Abstract
Background
Recent years have seen a growing interest in the appearance of the eyes among the concerns expressed by patients in cosmetic clinics. This has led to an increase in the frequency of diagnosis of tear trough deformity, and, as a result, the number of treatments performed by specialized professionals has also risen. Hyaluronic acid filler injection is a rapid, nonsurgical technique that gives good long‐lasting, but not permanent, results. However, to achieve optimal results, the attending physician must have good anatomical knowledge of the area and involvement of the structures in the tear trough, carry out proper clinical assessment of the patient, and use an appropriate injection technique with the right product.
Aims
To support good practice among the professionals who carry out these procedures, this interdisciplinary consensus document describes the relevant issues and recommendations, in order to improve safety standards and to help successfully resolve this aesthetic problem.
Keywords: filler injection, hyaluronic acid, recommendations, Tear trough deformity
1. INTRODUCTION
The eyes are the first thing we look at when we see a human face. 1 We search for information in them about basic emotions in our interactions, 2 , 3 but they also weigh heavily in our perception of the beauty of a face, more than any other facial component. 4 In fact, looking into someone's eyes activates the brain areas for both facial recognition and the emotions, as well as the areas allocated to the perception of beauty. 5 Thus, the presence of tear trough deformities will be an element that will influence both facial recognition (emotional aspects such as sadness or tiredness) and the perception of its attractiveness.
Recent years have seen a growing interest in the appearance of the eyes among the concerns expressed by patients in cosmetic clinics. This has increased the frequency of diagnosis of tear trough deformity, and consequently, the number of treatments performed has risen. The options have been developed significantly, and doctors and surgeons currently have various alternatives at their disposal, including hyaluronic acid (HA) gel fillers. 6 Nonsurgical techniques such as these are gaining widespread acceptance, 7 , 8 , 9 and this procedure has other desirable characteristics: It is quick to perform and has a long‐lasting, but not permanent, effect. 6 , 10 , 11 In fact, it is known that subcutaneously injected HA is absorbed within a year; however, volumizing effects partially persist due to neocollagenogenesis, angiogenesis, and proliferation of adipocytes in the area. 12 , 13
Nevertheless, this is one of the most challenging noninvasive aesthetic procedures for specialists, 6 , 14 because the possible adverse effects of the HA injection 15 are combined with the anatomical complexity of the area to be treated. 6
In short, the steady rise in procedures for the treatment of tear trough deformity with HA fillers, and the challenges in performing it satisfactorily, warrants a good practice proposal and compilation of recommendations by a multidisciplinary team, in order to improve safety standards and contribute to the successful resolution of this aesthetic problem.
2. THE GOALS OF HYALURONIC ACID INJECTION FOR THE TREATMENT OF TEAR TROUGH DEFORMITY
The addition or restoration of volume is part of a general strategy for rejuvenation and enhancement. 16 , 17 In tear trough deformity, there are several factors that contribute to the eyeball having a sunken appearance and a shadow appearing on the lower eyelid, 9 , 16 , 18 , 19 , 20 , 21 which we summarize in Table 1.
Table 1.
Structural characteristics that contribute to the appearance of the tear trough deformity
| Skin |
|
| Fat |
|
| Musculature |
|
| Bone |
|
In order to properly correct this deformity with HA injection and avoid undesirable effects, we must: (a) have good anatomical knowledge of the area and involvement of the structures in the tear trough; (b) carry out proper clinical assessment of the patient; (c) choose the correct product; and (d) use an appropriate injection technique.
3. ANATOMICAL ELEMENTS OF THE TEAR TROUGH AREA
The term “tear trough” refers to the depression formed in the medial lower eyelid, lateral to the lacrimal crest, and limited in its inferior aspect by the inferior orbital rim, 22 , 23 ending in a virtual vertical line projected from the pupillary axis. There are various structures in this area that, apart from contributing to the formation of the tear trough, should be carefully considered. They are shown in Figure 1 and described in more detail in Table 2.
Figure 1.
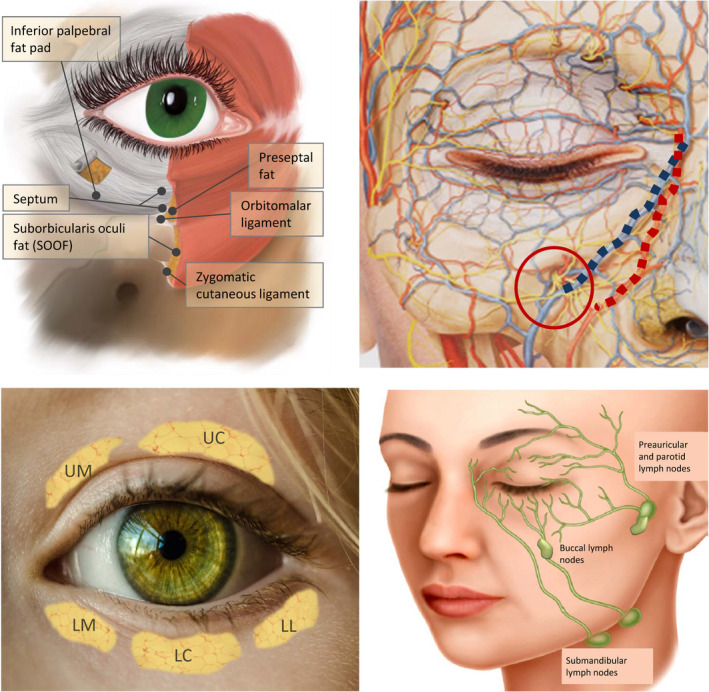
Anatomical structures involved in the formation of the tear trough deformity. This is located between the palpebral and orbital areas of the orbicularis oculi muscle, and the location of the nasojugal fold corresponds to the lower boundary of that muscle. Top left: Anatomy of the periocular area. Top right: Image of the vascular and nerve structures of the area. The blue line highlights the angular vein and the red the angular artery; the red circle indicates the emergence of the infraorbital nerve. Bottom left: Location of the fat pads: upper medial (UM), upper central (UC), lower medial (LM), lower central (LC), and lower lateral (LL). Bottom right: lymph drainage of the periocular area
Table 2.
Anatomical structures of the tear trough area and considerations for treatment
| Structure | Description | Considerations for treatment with HA injection |
|---|---|---|
| Orbicularis oculi muscle 39 | Forms a circle around the palpebral fissure and is divided into two parts: (a) orbital and (b) palpebral: in turn subdivided into pretarsal and preseptal | The region of the tear trough is located between the preseptal palpebral and orbital portion. In the transition between both, the muscle uses the orbitomalar ligament to insert itself into the bone, except for its innermost part, where it is inserted directly into it by means of small muscle fibers. It is not recommended to infiltrate this inner portion, as the fibers are so weak and fine that, even administering a submuscular injection, the HA could rise by positive pressure and compromise the lymphatic drainage of the region. |
| Malar septum 10 , 40 | Fibrous tissue that extends from the orbital rim, behind the orbicularis oculi muscle, to the tarsus, where it is inserted joining the capsulopalpebral fascia. | Extremely important as this is a barrier impermeable to the diffusion of fluids. The area anterior/superficial to this has a higher risk of suffering edema in the event of overload of lymphatic vessels. Do not inject superficial to the septum. |
| Fat pads 41 |
There are three lower pads: medial, central, and lateral. They are found behind the septum. |
In the management of tear trough deformity, the projection of the medial and central fat—which are separated by the inferior oblique muscle—is important. |
| Orbitomalar ligament | Originates at the level of the bone in the orbital rim and extends to the skin. | Where the inferior orbital rim is palpated is the upper limit of the area where we will inject the hyaluronic acid filler. |
| Preseptal‐preligamentous fat deposit 37 | A small amount of fat, which is not always present, in front of the septum and the orbitomalar ligament. | When it exists, it induces a relief that may make it difficult to determine the position of the orbitomalar ligament. If we inject above the latter and at the level of this fat by mistake, we could perforate the septum and enter at intraorbital level. |
| SOOF (suborbicularis oculi fat) 42 | Deposit of fatty tissue that is found between the orbitomalar ligament and the malar zygomatic. | It constitutes an ideal bed for the HA depots in the treatment of tear trough deformity, especially its highest part. |
| Zygomatic cutaneous ligament | Fibrous tissue that goes from the bone (between 0.2 and 1.1 mm below the inferior orbital rim) to the skin. | Defines the lower limit of the SOOF. |
| Vascular structures 43 | The angular artery emerges below the orbicularis oculi muscle and along the inner canthus of the eye, medial to the area where the HA treatment should be given. The angular vein, which drains into the facial vein, runs alongside it. The lacrimal vessels in the vicinity of the tear trough are superficial to the orbicularis | Although they are not the branches that vascularize the lower eyelid region, the angular artery is the most important structure to consider, due to its anatomical proximity to the injection zone. Both this and the angular vein should be taken into consideration to avoid intravascular injection. |
| Nerve structures 39 | The infraorbital nerve, which originates from the maxillary nerve (trigeminal branch), emerges through the infraorbital foramen to 0.6‐1 cm from the inferior orbital rim and at the level of the pupil. It is accompanied by the infraorbital artery and vein. | This nerve should be taken into account in the injection in the tear trough area since, although it is inferior and in many cases outside the injection area, poor practice on inserting the needle or cannula could damage it. |
| Lymphatic structures 37 , 44 |
The lymph vessels run at superficial level (dermis‐hypodermis). The outer third of the lower eyelid drains to the preauricular and parotid lymph nodes, while the inner two‐thirds drain to the submandibular lymph nodes. |
It is extremely important to perform deep injections so as not alter the lymph drainage. |
Figure 2 shows some examples of anatomical changes that can cause tear trough deformity. Among those that may be treatable with injection of the right HA product in well‐selected candidates after careful clinical and anatomical evaluation are excess fat in inferior palpebral fat pads with septal laxity (Figure 2B), receding of the inferior bony orbital rim (Figure 2C), hyperprojection of the intrinsic suborbicularis oculi fat (SOOF) caused by displacement of tissue with age or due to excess malar fat volume or muscle thickness defect (displacement of neighboring tissues) (Figure 2D) and thickness defect of skin or subcutaneous muscle tissue (Figure 2E). However, we should not confuse the presence of edema (Figure 2F) with tear trough deformity, since it requires a different approach or a complementary treatment if combined with tear trough deformity. Treating eye trough deformity means treating the change in volume, but also correcting the position of deep fat compartments, depending on the etiology of the deformity. In some cases, such as excess fat in inferior palpebral fat pads and septal laxity (Figure 2B) repositioning tissue by means of HA filler may improve the appearance. In any case, careful evaluation and patient selection are crucial.
Figure 2.
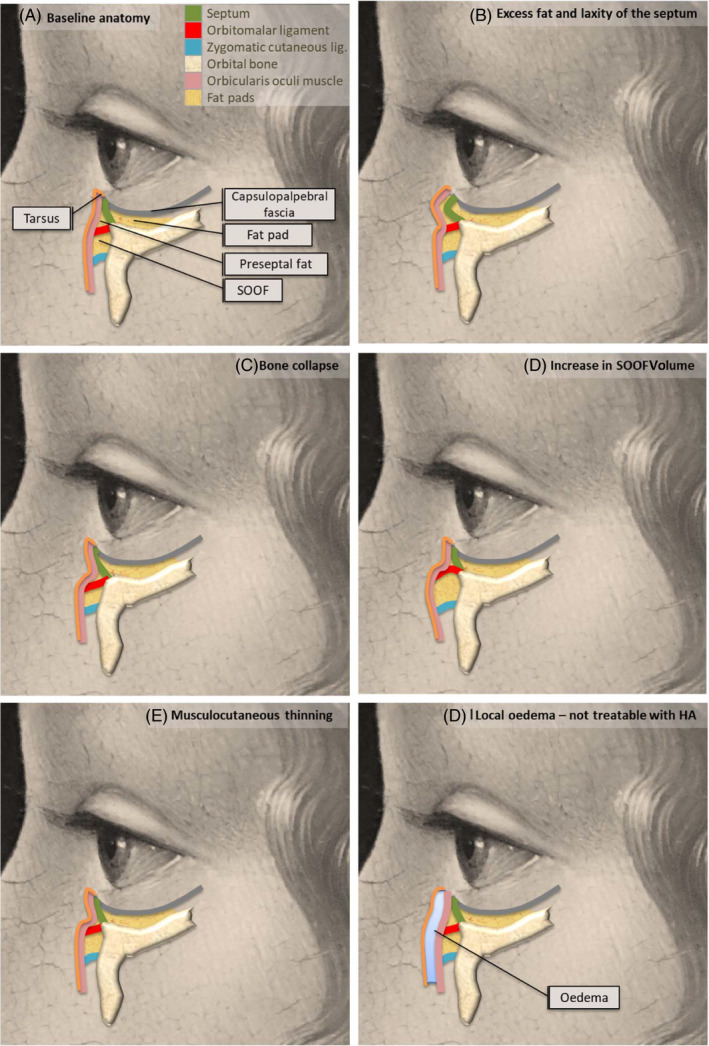
Examples of some anatomical causes of tear trough deformity. Panel A describes the baseline anatomy of the area. Cases B to E would be treatable with HA (in the absence of other characteristics that contradict it); Case F (local edema) would require another approach
4. PATIENT SELECTION
HA fillers are indicated for patients with appropriate anatomical conditions, 6 , 10 , 24 which may be an abstract concept, but a crucial point. To decide whether this is the best approach, it is useful to use a standard, objective classification system. Among the different classifications proposed 1 , 24 , 25 , 26 , 27 is the one described by Sadick et al, 28 who designed a numerical rating scale according to the clinical signs described in Table 3. Analysis of the clinical findings allows us to propose a quantification method to provide a guideline on the suitability of this treatment (Table 4). The ideal candidate for HA treatment is described in Figure 3. Nevertheless, the indication for treatment will depend on careful assessment and staging of the circumstances associated with the tear trough deformity. 27
Table 3.
Anatomical characteristics that affect the classification of the tear trough deformity and the hyaluronic acid treatment assessment
| Component | Description | Considerations for treatment | ||
|---|---|---|---|---|
| Pretarsal orbicularis oculi hypertrophy |
|
This is a diffuse muscle thickening, not greater than 4 mm, along the entire length of the lower eyelid, just below and parallel to the eyelashes that causes a fold |
This fold does not constitute a tear trough deformity per se. Treatment is not contraindicated, but it is very risky to improve it with HA. |
|
| Skin hyperpigmentation |

|
Darkening or coloration that does not contribute to the depth and extension of the tear trough deformity, but can be confused or accentuate the effects of a depression. |
Its presence may be due to various causes, and not all are treatable with HA. 27 The use of HA is indicated only when tear trough deformity (depression) and hyperpigmentation coexist. The reduction in the concavity may help the light to reflect more homogeneously. |
|
| Laxity/ skin wrinkles |
|
To evaluate the degree of laxity/photoaging, we can use the Glogau scale 45 |
The skin should be firm and of acceptable thickness. Marked skin laxity may lead to excess product use. If fluid retention by the HA occurs, the skin will be unable to contain it and it will become more obvious |
|
| Fat pad prolapse |
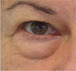
|
Anterior projection of the pad that contributes to the perception of depth of the tear trough deformity. The more pronounced the prolapse (medial and/or central), the greater this perception will be |
HA treatment is not contraindicated, but there are limitations: the greater the degree of prolapse, the harder it is to achieve a good result, as a higher volume needs to be injected into a very narrow area. |
|
| Lower eyelid/malar edema |
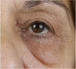
|
Periocular fluid retention that contributes to the tear trough deformity. It is essential to know the etiology to determine whether HA is the best approach. | Periocular edema should be addressed according to its etiology. 46 The HA could aggravate this accumulation of fluids due to its hygroscopic potential and the compression of lymph structures that could already be damaged beforehand. | |
| Extension of the depression | This will influence the amount of HA to inject, and therefore the limitations should be taken into account according to the quality of the skin and likelihood of lymphatic compression. | |||
Table 4.
Tear trough deformity clinical evaluation scale for hyaluronic filler treatment
| Points a | Orbicularis oculi hypertrophy | Hyperpigmentation | Myocutaneous laxity b | Fat pad prolapse | Fluid retention |
|---|---|---|---|---|---|
| 0 | None | None | Minimal | Minimal | None |
| 1 | Not very marked | Not very marked | Glogau 1 | Mild | Mild |
| 2 | Marked | Marked | Glogau 2 | Moderate | – |
| 3 | – | – | Glogau 3 | Severe | Moderate |
| 4 | – | – | Glogau 4 | – | Severe |
The patient score is obtained by adding the points obtained in each column: (i) The patient with values less than 2 is the ideal candidate for HA treatment. (ii) A tear trough deformity with a score between 2 and 8 could be treated with HA and should be assessed individually. (iii) We do not recommend treatment if the score is greater than 8 (assess surgery).
Points assigned in each column/factor that will be applied to the summation.
According to the Glogau scale. 45
Figure 3.
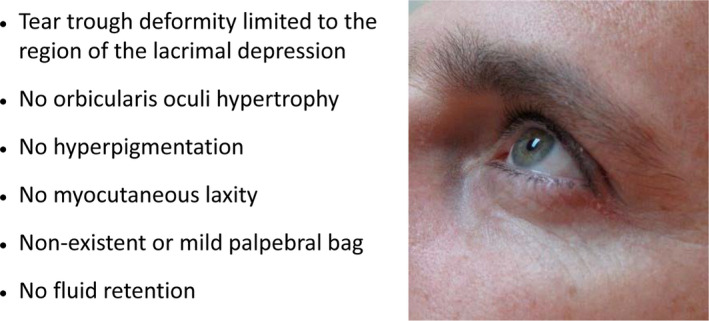
Characteristics of the ideal candidate for HA filler for tear trough deformity
5. CLINICAL EXAMINATION AND TREATMENT DECISION
5.1. Absolute contraindications
History of hypersensitivity to any of the components of the filler (detailed in the package leaflet of the recommended product 29 ).
History of diseases affecting the immune system.
Active dermatological disorders (herpes, acne, rosacea) or unhealed skin lesions.
Pregnancy or lactation.
Previous injection of permanent products in the area (silicon, acrylic polymers, etc).
The presence of permanent fillers in the area is an absolute contraindication for several reasons. First, the filler will have altered the anatomical structures of the periocular region. This is a very narrow area, and HA needs to be injected with great precision in depth. If another filler is present, it will be difficult to deposit HA in the periosteum and avoid the formation of lumps and irregularities. Additionally, potential incompatibilities should be borne in mind: Often the attending professional might not know the nature and characteristics of the previous filler used. The behavior of these fillers in combination cannot be foreseen, and the immunological response elicited by the previous filler is also unknown.
5.2. Diagnostic protocol
During the examination, we recommend that the patient is seated in a semi‐upright position. The characteristics described in Table 3 should be reviewed.
Determine whether there is lymphatic insufficiency: exert slight pressure on the eye, delimiting the inferior orbital rim, and assess periocular fluid retention. Pressure on the globe may allow orbital fat to become more visible and show a loose septum.
Delineate the depth of the tear trough deformity: The patient should look up.
Differentiate the presence of fat pad prolapse or periocular fluid retention, according to their physical and physiological characteristics. 23
Evaluate the grade of eyelid hyperlaxity using the snap‐back test.
Finding problems with any of these structures does not necessarily contraindicate the treatment (Table 3), but they will have to be taken into account, determining whether any intervention is needed before the HA injection. Very importantly, we must differentiate whether volume is caused by prolapse of the lower periocular fat or due to the presence of edema. If the cause is a slight prolapse of the fat, SOOF displacement, and atrophy, we will act as we recommend. If, on the other hand, we diagnose the presence of edema, we must exercise extreme caution in our treatment. We may decide that the patient is not a candidate for HA filler or, if we proceed with HA injection, we will reduce the amount in each session and increase the number of sessions in order to minimize the risk of increasing edema.
5.3. Examination and managing the patient's expectations
We need to talk to the patient about their expectations and to what extent the treatment may fulfill them, as well as making them aware of safety issues and potential complications. Although a good diagnosis, professionalism, and the use of top‐quality products are a combination with a high likelihood of success, the patient must understand that he or she is also an active part in the process. Having good communication and achieving maximum compliance with pretreatment and post‐treatment care will improve the result.
6. TREATMENT WITH HA FILLERS
Once HA injection is decided, the basic technique to prevent a poor outcome or minimize the risk of complications is independent of the etiology of the deformity.
6.1. Instructions prior to treatment
To prevent complications, particularly those related to potential hemorrhages, 11 , 30 it is important to check whether the patient is receiving chronic treatment with oral anticoagulants, antiplatelet agents, or nonsteroidal anti‐inflammatory drugs. If so, they should be instructed to discuss their intention to undergo this procedure with the attending physician, so that preventive measures can be taken. It is also recommended to review the patient's diet and possible use of supplements, 31 since certain foods—including various commonly eaten fruits, garlic, onion, and products like Ginkgo biloba—may contribute to bleeding due to their content of substances such as salicylates, vitamin C, or vitamin E, and their consumption should be reduced. Using vitamin K creams for a few days before the procedure will also help to prevent hematomas. 32 , 33 , 34 It is recommended that the patient does no strenuous exercise in the previous days/hours. 35 Physical exercise is physiologically a natural vasodilator, so it can increase the risk of bruises or their intensity. Blood pressure increases, favoring fluid extravasation and swelling. In addition, a greater dilation of the vascular system will produce a greater influx of anti‐inflammatory cellular elements, which could lead to changes in the characteristics and duration of HA filling.
6.2. Filling procedure
It is very important to select the appropriate filler and to know how to locate the entry point reproducibly in all patients. The characteristics of the area and its vascular complexity determine the choice of both technique and injection material to use, but the type of deformity and the patient's previous situation, as well as the treating professional's strategy, are also important.
6.2.1. Choice of product
To avoid complications, it is helpful to know the physiology of the fillers, the properties derived from the level of HA cross‐linking, particle sizes and concentrations, and the function of other active substances. For example, a high level of cross‐linking is associated with longer‐lasting fillers, as is larger particle size, but it also increases water absorption and subsequent edema. It has been postulated that products with these characteristics could produce more adverse reactions. 30
There is currently only one product formulated specifically to treat the eye contour, with approved indication in Europe: Redensity II (Teosyal® Puresense Redensity [II], Teoxane, Geneva). The formula for this gel combines a mixture of cross‐linked (70%) and noncross‐linked HA (30%), with a concentration of 15 mg/ml of product, resulting in a rheological profile that is suitable for filling this area. 29 Its hygroscopic behavior is low, and it retains little water. Moreover, due to its characteristics, it has been observed that it greatly reduces the likelihood of inflammation, 8 , 36 and a combination of vitamins, minerals, and amino acids has been added to help improve the quality of surrounding dermic structures. Several studies support its excellent efficacy and safety outcomes in well‐selected patients using a suitable technique such as that described in this document. 8 , 10 , 36 , 37
6.2.2. Location of the entry point
The protocol should provide the maximum safety and efficacy in all patients. It is essential to know how to correctly locate the entry point for each eye to be treated, and this is determined from its mathematical definition: the intersection between two lines. The entry points will depend on the choice of injection method.
Injection: Cannula or needle
Good results can be obtained with both cannula and needle. It is important to know the particular characteristics of each option and the appropriate technique and that the specialist must always perform the procedure carefully and slowly to produce the least trauma possible.
Cannula
The cannula offers a high level of safety in the process, taking into account the complexity of this anatomical region due to its vascularization, nerve supply, and combination of muscle and fatty structures. We describe the technique below:
-
1
The entry point is located as indicated in Figure 4. We describe two options to locate it: A) by determining the point at which the nasojugal fold projection line converges with the perpendicular line to the outer canthus of the eye or B) by locating the midpoint of the perpendicular projection from the outer canthus of the eye between the two virtual lines that delimit the upper and lower rim of the zygomatic bone.
Figure 4.
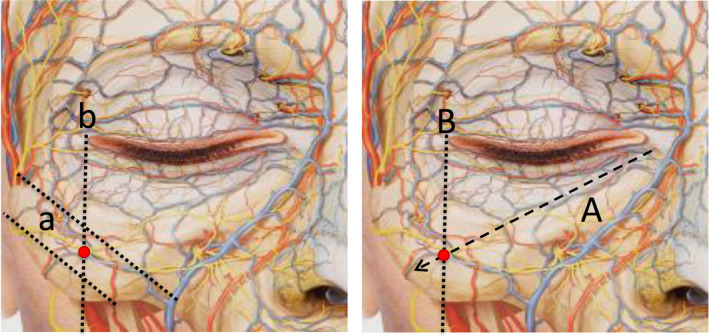
Two options for locating the entry point are shown. Option 1 (left): The a lines delimit the upper and lower boundaries of the zygomatic bone. Line b marks the perpendicular projection of the outer canthus of the eye. The entry point is located as the midpoint of line b between the two a lines. Option 2 (right): Line A represents the projection of the nasojugal fold. Line B marks the perpendicular projection of the outer canthus of the eye. The entry point is the intersection between these lines
The procedure is the same regardless of the method used to locate the entry point:
-
2
The skin is pierced with a 23G needle perpendicular to the skin (90°), inserting it deeply to go through the superficial musculoaponeurotic system (SMAS), after which it is removed.
-
3
A 25G or 27G cannula is inserted; it is important to observe that it reaches the periosteum and guarantee that it is situated in a deep plane.
-
4
The cannula is rotated from 90° to a 45° angle, continuing with this inclination smoothly and continuously in the direction of the valley that represents the tear trough to the injection position. A slight difficulty in passing should be noted, due to the presence of the orbitomalar ligament, which should not be crossed. At that point, the cannula will be in the right place.
-
5
The HA is then deposited in a retrograde manner until the depression is corrected. A gentle massage is performed to distribute the product within the eye trough. 35 In this regard, the eye trough is an anatomically limited area (Figure 5) and the product cannot escape when it is properly injected in the deep suborbicular fat package. If injection was well performed, product displacement does not occur, while the massage allows homogenization of the filling.
Figure 5.
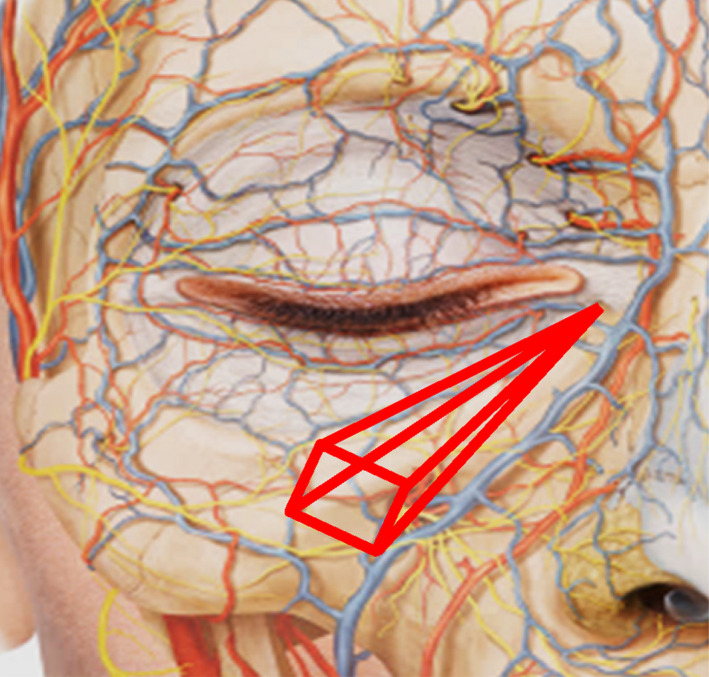
Anatomical limits of the eye trough. Internal limit: Lower lacrimal sac; bottom limit: bone; superior lateral limit: Curvature of the orbicular ligament; lower lateral limit: Facial vein and elevator muscle of the lip and nasal flap; superior limit: orbicularis muscle; lateral limit: zygomatic ligament
Needle
If the physician opts for injection by needle, it is recommended to use a 28G caliber, 19‐mm‐long needle, although the 30.5G needle supplied with the product can also be used. Since the needle is shorter than the cannula, two entry points are needed to reach appropriately the whole area. Safety is paramount, so location of entry points, as with cannula, takes into account the underlying structures.
-
1
The entry points are located as indicated in Figure 6. The skin is punctured with the needle perpendicular to the skin (90°), inserting it deeply until it reaches the periosteum.
-
2
It is reoriented horizontally so that it remains parallel to the underlying bone plane and advanced slowly to the injection area.
-
3
The HA can be applied using a linear retrograde fanning technique 38 (Figure 6), depositing the product at bone level, below the orbicularis oculi muscle.
-
4
If the patient requires treatment of the palpebromalar grooves, then the injection is given from the second needle insertion point, as described in Figure 5.
-
5
A gentle massage is performed to ensure that the filler stays in the right place.
Figure 6.
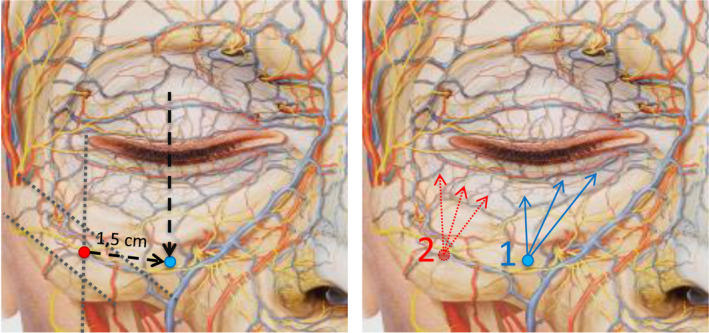
Left. Diagram locating the entry point for needle injection. Left. The initial reference point (red) is located using the references described for the cannula injection in Figure 4. Starting from it, the needle insertion point (blue) will be located by advancing toward the projection of the midpupillary line, at approximately 1.5 cm, tracing a downward diagonal parallel to the orbital rim. It is important not to advance more toward the vascular/nerve bundle. Right. The needle is inserted at point 1, and the product is deposited according to the linear retrograde fanning technique, starting with the points indicated by the blue arrows. If the patient also needs treatment of the palpebromalar groove, the needle will be inserted at point 2, injecting using the same technique (dotted red arrows)
6.2.3. Issues further to the injection technique
Regardless of the technique selected, some basic rules must be observed in the injection of HA:
-
6
It must be deep: to avoid compression in the lymphatics (situated in superficial planes) and to avoid the appearance of the Tyndall effect. We do not recommend the injection of the mini‐fat pad that exists in some patients, between the orbitomalar and zygomatic‐malar ligaments, due to the potential risk of altering lymphatic drainage. Neither, of course, injection at the level of the orbitomalar ligament or cranially to it. It is very important that it does not remain superficial to the malar septum. 10 , 37
-
7
It must be slow: to allow the product to accommodate properly to the depression and to avoid overcorrection by injecting too much product.
-
8
Unnecessary movements of the cannula or needle must be avoided: to prevent or reduce trauma to the tissues and, in particular, to the delicate lymph vessels.
-
9
The amount of filler used should be a maximum of 0.5 ml per tear trough and session. That is, it is advisable to initially perform undercorrection, as the HA injected will undergo a process of consolidation, drying, and subsequent rehydration in the next 30 days. 10 , 37 More HA may be added in subsequent sessions considering this time frame.
In all cases, we intend a “revolumization” of the area. The technique will be the same in each case, adapting to the intensity of the anatomical defect of each patient by adjusting the amount of final product, the volume per session, and, therefore, the number of sessions. We can suggest volume issues depending on the defect, and we add it in the article. It is estimated that it will be greater in cases of bone collapse, because a recovery of the projection must be made, and less in the case of increased SOOF, in which only a volume adjustment is necessary to achieve a smooth transition from cranial to flow. In case of muscle‐skin thinning, the amount required is also usually low. If the problem is excess fat and septum laxity, this amount will vary depending on the severity of the defect. Examples of correction of eye trough deformity of various etiologies are shown in Figure 7.
Figure 7.
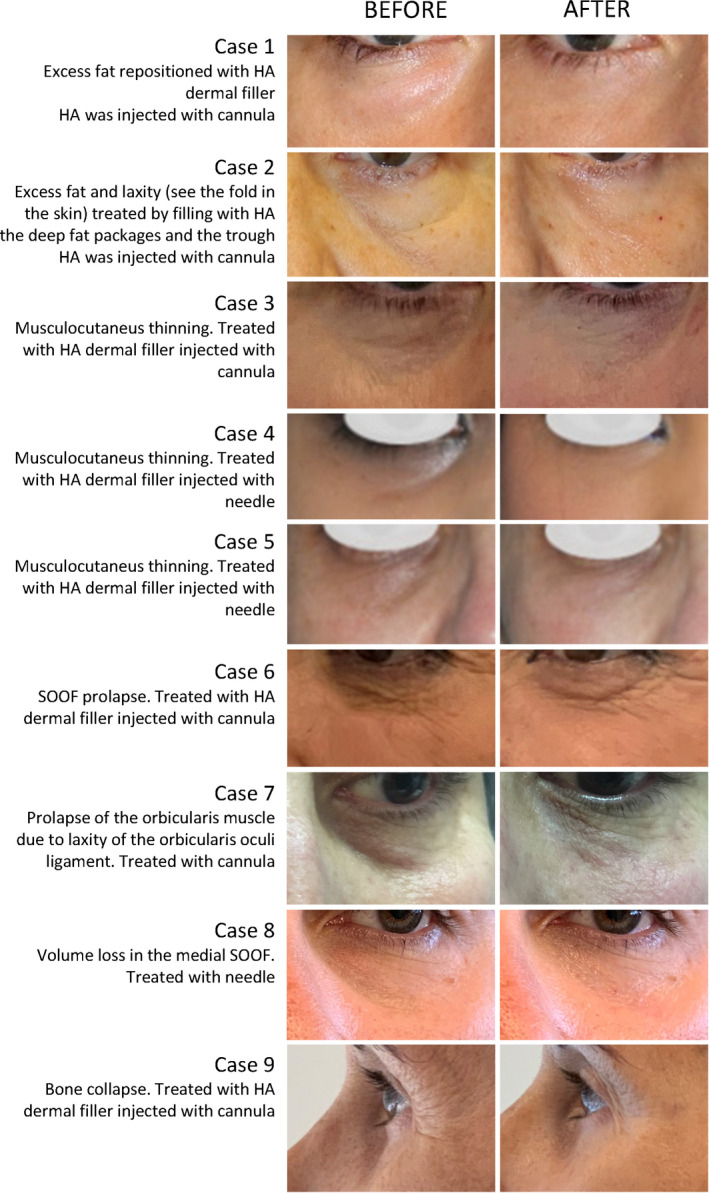
Examples of eye trough deformity of various etiologies and the outcome after HA filler injection
6.3. Recommendations following HA filler injections in the tear trough
A follow‐up visit is recommended 7‐15 days after the HA injections (the initial injection and after possible subsequent procedures). New injections must not be administered for 28 days, considering the consolidation and rehydration time of the HA, to avoid overcorrection. If no new HA injections are administered, the following check‐up will be at 6 months. To prevent or reduce possible hematomas, it may be useful to apply a vitamin K or liposomal lactoferrin cream to the area for a few days. 32 , 33 , 34 Other complications such as edema may be reduced or prevented by avoiding localized heat or wearing accessories that compress the treated area for a while. 35 It is prudent for the patient to avoid strenuous physical exercise and brusque movements that could cause trauma in the area in the week following the procedure. 35
Finally, the results achieved after treatment of tear trough deformity will be enhanced if we offer the patient education as regards health and prevention of aging through healthy habits such as avoiding consumption of alcohol, tobacco, and excess salt and favoring a diet with antioxidants and abundant water, as well as preventing sun exposure and using protection.
7. UNDESIRABLE SITUATIONS AND POSSIBLE CAUSES
The description of the approach, technique, and choice of product provided here are intended to avoid complications and other undesirable situations after applying HA fillers. The management of complications is outside the scope of the present document. As an additional guide for practice, in Table 5 we describe some situations that may arise and their potential cause.
Table 5.
Undesirable situations and adverse effects with their possible causes
| Situation or adverse effect | Frequency reported | Most common cause | Observations |
|---|---|---|---|
| Bruise | 10.7% a | Trauma at the time of treatment | Disappears spontaneously in 4‐7 days. Vitamin K reduces the likelihood of appearance of bruising, as well as its duration. |
| Irregular correction | 0% a , b | Injection too fast or poor patient selection (eg palpebral myocutaneous hyperlaxity) | Will require further action |
| Transient edema or inflammation | 11.6% a | Trauma at the time of treatment | Disappears spontaneously in 4‐7 days. |
| Severe edema | 0.9% a | Injection performed in a superficial plane, with too much filler or excessive extrusion force | Will require further action |
| Tyndall effect | 2.6% a , c | Injection was performed in superficial plane 47 | Will require further action |
Frequencies reported in the literature with the product recommended, specifically formulated to treat this area. 8
Irregular correction is reported in the literature to be up to 33% with some HA fillers, 47 , 48 but appropriate technique with the recommended product prevents such problem. 8
Tyndall effect was described as a temporary problem in safety study with the product recommended. 8 Deep injection following the present recommendations prevents this issue.
8. CONCLUSIONS
In summary, the use of filler for the treatment of tear trough deformity is a noninvasive procedure whose popularity continues to rise. The more its use increases, the higher too the likelihood of undesirable effects, so it is essential to be aware of its difficulty and to maintain good practice at all times so that the procedure is successful. This document has summarized the fundamental points of the anatomy of the area and offered recommendations for the right patient choice. It has also provided guidelines for performing a good technique with what is currently the only HA filler specifically formulated for the treatment of tear trough deformity, as it fulfills the right characteristics to be the filler of choice.
AUTHOR CONTRIBUTIONS
All the authors contributed scientific content to the manuscript and performed critical reviews until its final approval.
ACKNOWLEDGMENTS
The authors would like to thank Dr Blanca Piedrafita (MSC, Valencia) for compiling the author's contributions, writing, and editing. These services were funded by Teoxane Ibérica S. L. The authors would like to thank Marta Fernández Pérez for the drawing and preparation of the first panel of Figure 1, as well as the release by the editorial Quintessenz for the image used in Figures 1,4 and 5, from the book “The face” (Randlanski and Wesker; page 204; ISBN 978‐1‐85097‐289‐1).
Anido J, Fernández JM, Genol I, Ribé N, Pérez Sevilla G. Recommendations for the treatment of tear trough deformity with cross‐linked hyaluronic acid filler. J Cosmet Dermatol. 2021;20:6–17. 10.1111/jocd.13475
REFERENCES
- 1. Barton JJ, Radcliffe N, Cherkasova MV, Edelman J, Intriligator JM. Information processing during face recognition: the effects of familiarity, inversion, and morphing on scanning fixations. Perception. 2006;35(8):1089‐1105. [DOI] [PubMed] [Google Scholar]
- 2. Vaidya AR, Jin C, Fellows LK. Eye spy: the predictive value of fixation patterns in detecting subtle and extreme emotions from faces. Cognition. 2014;133(2):443‐456. [DOI] [PubMed] [Google Scholar]
- 3. Wegrzyn M, Vogt M, Kireclioglu B, Schneider J, Kissler J. Mapping the emotional face. How individual face parts contribute to successful emotion recognition. PLoS ONE. 2017;12(5):e0177239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saegusa C, Watanabe K. Judgments of facial attractiveness as a combination of facial parts information over time: Social and aesthetic factors. J Exp Psychol Hum Percept Perform. 2016;42(2):173‐179. [DOI] [PubMed] [Google Scholar]
- 5. Chatterjee A, Thomas A, Smith SE, Aguirre GK. The neural response to facial attractiveness. Neuropsychology. 2009;23(2):135‐143. [DOI] [PubMed] [Google Scholar]
- 6. Stutman RL, Codner MA. Tear trough deformity: review of anatomy and treatment options. Aesthet Surg J. 2012;32(4):426‐440. [DOI] [PubMed] [Google Scholar]
- 7. Alam M, Tung R. Injection technique in neurotoxins and fillers: planning and basic technique. J Am Acad Dermatol. 2018;79(3):407‐419. [DOI] [PubMed] [Google Scholar]
- 8. Berguiga M, Galatoire O. Tear trough rejuvenation: a safety evaluation of the treatment by a semi‐cross‐linked hyaluronic acid filler. Orbit. 2017;36(1):22‐26. [DOI] [PubMed] [Google Scholar]
- 9. Wollina U. Improvement of tear trough by monophasic hyaluronic Acid and calcium hydroxylapatite. J Clin Aesthet Dermatol. 2014;7(10):38‐43. [PMC free article] [PubMed] [Google Scholar]
- 10. Pascali M, Quarato D, Pagnoni M, Carinci F. Tear trough deformity: study of filling procedures for its correction. J Craniofac Surg. 2017;28(8):2012‐2015. [DOI] [PubMed] [Google Scholar]
- 11. Walker K, Pellegrini MV. Hyaluronic Acid. StatPearls. Treasure Island (FL): StatPearls Publishing StatPearls Publishing LLC. 2018.
- 12. Sisti A, Boczar D, Restrepo DJ, Nisi G, Forte AJ. Evaluation of the In Vivo Kinetics and Biostimulatory Effects of Subcutaneously Injected Hyaluronic Acid Filler. Plast Reconstr Surg. 2019;143(3):659e. [DOI] [PubMed] [Google Scholar]
- 13. Mochizuki M, Aoi N, Gonda K, Hirabayashi S, Komuro Y. Evaluation of the In vivo kinetics and biostimulatory effects of subcutaneously injected hyaluronic acid filler. Plast Reconstr Surg. 2018;142(1):112‐121. [DOI] [PubMed] [Google Scholar]
- 14. Matarasso SL, Carruthers JD, Jewell ML. Consensus recommendations for soft‐tissue augmentation with nonanimal stabilized hyaluronic acid (Restylane). Plast Reconstr Surg. 2006;117:3S‐34S. [DOI] [PubMed] [Google Scholar]
- 15. Duffy DM. Complications of fillers: overview. Dermatol Surg. 2005;31(11 Pt 2):1626‐1633. [DOI] [PubMed] [Google Scholar]
- 16. Donath AS, Glasgold RA, Glasgold MJ. Volume loss versus gravity: new concepts in facial aging. Curr Opin Otolaryngol Head Neck Surg. 2007;15(4):238‐243. [DOI] [PubMed] [Google Scholar]
- 17. Raspaldo H, Gassia V, Niforos FR, Michaud T. Global, 3‐dimensional approach to natural rejuvenation: part 1 ‐ recommendations for volume restoration and the periocular area. J Cosmet Dermatol. 2012;11(4):279‐289. [DOI] [PubMed] [Google Scholar]
- 18. de Maio M, DeBoulle K, Braz A, Rohrich RJ. Facial assessment and injection guide for botulinum toxin and injectable hyaluronic acid fillers: focus on the midface. Plast Reconstr Surg. 2017;140(4):540e‐e550. [DOI] [PubMed] [Google Scholar]
- 19. Kikkawa DO, Lemke BN, Dortzbach RK. Relations of the superficial musculoaponeurotic system to the orbit and characterization of the orbitomalar ligament. Ophthalmic Plast Reconstr Surg. 1996;12(2):77‐88. [DOI] [PubMed] [Google Scholar]
- 20. Lambros V. Observations on periorbital and midface aging. Plast Reconstr Surg. 2007;120(5):1367‐1376. [DOI] [PubMed] [Google Scholar]
- 21. Lee JH, Hong G. Definitions of groove and hollowness of the infraorbital region and clinical treatment using soft‐tissue filler. Arch Plast Surg. 2018;45(3):214‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bosniak S, Cantisano‐Zilkha M, Purewal BK, Torres JJ, Rubin M, Remington K. Defining the tear trough. Ophthalmic Plast Reconstr Surg. 2007;23(3):254‐255. [DOI] [PubMed] [Google Scholar]
- 23. Naik MN. Hills and valleys: understanding the under‐eye. J Cutan Aesthet Surg. 2016;9(2):61‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Turkmani MG. New classification system for tear trough deformity. Dermatol Surg. 2017;43(6):836‐840. [DOI] [PubMed] [Google Scholar]
- 25. Hirmand H. Anatomy and nonsurgical correction of the tear trough deformity. Plast Reconstr Surg. 2010;125(2):699‐708. [DOI] [PubMed] [Google Scholar]
- 26. Peng PH, Peng JH. Treating the tear trough: a new classification system, a 6‐step evaluation procedure, hyaluronic acid injection algorithm, and treatment sequences. J Cosmet Dermatol. 2018;17(3):333‐339. [DOI] [PubMed] [Google Scholar]
- 27. Roberts WE. Periorbital hyperpigmentation: review of etiology, medical evaluation, and aesthetic treatment. J Drugs Dermatol. 2014;13(4):472‐482. [PubMed] [Google Scholar]
- 28. Sadick NS, Bosniak SL, Cantisano‐Zilkha M, Glavas IP, Roy D. Definition of the tear trough and the tear trough rating scale. J Cosmet Dermatol. 2007;6(4):218‐222. [DOI] [PubMed] [Google Scholar]
- 29. Teoxane . Ficha técnica e instrucciones de Teosyal Puresense Redensity II. Disponible en: http://teoxane.com/sites/default/files/notices/Teosyal%20Redensity/Teosyal%20PureSense%20Redensity%20II.pdf. Acces date.
- 30. Vedamurthy M. Beware what you inject: complications of injectables‐dermal fillers. J Cutan Aesthet Surg. 2018;11(2):60‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Minno A, Frigerio B, Spadarella G, et al. Old and new oral anticoagulants: Food, herbal medicines and drug interactions. Blood Rev. 2017;31(4):193‐203. [DOI] [PubMed] [Google Scholar]
- 32. Cohen JL, Bhatia AC. The role of topical vitamin K oxide gel in the resolution of postprocedural purpura. J Drugs Dermatol. 2009;8(11):1020‐1024. [PubMed] [Google Scholar]
- 33. Karavani I. How vitamin K gels treat postoperative bruising. Body. Language. 2004;6:14‐15. [Google Scholar]
- 34. King M. The management of bruising following nonsurgical cosmetic treatment. J Clin Aesthet Dermatol. 2017;10(2):E1‐E4. [PMC free article] [PubMed] [Google Scholar]
- 35. Urdiales‐Gálvez F, Delgado NE, Figueiredo V, et al. Treatment of soft tissue filler complications: expert consensus recommendations. Aesthetic Plast Surg. 2018;42(2):498‐510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ardeleanu V, Berbece S, Petre Florescu I, Jecan C. The use of hyaluronic acid combined with teosyal redensity ii for the treatment of dark circles under the eyes. Mater Plast. 2017;54(1):37‐40. [Google Scholar]
- 37. Guisantes E, Beut J. Linfáticos faciales: cómo prevenir el linfedema en los rellenos del surco de la ojera. Eur Aesth Plast Surg J. 2015;5(1):36‐43. [Google Scholar]
- 38. Ribé SN. Relleno de ojeras con ácido hialurónico In: Rubi Sánchez MA, Hernández Lobertini CM, eds. Medicina Estética. Claves Abordajes y Tratamientos Actuales. Madrid, Spain: Formación Alcalá; 2017. [Google Scholar]
- 39. Randlanski RJ, La WKH. Cara ‐ Atlas Ilustrado de Anatomía Clínica (1a edn). (español) ed: Quintessence Publishing; 2018. 354 p.
- 40. Pessa JE, Garza JR. The malar septum: the anatomic basis of malar mounds and malar edema. Aesthet Surg J. 1997;17(1):11‐17. [DOI] [PubMed] [Google Scholar]
- 41. Gierloff M, Stohring C, Buder T, Wiltfang J. The subcutaneous fat compartments in relation to aesthetically important facial folds and rhytides. J Plast Reconstr Aesthet Surg. 2012;65(10):1292‐1297. [DOI] [PubMed] [Google Scholar]
- 42. Rohrich RJ, Arbique GM, Wong C, Brown S, Pessa JE. The anatomy of suborbicularis fat: implications for periorbital rejuvenation. Plast Reconstr Surg. 2009;124(3):946‐951. [DOI] [PubMed] [Google Scholar]
- 43. Hufschmidt K, Bronsard N, Foissac R, et al. The infraorbital artery: Clinical relevance in esthetic medicine and identification of danger zones of the midface. J Plast Reconstr Aesthet Surg. 2019;72(1):131‐136. [DOI] [PubMed] [Google Scholar]
- 44. Shoukath S, Taylor GI, Mendelson BC, et al. The lymphatic anatomy of the lower eyelid and conjunctiva and correlation with postoperative chemosis and edema. Plast Reconstr Surg. 2017;139(3):628e‐e637. [DOI] [PubMed] [Google Scholar]
- 45. Glogau RG. Physiologic and structural changes associated with aging skin. Dermatol Clin. 1997;15(4):555‐559. [DOI] [PubMed] [Google Scholar]
- 46. Sobel RK, Carter KD, Allen RC. Periorbital edema: a puzzle no more? Curr Opin Ophthalmol. 2012;23(5):405‐414. [DOI] [PubMed] [Google Scholar]
- 47. Morley AM, Malhotra R. Use of hyaluronic acid filler for tear‐trough rejuvenation as an alternative to lower eyelid surgery. Ophthalmic Plast Reconstr Surg. 2011;27(2):69‐73. [DOI] [PubMed] [Google Scholar]
- 48. Berros P. Periorbital contour abnormalities: hollow eye ring management with hyalurostructure. Orbit. 2010;29(2):119‐125. [DOI] [PubMed] [Google Scholar]


