Abstract
Background
Psoriatic arthritis (PsA) is a chronic, systemic immune‐mediated inflammatory musculoskeletal disease. The onset of dermatologic symptoms often precedes rheumatic manifestations. Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA that has been shown to improve dermatologic symptoms in patients with PsA.
Objectives
To investigate the efficacy of tofacitinib in improving dermatologic endpoints in adult patients with active PsA.
Methods
This analysis included data from two placebo‐controlled, double‐blind, phase 3 studies in patients with active PsA and an inadequate response (IR) to ≥1 conventional synthetic disease‐modifying antirheumatic drug (csDMARD) who were tumor necrosis factor inhibitor (TNFi)‐naïve (OPAL Broaden; NCT01877668) or an IR to ≥1 TNFi (OPAL Beyond; NCT01882439). Patients had active plaque psoriasis at screening and received a stable dose of one csDMARD during the study. Patients were randomized to tofacitinib 5 mg twice daily (BID), 10 mg BID, adalimumab 40 mg subcutaneous injection once every 2 weeks (OPAL Broaden only) or placebo (to Month 3). Dermatologic endpoints: Psoriasis Area and Severity Index (PASI) total score; PASI90 overall; PASI75 and PASI90 by baseline PASI severity; Physician’s Global Assessment of Psoriasis; Nail Psoriasis Severity Index; Dermatology Life Quality Index total and sub‐dimension scores; Itch Severity Item; and Patient’s Global Joint and Skin Assessment‐Visual Analog Scale‐Psoriasis question.
Results
In patients with active PsA, including those stratified by mild or moderate/severe dermatologic symptoms, greater improvements from baseline and percentage of responders were observed in tofacitinib‐treated patients vs. placebo for the majority of analyzed dermatologic endpoints at Months 1 and 3, and improvements were maintained to Month 12 in OPAL Broaden and Month 6 in OPAL Beyond. Similar effects were observed in adalimumab‐treated patients vs. placebo in OPAL Broaden across dermatologic endpoints.
Conclusions
Tofacitinib provides a treatment option for patients with active PsA, including the burdensome dermatologic symptoms of PsA.
Introduction
Psoriatic arthritis (PsA) is a chronic, systemic immune‐mediated inflammatory musculoskeletal disease, and symptoms can include peripheral arthritis, enthesitis, dactylitis, spondylitis, and skin and nail psoriasis, 1 , 2 all of which can impact health‐related quality of life (HRQoL). 3 PsA occurs in up to 30% of patients with psoriasis 4 —cutaneous signs and symptoms often precede skeletal manifestations, with the onset of psoriasis commonly occurring around 10 years prior to arthritic symptoms. 4
Considering the multi‐domain nature of PsA, efficacy across a range of both clinical measures and patient‐reported outcomes (PROs) is a requisite for an effective treatment. As new treatments for PsA emerge, further investigations into their efficacy on dermatologic outcome measures are warranted.
Tofacitinib is an oral Janus kinase inhibitor for the treatment of PsA. The efficacy and safety of tofacitinib 5 mg twice daily (BID; recommended dosage) 5 , 6 and 10 mg BID has been demonstrated in placebo‐controlled, double‐blind, phase 3 trials of up to 12 months’ duration in adult patients with active PsA. 7 , 8 In OPAL Broaden, patients were tumor necrosis factor inhibitor (TNFi)‐naïve and had an inadequate response (IR) to ≥1 conventional synthetic disease‐modifying antirheumatic drug (csDMARD). 7 In OPAL Beyond, patients had an IR to ≥1 TNFi therapy. 8 In both trials, a key secondary study endpoint was improvement of 75% or more from baseline in the Psoriasis Area and Severity Index (PASI75) among patients with at least 3% of their body surface area (BSA) affected and a PASI score >0 at baseline. 7 , 8 The percentage of patients achieving PASI75 response at Month 3 was significantly greater for both tofacitinib doses and adalimumab vs. placebo in OPAL Broaden and was significantly greater for tofacitinib 10 mg BID and numerically greater for tofacitinib 5 mg BID vs. placebo in OPAL Beyond. 7 , 8 While tofacitinib is not approved for psoriasis in most countries (only in Russia), it has also previously shown efficacy for improvement in dermatologic endpoints in phase 3 and long‐term extension studies in patients with psoriasis 9 , 10 , 11 , 12 and nail psoriasis. 13
This analysis reports the efficacy of tofacitinib for additional dermatologic endpoints and dermatology‐related PROs, using data from the PsA trials OPAL Broaden and OPAL Beyond. 7 , 8
Methods
Study design
This analysis included data from two global, phase 3, randomized controlled trials. OPAL Broaden (NCT01877668) was a 12‐month study in TNFi‐naïve patients with active PsA and IR to ≥1 csDMARD. 7 Patients were randomized 2 : 2 : 2 : 1 : 1 to receive tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg subcutaneous (SC) injection once every 2 weeks (Q2W), placebo advancing to tofacitinib 5 mg BID at Month 3 or placebo advancing to tofacitinib 10 mg BID at Month 3. OPAL Beyond (NCT01882439) 8 was a 6‐month study in patients with active PsA and IR to ≥1 TNFi. Patients were randomized 2 : 2 : 1 : 1 to receive tofacitinib 5 mg BID, tofacitinib 10 mg BID, placebo advancing to tofacitinib 5 mg BID at Month 3 or placebo advancing to tofacitinib 10 mg BID at Month 3 (data only shown for placebo to Month 3). Patients were required to receive a stable dose of one csDMARD (e.g. methotrexate, leflunomide or sulfasalazine) during both studies.
Patients
Details of eligibility criteria for OPAL Broaden and OPAL Beyond have been published previously. 7 , 8 Briefly, patients were required to have signs and symptoms consistent with a ClASsification criteria for Psoriatic ARthritis (CASPAR) diagnosis of PsA for ≥6 months, active arthritis (≥3 tender/painful and ≥3 swollen joints) at screening and baseline, and active plaque psoriasis at screening (diagnosed or confirmed by a dermatologist or rheumatologist). No minimum affected BSA was required.
Studies were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice Guidelines, along with applicable local country regulations and laws. The study protocols were approved by the Institutional Review Boards and/or Independent Ethics Committee at each center. All patients provided written informed consent.
Dermatologic endpoints
This study reports both prespecified and post hoc analyses of dermatologic endpoints at Months 1, 3, 6, 9 and 12 (OPAL Broaden), and Months 1, 3 and 6 (OPAL Beyond) (see Table 1 for endpoints included in the analyses).
Table 1.
Prespecified and post hoc dermatologic and dermatology‐related PRO endpoints included in the analysis
| Prespecified endpoint/analysis | Post hoc endpoint/analysis |
|---|---|
| Dermatologic | |
| Dermatologic‐related PROs | |
|
|
BSA, body surface area; DLQI, Dermatology Life Quality Index; ISI, Itch Severity Item; LSM, least squares mean; MCID, minimum clinically important difference; NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; PASI75, improvements from baseline in PASI ≥75%; PASI90, improvements from baseline in PASI ≥90%; PGA‐PsO, Physician’s Global Assessment of Psoriasis; PGJS‐VAS‐PsO, Patient’s Global Joint and Skin Assessment‐Visual Analog Scale‐Psoriasis question; PRO, patient‐reported outcome.
Among patients with baseline BSA ≥3% and baseline PASI >0.
As stratified by baseline PASI >0 to ≤10 (mild) or baseline PASI >10 (moderate–severe).
Among patients with baseline PGA‐PsO >0.
Among patients with baseline NAPSI >0 (based on one target fingernail affected).
Percentage of patients achieving PGA‐PsO response, defined as a score of 0 (clear)/1 (almost clear) on a 0–4 scale and ≥2‐point improvement from baseline in patients with baseline PGA‐PsO ≥2.
DLQI total score ranges from 0 to 30, with a higher score indicating greater impact on quality of life. 23
Among patients with baseline ISI >0. Scores range from 0–10, where 0 = no itching and 10 = worst possible itching. 14
DLQI question #1 on skin symptoms: ‘Over the last week, how itchy, sore, painful or stinging has your skin been?’. 23
Patients were asked to place a mark on a 0–100 mm line to indicate their response to the question ‘In all the ways your psoriasis affects you, how would you rate the way in which you felt over the past week?’ (0 = excellent and 100 = poor).
Defined as improvement from baseline ≥5 points 24 among patients with baseline DLQI total score >0, BSA ≥3% and PASI >0.
Based on improvements from baseline of ≥2, ≥3 or ≥4, among patients with baseline ISI >0.
Dermatology‐related patient‐reported outcomes
Dermatology‐related PROs were assessed at Months 1, 3, 6, 9 and 12 (OPAL Broaden), and Months 1, 3 and 6 (OPAL Beyond). HRQoL endpoints included in this analysis are presented in Table 1.
Statistical analysis
For efficacy analyses, all subjects in the full analysis set (FAS; all patients randomized who received ≥1 dose of study drug, including injection [adalimumab or placebo] in OPAL Broaden) were included, unless otherwise stated for some endpoints in which subjects meeting specified baseline criteria in the FAS were included. Safety events were analyzed using the safety analysis set, which was the same as the FAS. Treatment comparisons for binary endpoints at each time point were analyzed using the normal approximation for the difference in binomial proportions with non‐responder imputation for missing values, except for the analysis of minimum clinically important difference (MCID, ≥5 points) for change from baseline in Dermatology Life Quality Index (DLQI) total score. No imputation for missing values was performed for descriptive statistics by treatment. Treatment comparisons for continuous endpoints were analyzed using repeated measures models with no imputation for missing values. The repeated measures model included the fixed effects of treatment, visit, treatment‐by‐visit interaction, geographic region and baseline value. A common unstructured variance‐covariance matrix was used. Results up to Month 3 were based on a model including data up to Month 3 with the two placebo treatment sequences (placebo advancing to tofacitinib 5 mg or 10 mg BID), combined into a single placebo group. Results after Month 3 were based on another model including all data up to end of study with the two placebo sequences separate (the results of the two placebo treatment sequences after Month 3 were not reported). For all endpoints, nominal P values for the comparison between active treatments and placebo up to Month 3 are presented without adjustment for multiplicity.
Results
Patients
Overall, 816 randomized and treated patients from OPAL Broaden (n = 422) and OPAL Beyond (n = 394) were included in this analysis. Patient demographics and baseline disease characteristics have been published previously and were mostly similar across treatment groups in both studies. 7 , 8 In OPAL Broaden and OPAL Beyond, respectively, 73.9% and 62.7% of patients had ≥3% affected BSA, median PASI scores were 6.5 and 7.9 (among patients with baseline BSA ≥3% and PASI >0), 78.4% and 70.3% of patients had nail psoriasis (≥1 affected nail), 43.1% and 41.1% had mild disease severity, and 21.8% and 20.3% had moderate disease severity based on the PGA‐PsO.
Dermatologic patient baseline characteristics are shown in Table 2.
Table 2.
Baseline dermatologic characteristics of patients enrolled in the OPAL Broaden and OPAL Beyond studies
| OPAL Broaden (N = 422) | OPAL Beyond (N = 394) | ||||||
|---|---|---|---|---|---|---|---|
|
Tofacitinib 5 mg BID |
Tofacitinib 10 mg BID |
Adalimumab 40 mg SC Q2W |
Placebo |
Tofacitinib 5 mg BID |
Tofacitinib 10 mg BID |
Placebo | |
| N = 107 | N = 104 | N = 106 | N = 105 | N = 131 | N = 132 | N = 131 | |
| Affected BSA ≥3%, n (%) | 82 (76.6) | 70 (67.3) | 78 (73.6) | 82 (78.1) | 80 (61.1) | 81 (61.4) | 86 (65.6) |
| PASI score, † median (range) [N1] | 5.6 (0.4–46.0) [82] | 7.8 (0.3–24.3) [70] | 7.0 (2.0–47.1) [77] | 6.6 (0.8–41.4) [82] | 7.6 (0.6–32.2) [80] | 8.8 (0.8–41.6) [81] | 7.1 (1.6–66.0) [86] |
| PGA‐PsO, ‡ n (%) | |||||||
| Clear (0) | 4 (3.7) | 6 (5.8) | 3 (2.8) | 3 (2.9) | 7 (5.3) | 4 (3.0) | 4 (3.1) |
| Almost clear (1) | 31 (29.0) | 27 (26.0) | 35 (33.0) | 27 (25.7) | 36 (27.5) | 38 (28.8) | 42 (32.1) |
| Mild (2) | 46 (43.0) | 42 (40.4) | 43 (40.6) | 51 (48.6) | 56 (42.7) | 51 (38.6) | 55 (42.0) |
| Moderate (3) | 23 (21.5) | 27 (26.0) | 22 (20.8) | 20 (19.0) | 25 (19.1) | 33 (25.0) | 22 (16.8) |
| Severe (4) | 2 (1.9) | 2 (1.9) | 2 (1.9) | 3 (2.9) | 6 (4.6) | 5 (3.8) | 8 (6.1) |
| PGA‐PsO, § mean (SD) [N1] | 2 (0.8) [102] | 2 (0.8) [98] | 1.9 (0.8) [102] | 2 (0.8) [101] | 2 (0.8) [123] | 2 (0.9) [127] | 2 (0.9) [127] |
| PGA‐PsO ≥3, PASI ≥12 and BSA ≥10%, n (%) ¶ | 12 (11.2) | 13 (12.5) | 12 (11.3) | 11 (10.5) | 18 (13.7) | 16 (12.1) | 18 (13.7) |
| NAPSI, †† mean (SD) [N1] | 4.0 (1.9) [81] | 3.9 (2.0) [73] | 4.3 (2.2) [89] | 4.0 (2.2) [88] | 3.7 (2.0) [87] | 4.1 (2.1) [97] | 3.6 (2.0) [93] |
| Nail psoriasis, ‡‡ n (%) | 81 (75.7) | 73 (70.2) | 89 (84.0) | 88 (83.8) | 87 (66.4) | 97 (73.5) | 93 (71.0) |
| DLQI, mean (SD) | 8.8 (7.3) | 9.0 (7.7) | 7.7 (7.1) | 9.4 (7.6) | 7.8 (7.1) ††† | 10.2 (8.2) ††† | 8.4 (7.7) |
| ISI, §§ mean (SD) [N1] | 5.0 (2.6) [97] | 4.9 (2.5) [93] | 4.4 (2.5) [93] | 4.7 (2.5) [92] | 5.1 (2.6) [111] | 5.4 (2.8) [122] | 5.1 (2.8) [115] |
| PGJS‐VAS‐PsO, ¶¶¶ mean (SD) | 53.8 (28.4) | 54.8 (30.0) | 51.0 (30.2) | 51.4 (27.6) | 53.5 (29.5) ‡‡‡ | 58.9 (29.4) | 54.6 (28.7) |
BID, twice daily; BSA, body surface area; DLQI, Dermatology Life Quality Index; FAS, full analysis set; ISI, Itch Severity Item; N, number of patients in the FAS; N1, number of patients in the FAS meeting endpoint‐specific baseline criteria as described in footnotes; NAPSI, Nail Psoriasis Severity Index; PASI, Psoriasis Area and Severity Index; PGA‐PsO, Physician’s Global Assessment of Psoriasis; PGJS‐VAS‐PsO, Patient’s Global Joint and Skin Assessment‐Visual Analog Scale‐Psoriasis question; PsA, psoriatic arthritis; Q2W, once every 2 weeks; SC, subcutaneous; SD, standard deviation.
Assessed in patients with baseline BSA ≥3% and PASI >0.
Scored on a 5‐point severity scale (0–4).
Assessed in patients with baseline PGA‐PsO >0.
Represents the definition for moderate to severe plaque psoriasis.
Based on one target fingernail affected, assessed in patients with baseline NAPSI value >0.
Defined as baseline NAPSI >0 based on one target fingernail chosen at baseline.
Assessed in patients with baseline ISI >0.
Patients answered the question ‘In all the ways your psoriasis affects you, how would you rate the way in which you felt over the past week?’, and their response was recorded on a VAS of 0–100 mm (0 = excellent; 100 = poor).
Data not available for 1 patient who received tofacitinib 5 mg BID (N = 130) and for 1 patient who received tofacitinib 10 mg BID (N = 131) in OPAL Beyond.
Data not available for 1 patient who received tofacitinib 5 mg BID in OPAL Beyond (N = 130).
Dermatologic efficacy endpoints
In both studies, the percentage of patients achieving PASI90 increased to Month 3 with tofacitinib, with significant differences vs. placebo at Month 1 for tofacitinib 5 mg BID in OPAL Broaden (P < 0.001) and for tofacitinib 10 mg BID in OPAL Beyond (P ≤ 0.05) (Fig. 1a). No differences were observed for adalimumab vs. placebo at Month 1 (Fig. 1a). PASI90 response rates at Month 3 were greater for tofacitinib 5 mg BID, tofacitinib 10 mg BID and adalimumab vs. placebo (P < 0.01, P < 0.001 and P ≤ 0.05, respectively) in OPAL Broaden and for tofacitinib 10 mg BID vs. placebo (P ≤ 0.05) in OPAL Beyond (Fig. 1a).
Figure 1.
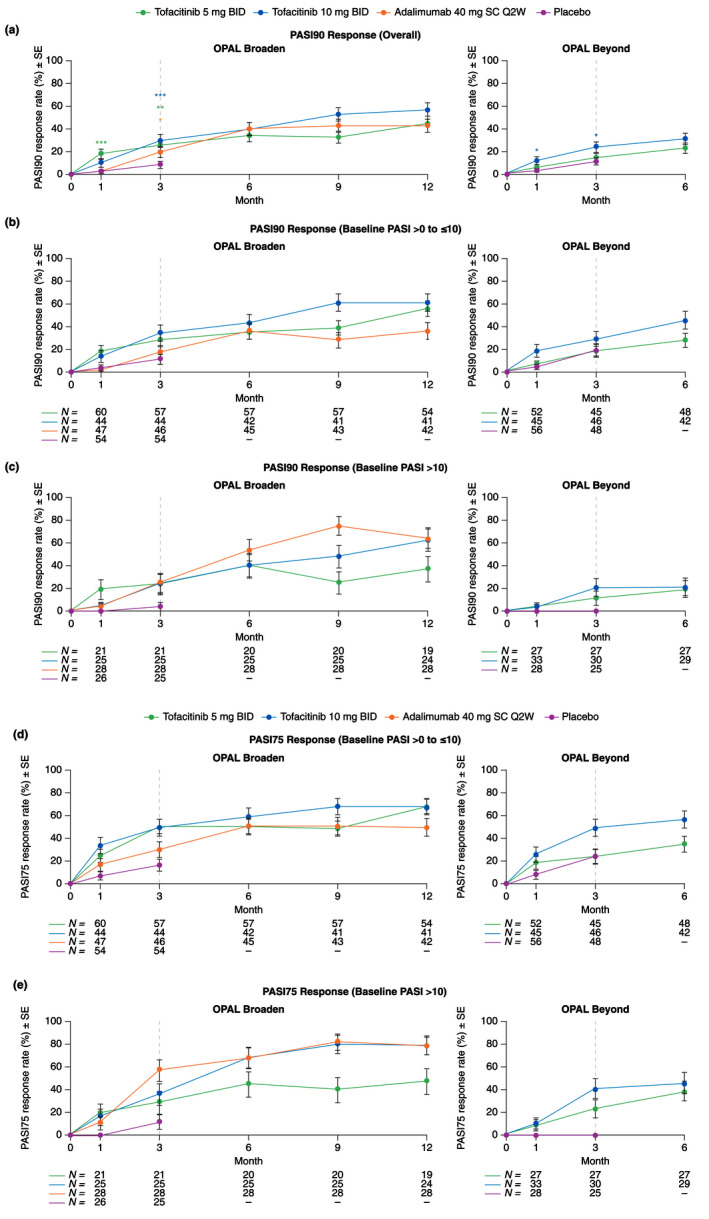
PASI90 and PASI75 response rates by baseline PASI severity (Assessed in patients with baseline BSA ≥3% and PASI >0; Post hoc analysis) (SE): (a) PASI90 (overall); (b) PASI90 (baseline PASI >0 to ≤10) (Baseline PASI >0 to ≤10, mild disease severity); (c) PASI90 (baseline PASI >10) (Baseline PASI >10, moderate–severe disease severity); (d) PASI75 (baseline PASI >0 to ≤10) (Baseline PASI >10, moderate–severe disease severity); (e) PASI75 (baseline PASI > 10) (Baseline PASI >10, moderate–severe disease severity). *Nominal P ≤ 0.05; **P < 0.01; ***P < 0.001 vs. placebo. Dashed lines indicate the end of the placebo‐controlled period (Month 3). For (a), treatment comparisons with placebo at Months 1 and 3 were based on normal approximation of the difference in binomial proportions. Missing responses were imputed as nonresponse at all time points. Patients included in the analysis had baseline BSA ≥3% and PASI >0 and patient numbers were the same across visits. In OPAL Broaden, the numbers of unique patients were 82, 70, 77 and 82 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively. In OPAL Beyond, the numbers of unique patients were 80, 81 and 86 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively. For (b) – (e), no imputation was performed as these were descriptive only. N is the number of patients evaluable at each visit in each subgroup. BID, twice daily; BSA, body surface area; PASI, Psoriasis Area and Severity Index; PASI75, ≥75% improvement from baseline in PASI; PASI90, ≥90% improvement from baseline in PASI; Q2W, once every 2 weeks; SC, subcutaneous; SE, standard error.
For all active treatment groups, PASI90 response rates increased from Month 3 to the end of each study (Fig. 1a). In OPAL Broaden, the percentages of patients achieving PASI90 were similar between both tofacitinib doses and adalimumab in the total patient population.
At Month 12 in OPAL Broaden, PASI90 response rates in patients with baseline PASI >0 to ≤10 were 55.6%, 61.0% or 35.7%, in patients who received tofacitinib 5 mg BID, tofacitinib 10 mg BID or adalimumab, respectively; comparatively, in patients with baseline PASI >10, response rates were 36.8%, 62.5% or 64.3%, respectively (Fig. 1b,c). At Month 6 in OPAL Beyond, PASI90 response rates in patients with baseline PASI >0 to ≤10 were 27.1% and 45.2% in patients who received tofacitinib 5 and 10 mg BID, respectively; comparatively, in patients with baseline PASI >10, response rates were 18.5% and 20.7%, respectively (Fig. 1b,c).
The percentage of patients achieving PASI75 have been reported previously for the total population. 7 , 8 In both studies, the percentage of patients with baseline PASI >0 to ≤10 or baseline PASI >10 achieving PASI75 were numerically higher with all active treatments vs. placebo at Month 1 and Month 3 (except tofacitinib 5 mg BID in OPAL Beyond at Month 3 in patients with baseline PASI >0 to ≤10) (Fig. 1d,e). Improvements in PASI75 response rates continued to increase to each study end for both baseline severities and were generally higher at study end than PASI90 response rates (Fig. 1b–e).
In OPAL Broaden, the percentage of patients with baseline PASI >0 to ≤10 achieving PASI75 were numerically higher for tofacitinib 10 mg BID vs. adalimumab (Fig. 1d). After Month 3, PASI75 response rates in the baseline PASI >10 subgroup were similar for tofacitinib 10 mg BID and adalimumab, and both were numerically higher than tofacitinib 5 mg BID (Fig. 1e). Least squares mean (LSM) % changes from baseline and LSM changes from baseline in PASI total score are reported in the online supplemental appendix (Table S1, Supporting Information).
A greater percentage of patients achieved PGA‐PsO response at Month 1 and Month 3 with both tofacitinib doses in both studies, and with adalimumab vs. placebo at Month 3 in OPAL Broaden (all P ≤ 0.05; Fig. 2). In all active treatment groups, the percentage of patients achieving PGA‐PsO response increased from Month 3 to study end in both OPAL Broaden and OPAL Beyond (Fig. 2). PGA‐PsO response rates in both studies were numerically greater with tofacitinib 10 mg BID compared with tofacitinib 5 mg BID at each time point (Fig. 2). Greater LSM changes from baseline in PGA‐PsO were seen at Month 1 and Month 3 with both tofacitinib doses in both studies, and with adalimumab vs. placebo in OPAL Broaden (all P < 0.01), and improvements were maintained to study end (Fig. S1, Supporting Information).
Figure 2.
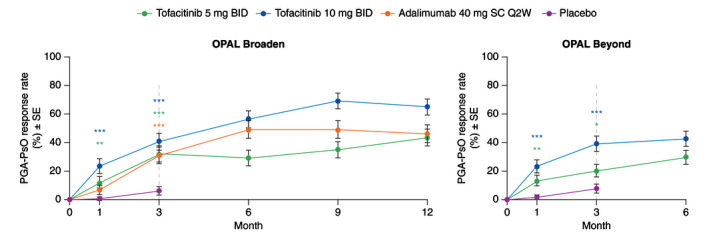
PGA‐PsO response rates (PGA‐PsO response is defined as a score of 0 (clear)/1 (almost clear) on a 0–4 scale and ≥2‐point improvement from baseline in patients with baseline PGA‐PsO ≥2; Post hoc analysis) (SE) among patients with baseline PGA‐PsO ≥2. *Nominal P ≤ 0.05; **P < 0.01; ***P < 0.001 vs. placebo. Dashed lines indicate the end of the placebo‐controlled period (Month 3). Treatment comparisons with placebo at Months 1 and 3 were based on normal approximation of the difference in binomial proportions. Missing responses were imputed as nonresponse. The numbers of patients included in the analysis were the same across visits. In OPAL Broaden, the numbers of unique patients were 71, 71, 67 and 74 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively. In OPAL Beyond, the numbers of unique patients were 87, 89 and 85 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively. BID, twice daily; PGA‐PsO, Physician’s Global Assessment of Psoriasis; Q2W, once every 2 weeks; SC, subcutaneous; SE, standard error.
While all active treatment groups demonstrated improvements to end of study in LSM change from baseline in Nail Psoriasis Severity Index (NAPSI), differences between tofacitinib or adalimumab and placebo did not achieve statistical significance at Month 3 in either study (Fig. 3).
Figure 3.
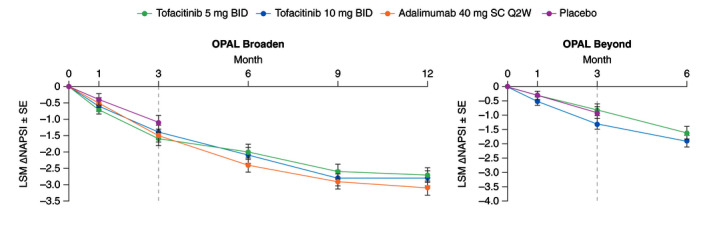
LSM (SE) change from baseline in NAPSI in patients with baseline NAPSI >0 (Data are based on one target fingernail affected; Prespecified analysis). Dashed lines indicate the end of the placebo‐controlled period (Month 3). In the analyses of change from baseline in NAPSI through the first 3 months, the two placebo‐to‐tofacitinib sequences were combined into a single placebo group (pooled placebo group); the results through Month 3 are from a repeated measures model. For results after Month 3, the two placebo‐to‐tofacitinib sequences were separate in another repeated measures model (data not shown). No imputation was applied for missing values. In OPAL Broaden, the numbers of unique patients included in the repeated measures model up to Month 3 were 79, 71, 88 and 86 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively; the number of patients included up to Month 12 were 80, 72 and 89 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and adalimumab 40 mg SC Q2W, respectively. In OPAL Beyond, the numbers of unique patients included in the repeated measures model were 85, 96 and 92 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively (same across all visits). ∆, change from baseline; BID, twice daily; LSM, least squares mean; NAPSI, Nail Psoriasis Severity Index; Q2W, once every 2 weeks; SC, subcutaneous; SE, standard error.
Dermatology‐related patient‐reported outcomes
Greater LSM changes from baseline in DLQI total score were seen at Month 1 and Month 3 with both tofacitinib doses vs. placebo in both studies (all P ≤ 0.05), and with adalimumab vs. placebo at Month 3 in OPAL Broaden (P ≤ 0.05; Fig. 4). Improvements in DLQI total score were maintained up to Month 12 in OPAL Broaden and Month 6 in OPAL Beyond (Fig. 4). At all time points, tofacitinib 10 mg BID demonstrated numerically greater LSM changes from baseline in DLQI total score vs. tofacitinib 5 mg BID and adalimumab in OPAL Broaden; tofacitinib 5 mg BID demonstrated similar improvements to adalimumab. LSM changes from baseline on DLQI sub‐dimensions and DLQI question #1 on skin symptoms are reported in the online supplemental appendix (Fig. S2, Supporting Information).
Figure 4.
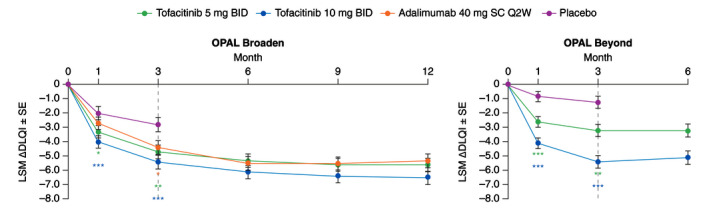
LSM (SE) change from baseline in DLQI total score (Prespecified analysis). *Nominal P ≤ 0.05; **P < 0.01; ***P < 0.001 vs. placebo. Dashed lines indicate the end of the placebo‐controlled period (Month 3). In the analyses of change from baseline in DLQI total score through the first 3 months, the two placebo‐to‐tofacitinib sequences were combined into a single placebo group (pooled placebo group); the results through Month 3 are from a repeated measures model. For results after Month 3, the two placebo‐to‐tofacitinib sequences were separate in another repeated measures model (data not shown). No imputation was applied for missing values. In OPAL Broaden, the numbers of unique patients included in the repeated measures model were 106, 104, 106 and 104 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively (same across all visits). In OPAL Beyond, the numbers of unique patients included in the repeated measures models were 128, 130 and 129 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively (same across all visits). ∆, change from baseline; BID, twice daily; DLQI, Dermatology Life Quality Index; LSM, least squares mean; Q2W, once every 2 weeks; SC, subcutaneous; SE, standard error.
Greater LSM changes from baseline in DLQI total score in patients with baseline BSA ≥3% and PASI >0 were seen at Month 1 and Month 3 with both tofacitinib doses in both studies, and with adalimumab vs. placebo at Month 3 in OPAL Broaden (all P ≤ 0.05; Table S2, Supporting Information).
The percentage of patients achieving the MCID (≥5 points) in change (improvement) from baseline in DLQI total score at Month 3 was higher with both doses of tofacitinib and adalimumab vs. placebo in OPAL Broaden (all P < 0.001) and OPAL Beyond (both P ≤ 0.05) (Table S3, Supporting Information).
Greater LSM changes (improvement) from baseline in Itch Severity Item (ISI) were seen at Month 1 and Month 3 with both tofacitinib doses in both studies (all P < 0.001), and with adalimumab vs. placebo in OPAL Broaden (P ≤ 0.05); improvements in ISI were maintained up to Month 12 in OPAL Broaden and Month 6 in OPAL Beyond (Fig. 5a). A greater percentage of patients achieved an ISI response (defined as an improvement from baseline ≥4 units) at Month 1 and Month 3 with both tofacitinib doses in both studies vs. placebo (all P < 0.01; Fig. 5b). In OPAL Beyond, LSM changes from baseline in ISI and percentage of patients achieving an ISI response were numerically greater with tofacitinib 10 mg BID compared with tofacitinib 5 mg BID. Similar results were observed with an ISI response threshold of ≥2 or ≥3 units (Fig. S3, Supporting Information). Note that the clinically important difference has been defined as 1.48. 14 The percentage of responders with an improvement from baseline on ISI in ≥2, ≥3 or ≥4 units was greater with adalimumab vs. placebo at Month 3 in OPAL Broaden (all P ≤ 0.05; Fig. 5b; Fig. S3, Supporting Information).
Figure 5.
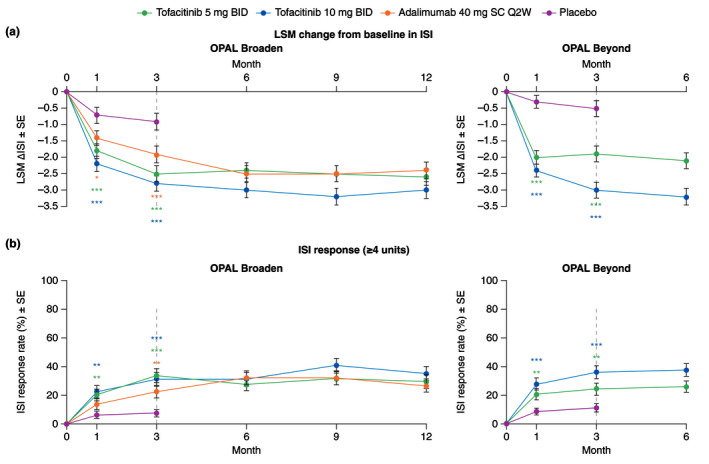
ISI in patients with baseline ISI >0, (a) LSM (SE) change from baseline (Prespecified analysis); (b) ISI response rate (≥4 units) (Post hoc analysis; Responders were patients with a ≥4 unit improvement from baseline. ISI score range 0–10, where 0 = no itching and 10 = worst possible itching). *Nominal P ≤ 0.05; **P < 0.01; ***P < 0.001 vs. placebo. Dashed lines indicate the end of the placebo‐controlled period (Month 3). For (a), in the analyses of change from baseline in ISI through the first 3 months, the two placebo‐to‐tofacitinib sequences were combined into a single placebo group (pooled placebo group); the results through Month 3 are from a repeated measures model. For results after Month 3, the two placebo‐to‐tofacitinib sequences were separate in another repeated measures model (data not shown). No imputation was applied for missing values. In OPAL Broaden, the numbers of unique patients included in the repeated measures model were 96, 93, 93 and 91 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively. In OPAL Beyond, the numbers of unique patients included in the repeated measures models were 109, 120 and 114 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively. For (b), in the ISI responder analysis, treatment comparisons with placebo at Months 1 and 3 were based on normal approximation of the difference in binomial proportions. Missing response was imputed as nonresponse. The numbers of patients included in the analysis were the same across visits. In OPAL Broaden, the numbers of unique patients were 97, 93, 93 and 92 for tofacitinib 5 mg BID, tofacitinib 10 mg BID, adalimumab 40 mg SC Q2W and placebo, respectively. In OPAL Beyond, the numbers of unique patients were 111, 122 and 115 for tofacitinib 5 mg BID, tofacitinib 10 mg BID and placebo, respectively. ∆, change from baseline; BID, twice daily; ISI, Itch Severity Item; LSM, least squares mean; Q2W, once every 2 weeks; SC, subcutaneous; SE, standard error.
Greater LSM change (improvement) from baseline in Patient’s Global Joint and Skin Assessment‐Visual Analog Scale‐Psoriasis question (PGJS‐VAS‐PsO) was seen at Months 1 and 3 with both tofacitinib doses in both studies (all P < 0.001), and with adalimumab vs. placebo in OPAL Broaden (P < 0.01) (Table S4, Supporting Information). The percentage of patients with ≥30%, ≥50% and ≥70% improvement from baseline in PGJS‐VAS‐PsO vs. placebo at Months 1 and 3 were greater with both tofacitinib doses in both studies (all P ≤ 0.05), and with adalimumab vs. placebo in OPAL Broaden (all P ≤ 0.05) (Table S5, Supporting Information).
Safety
Safety data have been previously reported for OPAL Broaden 7 and OPAL Beyond 8 and are summarized briefly in the online supplemental appendix.
Discussion
Using data from two phase 3 trials of tofacitinib in patients with active PsA (OPAL Broaden and OPAL Beyond), this analysis explored the efficacy of tofacitinib across a range of dermatologic outcomes and also assessed the effects of treatment by stratification of baseline severity.
The primary objectives of OPAL Broaden and OPAL Beyond were both met at Month 3; the primary trial publications reported that tofacitinib 5 and 10 mg BID were efficacious in reducing rheumatic signs and symptoms (American College of Rheumatology 20 [ACR20] response) and improving physical functioning (change from baseline in Health Assessment Questionnaire–Disability Index [HAQ‐DI]) score over 3 months. 7 , 8 A significantly higher rate of PASI75 response with tofacitinib vs. placebo at Month 3 was also reported for both trials, with the exception of the 5 mg dose in OPAL Beyond (P> 0.05). 7 , 8 The endpoints assessed in these trials represent PsA core domains. 15 Skin disease activity is also considered a PsA core domain and, while tofacitinib has demonstrated efficacy in patients with psoriasis 9 , 10 , 11 , 12 and nail psoriasis, 13 it is not approved for the treatment of psoriasis and further investigations in PsA are required.
Due to the heterogeneous presentation of PsA, it is critical that treatments are effective in controlling the range of signs and symptoms that can be experienced. 4 The symptomatic burden of PsA can negatively impact the physical and mental health of a patient, resulting in worse HRQoL. 4 , 16 Additionally, the presence of both active joint and skin disease in PsA has been associated with a more severe overall disease state, worse patient‐centered outcomes and increased healthcare resource utilization vs. patients with joint symptoms only. 17 For example, patients with both skin and joint involvement had a significantly higher current level of pain, a greater number of concomitant conditions and a higher number of PsA symptoms overall, including fatigue, compared to patients who had only joint involvement. Therefore, the effective treatment of joint and skin symptoms is necessary in order to improve HRQoL in patients with PsA and active psoriasis. 18 The Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey reported that in US patients with PsA, itching was second only to pain/swelling of the joints as the most important factor for contributing to disease severity. 19 Furthermore, a recent cross‐sectional patient survey reported that skin symptoms have a substantial emotional burden on patients, including feelings of shyness, embarrassment, shame and guilt. 20
According to the 2018 American College of Rheumatology/National Psoriasis Foundation (ACR/NPF) guideline for the treatment of PsA, a treat‐to‐target approach is recommended for patients with active PsA and should be based on a shared decision‐making between the patient and their physician, with consideration of the impact of symptoms across the domains, including dermatologic and rheumatologic. 21 An online survey found that for patients with symptoms of psoriatic disease, the most bothersome item was painful, inflamed or broken skin, followed closely by joint pain, soreness or tenderness, in contrast to dermatologists and rheumatologists, 22 suggesting that the successful treatment of dermatologic symptoms is a priority for patients, in addition to their rheumatologic symptoms. Therefore, there is a need to investigate how current therapies for PsA contribute to patient‐reported improvements of dermatologic symptoms.
Our results found that tofacitinib demonstrated improvements in PASI75 and PASI90 response rates in patients with both mild and moderate/severe skin disease, as well as improvements in PASI total score (LSM % change from baseline and LSM change from baseline) vs. placebo at Month 1 and Month 3. Both tofacitinib doses also demonstrated improvements in PGA‐PsO and HRQoL (assessed by DLQI), as well as improvements in itch (LSM change or improvements from baseline of ≥2, ≥3 or ≥4 units in ISI) and HRQoL associated with itchy and painful skin (as assessed by DLQI question #1 on skin symptoms) up to Month 3. Improvements in dermatologic symptoms were sustained up to Month 12 in OPAL Broaden and Month 6 in OPAL Beyond. Similar effects were observed with adalimumab vs. placebo in OPAL Broaden across these endpoints. Some differences between tofacitinib doses were observed. For example, in OPAL Beyond, tofacitinib 10 mg BID was associated with greater improvements compared with tofacitinib 5 mg BID across the dermatologic endpoints reported in this analysis, and in OPAL Broaden, a lower PASI75 response in patients with baseline PASI >10 was observed with tofacitinib 5 mg BID compared with tofacitinib 10 mg BID.
There were some limitations to this analysis. Due to the study designs, comparisons with placebo were limited to the 3‐month placebo‐controlled portion of the phase 3 studies. There was hierarchical testing in both OPAL Broaden and OPAL Beyond (see online supplemental appendix). Of the hierarchy, one skin endpoint (PASI75 response rate at Month 3) was included. With respect to PASI75 response, the comparison between tofacitinib 5 mg and 10 mg BID vs. placebo met statistical significance in OPAL Broaden, whereas only the comparison between tofacitinib 10 mg BID vs. placebo and not tofacitinib 5 mg BID vs. placebo was significant in OPAL Beyond. No other dermatologic endpoints were included in the hierarchical testing. Although numerical similarities and differences are reported, it should be noted that OPAL Broaden was not designed and was not powered for statistical comparisons between tofacitinib and adalimumab. Additionally, as some of the analyses were post hoc, without adjustment for multiplicity, this limits their interpretation. Finally, the PASI endpoint lacks sensitivity when BSA is <10%, which may mean that improvements were underestimated.
Conclusion
In conclusion, this analysis of dermatologic endpoints from OPAL Broaden and OPAL Beyond found that in patients with active PsA and IR to csDMARDs or TNFi, tofacitinib improved dermatologic signs and symptoms and dermatology‐related quality of life vs. placebo at Month 3. Improvements in PASI75 and PASI90 were observed regardless of baseline dermatologic disease severity. Importantly, clinically meaningful improvements in dermatologic endpoints were sustained to the end of each study. These data confirm that tofacitinib provides a treatment option for patients with active PsA including burdensome dermatologic symptoms.
Author contributions
JFM, JW, CW, DT and PY were involved in the conception and design of the study/analyses. JFM was involved in patient recruitment and/or data acquisition. JW and CW performed the data and statistical analyses. JFM, KAP, PN, JG, WHB, DT, DG, MAH, CW, JW and PY were involved in data interpretation. JFM, KAP, PN, JG, WHB, DT, DG, MAH, CW, JW and PY revised the manuscript for important intellectual content. JFM, KAP, PN, JG, WHB, DT, DG, MAH, CW, JW and PY gave final approval for publication.
Data‐sharing statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical‐trials/trial‐data‐and‐results for more information), Pfizer will provide access to individual de‐identified participant data from Pfizer‐sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de‐identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
Supporting information
Appendix S1. Supplementary material
Acknowledgments
This study was sponsored by Pfizer Inc. Medical writing support, under the guidance of the authors, was provided by Jennifer Higginson, PhD, CMC Connect, McCann Health Medical Communications and Carole Evans, PhD, on behalf of CMC Connect, and was funded by Pfizer Inc, New York, NY, USA in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med 2015;163:461–4).
Conflicts of interest
J. F. Merola is a consultant and/or investigator for AbbVie, Aclaris, Almirall, Biogen, Celgene, Dermavant, Eli Lilly, EMD Sorono, GSK, Incyte, Janssen, Kyowa Kirin Co, Leo Pharma, Merck Research Laboratories, Novartis, Pfizer Inc, Samumed, Sanofi Regeneron, Sun Pharma and UCB; and is a member of the Burrage Capital Management Boston Advisory Board. K.A. Papp has received personal fees and grant/research support from and has acted as a consultant, speaker and advisory board member for AbbVie, Akros, Amgen, Anacor, Arcutis, Astellas, Avillion, Baxalta, Biopharm, Boehringer Ingelheim, Botanix, Bristol‐Myers Squibb, Can‐Fite Biopharma, Celgene, Coherus, Dermavant, Dermira, Dow Pharma, Eli Lilly, EMD Serono, Galderma, Genentech, Gilead, Glenmark, GSK, Incyte, InflaRX GmbH, Janssen, Kyowa Hakko Kirin, Leo Pharma, MedImmune, Meiji Seika Pharma, Merck (MSD), Merck‐Serono, Mitsubishi Pharma, Moberg Pharma, Novartis, Pfizer Inc, PRCL Research, Regeneron, Roche, Sanofi, Genzyme, Sumitomo Dainippon, Sun Pharma, Takeda, UCB and Valeant (Bausch Health). P. Nash has received grant/research support from, has acted as a consultant and has participated in speaker’s bureaus for Pfizer Inc; and has received honoraria as a consultant or speaker for AbbVie, Bristol‐Myers Squibb, Celgene, Eli Lilly, Gilead, Janssen, MSD, Novartis, Roche, Sanofi and UCB. J. Gratacós has received grant/research support from, has acted as a consultant and has participated in speaker’s bureaus for Pfizer Inc. W.H. Boehncke has received honoraria as a consultant or speaker for Almirall, Bristol‐Myers Squibb, Celgene, Eli Lilly, Janssen‐Cilag, LEO Pharma, Novartis, Pfizer Inc and UCB; and has received a research grant from Pfizer Inc. D. Thaçi has acted as a consultant, investigator and speaker and has participated in advisory boards for AbbVie, Almirall, Amgen, Biogen Idec, Bristol‐Myers Squibb, Celgene Corp, Janssen‐Cilag, LEO Pharma, Lilly, MSD, Novartis, Pfizer Inc, Regeneron, Sanofi and UCB; and has received research/educational grants from AbbVie, Novartis and Celgene Corp. D. Graham, M‐A. Hsu, C. Wang, J. Wu and P. Young are employees of Pfizer Inc and hold stock/stock options in Pfizer Inc.
Funding sources
This study was sponsored by Pfizer Inc.
The copyright line for this article was changed on 19 August 2020 after original online publication
References
- 1. Merola JF, Espinoza LR, Fleischmann R. Distinguishing rheumatoid arthritis from psoriatic arthritis. RMD Open 2018; 4: e000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018; 391: 2273–2284. [DOI] [PubMed] [Google Scholar]
- 3. Gladman DD, Antoni C, Mease P, Clegg DO, Nash P. Psoriatic arthritis: epidemiology, clinical features, course, and outcome. Ann Rheum Dis 2005; 64: ii14–ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017; 376: 957–970. [DOI] [PubMed] [Google Scholar]
- 5. US Food and Drug Administration . Xeljanz® prescribing information. [WWW document] 2018. URL http://labeling.pfizer.com/showlabeling.aspx?id=959 (last accessed 22 April 2020).
- 6. European Medicines Agency . Xeljanz (tofacitinib) – summary of product characteristics. [WWW document] 2019. URL https://www.ema.europa.eu/en/documents/product‐information/xeljanz‐epar‐product‐information_en.pdf (last accessed 22 April 2020).
- 7. Mease P, Hall S, FitzGerald O et al Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017; 377: 1537–1550. [DOI] [PubMed] [Google Scholar]
- 8. Gladman D, Rigby W, Azevedo VF et al Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017; 377: 1525–1536. [DOI] [PubMed] [Google Scholar]
- 9. Bachelez H, van de Kerkhof PC, Strohal R et al Tofacitinib versus etanercept or placebo in moderate‐to‐severe chronic plaque psoriasis: a phase 3 randomised non‐inferiority trial. Lancet 2015; 386: 552–561. [DOI] [PubMed] [Google Scholar]
- 10. Bissonnette R, Iversen L, Sofen H et al Tofacitinib withdrawal and retreatment in moderate‐to‐severe chronic plaque psoriasis: a randomized controlled trial. Br J Dermatol 2015; 172: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 11. Papp KA, Menter MA, Abe M et al Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: results from two, randomized, placebo‐controlled, phase III trials. Br J Dermatol 2015; 173: 949–961. [DOI] [PubMed] [Google Scholar]
- 12. Papp KA, Krueger JG, Feldman SR et al Tofacitinib, an oral Janus kinase inhibitor, for the treatment of chronic plaque psoriasis: Long‐term efficacy and safety results from 2 randomized phase‐III studies and 1 open‐label long‐term extension study. J Am Acad Dermatol 2016; 74: 841–850. [DOI] [PubMed] [Google Scholar]
- 13. Merola JF, Elewski B, Tatulych S, Lan S, Tallman A, Kaur M. Efficacy of tofacitinib for the treatment of nail psoriasis: two 52‐week, randomized, controlled phase 3 studies in patients with moderate‐to‐severe plaque psoriasis. J Am Acad Dermatol 2017; 77: 79–87.e1. [DOI] [PubMed] [Google Scholar]
- 14. Ständer S, Luger T, Cappelleri JC et al Validation of the Itch Severity Item as a measurement tool for pruritus in patients with psoriasis: results from a phase 3 tofacitinib program. Acta Derm Venereol 2018; 98: 340–345. [DOI] [PubMed] [Google Scholar]
- 15. Orbai AM, de Wit M, Mease PJ et al Updating the Psoriatic Arthritis (PsA) core domain set: a report from the PsA Workshop at OMERACT 2016. J Rheumatol 2017; 44:1522–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018; 14: 405–417. [DOI] [PubMed] [Google Scholar]
- 17. de Vlam K, Merola JF, Birt JA et al Skin involvement in psoriatic arthritis worsens overall disease activity, patient‐reported outcomes, and increases healthcare resource utilization: an observational, cross‐sectional study. Rheumatol Ther 2018; 5: 423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gladman DD. Recent advances in understanding and managing psoriatic arthritis. F1000Res 2016; 5: 2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lebwohl MG, Kavanaugh A, Armstrong AW, Van Voorhees AS. US perspectives in the management of psoriasis and psoriatic arthritis: patient and physician results from the population‐based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) survey. Am J Clin Dermatol 2016; 17: 87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Merola JF, Shrom D, Eaton J et al Patient perspective on the burden of skin and joint symptoms of psoriatic arthritis: results of a multi‐national patient survey. Rheumatol Ther 2019; 6: 33–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Singh JA, Guyatt G, Ogdie A et al Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res (Hoboken) 2019; 71: 2–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Husni ME, Fernandez A, Hauber B et al Comparison of US patient, rheumatologist, and dermatologist perceptions of psoriatic disease symptoms: results from the DISCONNECT study. Arthritis Res Ther 2018; 20: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mease PJ. Measures of psoriatic arthritis: Tender and Swollen Joint Assessment, Psoriasis Area and Severity Index (PASI), Nail Psoriasis Severity Index (NAPSI), Modified Nail Psoriasis Severity Index (mNAPSI), Mander/Newcastle Enthesitis Index (MEI), Leeds Enthesitis Index (LEI), Spondyloarthritis Research Consortium of Canada (SPARCC), Maastricht Ankylosing Spondylitis Enthesis Score (MASES), Leeds Dactylitis Index (LDI), Patient Global for Psoriatic Arthritis, Dermatology Life Quality Index (DLQI), Psoriatic Arthritis Quality of Life (PsAQOL), Functional Assessment of Chronic Illness Therapy‐Fatigue (FACIT‐F), Psoriatic Arthritis Response Criteria (PsARC), Psoriatic Arthritis Joint Activity Index (PsAJAI), Disease Activity in Psoriatic Arthritis (DAPSA), and Composite Psoriatic Disease Activity Index (CPDAI). Arthritis Care Res (Hoboken) 2011; 63: S64–S85. [DOI] [PubMed] [Google Scholar]
- 24. Basra MK, Fenech R, Gatt RM, Salek MS, Finlay AY. The Dermatology Life Quality Index 1994–2007: a comprehensive review of validation data and clinical results. Br J Dermatol 2008; 159: 997–1035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary material


