Abstract
Head and neck cancer treatment can severely alter oral function and aesthetics, and reduce quality of life. The role of maxillofacial prosthodontists in multidisciplinary treatment of head and neck cancer patients is essential when it comes to oral rehabilitation and its planning. This role should preferably start on the day of first intake. Maxillofacial prosthodontists should be involved in the care pathway to shape and outline the prosthetic and dental rehabilitation in line with the reconstructive surgical options. With the progress of three‐dimensional technology, the pretreatment insight in overall prognosis and possibilities of surgical and/or prosthetic rehabilitation has tremendously increased. This increased insight has helped to improve quality of cancer care. This expert review addresses the involvement of maxillofacial prosthodontists in treatment planning, highlighting prosthodontic rehabilitation of head and neck cancer patients from start to finish.
Keywords: head and neck oncology, maxillofacial prosthodontics, multidisciplinary, oral rehabilitation, prosthetic pathway
1. INTRODUCTION
Head and neck cancer is the fifth most common cancer worldwide (Goon et al., 2009). The course of the disease and its treatment have major effects on psychological well‐being and functioning of the patients (Korfage et al., 2011). The treatment of head and neck cancers consists of different treatment modalities, typically being surgery, radiotherapy, chemotherapy or a combination of these modalities. Besides curing cancer, another important aim is to regain the oral function and aesthetics that got lost or altered due to the treatment.
Effects of primary oncology surgery can impede rehabilitation goals (Pace‐Balzan, Shaw, & Butterworth, 2011). These effects include an altered oral anatomy, compromised soft tissue conditions like missing or scarred tissues and bulky flaps, altered muscle attachments and muscle balance, sensitivity disorders, loss of lip competence and trismus, loss of anatomical structures, loss of bony structures and/or teeth, and alterations in facial appearance. Regaining oral function and aesthetics is a challenge because of limitations in the restorative treatment options due to, for example, poor support and lack of space for a prosthesis, impeded resilience of soft tissues, impaired tongue function, and loss of integrity and competence of the velopharyngeal complex (Nayar, 2019).
Posterior situated tumours, tumour size, adjuvant radiotherapy and extensive soft palate and tongue resections have been shown to be predictors for deterioration of oral functioning (Bohle et al., 2005; Brown, Rogers, & Lowe, 2006; Vissink, Jansma, Spijkervet, Burlage, & Coppes., 2003). Studies that looked into the quality of life of head and neck cancer patients after completion of oncologic treatment reported that regaining oral function, including prosthetic rehabilitation, is of great importance (Kamstra et al., 2011; Rogers, 2010; Tang, Rieger, & Wolfaardt., 2008). Therefore, the oncological team is in need of specially trained, experienced dental professionals, preferably maxillofacial prosthodontists, to support the team with planning of the oral rehabilitating head and neck patients. This planning and treatment may include the use of osseo‐integrated intra‐ and extra‐oral implants to retain oral and/or facial prostheses.
As mentioned, to achieve rehabilitation goals, a close and open collaboration between ablative surgeons, reconstructive surgeons, radiation oncologists, maxillofacial prosthodontists and medical engineers is of utmost importance to move towards an optimal rehabilitation of the head and neck cancer patient. The purpose of this expert review is to emphasize the role of the maxillofacial prosthodontist in the treatment planning and oral rehabilitation of head and neck cancer patients as well as to discuss challenges and new developments in the prosthodontic rehabilitation of these patients.
2. PRETREATMENT SCREENING
Multidisciplinary first‐day consultation intents to shorten time between diagnosis and treatment of oral cancer (van Huizen et al., 2018). Maxillofacial prosthodontics should be included in the multidisciplinary first‐day consultation. This first‐day consultation aims to provide a preliminary plan stating the required diagnostic procedures and prosthetic involvement (Figure 1) so that treatment can start as soon as and as effective as possible. The involvement of the maxillofacial prosthodontist includes a preradiation dental screening (Spijkervet, Schuurhuis, Stokman, Witjes, & Vissink, 2020), and a pretreatment dental and oral rehabilitation screening. During this screening, all available information is gathered with regard to self‐care, oral hygiene, dental situation, mouth opening, location of the suspected or confirmed tumour, presumed need for ablative surgery and/or radiotherapy, estimation of retention and bearing of a future (obturator, dental) prosthesis, and estimation of the pre‐existent level of oral function (Jensen, Vissink, Limesand, & Reyland, 2019; Spijkervet, Brennan, Peterson, Witjes, & Vissink, 2019). This information is needed to design the best prosthetic treatment plan. This plan should be designed taking the patients’ wishes, the tumour characteristics, extent of acquired resection for clean margins, possible types of reconstruction, need for (chemo)radiation, and dental and/or prosthetic possibilities into account.
FIGURE 1.
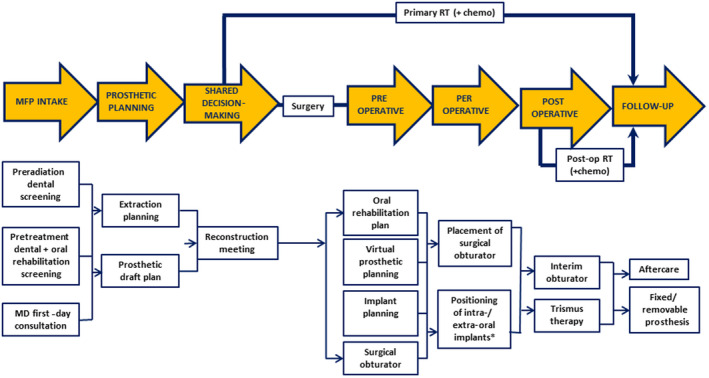
Involvement of the maxillofacial prosthodontist in treatment planning and rehabilitation of head and neck cancer patients focused on ablative surgery. chemo, chemotherapy; MD, multidisciplinary; MFP, maxillofacial prosthodontics; Post‐op, postoperative; RT, radiotherapy. *Preferably, implants are placed during ablative tumour surgery. When not feasible, implants can also be placed during follow‐up. For details, see Alberga et al. (2020)
2.1. Preradiation dental screening
In case radiotherapy might become involved, head and neck cancer patients in whom the oral cavity is within the radiation treatment portal are in need of a thorough dental examination. These patients have to complete any required dental treatment before the onset of radiotherapy (Schuurhuis et al., 2011). Preradiation dental screening aims to locate and eliminate oral foci of infection, such as unrestorable caries, periodontal disease with pockets ≥6 mm, periapical problems and (partially) impacted teeth (for details, see Spijkervet et al., 2020).
2.2. Pretreatment dental and oral rehabilitation screening
Although at the first‐day consultation the extent of the final oncologic treatment plan is uncertain, at this stage the maxillofacial prosthodontists should already estimate whether patients are in need of a prosthetic rehabilitation simultaneously with reconstructive surgery or after completion of cancer therapy, and what the patients’ desires are. Implementing the results of pretreatment screening into the prosthetic workflow ensures that all information is gathered and all needed care is provided to design a patient specific prosthetic rehabilitation draft plan. In some cases, prosthetic retentive considerations are critical to achieve successful prosthetic rehabilitation. The size of the defect and number of critical remaining teeth that may serve as anchorage for conventional clasp supported removable partial denture framework challenges the maxillofacial prosthodontists to obtain insight into the intended therapeutic isodosis fields in relation to the strategic important teeth. This sometimes results in a well‐considered decision to leave teeth which are considered an oral focus of infection in situ (including a thorough discussion of the risk on development of osteoradionecrosis).
With regard to the future prosthodontic rehabilitation, an early decision whether there is a need to place implants is important. This allows for the preferred prosthodontic rehabilitation of head and neck patients. For example, choices in planning, positioning and amount of endosseous oral implants or oncology zygomatic implants are key factors for retention of the prosthetic construction (Alberga et al., 2020; Hackett, El‐Wazani, & Butterworth, 2020). Literature emphasizes the importance of an immediate implant procedure as it has been shown that placement of mandibular implants in edentulous patients during ablative surgery results in a higher number of patients with functioning mandibular dentures after completion of oncologic therapy (Korfage et al., 2011; Mizbah et al., 2013; Wetzels et al., 2017). Furthermore, an increasing trend is observed to early complete the prosthodontic rehabilitation for which an immediate implant procedure is often a prerequisite (Alberga et al., 2020; Chuka et al., 2017). When implants are placed postradiation, the anatomical site where the implants are placed seems to effect implant survival, as the implant survival rate is higher in the mandible than in the maxilla and in grafted bone (Chrcanovic, Albrektsson, & Wennerberg, 2016; Nooh, 2013). Therefore, implant placement during ablative surgery is preferred, at least in selected cases (for details, see Alberga et al., 2020).
When there is a need for per‐operative prosthetics, the maxillofacial prosthodontist has to record the actual intra‐oral situation impression taking, intra‐oral scanning and/or cone beam computer tomography (CBCT) imaging all to capture the intra‐oral pretreatment situation and occlusal plane for fabrication of a surgical obturator, surgical guides and models, or an implant‐supported prosthesis. A huge advantage of working with three‐dimensional (3D) intra‐oral scanning is the ease to combine the data of the intra‐oral situation, like the position of teeth and occlusion, with (CB)CT and magnetic resonance imaging (MRI) data of the surrounding tissues in an augmented model. This 3D virtual model provides more insight into the implications and complexity of surgical and prosthetic rehabilitation. This insight allows the surgical team to analyse the surgical and rehabilitation outcome and plan the treatment (Kraeima et al., 2020; Witjes, Schepers, & Kraeima, 2018). Although intra‐oral scan techniques are widely used nowadays, some limitations can occur mostly due to poor intra‐oral access caused by, for example, the tumour, trismus or pain. In those situations, analogue impressions are the only feasible option. The produced plaster model can then in a second stage be digitalized in order to create the 3D virtual model.
When mutilating extra‐oral defects are expected as a result of ablative surgery, extra‐oral dimensions have to be recorded as well as to prepare for future extra‐oral prostheses. Although analogue workflows still meet the quality standards of prosthetic care, digital technology has demonstrated ease and utility in design and construction workflows in prosthodontics (Davis, 2010). The prosthodontic documentation can be completed by taking clinical photographs. In this way, skin, prosthetic and facial characteristics are captured and aid with communication between the head and neck team. With all gathered information, a prosthetic draft plan can be worked out in preparation of the necessary input of maxillofacial prosthodontists in choice of rehabilitation treatment.
3. MULTIDISCIPLINARY APPROACH
In the past, prosthodontic rehabilitation in the oncological treatment path was a stand‐alone final procedure after completion of oncological therapy. Nowadays, planning of surgical reconstruction starting with occlusion of teeth also safeguards a proper dental rehabilitation. This approach supports a thorough adjustment of the surgical and prosthetic planning and treatment before the oncologic treatment is started (Seikaly et al., 2019; Witjes et al., 2018). In a reconstruction meeting, the head and neck team can go through the available options of surgical, prosthetic or combined reconstruction. The input of maxillofacial prosthodontists in such a reconstruction meeting guards the feasibility from a prosthetic point of view, guided by a prosthetic draft plan, and includes the eventual need for implant placement. With the introduction of 3D planning and computer‐aided design (CAD) assistance, preoperative virtual augmented models provided by medical engineers at these meetings are a great asset to the surgical team and support shared decision‐making regarding favourable reconstruction option after oncology treatment.
3.1. Virtual planning
Once the final oncological treatment plan is agreed upon, having access to a preoperative virtual surgical planning (VSP) can be of importance for the surgical team (Kraeima et al., 2020). Three‐dimensional planning enables a high accuracy of guided resection surgery and prosthetic‐driven reconstruction planning (Kraeima et al., 2018; Tarsitano et al., 2017). Besides a reliable intended outcome, the concept of backwards planning from occlusion maximizes the chances of completing oral rehabilitation of the patient. A 3D VSP can be very precisely executed, with the use of 3D printed guides creating the possibility of completing a full ablative and reconstructive plan in one surgery (Seikaly et al., 2019; Witjes et al., 2018). However, soft tissues are not very reliably reproduced yet by digital techniques. This is still an uncertain factor to be taken into account when it comes to planning prosthetic treatment. The risk of losing prosthetic retention options due to compromised soft tissues means critically assessing choices such as preservation of a functional dental arch (shortened), planning a fixed or removable prosthesis, and indication of per‐operative insertion of endosseous oral implants or oncology zygomatic implants. Tools to better reproduce soft tissues are in development.
4. REHABILITATION OF MANDIBULAR DEFECTS
Smaller head and neck tumours can require resection of soft tissue only and can surgically be managed by primary closure. To overcome possible absence of vestibule or compromised neutral zone, provision of individualized adapted prostheses is required. With such an approach, oral function might reach a near normal level after ablative surgery and prosthetic rehabilitation (Tang et al., 2008).
Advanced tumours can result in large defects, requiring surgical reconstruction (Vaughan, Bainton, & Martin, 1992). The resulting altered anatomy can be unfavourable because of flap positioning and presence of scar tissue. Such unfavourable conditions may impair the ability to speak, masticate and swallow. Loss of sensibility, a shallow or absent buccal vestibule, radiation‐induced hyposalivation and trismus may further compromise oral function. Advanced tumour surgery requiring bone resection may further compromise oral function due to loss of the continuity of the mandible, loss of teeth and severe deformities. Most of all, an impaired mobility of the tongue challenges the fabrication of a functional mandibular resection prosthesis as it compromises stability of this prosthesis during speech and mastication (Petrovic et al., 2019).
Many of the aforementioned problems can, at least in part, be reduced by the use of endosseous oral implants to retain prostheses (Figure 2). These implants contribute to stabilization of prostheses and reduce loading of the compromised soft tissues and underlying bone (Schoen, Reintsema, Raghoebar, Vissink, & Roodenburg, 2004). In many patients, an almost normal masticatory function can be achieved with a rehabilitation of the reconstructed side with implant‐supported removable partial dental prostheses or implant‐retained mandibular overdentures (Kumar & Srinivasan, 2018). Maximization of dental rehabilitation significantly improves oral functioning, oral diet achievements and oral health related quality of life (Kansara et al., 2019; Korfage et al., 2011). Several authors reported that a relatively low percentage of reconstructed patients complete prosthetic rehabilitation (Barber, Butterworth, & Rogers, 2011). Causes of not completing the prosthetic treatment after implant placement are vertical discrepancy between the graft and the remaining mandible, which leads to an unfavourable implant–crown ratio, poor quality of soft tissues (hypertrophy often appears after the placement of the abutments) and the type of the prosthesis (fixed or removable; Anne‐Gaelle, Samuel, Julie, Renaud, & Pierre, 2011). As implant placement during primary reconstruction shortens the interval between surgery and dental rehabilitation, the number of orally rehabilitated patients will increase (Alberga et al., 2020; Urken et al., 1989).
FIGURE 2.
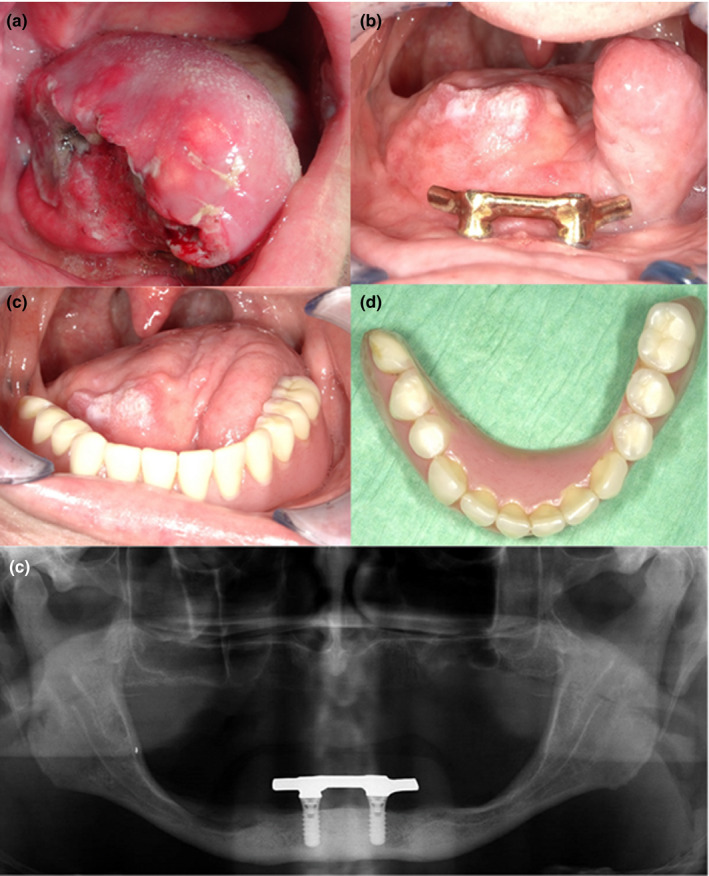
Patient diagnosed with squamous cell carcinoma of the tongue after hemiglossectomy and radial forearm free flap reconstruction. (a) Preoperative image of tumour (b) Intra‐oral view after ablative surgery and postoperative radiotherapy. Bar suprastructure with distal extensions fixed on two endosseous implants (c, d) Implant‐supported prosthesis with patient‐specific design to optimize tongue function during speech and mastication. (e) Orthopantomogram 2 years after reconstructive surgery showing good integration of endosseous implants
5. REHABILITATION OF MAXILLARY DEFECTS
Management of maxillary, midface and skull‐base tumours is challenging and complex when it comes to ablative surgery with a need for oral and facial reconstruction, and oral rehabilitation. Maxillary resections lead to a variety of oronasal defects, with a diversity of approaches for restoring oral functioning. Manifold maxillectomy classification schemes are mentioned in literature, all originating from the Brown classification published in 2000 (Brown, Rogers, McNally, & Boyle, 2000). These schemes categorize the range of maxillary defects by location, extension like the vertical and horizontal components, and biomechanical forces, and provide guidelines for surgical and prosthetic rehabilitation choices.
5.1. Restorative decision‐making
When tumour resection causes a minor oronasal fistula and primary closure is not feasible, surgical reconstruction with soft tissue flaps alone can lead to excellent functional and aesthetic results, as long as prosthetic retention of teeth replacement is guaranteed. For larger maxillary defects, the option of prosthetic rehabilitation with an obturator prosthesis is the standard of care in many institutions since decades (Aramany, 2001; Desjardins, 1978). This approach includes maxillary obturators for defects of the hard palate, pharyngeal obturators for defects of the soft palate and maxillopharyngeal obturators for defects that include both structures. However, the discomfort of wearing, removing and cleaning such a prosthesis, its poor retention in large defects and the frequent need for readjustments often limit the value of this cost‐effective method of restoring speech and mastication (Andrades, Militsakh, Hanasono, Rieger, & Rosenthal, 2011).
In case of even larger tumours, the defect size increases and the remaining dentition and supporting palatal bone will be more limited. Due to lack of retention and stability of a prosthesis, the interplay of forces further compromises functional rehabilitation and thereby overall success of treatment (Moreno, Skoracki, Hanna, & Hanasono, 2010). Placing endosseous implants in the native bone of the maxilla will allow to improve retention of the obturator prosthesis and thereby increase the success of prosthetic rehabilitation. Patients with implant‐supported obturator prostheses have significantly better masticatory and oral function, and less discomfort during food intake than patients with a conventional obturator (Buurman, Speksnijder, Engelen, & Kessler, 2020). Studies which compared prosthetic obturation with reconstruction of a palatomaxillary defect demonstrated that there are some advantages to reconstruct the defects above obturation of these defects, in particular with regard to quality‐of‐life issues such as comfort, convenience and feelings of self‐consciousness (Rogers, Lowe, McNally, Brown, & Vaughan, 2003). However, especially in medically compromised and older patients, implant‐supported obturator treatment is a viable alternative to surgical reconstruction after maxillectomy (Buurman et al., 2020), although an obturator prosthesis is not obsolete and is still standard care in low‐income and middle‐income countries. With the benefits of digital techniques and surgical reconstruction options, the obturator prosthesis has increasingly gained a temporary function by bridging time to secondary surgical reconstruction of the defect.
New workflows are rising in processing surgical obturators. Several case reports describe production of 3D obturator prostheses (Bartellas, Tibbo, Angel, Rideout, & Gillis, 2018; Rodney & Chicchon, 2017). Three‐dimensional knowledge of resection planes provides a better knowledge of the dimensions of the postresection defect, giving the option of preoperative production of a surgical obturator. With proper tumour visualization and insight in the remaining anatomic structures, a surgical obturator prosthesis can be digitally designed and printed prior to ablative surgery. A nearby fit can be achieved, and only minor per‐operative adjustments are needed (Figure 3).
FIGURE 3.
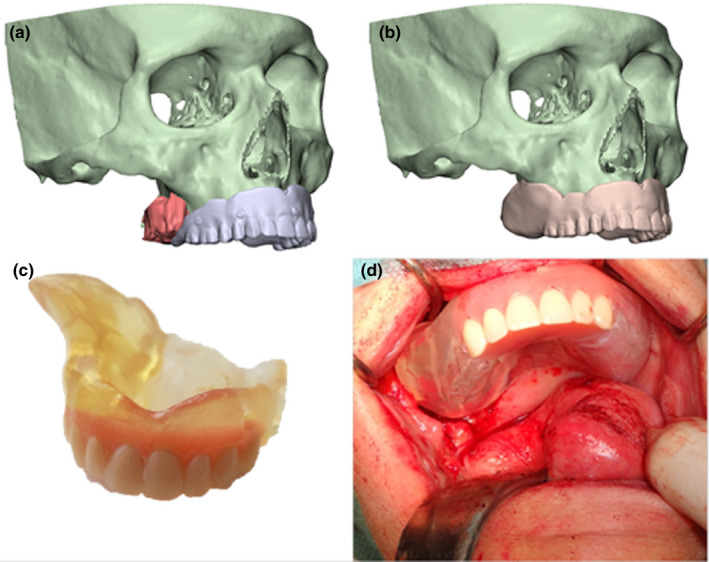
Patient diagnosed with mucoepidermoid carcinoma of the maxilla with prosthetic rehabilitation using a three dimensional printed obturator prosthesis based on a three dimensional virtual surgical planning workflow. (a) Tumour visualization based on CT and magnetic resonance imaging data fusion related to position of digitalized conventional prosthesis. (b) Virtual design of surgical obturator. (c) Image showing preoperative printed surgical obturator. (d) Digital designed and printed obturator prosthesis with nearby fit during ablative surgery
If the defect overextends in size and vertical dimension, obturation of the defect cannot be adequately addressed with prosthetic management alone (Urken et al., 2018). Surgical reconstruction combined with dental rehabilitation is then preferred. Zygomatic implants can, for example, provide a predictable in‐defect support for prosthetic rehabilitation of the maxilla if placed at the time of primary surgery (Butterworth, 2019). The zygomatic implant perforated flap procedure combines autogenous soft tissue reconstruction with zygomatic implant‐supported fixed dental rehabilitation (Butterworth & Rogers, 2017; Hackett et al., 2020). Furthermore, using the Rohner technique in combination with VSP it is possible to reconstruct high level maxillectomy cases with a reliable single‐stage approach (Figure 4) in a secondary stage procedure (Rohner et al., 2002; Runyan et al., 2016; Schepers et al., 2013; Seikaly et al., 2019).
FIGURE 4.
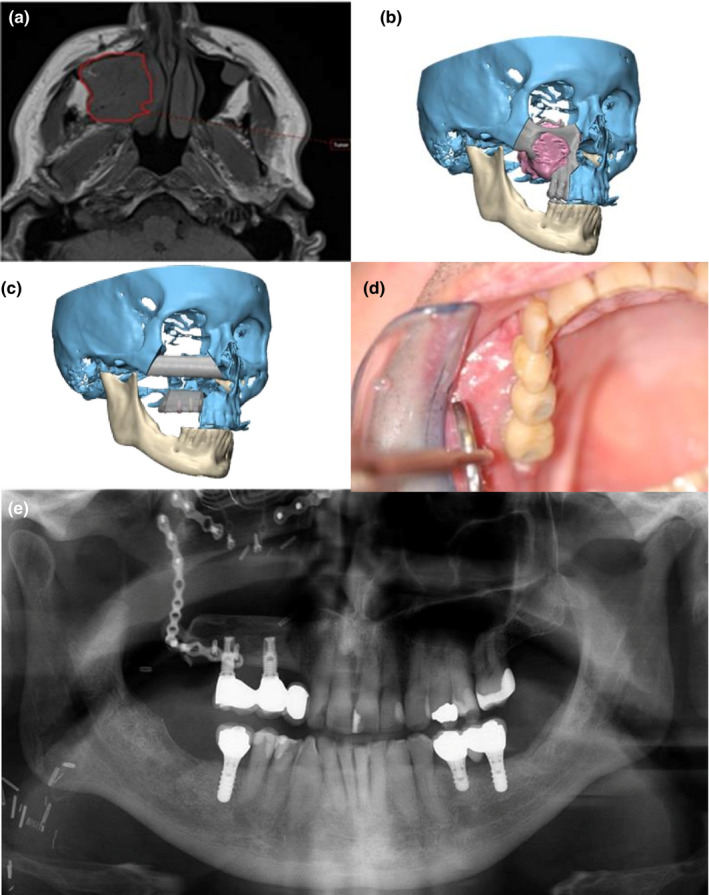
Jaw reconstruction of patient diagnosed with ameloblastoma treated with maxillectomy and reconstruction with fibular free flap. (a) The tumour was delineated on the magnetic resonance imaging using radiotherapeutic planning software. (b) Three dimensional virtual surgical planning for tumour ablation surgery. (c) Virtual surgical planning of the maxilla and orbital floor reconstruction with fibula bone and implant planning. (d) Suprastructure fixed on 2 endosseous implants placed in the fibula bone segment. (e) Orthopantomogram 4 years after reconstructive surgery showing good integration of fibula bone segment and implants
6. CONCLUSION
Oral rehabilitation is an encompassing component of the treatment of head and neck cancer patients and is a major contributor to enhance the quality of life of cancer survivors. Involvement in a multidisciplinary team to prepare and execute the rehabilitation treatment is of utmost importance. Maxillofacial prosthodontists should be involved from the beginning, and their role in this process is essential and guiding. The rise of 3D techniques in diagnostics, planning and oral rehabilitation is enormous, and is expected to evolve to the standard of care.
ACKNOWLEDGEMENTS
We would like to thank our colleagues H.H. Glas, MSc, B.J. Merema, BSc, and J Kraeima, PhD for their contribution.
Vosselman N, Alberga J, Witjes MHJ, et al. Prosthodontic rehabilitation of head and neck cancer patients—Challenges and new developments. Oral Dis. 2020;27:64–72. 10.1111/odi.13374
REFERENCES
- Alberga, J. M. , Vosselman, N. , Korfage, A. , Witjes, M. J. A. , Raghoebar, G. M. , & Vissink, A. (2020). What is the optimal timing for implant placement in oral cancer patients? Oral Diseases. 10.1111/odi.13312 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrades, P. , Militsakh, O. , Hanasono, M. M. , Rieger, J. , & Rosenthal, E. L. (2011). Current strategies in reconstruction of maxillectomy defects. Archives of Otolaryngology‐Head & Neck Surgery, 137(8), 806–812. 10.1001/archoto.2011.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anne‐Gaelle, B. , Samuel, S. , Julie, B. , Renaud, L. , & Pierre, B. (2011). Dental implant placement after mandibular reconstruction by microvascular free fibula flap: Current knowledge and remaining questions. Oral Oncology, 47(12), 1099–1104. 10.1016/j.oraloncology.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Aramany, M. A. (2001). Basic principles of obturator design for partially edentulous patients. Part II: Design principles. 1978 [classical article. The Journal of Prosthetic Dentistry, 86(6), 562–568. S0022391301307965 [DOI] [PubMed] [Google Scholar]
- Barber, A. J. , Butterworth, C. J. , & Rogers, S. N. (2011). Systematic review of primary osseointegrated dental implants in head and neck oncology. The British Journal of Oral & Maxillofacial Surgery, 49(1), 29–36. 10.1016/j.bjoms.2009.12.007 [DOI] [PubMed] [Google Scholar]
- Bartellas, M. , Tibbo, J. , Angel, D. , Rideout, A. , & Gillis, J. (2018). Three‐dimensional printing: A novel approach to the creation of obturator prostheses following palatal resection for malignant palate tumors. The Journal of Craniofacial Surgery, 29(1), e12–e15. 10.1097/SCS.0000000000003987 [DOI] [PubMed] [Google Scholar]
- Bohle, G. 3rd , Rieger, J. , Huryn, J. , Verbel, D. , Hwang, F. , & Zlotolow, I. (2005). Efficacy of speech aid prostheses for acquired defects of the soft palate and velopharyngeal inadequacy – Clinical assessments and cephalometric analysis: A Memorial Sloan‐Kettering Study. Head & Neck, 27(3), 195–207. 10.1002/hed.10360 [DOI] [PubMed] [Google Scholar]
- Brown, J. S. , Rogers, S. N. , & Lowe, D. (2006). A comparison of tongue and soft palate squamous cell carcinoma treated by primary surgery in terms of survival and quality of life outcomes. International Journal of Oral and Maxillofacial Surgery, 35(3), 208–214. S0901-5027(05)00324-3 [DOI] [PubMed] [Google Scholar]
- Brown, J. S. , Rogers, S. N. , McNally, D. N. , & Boyle, M. (2000). A modified classification for the maxillectomy defect. Head & Neck, 22(1), 17–26. [DOI] [PubMed] [Google Scholar]
- Butterworth, C. J. (2019). Primary vs secondary zygomatic implant placement in patients with head and neck cancer – A 10‐year prospective study. Head & Neck, 41(6), 1687–1695. 10.1002/hed.25645 [DOI] [PubMed] [Google Scholar]
- Butterworth, C. J. , & Rogers, S. N. (2017). The zygomatic implant perforated (ZIP) flap: A new technique for combined surgical reconstruction and rapid fixed dental rehabilitation following low‐level maxillectomy. International Journal of Implant Dentistry, 3(1). 10.1186/s40729-017-0100-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buurman, D. J. M. , Speksnijder, C. M. , Engelen, B. H. B. T. , & Kessler, P. (2020). Masticatory performance and oral health‐related quality of life in edentulous maxillectomy patients: A cross‐sectional study to compare implant‐supported obturators and conventional obturators. Clinical Oral Implants Research, 10.1111/clr.13577 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrcanovic, B. R. , Albrektsson, T. , & Wennerberg, A. (2016). Dental implants in irradiated versus nonirradiated patients: A meta‐analysis. Head & Neck, 38(3), 448–481. 10.1002/hed.23875 [DOI] [PubMed] [Google Scholar]
- Chuka, R. , Abdullah, W. , Rieger, J. , Nayar, S. , Seikaly, H. , Osswald, M. , & Wolfaardt, J. (2017). Implant utilization and time to prosthetic rehabilitation in conventional and advanced fibular free flap reconstruction of the maxilla and mandible. The International Journal of Prosthodontics, 30, 289–294. 10.11607/ijp.5161 [DOI] [PubMed] [Google Scholar]
- Davis, B. K. (2010). The role of technology in facial prosthetics. Current Opinion in Otolaryngology & Head and Neck Surgery, 18(4), 332–340. 10.1097/MOO.0b013e32833bb38c [DOI] [PubMed] [Google Scholar]
- Desjardins, R. P. (1978). Obturator prosthesis design for acquired maxillary defects. The Journal of Prosthetic Dentistry, 39(4), 424–435. S0022-3913(78)80161-9 [DOI] [PubMed] [Google Scholar]
- Goon, P. K. , Stanley, M. A. , Ebmeyer, J. , Steinstrasser, L. , Upile, T. , Jerjes, W. , & Sudhoff, H. H. (2009). HPV & head and neck cancer: A descriptive update. Head & Neck Oncology, 1 10.1186/1758-3284-1-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett, S. , El‐Wasani, B. , & Butterworth, C. (2020). Zygomatic implant based rehabilitation for patients with maxillary and mid‐facial oncology defects. Oral Diseases. 10.1111/odi.13305 [Epub ahead of print ]. [DOI] [PubMed] [Google Scholar]
- Jensen, S. B. , Vissink, A. , Limesand, K. H. , & Reyland, M. E. (2019). Salivary gland hypofunction and xerostomia in head and neck radiation patients. Journal of the National Cancer Institute Monographs, 2019(53). 10.1093/jncimonographs/lgz016 [DOI] [PubMed] [Google Scholar]
- Kamstra, J. I. , Jager‐Wittenaar, H. , Dijkstra, P. U. , Huisman, P. M. , van Oort, R. P. , van der Laan, B. F. , & Roodenburg, J. L. (2011). Oral symptoms and functional outcome related to oral and oropharyngeal cancer. Supportive Care in Cancer, 19(9), 1327–1333. 10.1007/s00520-010-0952-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kansara, S. , Wang, T. , Koochakzadeh, S. , Liou, N. E. , Graboyes, E. M. , Skoner, J. M. , … Huang, A. T. (2019). Prognostic factors associated with achieving total oral diet following osteocutaneous microvascular free tissue transfer reconstruction of the oral cavity. Oral Oncology, 98, 1–7. S1368-8375(19)30303-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korfage, A. , Schoen, P. J. , Raghoebar, G. M. , Bouma, J. , Burlage, F. R. , Roodenburg, J. L. , … Reintsema, H. (2011). Five‐year follow‐up of oral functioning and quality of life in patients with oral cancer with implant‐retained mandibular overdentures. Head & Neck, 33(6), 831–839. 10.1002/hed.21544 [DOI] [PubMed] [Google Scholar]
- Kraeima, J. , Dorgelo, B. , Gulbitti, H. A. , Steenbakkers, R. J. H. M. , Schepman, K. P. , Roodenburg, J. L. , … Witjes, M. J. H. (2018). Multi‐modality 3D mandibular resection planning in head and neck cancer using CT and MRI data fusion: A clinical series. Oral Oncology, 81, 22–28. S1368-8375(18)30115-5 [DOI] [PubMed] [Google Scholar]
- Kraeima, J. , Glas, H. H. , Merema, B. J. , Vissink, A. , Spijkervet, F. K. L. , & Witjes, M. J. H. (2020). Three dimensional virtual surgical planning in the oncologic treatment of the mandible – Current routines and clues for optimization. Oral Diseases, (in press). [DOI] [PubMed] [Google Scholar]
- Kumar, V. V. , & Srinivasan, M. (2018). Masticatory efficiency of implant‐supported removable partial dental prostheses in patients with free fibula flap reconstructed mandibles: A split‐mouth, observational study. Clinical Oral Implants Research, 29(8), 855–863. 10.1111/clr.13304 [DOI] [PubMed] [Google Scholar]
- Mizbah, K. , Dings, J. P. , Kaanders, J. H. A. M. , van den Hoogen, F. J. A. , Koole, R. , Meijer, G. J. , & Merkx, M. A. W. (2013). Interforaminal implant placement in oral cancer patients: During ablative surgery or delayed? A 5‐year retrospective study. International Journal of Oral and Maxillofacillofacial Surgery, 42, 651–655. 10.1016/j.ijom.2012/09.013 [DOI] [PubMed] [Google Scholar]
- Moreno, M. A. , Skoracki, R. J. , Hanna, E. Y. , & Hanasono, M. M. (2010). Microvascular free flap reconstruction versus palatal obturation for maxillectomy defects. Head & Neck, 32(7), 860–868. 10.1002/hed.21264 [DOI] [PubMed] [Google Scholar]
- Nayar, S. (2019). Current concepts and novel techniques in the prosthodontic management of head and neck cancer patients. British Dental Journal, 226(10), 725–737. 10.1038/s41415-019-0318-3 [DOI] [PubMed] [Google Scholar]
- Nooh, N. (2013). Dental implant survival in irradiated oral cancer patients: A systematic review of the literature. International Journal of Oral & Maxillofacial Implants, 28, 1233–1242.https://doi.org/10.11607/jomi.3045. [DOI] [PubMed] [Google Scholar]
- Pace‐Balzan, A. , Shaw, R. J. , & Butterworth, C. (2011). Oral rehabilitation following treatment for oral cancer. Periodontology 2000, 57(1), 102–117. 10.1111/j.1600-0757.2011.00384.x [DOI] [PubMed] [Google Scholar]
- Petrovic, I. , Ahmed, Z. U. , Huryn, J. M. , Nelson, J. , Allen, R. J. Jr , Matros, E. , & Rosen, E. B. (2019). Oral rehabilitation for patients with marginal and segmental mandibulectomy: A retrospective review of 111 mandibular resection prostheses. The Journal of Prosthetic Dentistry, 122(1), 82–87. S0022-3913(18)30910-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodney, J. , & Chicchon, I. (2017). Digital design and fabrication of surgical obturators based only on preoperative computed tomography data. The International Journal of Prosthodontics, 30(2), 111–112. 10.11607/ijp.5066 [DOI] [PubMed] [Google Scholar]
- Rogers, S. N. (2010). Quality of life perspectives in patients with oral cancer. Oral Oncology, 46(6), 445–447. 10.1016/j.oraloncology.2010.02.021 [DOI] [PubMed] [Google Scholar]
- Rogers, S. N. , Lowe, D. , McNally, D. , Brown, J. S. , & Vaughan, E. D. (2003). Health‐related quality of life after maxillectomy: A comparison between prosthetic obturation and free flap. Journal of Oral and Maxillofacial Surgery, 61(2), 174–181. 10.1053/joms.2003.50044 [DOI] [PubMed] [Google Scholar]
- Rohner, D. , Bucher, P. , Kunz, C. , Hammer, B. , Schenk, R. K. , & Prein, J. (2002). Treatment of severe atrophy of the maxilla with the prefabricated free vascularized fibula flap. Clinical Oral Implants Research, 13(1), 44–52. 10.1034/j.1600-0501.2002.130105.x [DOI] [PubMed] [Google Scholar]
- Runyan, C. M. , Sharma, V. , Staffenberg, D. A. , Levine, J. P. , Brecht, L. E. , Wexler, L. H. , & Hirsch, D. L. (2016). Jaw in a day: State of the art in maxillary reconstruction. The Journal of Craniofacial Surgery, 27(8), 2101–2104. 10.1097/SCS.0000000000003139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schepers, R. H. , Raghoebar, G. M. , Vissink, A. , Lahoda, L. U. , Van der Meer, W. J. , Roodenburg, J. L. , … Witjes, M. J. (2013). Fully 3‐dimensional digitally planned reconstruction of a mandible with a free vascularized fibula and immediate placement of an implant‐supported prosthetic construction. Head & Neck, 35(4), E109–E114. 10.1002/hed.21922 [DOI] [PubMed] [Google Scholar]
- Schoen, P. J. , Reintsema, H. , Raghoebar, G. M. , Vissink, A. , & Roodenburg, J. L. (2004). The use of implant retained mandibular prostheses in the oral rehabilitation of head and neck cancer patients. A review and rationale for treatment planning. Oral Oncology, 40(9), 862–871. 10.1016/j.oraloncology.2003.08.024 [DOI] [PubMed] [Google Scholar]
- Schuurhuis, J. M. , Stokman, M. A. , Roodenburg, J. L. , Reintsema, H. , Langendijk, J. A. , Vissink, A. , & Spijkervet, F. K. (2011). Efficacy of routine pre‐radiation dental screening and dental follow‐up in head and neck oncology patients on intermediate and late radiation effects. A retrospective evaluation. Radiology and Oncology, 101(3), 403–409. 10.1016/j.radonc.2011.09.018 [DOI] [PubMed] [Google Scholar]
- Seikaly, H. , Idris, S. , Chuka, R. , Jeffery, C. , Dzioba, A. , Makki, F. , … Wolfaardt, J. (2019). The Alberta reconstructive technique: An occlusion‐driven and digitally based jaw reconstruction. The Laryngoscope, 129(Suppl. 4), S1–S14. 10.1002/lary.28064 [DOI] [PubMed] [Google Scholar]
- Spijkervet, F. K. , Brennan, M. T. , Peterson, D. E. , Witjes, M. J. , & Vissink, A. (2019). Research frontiers in oral toxicities of cancer therapies: Osteoradionecrosis of the jaws. Journal of the National Cancer Institute Monographs, 2019(53). pii: lgz006. 10.1093/jncimonographs/lgz006 [DOI] [PubMed] [Google Scholar]
- Spijkervet, F. K. , Schuurhuis, J. M. , Stokman, M. A. , Witjes, M. H. J. , & Vissink, A. (2020). Should oral foci of infection be removed before onset of radiotherapy or chemotherapy? Oral Diseases. 10.1111/odi.13329 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, J. A. , Rieger, J. M. , & Wolfaardt, J. F. (2008). A review of functional outcomes related to prosthetic treatment after maxillary and mandibular reconstruction in patients with head and neck cancer. The International Journal of Prosthodontics, 21(4), 337–354. [PubMed] [Google Scholar]
- Tarsitano, A. , Ricotta, F. , Baldino, G. , Badiali, G. , Pizzigallo, A. , Ramieri, V. , … Marchetti, C. (2017). Navigation‐guided resection of maxillary tumours: The accuracy of computer‐assisted surgery in terms of control of resection margins ‐ A feasibility study. Journal of Cranio‐Maxillo‐Facial Surgery, 45(12), 2109–2114. S1010-5182(17)30332-3 [DOI] [PubMed] [Google Scholar]
- Urken, M. L. , Buchbinder, D. , Weinberg, H. , Vickery, C. , Sheiner, A. , & Biller, H. F. (1989). Primary placement of osseointegrated implants in microvascular mandibular reconstruction. Otolaryngology‐Head and Neck Surgery, 101(1), 56–73. 10.1177/019459988910100111 [DOI] [PubMed] [Google Scholar]
- Urken, M. L. , Roche, A. M. , Kiplagat, K. J. , Dewey, E. H. , Lazarus, C. , Likhterov, I. , … Okay, D. J. (2018). Comprehensive approach to functional palatomaxillary reconstruction using regional and free tissue transfer: Report of reconstructive and prosthodontic outcomes of 140 patients. Head & Neck, 40(8), 1639–1666. 10.1002/hed.25134 [DOI] [PubMed] [Google Scholar]
- van Huizen, L. S. , Dijkstra, P. U. , van der Laan, B. F. , Reintsema, H. , Ahaus, K. T. , Bijl, H. P. , & Roodenburg, J. L. (2018). Multidisciplinary first‐day consultation accelerates diagnostic procedures and throughput times of patients in a head‐and‐neck cancer care pathway, a mixed method study. BMC Health Services Research, 18(1), 820 10.1186/s12913-018-3637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan, E. D. , Bainton, R. , & Martin, I. C. (1992). Improvements in morbidity of mouth cancer using microvascular free flap reconstructions. Journal of Cranio‐Maxillo‐Facial Surgery, 20(3), 132–134. S1010‐5182(05)80095‐2 [DOI] [PubMed] [Google Scholar]
- Vissink, A. , Jansma, J. , Spijkervet, F. K. , Burlage, F. R. , & Coppes, R. P. (2003). Oral sequelae of head and neck radiotherapy. Critical Reviews in Oral Biology and Medicine, 14(3), 199–212. [DOI] [PubMed] [Google Scholar]
- Wetzels, J.‐W. , Meijer, G. J. , Koole, R. , Adang, E. M. , Merkx, M. A. W. , & Speksnijder, C. M. (2017). Costs and clinical outcomes of implant placement during ablative surgery and postponed implant placement in curative oral oncology: A five‐year retrospective cohort study. Clinical Oral Implants Research, 28, 1433–1442. 10.1111/clr.13008 [DOI] [PubMed] [Google Scholar]
- Witjes, M. J. H. , Schepers, R. H. , & Kraeima, J. (2018). Impact of 3D virtual planning on reconstruction of mandibular and maxillary surgical defects in head and neck oncology. Current Opinion in Otolaryngology & Head and Neck Surgery, 26(2), 108–114. 10.1097/MOO.0000000000000437 [DOI] [PubMed] [Google Scholar]


