Abstract
Background
In sacral neuromodulation (SNM), stimulation programming plays a key role to achieve success of the therapy. However to date, little attention has been given to the best ways to set and optimize SNM programming during the test and chronic stimulation phases of the procedure.
Objective
Standardize and make SNM programming easier and more efficient for the several conditions for which SNM is proposed.
Methods
Systematic literature review and collective clinical experience report.
Results
The basic principles of SNM programming are described. It covers choice of electrode configuration, stimulation amplitude, pulse frequency and pulse widths, while use of cycling is also briefly discussed. Step‐by‐step practical flow charts developed by a group of 13 European experts are presented.
Conclusions
Programming of SNM therapy is not complex. There are few programming settings that seem beneficial or significantly impact patient outcomes. Only four basic electrode configurations could be identified according to four different options to define the cathode. In a majority of patients, the proposed stimulation parameters will allow a satisfactory improvement for long periods of time. A regular follow‐up is, however, necessary to assess and eventually optimize results, as well as to reassure patients.
Keywords: Basic programming, electric stimulation, fecal incontinence, pelvic organ dysfunction, sacral neuromodulation, urinary incontinence
INTRODUCTION
The success of sacral neuromodulation (SNM) to treat a number of functional bladder and/or bowel disorders represents the combined result of accurate placement of the electrode lead (a temporary electrode wire or the quadripolar tined lead) in close proximity to the S3 or S4 sacral spinal nerves as addressed in a previous paper of our group (1) and a logical approach to programming the implanted neurostimulator (implanted pulse generator [IPG]).
Little attention has been so far given to this often delegated part of the therapy, although there is an obvious need to improve expertise on programming. A recent survey among 99 urogynecologic surgeons who offer SNM treatment showed that about 70% of the respondents were able to answer correctly all knowledge‐based questions on programming but the majority did have knowledge of basic programming parameters. However, they felt less familiar with the practical usage of those parameters, and only 22% performed postimplantation programming themselves. Responders were significantly more likely to report performing their own programming if they have had formal training on SNM programming (2).
SCOPE
Specific goals for a standardized programming algorithm are to optimize clinical benefit, minimize adverse effects, and reduce current consumption to prolong battery life as the need for follow‐up. This publication summarizes recent literature on programming methods and usage. It suggests some basic principles and practical ways to standardize and make SNM programming easier and more efficient at the different phases of the therapy. The article does not address the 20% patients who fail for a variety of reasons to benefit clinically. These form the subject of trouble‐shooting which includes what to do in situations of: loss or lack of efficacy, technical failure of components, for example, lead breakages, end of battery life; management of complications, for example, adverse stimulation effects.
CURRENT PRACTICE OF PROGRAMMING
Clinical Evidence on SNM Programming: Systematic Literature Review
Since the beginning of SNM use in the 1990s, four main programming parameters of SNM have been considered as essential to obtain the expected effect of the therapy. These are the electrode configuration (selection of the anode and cathode), the amplitude of the electrical pulses (mA or V), the pulse frequency (Hz), and the duration of each electrical pulse, termed the pulse width (μs). A systematic literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines (3). The search was conducted using Medline and Embase data bases, restricted to a publication date before May 6, 2019. The PRISMA flow diagram is shown in Figure 1. Exclusion criteria were publications only available as abstracts, nonhuman studies, non‐English papers, computational models, age < 18, treatment modalities other than SNM, and diseases outside established indications of overactive bladder, urinary retention, fecal incontinence, or pelvic pain. 1
Figure 1.
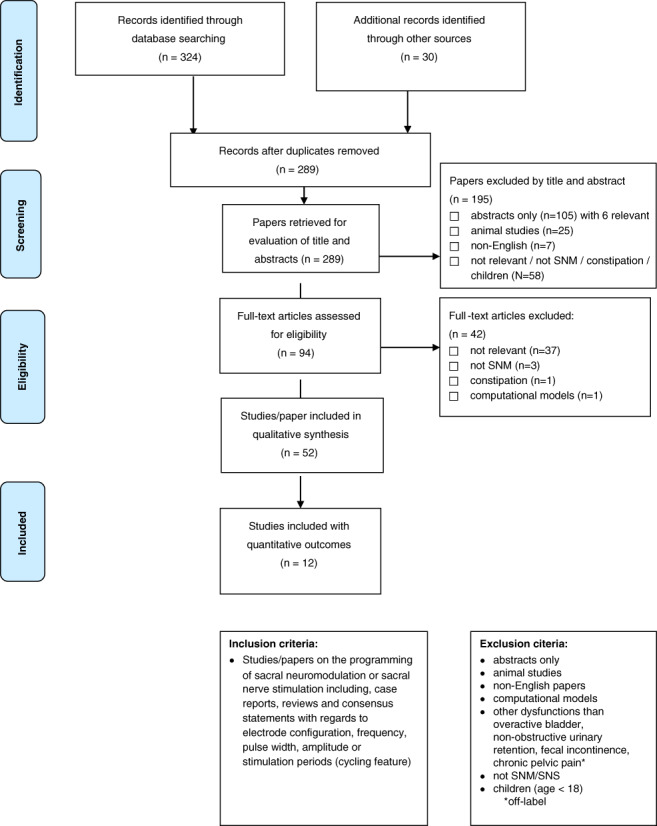
Prisma flow diagram—systematic review of the literature on SNM programming. [Color figure can be viewed at wileyonlinelibrary.com]
Electrode Configuration
As a general rule for stimulation parameter setting, the electrode configuration with the best sensory response at the lowest amplitude is chosen for therapeutic stimulation (4). Although there is no consistent definition for the best sensation site, midline sensation such as anal, perineal, or vaginal sensations are considered optimal responses irrespective of the SNM indication (4, 5, 6). These sensation sites are consistent with the anatomical course of the sacral spinal nerves and their branches. Conversely, there is agreement that leg, buttock, or back sensations represent a poor response regardless of indication (2). Recently, “sensation” maps have been described for SNM programming to help record over time an individual patient's stimulation site (Fig. 2) (7).
Figure 2.
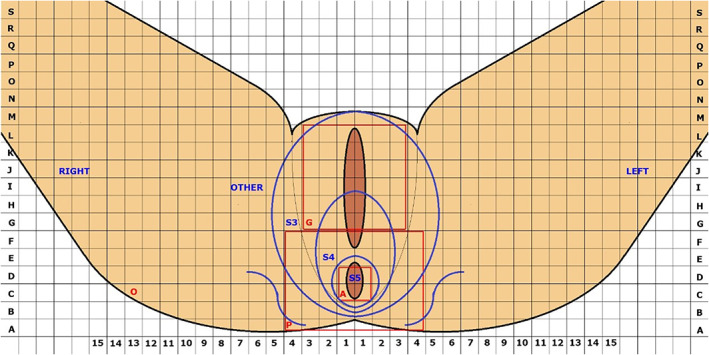
Recording document for SNM therapy. Reproduced with permission from Reference 7. [Color figure can be viewed at wileyonlinelibrary.com]
With four electrodes (also named poles) available to serve as contact points for the cathode, several programs can be used for electric stimulation of the sacral spinal nerves. In a series of 38 permanently implanted SNM patients with 102 program changes, the most commonly selected electrode configuration was bipolar with electrode “2” as a cathode [−] and “1” as an anode [+], when three of the four electrodes were typically beyond the anterior border of the sacrum on lateral x‐ray (8). Not surprisingly, electrode “2” is most often the one with the lowest motor/sensory threshold (9) (the electrodes are counted “0” to “3,” with “0” being the most distal one). However, with optimized lead placement “along the nerve” as recently described, more programming options are available and several programs can elicit similar positive responses (1, 10). Moreover, it has been reported that an optimized lead placement with the curved stylet reduces the need for reprogramming and for changes in the stimulation amplitude (11).
There is still an ongoing controversy, whether a monopolar (when IPG is used as the anode [+]) or bipolar electrode setting (two contact points used) should be the preferred electrode configuration at the initial programming following the IPG implantation. At the advent of SNM with the InterStim® device, monopolar configuration was suggested as the standard, because it was assumed to be more effective at lower amplitudes (12). Later, a bipolar configuration was considered to be preferable based on the assumption (which subsequently was revealed to be wrong) that bipolar settings yielded better battery longevity (5). There is no indication from the manufacturer of the InterStim™ system (Medtronic, MN, USA) whether either a monopolar or a bipolar configuration is associated with a longer battery life, which has been recently estimated at about five to seven years for the InterStim II device (13, 14, 15), and data from the literature are currently inconclusive. In a series of 47 patients followed‐up at a mean 20 months, 16 (34%) had a monopolar configuration used as their final setting, although all patients started with a bipolar configuration (16). In this study, the decision of whether the patient was switched to a bipolar or monopolar setting was based on the patient's improvement of symptoms at the time of the reprogramming visit. Similarly, it was observed in patients with fecal incontinence that a monopolar setting was more likely to be used in patients with longer follow‐up (8). Furthermore, a monopolar setting delivers lower sensory thresholds than bipolar configurations (9).
Stimulation Amplitude
The amplitude of stimulation determines the energy delivered to the sacral spinal nerve, and probability of axon depolarization (17). Precise lead placement allows for lower amplitude stimulation and thence low energy consumption to obtain the desired effect (1, 18, 19). At present, the InterStim™ system is based on two different energy delivery technologies. Although the external test stimulator (Verify™) works with constant current (mA), the InterStim™ II IPG delivers the energy on a constant voltage basis (V). There is no evidence that one stimulation modality is clinically superior to the other. As long as the impedance is stable, both systems deliver the same amount of energy to the sacral nerve. There are no data to suggest that constant current systems require significantly fewer amplitude adjustments than constant voltage systems (20, 21).
Present and historical practice suggests strongly the use of subsensory stimulation with two objectives: to prolong battery life and to reduce adverse effects associated with stimulation. Furthermore, the decreased amplitude required for subsensory stimulation diminishes the theoretical risk of minor nerve damage or habituation of stimulation associated with continuous electrical nerve stimulation (22, 23, 24). For patients suffering from fecal incontinence, subsensory stimulation as low as 50% of the sensory threshold did not have a negative impact on clinical outcome (24). Likewise, a recent study with a one‐year follow‐up showed that the median amplitude could be reduced from 1.5 to 0.75 V by using subsensory stimulation without compromising clinical effectiveness (25). In urology indications, subsensory stimulation is also widely used (22).
Pulse Frequency (or Rate)
It has been suggested that from a physiological perspective, the pulse rate may be an important parameter for the success of treatment (17). Since the early days of SNM, the standard pulse frequency has been 14–16 Hz (22, 26) for both urological and bowel dysfunctions. Nevertheless, it has been shown that frequency changes can lead to significant clinical improvements (27, 28, 29, 30, 31). Two double‐blind studies with random parameter selection investigated the impact of changing the frequency or the pulse width in patients with fecal incontinence (28, 29). Both studies demonstrated that increasing the frequency to 31 Hz improved clinical outcomes in about half of the patients with loss of efficacy. Likewise, in a more recent study with a one‐year follow‐up, functional results in 28% of patients have been ameliorated to a satisfactory level by stimulation with 31 Hz (25).
Although it has been shown that frequency changes can lead to significant clinical improvements (27, 28, 29, 30, 31), 10–14 Hz remains the most frequently used pulse rate range for basic programming (32).
Pulse Width
The standard pulse width is 210 μs (5, 33). When the pulse width is increased, the current or voltage required to stimulate a neural tissue decreases (34). However, changes of the pulse width from 210 μs are rare in real‐life practice (12, 33), because with currently available IPGs the amplitude can be adjusted on a much finer scale than the pulse width, if more electrical charges have to be applied to the nerve fibers to achieve a clinical response (35). In a preclinical ovine model, shorter pulse widths have been suggested to be potentially advantageous in terms of battery consumption and higher nerve fiber selectivity (36). In the aforementioned randomized studies, there were only four patients who preferred short pulse widths of 90 μs (28, 29). Since the corresponding amplitudes have not been reported in those studies, it is impossible to draw any further conclusions.
Cycling Mode
Cycling mode is a less frequently used programming parameter. With cyclic (intermittent) SNM, the stimulation is automatically switched “on and off” in a periodic manner. With the present InterStim™ system cycle times can vary from 0.1 sec to 24 hours. The purpose of cyclic SNM is to extend battery life with subsequent cost savings and to minimize the theoretical risk for minor neural damage or accommodation of stimulation, thereby increasing the effectiveness of the therapy (13, 23). Several observational but also randomized studies have demonstrated in both overactive bladder and fecal incontinence indications that up to approximately 60% of patients can be treated satisfactorily with cycling SNM (13, 23, 37, 38, 39). However, caution has to be given to the duration of cycle times, since when short they may even reduce the longevity of the IPG. In order to prolong battery life, the manufacturer recommends for patients under cycling mode to enable cycling intervals greater than 60 sec. for both the “on and off” periods, if the SoftStart/Stop function (a gradual ramp‐up of power) is activated. This SoftStart/Stop feature is intended to increase patient comfort by providing a gentle or “soft” start as the IPG is switched on and reduces the risk that the patient will be startled by the start of a stimulation cycle.
Results of the European SNM Programming Case Control Study
As evidence on programming algorithms was scarce (as shown in the above systematic literature review), the expert group decided to illustrate the programming practice with their own (unpublished) data. Programming details from a consecutive series of 90 patients were collected. These patients underwent implantation in 2017 for mixed indications (urology and colorectal) after the introduction of standardized lead placement (Table 1) (1).
Table 1.
SNM Settings at Initial (First) Programming Following IPG Implantation (Year 2017—European Expert Group)
| Programming data | 90 patients |
|---|---|
| Electrode configuration | |
| Monopolar N (%) | 11 (12) |
| Anode [+] adjacent to cathode [−] N (%) | 46 (51) |
| Anode [+] most distant from cathode [−] N (%) | 25 (28) |
| Longer (extended) cathode* N (%) | 4 (4) |
| Other settings | |
| Mean amplitude in V (± SD) | 0.95 (± 0.5) |
| Mean pulse frequency/rate (Hz) | 14.6 |
| Mean pulse width (μs) | 215 |
| Cycling mode N (%) | 6/90 (7) |
A longer cathode can be obtained on selecting two adjacent electrodes as cathode [−].
IPG, implanted pulse generator; SD, standard deviation.
These real‐life programming data show selected values of the four parameters discussed above. As an initial programming following IPG implantation, the chosen electrode configuration was an anode [+] adjacent to the cathode [−] in 51% of patients whereas an anode [+] most distant from the cathode [−] was the choice made in 28%. A monopolar configuration was infrequently chosen (11/90–12%). Regarding stimulation amplitude, with optimized lead placement, the mean amplitude was 0.95 V and for 87 patients (97%) the amplitude setting was less than <2 V. In historical patient case series, using nonoptimized lead placement, higher stimulation amplitudes have been observed with an average 2.0 V at three months follow‐up (40). The other parameters (pulse frequency and width) were those recommended by the manufacturer as a standard and a continuous mode of stimulation was prescribed for a majority of patients. Although of limited significance these results have to be seen as a range of what could be expected to initiate a SNM initial program once a patient has received an IPG.
FLOWCHARTS FOR BASIC PROGRAMMING
Based on available evidence from preclinical and clinical studies as well as expert opinion, practical recommendations from 13 experts from 9 European countries with cumulative 251 years SNM experience have been developed. The following recommendations are presented with day‐to‐day utility and simplicity in mind.
Programming During the Test Phase
The test phase is the initial entry into SNM therapy. It is seen by many as one of the major advantages of SNM over other therapies, allowing for selection of patients who may benefit from long‐term treatment before an expensive battery is implanted. There are two ways to perform nerve stimulation during the test phase: either the test with the temporary helical wire monopolar electrode (removed after the test period as it is not designed for long‐term use) or the “staged tined lead procedure” that uses the quadripolar lead designed for long‐term therapy if the test period turns out to be successful. Pros and cons of these two approaches have been already debated in detail (41).
Testing With a Temporary Wire
This type of test stimulation utilizes a temporary helical monopolar electrode (Medtronic model 3059) and ground pad placed on the patient's buttock or flank. Standard parameters are a pulse frequency of 14 Hz and 210 μsec pulse width regardless of clinical indication. With a monopolar configuration, there is only one “preset” electrode configuration possible. Multiple temporary leads in two or more foramina can be placed in order to find the best sensory response or to have a backup, if one temporary wire dislodges (6). Fluoroscopic guidance is optional. The recommended typical test period with a temporary wire is about 7 days in urology (41). Secure fixation of the temporary lead may be achieved by a double layer of adhesive polyurethane dressings. To minimize the potential risk of lead migration, a new temporary lead with a lower‐spring constant has been developed by Medtronic and received recently CE mark.
Staged Tined Lead Procedure (First SNM Stage)
Initial programming at the test phase
Initial programming at the beginning of the test phase can be performed immediately after the lead implantation if done under local anesthesia, or some hours or the day after when performed under general anesthesia, ensuring that the patient has fully recovered (Fig. 3). Recommendations are made to keep wounds covered and perfectly clean during the whole test period to avoid lead infection. The Verify™ system only allows for bipolar electrode configurations during the test phase (the battery being external cannot act as an anode). The use of four programs, using a wide stimulation field, allows for finding the cathodic electrode configuration that produces the best sensory response at the lowest amplitude. Use of sensory maps is recommended to help the patient to localize sensation, to confirm which is the stimulated nerve and to record the results as reference for later programming (Fig. 2) (7). If some discomfort occurs during stimulation, it is worth narrowing the stimulation field by moving the anode closer to the cathode (as an example, if 3–/0+ is painful, try 3–/2+). Reproducibility of both sensation site and sensory threshold are good indicators that settings are correct. In the test phase, many practitioners opt for suprasensory stimulation since the patients who are not yet familiar with the therapy prefer to have the reassurance of a perceptibly functioning device. However, other ways to confirm active stimulation (described below) exist and should be probably preferred.
Figure 3.
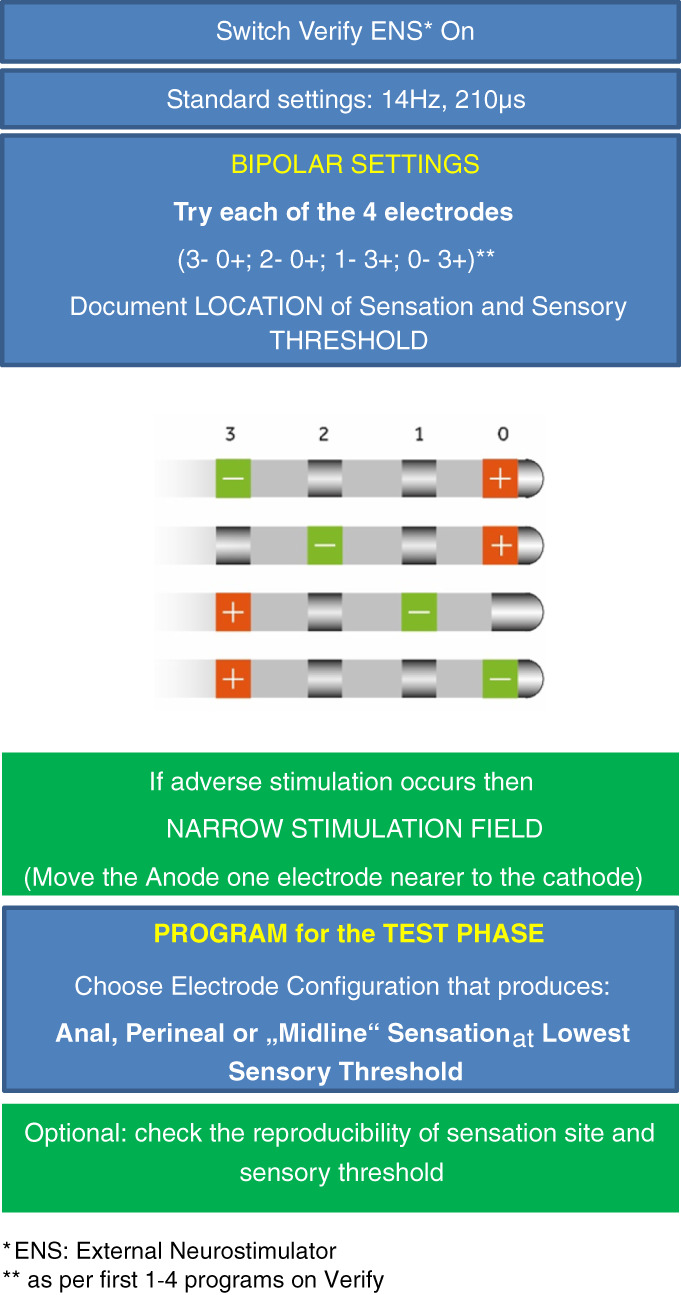
Programming at the test phase—staged tined‐lead procedure (first SNM stage). [Color figure can be viewed at wileyonlinelibrary.com]
Follow‐up during the test phase
It is of utmost importance to stay in touch with the patient during the test phase. This allows modification of settings in the case of absence of symptom improvement with initial parameter settings. With an extended test period of up to four weeks (42, 43), the staged procedure allows one to two programming adjustments if clinical outcome is not satisfactory. Potential programming adjustments can include changes to electrode configuration and/or pulse frequency (8, 40, 44). Reprogramming can be done either in the hospital center or, if the patient is staying far away from the center, he/she can be taught how to switch to another preset program. However, in the majority of patients only one program, set with optimal sensory response and low threshold is sufficient. The need for “early” programming adjustments has been reported as a predictor for long‐term failures (8).
With the current Verify™ enhanced device, up to four individual patient programs and seven predefined settings can be programmed by the physician or the Health Care Professional (HCP). There is no unanimous recommendation from the expert group whether one or several patient programs are preferable to simplify this step. This, however, offers the advantage that patients can change predefined programming settings by themselves if they experience no benefit or undesirable stimulation during the test phase. Patients should be instructed to use a program for one to two weeks, that is, the length of time required to assess the therapeutic success with adequate validity (2, 43). Either way, a telephone hotline provided by the implanting center can be critical for the success of SNM therapy.
Final Evaluation of the Test Phase
During the test phase, patients complete bladder and/or bowel diaries in order to record improvement of their symptoms when compared with baseline. In the vast majority of published studies, test stimulation was considered successful if there was a symptom improvement of ≥50%. For overactive bladder, symptom reduction can be measured by the number of incontinence episodes per day, pad usage per day, number of voids per day, or the average volume voided per void. In patients with nonobstructive urinary retention, the number of catheterizations per day or the average catheterized volume per catheterization is the usual variable measured. For patients with fecal incontinence, incontinence episodes per unit time (e.g., per week) or number of affected days per unit time (e.g., days per week) have been most commonly employed. Patient diaries can, however, be confounded by patient behavior, variations in severity of symptoms and misinterpretation (i.e., fecal soiling vs. incontinence). Therefore, subjective assessment based on patient satisfaction and visual analog scales are recommended as supplementary tools to assess the “true” benefit for the patients (6). In case of a doubtful test, the test period may be prolonged or a retest may be performed with a different stimulation site on another sacral spinal nerve. In the event of a test failure, the tined lead has to be removed.
Initial (First) Programming Following Battery Implantation
Once the IPG is implanted and connected to the tined lead, initial programming can be undertaken (Fig. 4). It differs from the test‐phase programming as the IPG (or case) can be used as an anode (+). The electrode with the best sensory response as defined above (midline sensation, lowest amplitude, no side‐effects) can be easily identified by testing successively the four electrodes (0, 1, 2, 3) as a cathode against the IPG in a monopolar stimulation mode. Typically, this corresponds with the best electrode response observed during intraoperative lead placement. Often the electrode close to the ventral surface of the sacrum (e.g., electrode 2) provides the lowest motor/sensory thresholds and can be set as the cathode. The amplitude of stimulation (V) is then initially set at the sensation threshold along with standard pulse frequency (14–16 Hz) and width (210 μs). To set up a bipolar stimulation mode, one of the other electrodes can be selected as an anode in either a wide or narrow stimulation field. A narrow field may reduce the stimulation amplitude required to achieve the sensory threshold and minimize the risk of adverse effects of stimulation. Impedance measurement does not seem beneficial for determining the best electrode configuration, because these values differ significantly between monopolar and bipolar settings and the electrical resistance is not a biomarker for the proximity of an electrode to a nerve.
Figure 4.
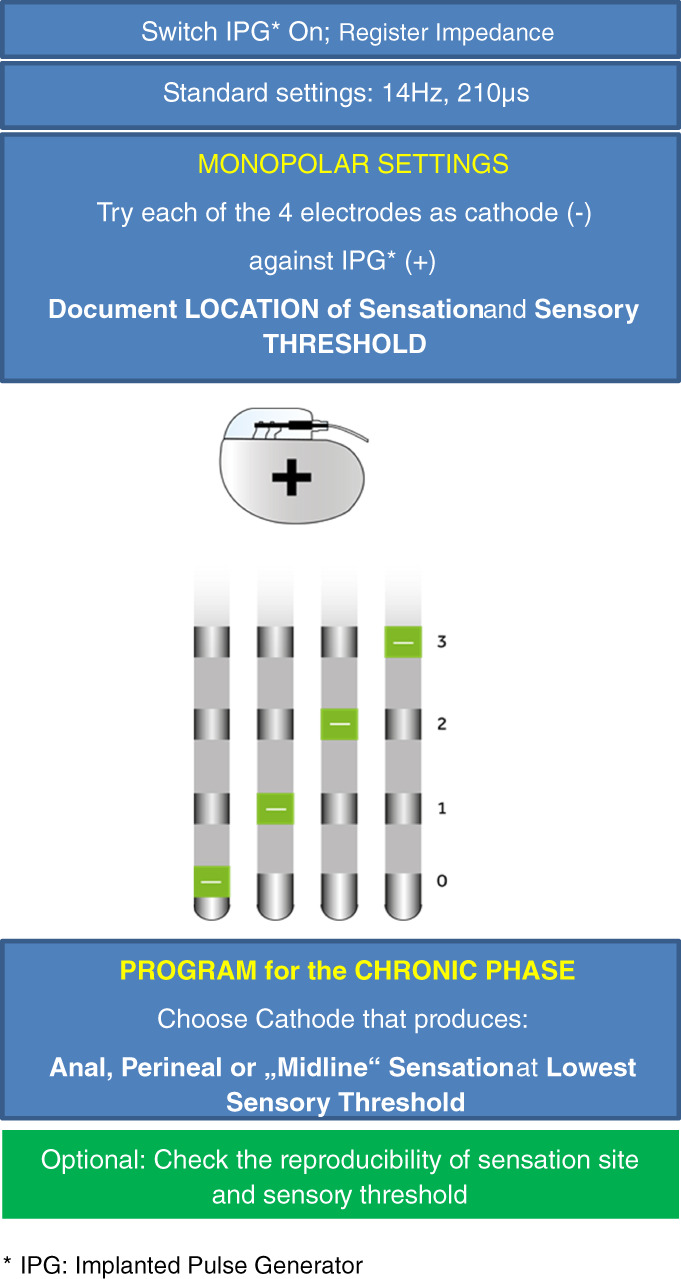
Initial (first) programming following IPG implantation (second SNM stage). [Color figure can be viewed at wileyonlinelibrary.com]
Although a bipolar configuration has been preferred by the expert group as the initial setting (Table 1) due to concerns with regard to battery consumption, some other experts have changed their practice and use now the monopolar configuration as standard in order to simplify the programming. A potential side‐effect of a monopolar setting is an adverse stimulation at the IPG pocket (45), but it seems to be rare. In a bipolar configuration, both the adjacent and most distant anodes can be tested for best sensation site and lowest sensory threshold (7). At present, there is insufficient evidence to recommend the use of complex electrode configurations such as a longer (extended) cathode (two adjacent electrodes) or a “guarded” cathode (two anodes separated by a cathode). Although such electrode configurations have been suggested in SNM (16, 46), there is no evidence that superior clinical outcomes can be achieved in this way. Moreover, recently published computational models on SNM have suggested that a longer cathode even reduces the activated tissue volume (47). Similarly, no clinical benefit has been reported for a “guarded” cathode (16). Selecting the most comfortable program setting for the patient is of key importance (2) and all programming settings should be carefully recorded in the patient's medical records for reference.
After a period of time, patients may experience a loss of the stimulation perception meaning that the IPG is operating at a subsensory level. If clinical benefits are still present, patients should be educated not to increase the amplitude permanently, even if they lose the constant stimulation perception. In case of doubt whether the stimulation system is still working, patients can check the amplitude and the device status with their patient programmer. They can also temporarily increase the amplitude or intentionally deactivate and then reactivate the system as typically reactivation causes the stimulation to be perceived.
There is no unanimous consensus from the expert group whether the IPG has to be switched off during micturition or defecation, although single cases have been reported where patients needed to deactivate the IPG in order to be able to void (48). It has been reported that SNM has been successfully used for treating urinary retention, overactive bladder and fecal incontinence at most centers without switching off the IPG before voiding/defecation (49).
Recommendations for Follow‐Up
With the InterStim™ II system, up to four individual patient programs and seven predefined programs can be activated by the physician or HCP for both the test and chronic stimulation phases. There is no unanimous recommendation from the expert group, whether one or several patient programs are preferable to simplify the follow‐up. On one hand, several programs will offer those patients living remotely from the hospital the advantage of changing predefined programming settings by themselves if they experience loss of efficacy or undesirable stimulation. Furthermore, some complex cases may require more frequent reprogramming. In case of lack or loss of efficacy, each program should be tried at least for a period of two to four weeks to draw any valid conclusions (2). On the other hand, some patients may overuse different programs be confused about which setting to choose and this can make it difficult to assess the therapeutic success.
The majority of programming can be done as a part of routine follow‐up (16). Service provision must ensure routine follow‐up and programming in the long term (6). Two programming visits per year are reported as average in subjects with overactive bladder and interstitial cystitis (16). Similarly, an average of 2.15 reprogramming sessions during the first year following implantation of the IPG has been reported in a urological Medicare patient population with that number decreasing over subsequent years (50). In accordance, most reprogramming was performed in the first year after implantation in patients treated for fecal incontinence with long‐term follow‐up (51).
LIMITATIONS
There is scarce literature on the impact of programming changes on the clinical effectiveness of SNM. From 12 studies on programming, which met the inclusion criteria of this review, six studies reported on the effects of cycling and four reported on the effects of changing the pulse frequency. Sample sizes in the few published clinical studies were small and conclusions of this article therefore reflect mainly expert opinion. It is noteworthy that there are more animal studies on the optimal stimulation parameters for SNM than well‐designed clinical studies. Further good‐quality studies are needed to identify the optimal programming parameters to apply a systematic approach to programming changes of SNM for both bladder and bowel dysfunctions. However, these are difficult to perform due to the length of time and patient input required to obtain data on sometimes subtle changes in efficacy which can be easily confounded or biased by patient behavior, medication use, or day‐to‐day variations in the severity of the underlying condition.
PROGRAMMING ON A PRACTICAL BASIS
Programming a key step of SNM therapy is not complex. Throughout the various steps of SNM therapy from start to follow‐up, what messages could we keep in mind for day‐to‐day practice with a value of simplicity and reinforced efficacy?
There are few programming settings which seem beneficial or significantly impact patient outcomes.
Only four basic electrode configurations could be identified according to four different options to define the cathode [− pole].
Although continuous stimulation is still the standard, cyclic or intermittent stimulation may be used in some patients to extend battery life without compromising the clinical efficacy or to avoid accommodation.
In a majority of patients, the proposed stimulation parameters will allow a satisfactory improvement for long periods of time.
The effects of changing other parameters of stimulation (e.g., pulse frequency) are unclear; however, doing so may benefit some patients.
A regular follow‐up (at least once per year) is, however, necessary to assess and eventually optimize results, as well as to reassure patients.
In a few patients, loss of efficacy or side‐effects may require specific management.
Authorship Statement
Drs. Sørensen and Dudding designed the study. All authors contributed to the content of the manuscript. Drs. Lehur, Sørensen, and Dudding and Mr. Engelberg drafted the manuscript. All authors approved the final version of the manuscript.
COMMENTS
This paper by the European SNM Group is a very welcome documentation of programming strategies not only from their own practices but from their conducted literature review. Too often I suspect physicians have had their accumulated tips and tricks gained from colleagues who trained them or from the empirical experience of senior colleague large volume implanters. It certainly was so for me. A more rigorous scientific approach is one that can be embraced by most of us in this field to considerable benefit. If everyone thinks that their company programming representative is state of the art then that is mathematically impossible. The Lake Wobegon Effect cannot apply in real life (we are not all ‘taller than average‘!) and thus ensuring there is physician involvement and physician oversight is critical in optimizing results. Remember most patients are ‘happy’ with a 30% improvement in their symptoms but the therapy should be explored to provide them with the optimal outcome, whatever that may be.
I am convinced, based on the recent breakthroughs in exploring subsets of SCS programming, that more options or rescue programs exist out there as yet untried. We should continue to explore the programming landscape and tie that into critical insights from animal models. I look forward to an update from this group in the years to come.
Marc Russo, MBBS
Newcastle, NSW, Australia
***
I feel this article is appropriate and timely. The article is one of expert opinion and is the best we have currently. I feel this will open discussion regarding the “voodoo” of programming that exists currently.
Kevin Benson, MD
Sioux Falls, SD, USA
***
This monograph from an expert group on neuromodulation provides useful guidance on initial programming of the Interstim II device. The scientific basis underlying the recommendations is published in an accompanying paper. Those familiar with programming will be aware that no one electrode combination is reliable and reprogramming with electrode adjustment or voltage change is often required. The advice regarding the importance of sensory localisation in the midline is valuable.
Ronan P. O'Connell, MB BCh, BAO, MD
Dublin, Ireland
***
Summarizing the programming parameter definitions and data behind established standard settings is helpful for the implanter of current sacral neuromodulation systems. One can feel confident that the standard settings for pulse width and frequency have science behind them and were not arbitrarily picked. Standardizing lead placement is invaluable in order to improve patient outcome and extend battery life.
Jannah Thompson, MD
Grand Rapids, MI, USA
Acknowledgement
The authors are grateful to Lance Zirpel for the review of the manuscript.
Composition of European SNM Expert Group: Maria Paola Bertapelle (Torino, Italy), Emmanuel Chartier‐Kastler (Paris, France), Tom Dudding (Southampton, UK), Karel Everaert (Ghent, Belgium), Philip van Kerrebroeck (Maastricht, the Netherlands), Charles H. Knowles (London, UK), Paul A. Lehur (Lugano, Switzerland), Lilli Lundby (Aarhus, Denmark), Klaus E. Matzel (Erlangen, Germany), Arantxa Muñoz‐Duyos (Barcelona, Spain), Mona B. Rydningen (Tromsö, Norway), Michael Sørensen (Hvidovre, Denmark), Stefan de Wachter (Antwerpen, Belgium).
For more information on author guidelines, an explanation of our peer review process, and conflict of interest informed consent policies, please go to http://www.wiley.com/WileyCDA/Section/id-301854.html
Source(s) of financial support: Medtronic International Trading Sarl, Switzerland, provided logistical support for the SNM expert group meetings and Open Access Fee of this article.
Conflict of Interest: All the authors have attended meetings of a European standardization group (Medtronic, directly relating to this manuscript). Paul A. Lehur has consulting agreements with Medtronic and B. Braun. Michael Sørensen serves on a Nordic standardization group and receives paid consulting fees from Medtronic. Thomas Dudding has a consultancy agreement and has received honoraria from Medtronic and Axonics. Charles Knowles and Klaus E. Matzel have management/advisory affiliations with a Medtronic global advisory panel regarding SNM for pelvic indications (directly relating to this manuscript). Charles Knowles and Klaus E. Matzel have paid consulting agreements with Medtronic (lectures). Charles Knowles also receives research support from Medtronic. Stefan De Wachter receives paid consulting fees from Medtronic as honoraria for invited speaker. Stefan Engelberg is a Medtronic employee.
Endnote
Off label.
REFERENCES
- 1. Matzel KE, Chartier‐Kastler E, Knowles CH et al. Sacral neuromodulation: standardized electrode placement technique. Neuromodulation 2017;20:816–824. [DOI] [PubMed] [Google Scholar]
- 2. Hobson DTG, Gaskins JT, Frazier L, Francis SL, Kinman CL, Meriwether KV. Current practice patterns and knowledge among gynecologic surgeons of InterStim® programming after implantation. Int Urogynecol J 2018;29:1135–1140. [DOI] [PubMed] [Google Scholar]
- 3. Moher D, Shamseer L, Clarke M et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015;4:1 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Van Kerrebroeck PE, Marcelissen TA. Sacral neuromodulation for lower urinary tract dysfunction. World J Urol 2012;30:445–450. [DOI] [PubMed] [Google Scholar]
- 5. Dudding TC, Hollingshead JR, Nicholls RJ, Vaizey CJ. Sacral nerve stimulation for faecal incontinence: optimizing outcome and managing complications. Colorectal Dis 2011;13:e196–e202. [DOI] [PubMed] [Google Scholar]
- 6. Dudding TC, Hollingshead JR, Nicholls RJ, Vaizey CJ. Sacral nerve stimulation for faecal incontinence: patient selection, service provision and operative technique. Colorectal Dis 2011;13:e187–e195. [DOI] [PubMed] [Google Scholar]
- 7. Vaganée D, Van de Borne S, Fransen E, Voorham J, Voorham‐van der Zalm P, De Wachter S. Repeatability of tools to assist in the follow up and troubleshooting of sacral neuromodulation patients using the sensory response. Neurourol Urodyn 2019;38:801–818. [DOI] [PubMed] [Google Scholar]
- 8. Cattle KR, Douglas L, Kiff ES. Programming InterStim for faecal incontinence. Colorectal Dis 2009;11:485–488. [DOI] [PubMed] [Google Scholar]
- 9. Vaganée D, Kessler TM, Van de Borne S, De Win G, De Wachter S. Sacral neuromodulation using the standardized tined lead implantation technique with a curved vs a straight stylet: 2‐year clinical outcomes and sensory responses to lead stimulation. BJU Int 2019;123:E7–E13. [DOI] [PubMed] [Google Scholar]
- 10. Liberman D, Ehlert MJ, Siegel SW. Sacral neuromodulation in urological practice. Urology 2017;99:14–22. [DOI] [PubMed] [Google Scholar]
- 11. Duelund‐Jakobsen J, Laurberg S, Lundby L. The functional outcome of sacral nerve stimulation for faecal incontinence can be improved by using lead model 3889 and a standardized implantation technique. Colorectal Dis 2018;20:O152–O157. [DOI] [PubMed] [Google Scholar]
- 12. Mahfooz AB, Elmayergi N, Abdelhady M, Wang Y, Hassouna M. Parameters of successful sacral root neuromodulation of the pelvic floor: a retrospective study. Can J Urol 2004;11:2303–2308. [PubMed] [Google Scholar]
- 13. Siegel S, Kreder K, Takacs E, McNamara R, Kan F. Prospective randomized feasibility study assessing the effect of cyclic sacral neuromodulation on urinary urge incontinence in women. Female Pelvic Med Reconstr Surg 2018;24:267–271. [DOI] [PubMed] [Google Scholar]
- 14. Duchalais E, Meurette G, Perrot B, Wyart V, Kubis C, Lehur PA. Exhausted implanted pulse generator in sacral nerve stimulation for faecal incontinence: What next in daily practice for patients? Int J Colorectal Dis 2016;31:439–444. [DOI] [PubMed] [Google Scholar]
- 15. Widmann B, Galata C, Warschkow R et al. Success and complication rates after sacral neuromodulation for fecal incontinence and constipation: A single‐center follow‐up study. J Neurogastroenterol Motil 2019;25:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burks FN, Diokno AC, Lajiness MJ, Ibrahim IA, Peters KM. Sacral neuromodulation reprogramming: Is it an office burden? Int Urogynecol J Pelvic Floor Dysfunct 2008;19:1137–1140. [DOI] [PubMed] [Google Scholar]
- 17. Devane LA, Evers J, Jones JF, Ronan O'Connell P. A review of sacral nerve stimulation parameters used in the treatment of faecal incontinence. Surgeon 2015;13:156–162. [DOI] [PubMed] [Google Scholar]
- 18. Rydningen MB, Dehli T, Wilsgaard T et al. Sacral neuromodulation for faecal incontinence following obstetric sphincter injury—outcome of percutaneous nerve evaluation. Colorectal Dis 2017;19:274–282. [DOI] [PubMed] [Google Scholar]
- 19. Duelund‐Jakobsen J, Lundby L, Lehur PA, Wyart V, Laurberg S, Buntzen S. Is the efficacy of sacral nerve stimulation for faecal incontinence dependent on the number of active electrode poles achieved during permanent lead insertion? Colorectal Dis 2016;18:O414–O419. [DOI] [PubMed] [Google Scholar]
- 20. Blok B, Van Kerrebroeck P, de Wachter S et al. Programming settings and recharge interval in a prospective study of a rechargeable sacral neuromodulation system for the treatment of overactive bladder. Neurourol Urodyn 2018;37:S17–S22. [DOI] [PubMed] [Google Scholar]
- 21. Noblett KL, Mangel J, Bennett J et al. Implantable neurostimulator programming at implant and follow‐up in a large prospective trial of sacral neuromodulation therapy for overactive bladder patients. Female Pelvic Med Reconstruct Surg 2014;20:S367–S368. [Google Scholar]
- 22. Amend B, Khalil M, Kessler TM, Sievert KD. How does sacral modulation work best? Placement and programming techniques to maximize efficacy. Curr Urol Rep 2011;12:327–335. [DOI] [PubMed] [Google Scholar]
- 23. 't Hoen LA, Groen J, Scheepe JR, Blok BF. Intermittent sacral neuromodulation for idiopathic urgency urinary incontinence in women. Neurourol Urodyn 2017;36:385–389. [DOI] [PubMed] [Google Scholar]
- 24. Duelund‐Jakobsen J, Buntzen S, Lundby L, Laurberg S. Sacral nerve stimulation at subsensory threshold does not compromise treatment efficacy: results from a randomized, blinded crossover study. Ann Surg 2013;257:219–223. [DOI] [PubMed] [Google Scholar]
- 25. Duelund‐Jakobsen J, Buntzen S, Laurberg S, Lundby L. Improved longevity and efficacy of sacral nerve stimulation by simple adjustments at follow‐up. Colorectal Dis 2019. Oct 13. 10.1111/codi.14874 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Matzel KE, Stadelmaier U, Hohenfellner M, Gall FP. Electrical stimulation of sacral spinal nerves for treatment of faecal incontinence. Lancet 1995;346:1124–1127. [DOI] [PubMed] [Google Scholar]
- 27. Marcelissen TA, Leong RK, Nieman FH, de Bie RA, van Kerrebroeck PE, de Wachter SG. The effect of pulse rate changes on the clinical outcome of sacral neuromodulation. J Urol 2011;185:1781–1785. [DOI] [PubMed] [Google Scholar]
- 28. Duelund‐Jakobsen J, Dudding T, Bradshaw E et al. Randomized double‐blind crossover study of alternative stimulator settings in sacral nerve stimulation for faecal incontinence. Br J Surg 2012;99:1445–1452. [DOI] [PubMed] [Google Scholar]
- 29. Dudding TC, Vaizey CJ, Gibbs A, Kamm MA. Improving the efficacy of sacral nerve stimulation for faecal incontinence by alteration of stimulation parameters. Br J Surg 2009;96:778–784. [DOI] [PubMed] [Google Scholar]
- 30. Cappellano F, Ciotti GV, Tafuri A et al. Cycling sacral root neuromodulation: pilot study to assess the effectiveness of this mode in neuromodulator programming for the treatment of chronic pelvic pain syndrome. Med Sur Urol 2017;6 10.4172/2168-9857.1000193. [DOI] [Google Scholar]
- 31. Peters KM, Shen L, McGuire M. Effect of sacral neuromodulation rate on overactive bladder symptoms: a randomized crossover feasibility study. Low Urin Tract Symptoms 2013;5:129–133. [DOI] [PubMed] [Google Scholar]
- 32. Noblett K. Neuromodulation and female pelvic disorders. Curr Opin Urol 2016;26:321–327. [DOI] [PubMed] [Google Scholar]
- 33. Siegel S, Noblett K, Mangel J et al. Five‐year follow‐up results of a prospective, multicenter study of patients with overactive bladder treated with sacral neuromodulation. J Urol 2018;199:229–236. [DOI] [PubMed] [Google Scholar]
- 34. Matias C, Lempka S, Machado A. Basic principles of deep brain and cortical stimulation In: Hamani C, Holtzheimer P, Lozano AM, Mayberg H, eds. Neuromodulation psychiatry. Hoboken, NJ: Wiley; 2016:101–110. 10.1002/9781118801086.ch6. [DOI] [Google Scholar]
- 35. Volkmann J, Moro E, Pahwa R. Basic algorithms for the programming of deep brain stimulation in Parkinson's disease. Mov Disord 2006;21:S284–S289. [DOI] [PubMed] [Google Scholar]
- 36. Su X, Cutinella M, Koppes S, Agran JE, Dinsmoor DA. Electromyographic responses across different pulse‐widths of sacral neuromodulation in sheep. Neuromodulation 2019;22:684–689. [DOI] [PubMed] [Google Scholar]
- 37. Price DM, Noblett K. Prospective randomized crossover trial comparing continuous and cyclic stimulation in InterStim therapy. Female Pelvic Med Reconstr Surg 2015;21:355–358. [DOI] [PubMed] [Google Scholar]
- 38. Norderval S, Behrenbruch C, Brouwer R, Keck JO. Efficacy of cyclic sacral nerve stimulation for faecal incontinence. Tech Coloproctol 2013;17:511–516. [DOI] [PubMed] [Google Scholar]
- 39. Michelsen HB, Krogh K, Buntzen S, Laurberg S. A prospective, randomized study: Switch off the sacral nerve stimulator during the night? Dis Colon Rectum 2008;51:538–540. [DOI] [PubMed] [Google Scholar]
- 40. Govaert B, Rietveld MP, van Gemert WG, Baeten CG. The role of reprogramming in sacral nerve modulation for faecal incontinence. Colorectal Dis 2011;13:78–81. [DOI] [PubMed] [Google Scholar]
- 41. Goldman HB, Lloyd JC, Noblett KL et al. International Continence Society best practice statement for use of sacral neuromodulation. NeurourolUrodyn 2018;37:1823–1848. [DOI] [PubMed] [Google Scholar]
- 42. Occhino JA, Siegel SW. Sacral nerve modulation in overactive bladder. Curr Urol Rep 2010;11:348–352. [DOI] [PubMed] [Google Scholar]
- 43. Falletto E, Ganio E, Naldini G, Ratto C, Altomare DF. Sacral neuromodulation for bowel dysfunction: a consensus statement from the Italian group. Tech Coloproctol 2014;18:53–64. [DOI] [PubMed] [Google Scholar]
- 44. Marchand C. Sacral neuromodulation for refractory urinary urgency/frequency: utility of reprogramming during the initial test phase. Neurourol Urodyn 2011;30:838. [Google Scholar]
- 45.Banakhar M, Al‐Shaiji T, Hassouna M. Challenges in sacral neuromodulation. In: Carrillo‐Ruiz J, ed. Topics in neuromodulation treatment. London, UK: InTech; 2012. http://www.intechopen.com/books/topics-in-neuromodulation-treatment/challenges-in-sacral-neuromodulation [Google Scholar]
- 46. Powell CR. Troubleshooting interstim sacral neuromodulation generators to recover function. Curr Urol Rep 2018;19:86 10.1007/s11934-018-0837-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yousif N, Vaizey CJ, Maeda Y. Mapping the current flow in sacral nerve stimulation using computational modelling. Health Technol Lett 2019;6:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Michelsen HB, Buntzen S, Krogh K, Laurberg S. Urinary retention during sacral nerve stimulation for faecal incontinence: Report of a case. Int J Colorectal Dis 2006;21:721–723. [DOI] [PubMed] [Google Scholar]
- 49. Kessler TM, Fowler CJ. Sacral neuromodulation for urinary retention. Nat Clin Pract Urol 2008;5:657–666. [DOI] [PubMed] [Google Scholar]
- 50. Cameron AP, Anger JT, Madison R, Saigal CS, Clemens JQ. Urologic diseases in America project. Battery explantation after sacral neuromodulation in the Medicare population. Neurourol Urodyn 2013;32:238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Matzel KE, Lux P, Heuer S, Besendorfer M, Zhang W. Sacral nerve stimulation for faecal incontinence: Long‐term outcome. Colorectal Dis 2009;11:636–641. [DOI] [PubMed] [Google Scholar]


