB, Kavanaugh A, Gottlieb AB et al Efficacy and safety of ustekinumab in patients with active psoriatic arthritis: 1 year results of the phase 3, multicentre, double‐blind, placebo‐controlled PSUMMIT 1 trial. Lancet 2013; 382: 780–789. [DOI] [PubMed] [Google Scholar]
- 11. Ritchlin C, Rahman P, Kavanaugh A et al Efficacy and safety of the anti‐IL‐12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non‐biological and biological anti‐tumour necrosis factor therapy: 6‐month and 1‐year results of the phase 3, multicentre, double‐blind, placebo‐controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014; 73: 990–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Gasink C, Gao LL et al Ustekinumab induction and maintenance therapy in refractory Crohn's disease. N Engl J Med 2012; 367: 1519–1528. [DOI] [PubMed] [Google Scholar]
- 13. Sandborn WJ, Feagan BG, Fedorak RN et al A randomized trial of ustekinumab, a human interleukin‐12/23 monoclonal antibody, in patients with moderate‐to‐severe Crohn's disease. Gastroenterology 2008; 135: 1130–1141. [DOI] [PubMed] [Google Scholar]
- 14. Feagan BG, Sandborn WJ, Gasink C et al Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med 2016; 375: 1946–1960. [DOI] [PubMed] [Google Scholar]
- 15. Griffiths CE, Strober BE, van de Kerkhof P et al Comparison of ustekinumab and etanercept for moderate‐to‐severe psoriasis. N Engl J Med 2010; 362: 118–128. [DOI] [PubMed] [Google Scholar]
- 16. Griffiths CE, Reich K, Lebwohl M et al Comparison of ixekizumab with etanercept or placebo in moderate‐to‐severe psoriasis (UNCOVER‐2 and UNCOVER‐3): results from two phase 3 randomised trials. Lancet 2015; 386: 541–551. [DOI] [PubMed] [Google Scholar]
- 17. Langley RG, Elewski BE, Lebwohl M et al Secukinumab in plaque psoriasis–results of two phase 3 trials. N Engl J Med 2014; 371: 326–338. [DOI] [PubMed] [Google Scholar]
- 18. Thaci D, Blauvelt A, Reich K et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate to severe plaque psoriasis: CLEAR, a randomized controlled trial. J Am Acad Dermatol 2015; 73: 400–409. [DOI] [PubMed] [Google Scholar]
- 19. Kivitz AJ, Nash P, Tahir H et al Efficacy and safety of subcutaneous secukinumab 150 mg with or without loading regimen in psoriatic arthritis: results from the FUTURE 4 study. Rheumatol Ther 2019; 6: 393–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McInnes IB, Mease PJ, Kirkham B et al Secukinumab, a human anti‐interleukin‐17A monoclonal antibody, in patients with psoriatic arthritis (FUTURE 2): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2015; 386: 1137–1146. [DOI] [PubMed] [Google Scholar]
- 21. Mease PJ, McInnes IB, Kirkham B et al Secukinumab inhibition of interleukin‐17A in patients with psoriatic arthritis. N Engl J Med 2015; 373: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 22. Nash P, Mease PJ, McInnes IB et al Efficacy and safety of secukinumab administration by autoinjector in patients with psoriatic arthritis: results from a randomized, placebo‐controlled trial (FUTURE 3). Arthritis Res Ther 2018; 20: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marzo‐Ortega H, Sieper J, Kivitz A et al Secukinumab provides sustained improvements in the signs and symptoms of active ankylosing spondylitis with high retention rate: 3‐year results from the phase III trial, MEASURE 2. RMD Open 2017; 3: e000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baeten D, Sieper J, Braun J et al Secukinumab, an interleukin‐17A inhibitor, in ankylosing spondylitis. N Engl J Med 2015; 373: 2534–2548. [DOI] [PubMed] [Google Scholar]
- 25. Bissonnette R, Luger T, Thaci D et al Secukinumab demonstrates high sustained efficacy and a favourable safety profile in patients with moderate‐to‐severe psoriasis through 5 years of treatment (SCULPTURE Extension Study). J Eur Acad Dermatol Venereol 2018; 32: 1507–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Blauvelt A, Reich K, Tsai TF et al Secukinumab is superior to ustekinumab in clearing skin of subjects with moderate‐to‐severe plaque psoriasis up to 1 year: results from the CLEAR study. J Am Acad Dermatol 2017; 76: 60–69.e69. [DOI] [PubMed] [Google Scholar]
- 27. Bagel J, Nia J, Hashim PW et al Secukinumab is superior to ustekinumab in clearing skin in patients with moderate to severe plaque psoriasis (16‐week CLARITY results). Dermatol Ther (Heidelb) 2018; 8: 571–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deodhar A, Mease PJ, McInnes IB et al Long‐term safety of secukinumab in patients with moderate‐to‐severe plaque psoriasis, psoriatic arthritis, and ankylosing spondylitis: integrated pooled clinical trial and post‐marketing surveillance data. Arthritis Res Ther 2019; 21: 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langley RG, Lebwohl M, Krueger GG et al Long‐term efficacy and safety of ustekinumab, with and without dosing adjustment, in patients with moderate‐to‐severe psoriasis: results from the PHOENIX 2 study through 5 years of follow‐up. Br J Dermatol 2015; 172: 1371–1383. [DOI] [PubMed] [Google Scholar]
- 30. Papp KA, Griffiths CEM, Gordon K et al Long‐term safety of ustekinumab in patients with moderate‐to‐severe psoriasis: final results from 5 years of follow‐up. Br J Dermatol 2013; 168: 844–854. [DOI] [PubMed] [Google Scholar]
- 31. Mease P, van der Heijde D, Landewe R et al Secukinumab improves active psoriatic arthritis symptoms and inhibits radiographic progression: primary results from the randomised, double‐blind, phase III FUTURE 5 study. Ann Rheum Dis 2018; 77: 890–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mease PJ, van der Heijde D, Ritchlin CT et al Ixekizumab, an interleukin‐17A specific monoclonal antibody, for the treatment of biologic‐naive patients with active psoriatic arthritis: results from the 24‐week randomised, double‐blind, placebo‐controlled and active (adalimumab)‐controlled period of the phase III trial SPIRIT‐P1. Ann Rheum Dis 2017; 76: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nash P, Kirkham B, Okada M et al Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24‐week randomised, double‐blind, placebo‐controlled period of the SPIRIT‐P2 phase 3 trial. Lancet 2017; 389: 2317–2327. [DOI] [PubMed] [Google Scholar]
- 34. Reich K, Armstrong AW, Langley RG et al Guselkumab versus secukinumab for the treatment of moderate‐to‐severe psoriasis (ECLIPSE): results from a phase 3, randomised controlled trial. Lancet 2019; 394: 831–839. [DOI] [PubMed] [Google Scholar]
- 35. López Jiménez P, Suárez Pérez J, Herrera Acosta E et al Secukinumab versus ustekinumab for skin clearance in patients with moderate to severe psoriasis after a year of treatment: real‐world practice. Dermatol Ther 2019; 32: e12937. [DOI] [PubMed] [Google Scholar]


